Introduction
Ischemia/reperfusion (IR) injury occurs when the
cellular damage of a hypoxic organ is increased subsequent to the
restoration of oxygen delivery. Warm IR injury may arise clinically
in hepatic surgery, liver transplantation, hypovolemic shock and
various types of toxic liver injury. The common denominator of the
aforementioned conditions is the ‘low-flow’ state induced by liver
hypoxia, which may lead to ischemic hepatitis. The Pringle maneuver
has been established as the occlusion of the hepatic artery and the
portal vein by cross-clamping the porta hepatis with a vascular
clamp. It is frequently used to control bleeding during the repair
of extensive liver wounds, hepatic resection and liver
transplantation. However, this may result in hepatic IR injury, in
the event that the clamping the hepatic vasculature is performed
for a long period of time.
Two distinct phases of liver injury have been
identified following warm IR injury. The initial phase (<2 h
after reperfusion) is characterized by oxidant stress, which
directly results in hepatocellular injury. The late phase (6–48 h
after hepatic reperfusion) is an inflammatory disorder mediated by
recruited neutrophils (1).
Cellular hypoxia has been identified as the key
factor that leads to the activation of the transcriptional
regulator, nuclear factor-κB (NF-κB), and triggers the release of
other inflammatory mediators, including activated Kupffer cells and
neutrophils, tumor necrosis factor-α (TNF-α), interleukin (IL)-1
and nitric oxide (NO), possibly in order to mediate the reperfusion
injury process (2,3). The production of inflammatory
mediators following the activation of Kupffer cells has been
determined to be an important component for the pathophysiology of
neutrophil-mediated injury during liver IR (4–6). In
addition, previous studies have identified that apoptosis in
hepatocytes is increased, and a decline in their regeneration
during hepatic IR injury has been observed (4,7).
The importance of apoptotic cell death in hepatic IR
has been investigated previously. According to previous studies,
40–60% of hepatocytes may undergo apoptosis during IR (8–10).
Ischemic preconditioning is a technique in which
organs are exposed to brief periods of IR in order to prepare an
organ for the subsequent effects of a longer period of IR exposure.
Initially, it was used for coronary ischemia and protection against
myocardial infarction; however, at present, it used in various
organs, including the brain, intestine, skeletal muscle and liver
(11). The underlying
cytoprotective mechanisms remain to be elucidated and may include
an increase in the adenosine levels in the cell microenvironment, a
decrease in NO levels, a reduction in TNF-α release, changes in
energy metabolism and accelerated cell-cycle progression (12).
Previous studies that conducted experimental models
of partial liver IR injury by 5–15 min of ischemia followed by 10
min of reperfusion led to hepatoprotective conditions, as a
significant reduction in areas of hepatocyte necrosis occurred and
the increase in transaminase levels was limited (13,14).
A previous study determined that this narrowed hepatoprotective
time window for ischemia is defined by the relative tissue
concentrations of adenosine and xanthine (15). Additionally, purinergic signaling
may have a protective role during hepatic IR injury (16).
Adenosine accumulates in the extracellular space
following ischemia by binding to selective G-protein-associated
membrane receptors, termed A1, A2A,
A2B and A3, and has been established to
promote cytoprotection. In a model of IR injury in mice, adenosine
administration was observed to induce a protective effect on the
liver (17). Additionally, the
activation of the A3 adenosine receptor
(A3AR) has been identified to exert cardioprotection,
neuroprotection and pulmonoprotection against IR injury (18–22).
Upon activation of the A3AR, specific
signal transduction pathways are modulated, including the Wnt and
NF-κB signaling pathways (23,24).
The activation of A3AR in inflammatory cells and tissues
may lead to deregulation of the NF-κB signaling pathway and may
result in reduced expression levels of phosphoinositide 3-kinase
(PI3K), protein kinase B/Akt (PKB/Akt), IκB kinase, IκB and NF-κB,
resulting in an anti-inflammatory effect (24–26).
Additionally, apoptosis of inflammatory cells was observed
downstream of PKB/Akt modulation, leading to upregulation of
caspase 3 expression levels (27).
It is of note that activation of the A3AR
resulted in increased differentiation of tumor cells compared with
normal cells (28). CF102, a
selective agonist to A3AR, induced the differentiation
of normal and tumor cells and inhibited their proliferation, along
with the induction of apoptosis in inflammatory cells, whereas it
had a protective effect on normal cells (29).
Based on the aforementioned previous studies, the
present study aimed to determine the ability of CF102 to protect
the liver against IR injury.
Materials and methods
Reagents
The A3AR agonist, CF102, a compound also termed
2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyl-uronamide
(Cl-IB-MECA),was synthesized for Can-Fite BioPharma (Petah Tikva,
Israel) by Albany Molecular Research, Inc. (Albany, NY, USA). A
stock solution of 5 mg/ml was prepared in dimethylsulphoxide and
further diluted in phosphate-buffered saline (PBS).
Animals
Animal handling was conducted according to the
guidelines of the National Institutes of Health (Bethesda, MD, USA)
and the Association for Assessment and Accreditation of Laboratory
Animal Care (Frederick, MD, USA).
