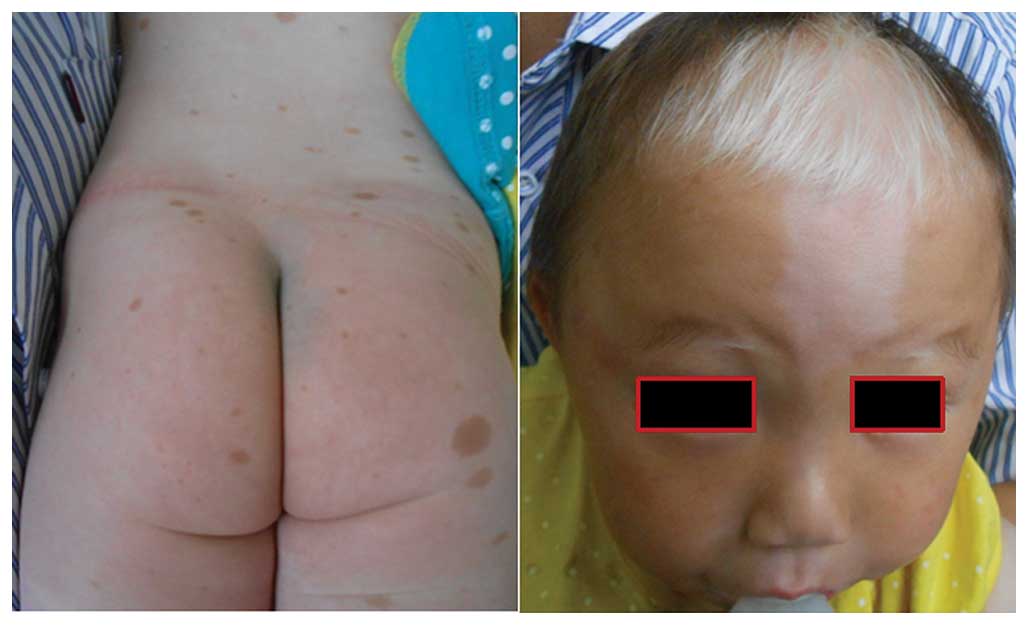
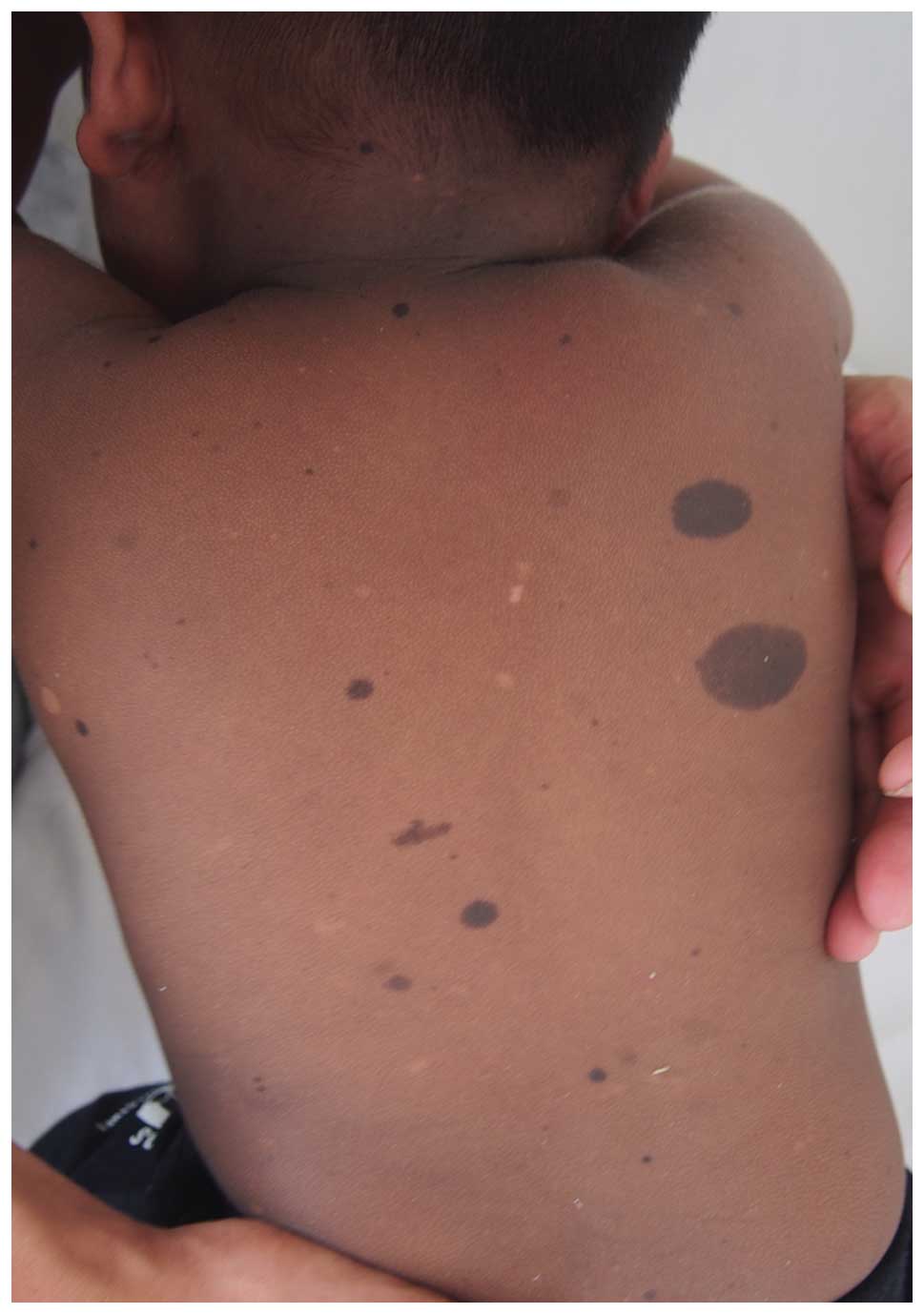
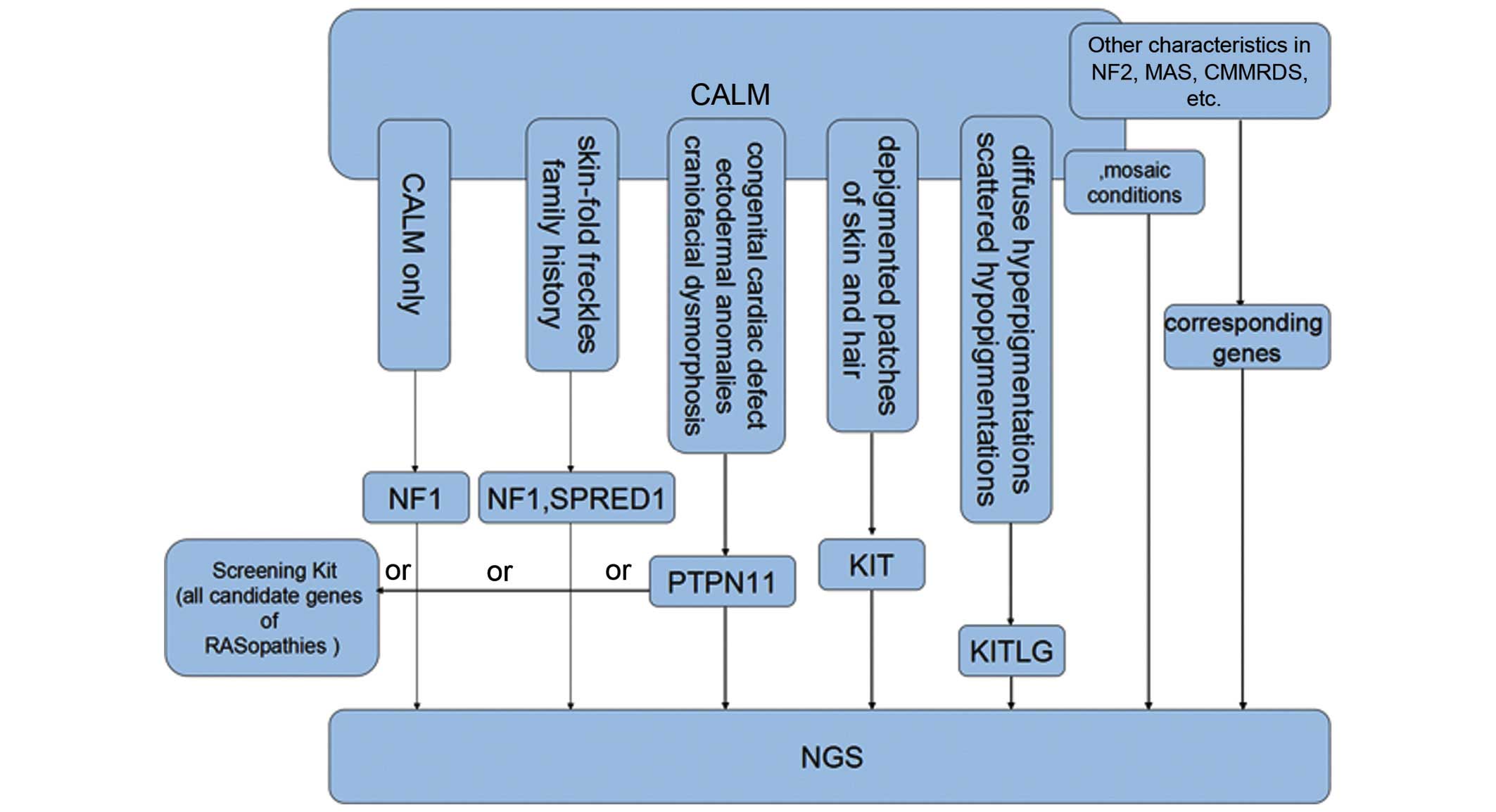
|
1
|
Messiaen LM, Callens T, Mortier G, Beysen
D, Vandenbroucke I, Van Roy N, Speleman F and Paepe AD: Exhaustive
mutation analysis of the NF1 gene allows identification of 95% of
mutations and reveals a high frequency of unusual splicing defects.
Hum Mutat. 15:541–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valero MC, Martin Y, Hernández-Imaz E,
Marina Hernández A, Meleán G, Valero AM, Javier Rodríguez-Álvarez
F, Tellería D and Hernández-Chico C: A highly sensitive genetic
protocol to detect NF1 mutations. J Mol Diagn. 13:113–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Picardo M and Cardinali G: The genetic
determination of skin pigmentation: KITLG and the KITLG/c-Kit
pathway as key players in the onset of human familial pigmentary
diseases. J Invest Dermatol. 131:1182–1185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Tong H, Fu X, Zhang Y, Liu J,
Cheng R, Liang J, Peng J, Sun Z, Liu H, et al: Molecular
characterization of NF1 and neurofibromatosis type 1
genotype-phenotype correlations in a Chinese population. Sci Rep.
5:112912015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Cheng R, Liang J, Ni C, Li M and
Yao Z: Lentiginous phenotypes caused by diverse pathogenic genes
(SASH1 and PTPN11): Clinical and molecular discrimination. Clin
Genet. Feb 3–2016.Epub ahead of print. View Article : Google Scholar
|
|
6
|
Zhang J, Cheng R, Liang J, Ni C, Li M and
Yao Z: Report of a sporadic familial progressive hyper- and
hypopigmentation child caused by a novel KITLG mutation. Br J
Dermatol. Apr 23–2016.Epub ahead of print. View Article : Google Scholar
|
|
7
|
Messiaen L, Yao S, Brems H, Callens T,
Sathienkijkanchai A, Denayer E, Spencer E, Arn P,
Babovic-Vuksanovic D, Bay C, et al: Clinical and mutational
spectrum of neurofibromatosis type 1-like syndrome. JAMA.