Male Wistar rats (n=76; age, 10 weeks; Harlan
Laboratories, Inc., Jerusalem, Israel) were used in the present
study. The body weights of the rats ranged from 275 to 300 g. The
animals were provided with a nutritionally balanced rodent diet and
water ad libitum. They were maintained under constant
environmental conditions, temperatures range was 18–24°C and
relative humidity range was 30–70% with 12 h light and 12 h dark
cycle. The present study was performed following approval by the
Can-Fite Biopharma, Institutional Ethic committee (Petah Tikva,
Israel), and in compliance with the Israel Animal Welfare Act,
approval nos. 029_a123 and 029_a128.
IR injury procedure
A total of 28 male Wistar rats (275–300 g) were used
for the IR injury procedure in the following groups: i) Naïve
(n=4), laparatomy was performed without IR procedure; ii) control
(n=12) no treatment or surgery; and iii) CF102 (n=12) laparatomy
and IR were performed, followed by treatment with CF102. Rats were
fasted 12 h prior to surgery, then anesthetized with ketamine (90
mg/kg) and xylazine (10 mg/kg). Laparatomy was performed via a
subcostal, bilateral incision. The main portal pedicle to the total
liver was clamped for a period of 30 min, and the blood flow was
restored by de-clamping. Subsequently, the rats were injected
subcutaneously with 100 µg/kg CF102 (in 0.1 ml PBS) during
reperfusion, and the compound was orally administered three times
per day thereafter. The control rats received normal saline of the
identical volume. All animals received 5 ml 5% glucose and 0.2 ml
penicillin subcutaneously immediately following the operation.
Postoperatively, the animals were maintained on a standardized
pelleted diet and were supplied with water ad libitum. The
present study was conducted over the course of 48 h.
The rats were anesthetized using isoflurane
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) 48 h after the
operation, 2 ml blood samples were collected from the inferior vena
cava and were centrifuged at 24, 000 × g for 15 min at 25°C. The
supernatant was collected and serum aspartate aminotransferase
(SGOT) and alanine aminotransferase (SGPT) levels were analyzed in
the Medical Center Laboratories (Herzelia, Israel) to evaluate the
extent of liver injury. The rats were sacrificed using
CO2 in a gas chamber. The livers were removed and fixed
in 10% buffered formalin for pathological analysis and
staining.
Partial hepatectomy
A total of 48 male Wistar rats (300–350 g) were used
in the present study: The control group contained 22 individuals,
the CF102 group had 22 and the naive group had 4 rats. Rats were
fasted 12 h prior to surgery and anesthetized by intraperitoneal
injection of ketamine (45 mg/kg) and xylazine (5 mg/kg). Laparatomy
was performed via a subcostal, bilateral incision. The primary
portal pedicle to the liver was identified and clamped prior to the
hepatectomy procedure. A period of 10 min total liver ischemia was
initiated, during which a 70% hepatectomy was performed. This was
achieved by resection of the median and left lateral lobes,
according to the technique previously described by Higgins and
Anderson (30). Following 10 min
of ischemia and completion of 70% hepatectomy, blood flow to the
remaining liver was restored by de-clamping. All animals received 5
ml 5% glucose and 0.2 ml penicillin subcutaneously immediately
following the operation. Postoperatively, the animals were
maintained on a standardized pelleted diet and were supplied with
tap-water. Rats were injected subcutaneously with 100 µg/kg CF102
(in 0.1 ml PBS) during reperfusion, and the compound was
administered orally three times per day thereafter. The control
rats received normal saline at the identical volume. At 2, 4 and 48
h after surgery, rats were anesthetized with isoflurane and blood
samples (2 ml) were collected from the rats' tails for analysis of
SGOT and SGPT levels. Rats were sacrificed using CO2
after 24 and 48 h, and the liver tissues were resected and weighed
in order to calculate the hepatic regeneration rate, with
subsequent fixing in 10% buffered formalin for further pathological
analysis and staining.
Immunohistochemical staining and
histology
Liver tissue specimens were fixed in 10% formalin,
embedded in paraffin and cut into 5 mm thick sections.
Hematoxylin and eosin staining was performed in
order to obtain a mitotic index (MI) by dividing the number of
cells undergoing mitosis by the total cell number in 30 high-power
fields. Additionally, the presence of hepatic injury and necrosis
was also observed.
Apoptosis was examined by observation of DNA
fragmentation using the ApopTag Peroxidase in Situ Detection kit
(Chemicon; EMD Millipore, Billerica, MA, USA) for a terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
following the manufacturer's protocol. Next, the sections were
deparaffinized, re-hydrated and endogenous peroxidase was quenched
with 20% hydrogen peroxidase in methanol for 20 min. The slides
were then pretreated with proteinase K (BioVision, Inc., Milpitas,
CA, USA) for 15 min at room temperature, and treated with an
equilibration buffer for 10 s and terminal deoxynucleotidyl
transferase enzyme (Sigma-Aldrich; Merck Millipore) for 1 h.
Sections were incubated with an anti-digoxigenin conjugated
antibody (cat. no. ab119345; 1:1,000; Abcam, Cambridge, UK), washed
with PBS and developed with 3,3′-diaminobenzidine, counterstained
with hematoxylin and eosin and then mounted.
To calculate the proliferation cell nuclear antigen
(PCNA) labeling index, 5 mm sections were incubated with anti-PCNA
antibody (cat. no. ab29; 1:1,000; Abcam). The proliferation of
hepatocytes was calculated as the number of cells in the S-phase.
PCNA-positive cells exhibited brownish-yellow or yellow nuclei. If
brown particles were observed in the nucleus, the cells were
considered to be positive.
Hepatic regeneration rate
The growth of residual liver lobes was assessed
using the following equation: Hepatic regeneration rate (%) =
C-(A-B) / A ×100, where A is the estimated total liver weight prior
to the hepatectomy (A was considered to be 3.4% of a rat's total
weight) (31), B is the weight of
the resected liver during the hepatectomy and C is the weight of
the regenerated liver at the final resection (32).
Statistical analysis
The results were evaluated using Student's t-test.
Comparisons between the mean values of the different experiments
were performed. Data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
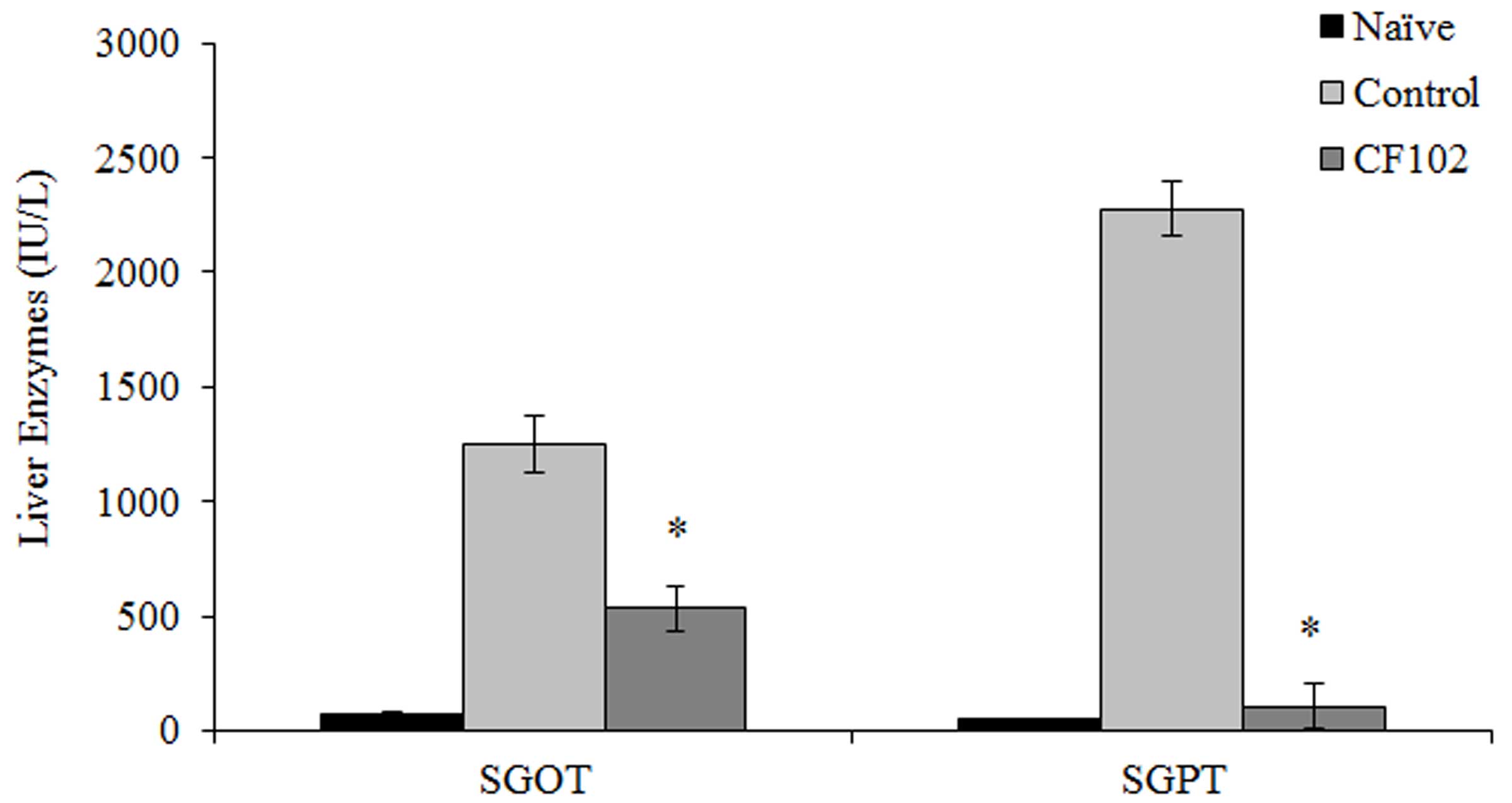
CF102 attenuates liver IR injury in
rats
In the present study, rats were exposed to 30 min of
ischemia. The serum levels of SGOT and SGPT, which may be used as a
proxy of the extent of liver damage, were significantly reduced in
the CF102 treatment group when compared with the control group 48 h
after surgery (P<0.05;Fig.