302:2111–2118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brems H, Pasmant E, Van Minkelen R, Wimmer
K, Upadhyaya M, Legius E and Messiaen L: Review and update of
SPRED1 mutations causing Legius syndrome. Hum Mutat. 33:1538–1546.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brems H and Legius E: Legius syndrome, an
update. Molecular pathology of mutations in SPRED1. Keio J Med.
62:107–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
[No authors lsited]: Neurofibromatosis.
Conference statement. National Institutes of Health Consensus
Development Conference, . Arch Neurol. 45:575–578. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brems H, Chmara M, Sahbatou M, Denayer E,
Taniguchi K, Kato R, Somers R, Messiaen L, De Schepper S, Fryns JP,
et al: Germline loss-of-function mutations in SPRED1 cause a
neurofibromatosis 1-like phenotype. Nat Genet. 39:1120–1126. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasmant E, Gilbert-Dussardier B, Petit A,
de Laval B, Luscan A, Gruber A, Lapillonne H, Deswarte C, Goussard
P, Laurendeau I, et al: SPRED1, a RAS MAPK pathway inhibitor that
causes Legius syndrome, is a tumour suppressor downregulated in
paediatric acute myeloblastic leukaemia. Oncogene. 34:631–638.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasmant E, Ballerini P, Lapillonne H,
Perot C, Vidaud D, Leverger G and Landman-Parker J: SPRED1 disorder
and predisposition to leukemia in children. Blood. 114:11312009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tartaglia M, Mehler EL, Goldberg R,
Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A,
Jeffery S, et al: Mutations in PTPN11, encoding the protein
tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet.
29:465–468. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conti E, Dottorini T, Sarkozy A, Tiller
GE, Esposito G, Pizzuti A and Dallapiccola B: A novel PTPN11
mutation in LEOPARD syndrome. Hum Mutat. 21:6542003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martínez-Quintana E and Rodríguez-González
F: LEOPARD Syndrome: Clinical features and gene mutations. Mol
Syndromol. 3:145–157. 2012.PubMed/NCBI
|
|
17
|
Wang Y, Chen C and Wang DW: Leopard
syndrome caused by heterozygous missense mutation of Tyr 279 Cys in
the PTPN11 gene in a sporadic case of Chinese Han. Int J Cardiol.
174:e101–e104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osawa R, Akiyama M, Yamanaka Y, Ujiie H,
Nemoto-Hasebe I, Takeda A, Yanagi T and Shimizu H: A novel PTPN11
missense mutation in a patient with LEOPARD syndrome. Brit J
Dermatol. 161:1202–1204. 2009. View Article : Google Scholar
|
|
19
|
Digilio MC, Conti E, Sarkozy A, Mingarelli
R, Dottorini T, Marino B, Pizzuti A and Dallapiccola B: Grouping of
multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11
gene. Am J Hum Genet. 71:389–394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida R, Hasegawa T, Hasegawa Y, Nagai
T, Kinoshita E, Tanaka Y, Kanegane H, Ohyama K, Onishi T, Hanew K,
et al: Protein-tyrosine phosphatase, nonreceptor type 11 mutation
analysis and clinical assessment in 45 patients with Noonan
syndrome. J Clin Endocrinol Metab. 89:3359–3364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tartaglia M, Martinelli S, Stella L,
Bocchinfuso G, Flex E, Cordeddu V, Zampino G, Burgt Iv, Palleschi
A, Petrucci TC, et al: Diversity and functional consequences of
germline and somatic PTPN11 mutations in human disease. Am J Hum
Genet. 78:279–290. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kontaridis MI, Swanson KD, David FS,
Barford D and Neel BG: PTPN11 (Shp2) mutations in LEOPARD syndrome
have dominant negative, not activating, effects. J Biol Chem.
281:6785–6792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motegi S, Yokoyama Y, Ogino S, Yamada K,
Uchiyama A, Perera B, Takeuchi Y, Ohnishi H and Ishikawa O:
Pathogenesis of multiple lentigines in LEOPARD syndrome with PTPN11
gene mutation. Acta Derm Venereol. 95:978–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishi E, Mizuno S, Nanjo Y, Niihori T,
Fukushima Y, Matsubara Y, Aoki Y and Kosho T: A novel heterozygous
MAP2K1 mutation in a patient with Noonan syndrome with multiple
lentigines. Am J Med Genet A 167A. 407–411. 2015. View Article : Google Scholar
|
|
25
|
Nava C, Hanna N, Michot C, Pereira S,
Pouvreau N, Niihori T, Aoki Y, Matsubara Y, Arveiler B, Lacombe D,
et al: Cardio-facio-cutaneous and Noonan syndromes due to mutations
in the RAS/MAPK signalling pathway: Genotype-phenotype
relationships and overlap with Costello syndrome. J Med Genet.
44:763–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allanson JE: Noonan syndrome. J Med Genet.
24:9–13. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Digilio MC, Sarkozy A, de Zorzi A, Pacileo
G, Limongelli G, Mingarelli R, Calabrò R, Marino B and Dallapiccola
B: LEOPARD syndrome: Clinical diagnosis in the first year of life.
Am J Med Genet A. 140:740–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carcavilla A, Pinto I, Muñoz-Pacheco R,
Barrio R, Martin-Frias M and Ezquieta B: LEOPARD syndrome (PTPN11,
T468 M) in three boys fulfilling neurofibromatosis type 1 clinical
criteria. Eur J Pediatr. 170:1069–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tartaglia M, Niemeyer CM, Fragale A, Song
X, Buechner J, Jung A, Hählen K, Hasle H, Licht JD and Gelb BD:
Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia,
myelodysplastic syndromes and acute myeloid leukemia. Nat Genet.
34:148–150. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rankin J, Short J, Turnpenny P, Castle B
and Hanemann CO: Medulloblastoma in a patient with the PTPN11
p.Thr468Met mutation. Am J Med Genet A 161A. 2027–2029. 2013.
View Article : Google Scholar
|
|
31
|
Hennekam RC: Costello syndrome: An
overview. Am J Med Genet Part C Semin Med Genet 117C. 42–48. 2003.
View Article : Google Scholar
|
|
32
|
Bryan ZT, Missall TA, Stieren S, Siegfried
E and Burkemper NM: Clinicopathologic evaluation of
cardiofaciocutaneous syndrome: Overcoming the challenges of
diagnosing a rare genodermatosis. Pediatr Dermatol. 32:e23–e28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alper J, Holmes LB and Mihm MC Jr:
Birthmarks with serious medical significance: Nevocellular nevi,
sebaceous nevi, and multiple café au lait spots. J Pediatr.
95:696–700. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siegel DH, McKenzie J, Frieden IJ and
Rauen KA: Dermatological findings in 61 mutation-positive
individuals with cardiofaciocutaneous syndrome. Brit J Dermatol.
164:521–529. 2011.