1).
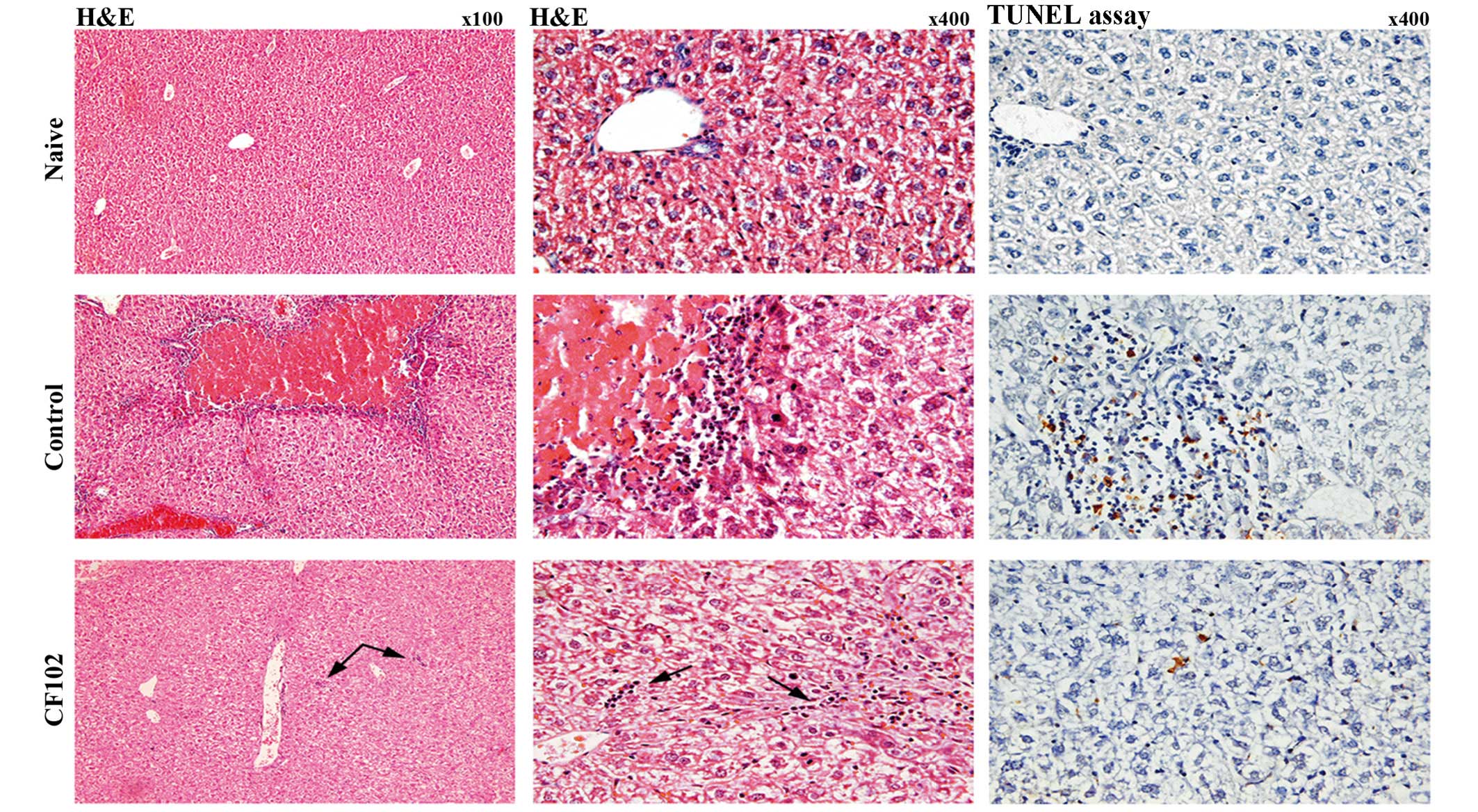
Pathological analyses were performed in order to
obtain additional qualitative assessment of liver injury.
Paraffin-embedded slides of liver tissues were stained by
hematoxylin and eosin 48 h after liver reperfusion. The upper panel
of Fig. 2 shows healthy
hepatocytes and no signs of necrosis or inflammation in the naïve
group, whereas large areas of necrosis surrounded by numerous
inflammatory cells and pyknotic nuclei were observed in liver
tissues from the control group (middle panel). By contrast, liver
tissues from the CF102-treated group did not exhibit areas of
necrosis, and only a few inflammatory cells were visible (lower
panel). These findings confirm that the A3AR agonist,
CF102, allowed for substantial protection against IR injury from
occurring (Fig. 2).
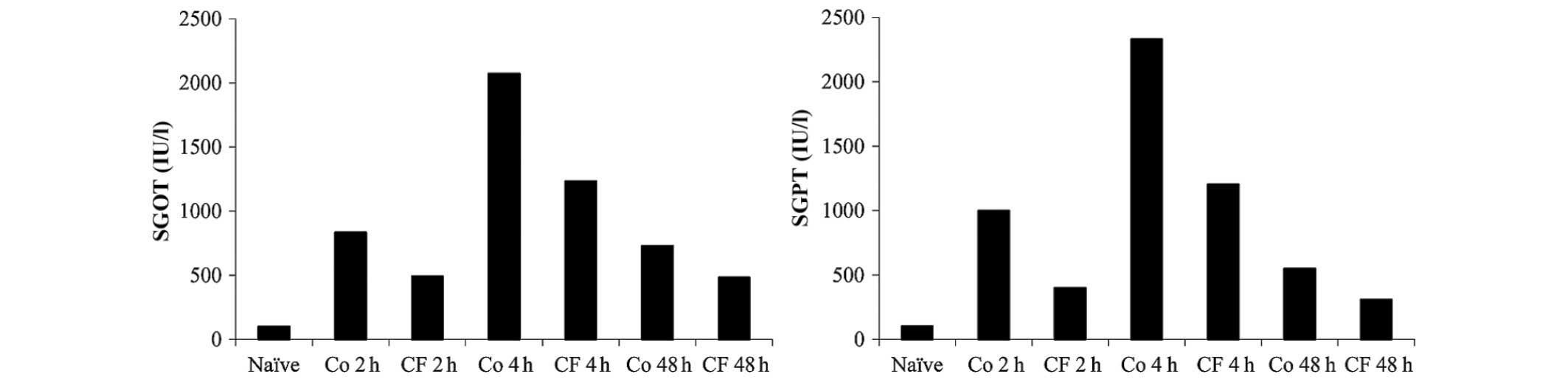
CF102 protects against IR injury in
hepatectomized rats
Liver hepatectomy was performed on 70% of the rat's
liver, whilst being exposed to 10 min of ischemia. Liver enzymic
analysis revealed a marked increase in the levels of SGOT and SGPT
2 h after the hepatectomy, which peaked at 4 h and started to
decrease after 48 h in the control group. CF102 treatment applied
following reperfusion induced a reduction in the levels of SGOT and
SGPT, which was maintained for 48 h (Fig. 3).
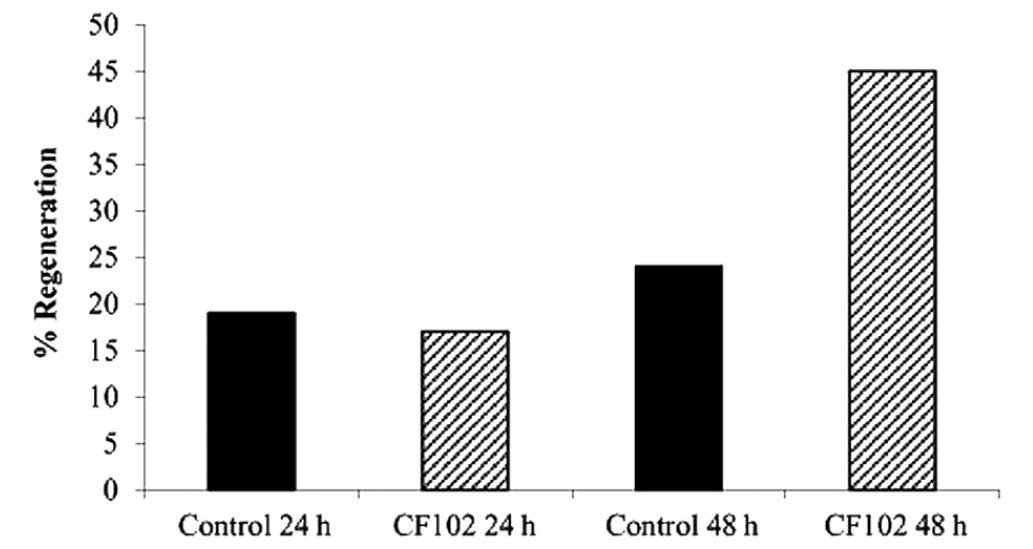
A3AR agonist enhances liver
regeneration following partial hepatectomy
Hepatic regeneration rates were higher in the CF102
treatment group compared with the saline-treated control group 48 h
after the hepatectomy (Fig. 4).