|
|
35
|
Zenker M, Lehmann K, Schulz AL, Barth H,
Hansmann D, Koenig R, Korinthenberg R, Kreiss-Nachtsheim M,
Meinecke P, Morlot S, et al: Expansion of the genotypic and
phenotypic spectrum in patients with KRAS germline mutations. J Med
Genet. 44:131–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fryer AE, Holt PJ and Hughes HE: The
cardio-facio-cutaneous (CFC) syndrome and Noonan syndrome: Are they
the same? Am J Med Genet. 38:548–551. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tidyman WE and Rauen KA: Noonan, Costello
and cardio-facio-cutaneous syndromes: Dysregulation of the Ras-MAPK
pathway. Expert Rev Mol Med. 10:e372008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bertola DR, Pereira AC, Brasil AS, Albano
LM, Kim CA and Krieger JE: Further evidence of genetic
heterogeneity in Costello syndrome: Involvement of the KRAS gene. J
Hum Genet. 52:521–526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niihori T, Aoki Y, Narumi Y, Neri G, Cavé
H, Verloes A, Okamoto N, Hennekam RC, Gillessen-Kaesbach G,
Wieczorek D, et al: Germline KRAS and BRAF mutations in
cardio-facio-cutaneous syndrome. Nat Genet. 38:294–296. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schubbert S, Zenker M, Rowe SL, Böll S,
Klein C, Bollag G, van der Burgt I, Musante L, Kalscheuer V, Wehner
LE, et al: Germline KRAS mutations cause Noonan syndrome. Nat
Genet. 38:331–336. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abe Y, Aoki Y, Kuriyama S, Kawame H,
Okamoto N, Kurosawa K, Ohashi H, Mizuno S, Ogata T, Kure S, et al:
Prevalence and clinical features of Costello syndrome and
cardio-facio-cutaneous syndrome in Japan: Findings from a
nationwide epidemiological survey. Am J Med Genet A 158A.
1083–1094. 2012. View Article : Google Scholar
|
|
42
|
Sarkozy A, Carta C, Moretti S, Zampino G,
Digilio MC, Pantaleoni F, Scioletti AP, Esposito G, Cordeddu V,
Lepri F, et al: Germline BRAF mutations in Noonan, LEOPARD, and
cardiofaciocutaneous syndromes: Molecular diversity and associated
phenotypic spectrum. Hum Mutat. 30:695–702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neri G and Zollino M: More on the
Noonan-CFC controversy. Am J Med Genet. 65:1001996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Luca A, Bottillo I, Sarkozy A, Carta C,
Neri C, Bellacchio E, Schirinzi A, Conti E, Zampino G, Battaglia A,
et al: NF1 gene mutations represent the major molecular event
underlying neurofibromatosis-Noonan syndrome. Am J Hum Genet.
77:1092–1101. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watson GH: Pulmonary stenosis,
café-au-lait spots, and dull intelligence. Arch Dis Child.
42:303–307. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Allanson JE, Upadhyaya M, Watson GH,
Partington M, MacKenzie A, Lahey D, MacLeod H, Sarfarazi M,
Broadhead W, Harper PS, et al: Watson syndrome: Is it a subtype of
type 1 neurofibromatosis? J Med Genet. 28:752–756. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Upadhyaya M, Shen M, Cherryson A, Farnham
J, Maynard J, Huson SM and Harper PS: Analysis of mutations at the
neurofibromatosis 1 (NF1) locus. Hum Mol Genet. 1:735–740. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tassabehji M, Strachan T, Sharland M,
Colley A, Donnai D, Harris R and Thakker N: Tandem duplication
within a neurofibromatosis type 1 (NF1) gene exon in a family with
features of Watson syndrome and Noonan syndrome. Am J Hum Genet.
53:90–95. 1993.PubMed/NCBI
|
|
49
|
Thiel C, Wilken M, Zenker M, Sticht H,
Fahsold R, Gusek-Schneider GC and Rauch A: Independent NF1 and
PTPN11 mutations in a family with neurofibromatosis-Noonan
syndrome. Am J Med Genet A 149A. 1263–1267. 2009. View Article : Google Scholar
|
|
50
|
Nyström AM, Ekvall S, Strömberg B,
Holmström G, Thuresson AC, Annerén G and Bondeson ML: A severe form
of Noonan syndrome and autosomal dominant café-au-lait
spots-evidence for different genetic origins. Acta Paediatr.