CF102 treatment increased the regeneration rate of the remnant
liver by 45% at 48 h following the 70% hepatectomy (Fig. 4). Additionally, in the CF102
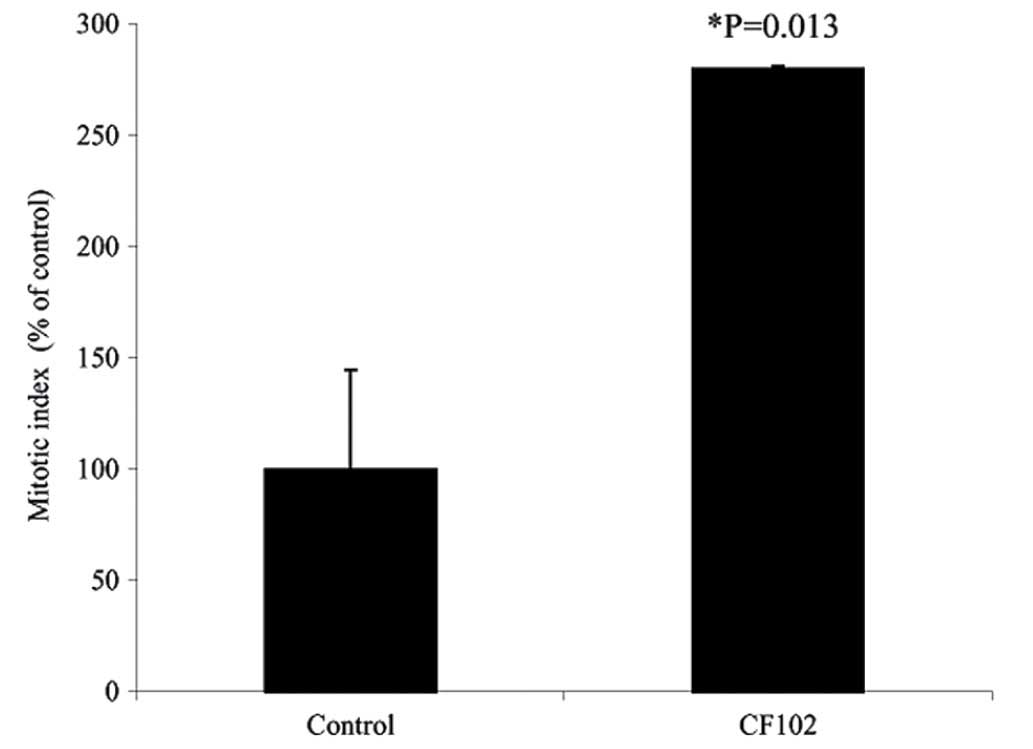
treatment group, the PCNA labeling index (Fig. 5) and the MI (P=0.013; Fig. 6) were higher compared with the
saline-treated control group.
Discussion
The present study determined that the selective
A3AR agonist, CF102, when administered during liver
reperfusion, may attenuate cellular injury, apoptosis and the
extent of necrosis in liver subjected to IR. Additionally, CF102
stimulated liver regeneration, which favored the survival of
hepatocytes during IR injury. A rat model combining 70% partial
hepatectomy with 10 min of Pringle maneuver was used, in order to
imitate the surgical procedure performed in humans. In this model,
30% of the liver remained, which was exposed to IR injury.
Following partial hepatectomy and liver reperfusion, serum levels
of SGOT and SGPT, which may be used as a proxy of liver damage,
were reduced in the CF102 treatment group when compared with the
control group at 2, 4, and 48 h after the hepatectomy. Reduced
liver necrosis and cell apoptosis were observed in the remaining
liver obtained from the CF102-treated rats 48 h after the operation
when compared with the control group. It is of note that the
remnant liver in the CF102-treated rats exhibited a 45% increase in
the hepatic regeneration rate when compared with the untreated
control group rats. This was confirmed, as the MI and the PCNA
labeling index were higher in the CF102 treatment group compared
with the control (Figs. 5 and
6). Therefore, CF102, a highly
selective A3AR agonist with high receptor affinity, may
be able to protect against liver IR injury at low concentrations.
Previous studies have extensively reported that A3AR may
be used as a target to mediate different protective functions,
including neuroprotection, chemoprotection and cardioprotection
(21,33,34).
Auchampach et al (33)
revealed that IB-MECA (CF102), administered at a concentration of
100 µg/kg intravenously 5 min prior to the onset of reperfusion,
reduced the size of myocardial infarct in dogs (33). Similarly, 2-CL-IB-MECA administered
during reperfusion reduced infarct size in isolated rat hearts and
prevented apoptosis in isolated rat cardiomyocytes (35). The PI3K-PKB/Akt signal transduction
pathway was identified to be responsible for IB-MECA-induced
glycogen synthase kinase-3b inactivation, which is considered to be
involved in cardioprotection (15).
Inhibition of the apoptotic response to IR injury in
the lungs by the activation of A3AR has also been
previously described (36). Rivo
et al (36) have
established a spontaneously breathing cat model where the
administration of IB-MECA (300 µg/kg) during reperfusion markedly
attenuated indices of injury and apoptosis (36). Adenosine has been established as a
regulator of cell viability (37),
and A3AR has been specifically identified to contribute
to the modulation of cell survival. Activation of A3AR
may induce cell protection or death, depending on the degree of
receptor activation (37,38).
Activation of the A3AR has also been
described to trigger neuroprotection. Chen et al (21) have determined that, when A3R
knockout mice are treated with a high dose of CL-IB-MECA (0.2
mg/kg), reduced cerebral infarction through the activation of
A3AR and suppression of apoptosis occurred (21).
Apoptosis has been implicated in the pathophysiology
of IR liver injury (39); however,
an association with A3AR activation remains to be
described.
The present study determined that treatment with
CF102 accelerated liver regeneration following a 70% hepatectomy
and IR injury. Zhang et al (40) have determined that, in a
regenerative state, the liver was protective against carbon
tetrachloride-induced hepatotoxicity via a mechanism involving
increased mitochondrial respiratory activity and plasma membrane
fluidity (40).
Our previous study determined that activation of
A3AR initiates a molecular mechanism that involves the
deregulation of the PI3K-NF-κB signaling pathway, which results in
an effective anti-inflammatory effect (26). Therefore, this may be the possible
mechanism through which CF102 exerts its hepatoprotection against
IR injury, as the late phase of IR liver injury involved an
inflammatory process.
In conclusion, the ability of CF102, an
A3AR agonist, to protect against hepatic IR injury, may
include an anti-inflammatory and an anti-apoptotic effect, combined
with an increased rate of liver regeneration. However, further
studies should be conducted in order to elucidate the molecular
basis for these clinically important effects of CF102.
Glossary
Abbreviations
Abbreviations:
|
A3AR
|
A3 adenosine receptor
|
|
IL-1
|
interleukin 1
|
|
I/R
|
ischemia-reperfusion injury
|
|
NF-κB
|
nuclear factor-κB
|
|
NO
|
nitric oxide
|
|
PKB/Akt
|
protein kinase B/Akt
|
|
PCNA
|
proliferation cell nuclear antigen
|
|
SGOT
|
aspartate aminotransferase
|
|
SGPT
|
alanine aminotransferase
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Lentsch AB, Atsushi K, Yoshidome H,
MacMasters KM and Edwards MJ: Inflammatory mechanisms and
therapeutic strategies for warm hepatic ischemia/reperfusion
injury. Hepatology. 32:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological response to hypoxia. J Appl
Physiol (1985). 88:1474–1480. 2000.PubMed/NCBI
|
|
3
|
Li C and Jackson RM: Reactive species
mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol
Cell Physiol. 282:C227–C241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jaeschke H: Mechanisms of reperfusion
injury after warm ischemia of the liver. J Hepatobiliary Pancreat
Surg. 5:402–408. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okuaki Y, Miyazaki H, Zeniya M, Ishikawa
T, Ohkawa Y, Teuno S, Sakaguchi M, Hara M, Takahashi H and Toda G:
Splenectomy-reduced hepatic injury induced by ischemia/reperfusion
in the rat. Liver. 16:188–194. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zwacka RM, Zhang Y, Halldorson J,
Schlossberg H, Dudus L and Ehgelhardt JF: CD4(+)
T-lymphocytes mediate ischemia/reperfusion-induced inflammatory
responses in mouse liver. J Clin Invest. 100:279–289. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu P, McGuire GM, Fischer MA, Farhood A,
Smith CW and Jaeschke H: Activation of Kupffer cells and
neutrophils for reactive oxygen formation is responsible for
endotoxin-enhanced liver injury after hepatic ischemia. Shock.
3:56–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao W, Bentely RC, Madden JF and Clavien
PA: Apoptosis of sinusoidal endothelial cells is a critical
mechanism of prevention of injury in rat liver transplantation.
Hepatology. 27:1652–1660. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cursio R, Gugenheim J, Ricci JE, Crenesse
D, Rostagno P, Maulon L, Saint-Paul MC, Ferrua B and Auberger AP: A
caspase inhibitor fully protects rats against lethal normothermic
liver ischemia by inhibition of liver apoptosis. FASEB J.
13:253–261. 1999.PubMed/NCBI
|
|
10
|
Kohli V, Selzner M, Madden JF, Bentley RC
and Clavien PA: Endothelial cell and hepatocyte deaths occur by
apoptosis after ischemia-reperfusion injury in the rat liver.
Transplantation. 67:1099–1105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murry CE, Jenning RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teoh NC and Farrell GC: Hepatic ischemia
reperfusion injury: Pathogenic mechanisms and basis for
hepatoprotection. J Gastroenterol Hepatol. 18:891–902. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peralta C, Closa D, Xaus C, Gelpi E,
Roselló-Catafau J and Hotter G: Hepatic preconditioning in rats is
defined by a balance of adenosine and xanthine. Hepatology.
28:768–773. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teoh N, Pena A Dela and Farrel G: Hepatic
ischemia preconditioning in mice is associated with activation of
NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 36:94–102.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gross ER, Hsu AK and Gross GJ:
Opioid-induced cardioprotection occurs via glycogen synthase kinase
beta inhibition during reperfusion in intact rat hearts. Circ Res.