98:693–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Spritz RA, Itin PH and Gutmann DH:
Piebaldism and neurofibromatosis type 1: Horses of very different
colors. J Invest Dermatol. 122:xxxiv–xxxv. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Duarte AF, Mota A, Baudrier T, Morais P,
Santos A, Cerqueira R, Tavares P and Azevedo F: Piebaldism and
neurofibromatosis type 1: Family report. Dermatol Online J.
16:112010.PubMed/NCBI
|
|
53
|
Chiu YE, Dugan S, Basel D and Siegel DH:
Association of Piebaldism, multiple café-au-lait macules, and
intertriginous freckling: Clinical evidence of a common pathway
between KIT and sprouty-related, ena/vasodilator-stimulated
phosphoprotein homology-1 domain containing protein 1 (SPRED1).
Pediatr Dermatol. 30:379–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Amyere M, Vogt T, Hoo J, Brandrup F, Bygum
A, Boon L and Vikkula M: KITLG mutations cause familial progressive
hyper- and hypopigmentation. J Invest Dermatol. 131:1234–1239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oiso N, Fukai K, Kawada A and Suzuki T:
Piebaldism. J Dermatol. 40:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Spritz RA: Molecular basis of human
piebaldism. J Invest Dermatol. 103:(Suppl 5). S137–S140. 1994.
View Article : Google Scholar
|
|
57
|
Angelo C, Cianchini G, Grosso MG, Zambruno
G, Cavalieri R and Paradisi M: Association of piebaldism and
neurofibromatosis type 1 in a girl. Pediatr Dermatol. 18:490–493.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Duarte A, Mota A, Baudrier T, Morais P,
Santos A, Cerqueira R, Tavares P and Azevedo F: Piebaldism and
neurofibromatosis: State of knowledge. Dermatol Online J.
19:172013.PubMed/NCBI
|
|
59
|
Wang ZQ, Si L, Tang Q, Lin D, Fu Z, Zhang
J, Cui B, Zhu Y, Kong X, Deng M, et al: Gain-of-function mutation
of KIT ligand on melanin synthesis causes familial progressive
hyperpigmentation. Am J Hum Genet. 84:672–677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Betz RC, Planko L, Eigelshoven S, Hanneken
S, Pasternack SM, Bussow H, Van Den Bogaert K, Wenzel J,
Braun-Falco M, Rutten A, et al: Loss-of-function mutations in the
keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet.
78:510–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pickup TL and Mutasim DF: Dowling-Degos
disease presenting as hypopigmented macules. J Am Acad Dermatol.
64:1224–1225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li M, Cheng R, Liang J, Yan H, Zhang H,
Yang L, Li C, Jiao Q, Lu Z, He J, et al: Mutations in POFUT1,
encoding protein O-fucosyltransferase 1, cause generalized
Dowling-Degos disease. Am J Hum Genet. 92:895–903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Basmanav FB, Oprisoreanu AM, Pasternack
SM, Thiele H, Fritz G, Wenzel J, Größer L, Wehner M, Wolf S,
Fagerberg C, et al: Mutations in POGLUT1, encoding protein
O-glucosyltransferase 1, cause autosomal-dominant Dowling-Degos
disease. Am J Hum Genet. 94:135–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pérez B, Mechinaud F, Galambrun C, Ben
Romdhane N, Isidor B, Philip N, Derain-Court J, Cassinat B,
Lachenaud J, Kaltenbach S, et al: Germline mutations of the CBL
gene define a new genetic syndrome with predisposition to juvenile
myelomonocytic leukaemia. J Med Genet. 47:686–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Aoki Y, Niihori T, Banjo T, Okamoto N,
Mizuno S, Kurosawa K, Ogata T, Takada F, Yano M, Ando T, et al:
Gain-of-function mutations in RIT1 cause Noonan syndrome, a
RAS/MAPK pathway syndrome. Am J Hum Genet. 93:173–180. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shah KN: The diagnostic and clinical
significance of café-au-lait macules. Pediatr Clin North Am.
57:1131–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|