94:960–966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaughn BP, Robson SC and Longhi MS:
Purinergic signaling in liver disease. Dig Dis. 32:516–24. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun X, Imai M, Nowak-Machen M,
Guckelberger O, Enjyoj K, Wu Y, Khalpey Z, Berberat P, Munasinghe J
and Robson SC: Liver damage and systemic inflammatory responses are
exacerbated by the genetic deletion of CD39 in total hepatic
ischemia. Purinergic Signal. 7:427–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Black RG Jr, Guo Y, Ge ZD, Murphree SS,
Prabhu SD, Jones WK, Bolli R and Auchampach JA: Gene
dosage-dependent effects of cardiac-specific overexpression of the
A3 adenosine receptor. Circ Res. 91:165–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cross HR, Murphy E, Black RG, Auchampach J
and Steenbergen C: Overexpression of A(3) adenosine receptors
decreases heart rate, preserves energetics, and protects ischemic
hearts. Am J Physiol Heart Circ Physiol. 283:H1562–H1568. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matot I, Weiniger CF, Zeira E, Galun E,
Joshi BV and Jacobson KA: A3 adenosine receptors and
mitogen-activated protein kinases in lung injury following in vivo
reperfusion. Crit Care. 10:R652006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen GJ, Harvey BK, Shen H, Chou J, Victor
A and Wang Y: Activation of A3 adenosine receptors reduces ischemic
brain injury in rodents. J Neurosci Res. 84:1848–1855. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borea PA, Varani K, Vincenzi F, Baraldi
PG, Tabrizi MA, Merighi S and Gessi S: The A3 adenosine receptor:
History and perspectives. Pharmacol Rev. 67:74–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fishman P, Bar-Yehuda S, Ohana G, Barer F,
Ochaion A, Erlanger A and Madi L: An agonist to the A3 adenosine
receptor inhibits colon carcinoma growth in mice via modulation of
GSK-3 beta and NF-kappa B. Oncogene. 23:2465–2471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fishman P, Madi L, Bar-Yehuda S, Barer F,
Del Valle L and Khalili K: Evidence for involvement of Wnt
signaling pathway in IB-MECA mediated suppression of melanoma
cells. Oncogene. 21:4060–4064. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanada T and Yoshimura A: Regulation of
cytokine signaling and inflammation. Cytokine Growth Factor Rev.
13:413–421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fishman P, Bar-Yehuda S, Madi L,
Rath-Wolfson L, Ochaion A, Cohen S and Baharav E: The PI3K-NF-kappa
B signal transduction pathway is involved in mediating the
anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis.
Arthritis Res Ther. 8:R332006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shackelford RE, Alford BP, Xue Y, Thai SF,
Adams DO and Pizzo S: Aspirin inhibits tumor necrosis factor-alpha
gene expression in murine tissue macrophages. Mol Pharmacol.
52:421–429. 1997.PubMed/NCBI
|
|
28
|
Ohana G, Bar-Yehuda S, Barer F and Fishman
P: Differential effect of adenosine on tumor and normal cell
growth: Focus on the A3 adenosine receptor. J Cell Physiol.
186:19–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cohen S, Stemmer SM, Zozulya G, Ochaion A,
Patoka R, Barer F, Bar-Yehuda S, Rath-Wolfson L, Jacobson KA and
Fishman P: CF102 an A3 adenosine receptor agonist mediates
anti-tumor and anti-inflammatory effects in the liver. J Cell
Physiol. 226:2438–2447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higgins G and Anderson R: Experimental
pathology of the liver: restoration of the liver of the white rat
following partial surgical removal. Arch Pathol. 12:186–202.
1931.
|
|
31
|
Kogure K, Zhang YQ, Shibata H and Kojima
I: Immediate onset of DNA synthesis in remnant rat liver after 90%
hepatectomy by administration of follistatin. J Hepatol.
29:977–984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Selzner M and Clavien PA: Failure of
regeneration of the steatotic rat liver: Disruption at two
different levels in the regeneration pathway. Hepatology. 31:35–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Auchampach JA, Ge ZD, Wan TC, Moore J and
Gross GJ: A3 adenosine receptor agonist IB-MECA reduces myocardial
ischemia-reperfusion injury in dogs. Am J Physiol Heart Circ
Physiol. 285:H607–H613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fishman P, Bar-Yehuda S, Barer F, Madi L,
Multani AS and Pathak S: The A3 adenosine receptor as a new target
for cancer therapy and chemoprotection. Exp Cell Res. 269:230–236.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maddock HL, Mocanu MM and Yellon DM:
Adenosine A(3) receptor activation protects the myocardium from
reperfusion/reoxygenation injury. Am J Physiol Heart Circ Physiol.
283:H1307–H1313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rivo J, Zeira E, Galun E and Matot I:
Activation of A3 adenosine receptors provides lung protection
against ischemia reperfusion injury associated with reduction in
apoptosis. Am J Transplant. 4:1941–1948. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jacobson KA, Hoffmann C, Cattabeni F and
Abbracchio MP: Adenosine-induced cell death: Evidence for
receptor-mediated signaling. Apoptosis. 4:197–211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abbracchio MP, Ceruti S, Brambilla R,
Franceschi C, Malorni W, Jacobson KA, von Lubitz DK and Cattabeni
F: Modulation of apoptsis by adenosine in the central nervous
system: A possible role for the A3 adenosine receptor.
Pathophysiological significance and therapeutic implications for
neurodegenerative disorders. Ann NY Acad Sci. 825:11–22. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borghi-Scoazec G, Scoazec JY, Durand F,
Bernuau J, Belghiti J, Feldmann G, Henin D and Degott C: Apoptosis
after ischemia-reperfusion in human liver allografts. Liver Transpl
Surg. 3:407–415. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang BH, Gong DZ and Mei MH: Protection
of regenerating liver after partial hepatectomy from carbon
tetrachloride hepatotoxicity in rats: Role of hepatic stimulator
substance. J Gastroenterol Hepatol. 14:1010–1017. 1999. View Article : Google Scholar : PubMed/NCBI
|