Introduction
Vibrio vulnificus is a Gram-negative,
comma-shaped bacterium found in warm seawater and brackish waters.
V. vulnificus occasionally causes primary septicemia,
gastroenteritis and wound infections in humans. Primary septicemia
and gastroenteritis can occur following ingestion of uncooked
seafood contaminated with V. vulnificus (1), whereas wound infections are often
caused by direct contact of an open wound with seawater during
marine activities or in the processing of seafood (2). V. vulnificus rarely infects
healthy individuals, however, immunocompromised patients with an
underlying illness, including hepatic disorder, diabetes or
immunodeficiency, are susceptible to infection by this bacterium
(3). Among the aforementioned
clinical states of V. vulnificus infection, primary
septicemia is the most life-threatening, with a mean mortality rate
of >50% worldwide (4). There is
no official national system for surveying V. vulnificus
infections in Japan, however, it was reported that 117 individuals
succumbed to mortality between 1975 and 2005 (5), and the annual number of cases of
V. vulnificus-septicemia has been estimated to be >200
(6). Cases of V. vulnificus
infection have also been reported annually in other countries. The
Centers for Disease Control (Atlanta, GA, USA) reported 32 cases of
V. vulnificus infection-associated mortality in the US in
2012 (7). In Europe, V.
vulnificus is a known fish pathogen, particularly in eels, and
V. vulnificus infection in humans is relatively rare
(8). However, due to the global
warming, there is concern of an increasing number of cases of V.
vulnificus infection as this bacterium grows rapidly in warm
seawater (9).
Several studies have reported on V.
vulnificus virulence genes (10–12),
however, the mechanism underlying the infection remains to be fully
elucidated. Mice have been used as experimental animals to
investigate the host-pathogen interactions of V. vulnificus
infections, however, using a large number of mammals is expensive
and raises ethical considerations (13,14).
Previously, invertebrate infection models using
nematodes, including Caenorhabditis elegans (15), and insects, including Galleria
mellonella (16), have being
applied to investigate bacterial virulence genes (17). In addition, an infection model
using silkworms (Bombyx mori) for the investigation of
bacterial pathogenesis has been reported (18). Silkworms are relatively easy to
breed in laboratories, and the animal is sufficient large enough
for the injection of samples into the hemolymph. The objective of
the present study was to use a silkworm model to investigate the
virulence of V. vulnificus. This model may be aid in the
investigation of V. vulnificus virulence factors and may
assist in the development of effective therapies to protect against
V. vulnificus infection
Materials and methods
Bacterial strains, plasmids and
medium
The V. vulnificus OPU1 strain was clinically
isolated, and its rifampicin (Rf)-resistant variant, V.
vulnificus OPU1-Rf, was used in the present study.
Escherichia coli BW19795 was provided by Dr Barry L. Wanner
(Purdue University, West Lafayette, IN, USA (19), which was used as a pUT donor for
conjugation. E. coli DH10BTM-competent cells were purchased
from Life Technologies; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). The signature-tagged mini-Tn5Km2-y67 transposon in
the pUT delivery suicide plasmid pool was provided by Dr David W.
Holden (Imperial College London, London, UK) (20). Bacterial cells were grown at 37°C
in Luria-Bertani (LB) medium containing 10 g tryptone (BD
Biosciences, Tokyo, Japan), 5 g yeast extract (BD Biosciences) and
10 g NaCl/l (21), unless
otherwise described. M9 minimal medium without glucose was prepared
as previously described (21) and
used for bacterial conjugation. The following antibiotics were
added to the medium at the indicated concentrations: Rf (100 µg/ml;
Wako Pure Chemical Industries, Ltd., Osaka, Japan), kanamycin (Km;
50 µg/ml; Meiji Seika Pharma Co., Ltd., Tokyo, Japan) and
ampicillin (Am; 100 µg/ml; Meiji Seika Pharma Co., Ltd.). Bacterial
growth was monitored by turbidity using a spectrophotometer
(Spectronic 20A; Shimadzu Corporation, Kyoto, Japan).
Silkworm lethality assay
The bacteria were inoculated into silkworms using
the protocol described by Hamamoto et al (22). The silkworms were raised from
fertilized silkworm eggs (Hu·Yo × Tsukuba·Ne) purchased from Ehime
Sansyu Co., Ltd. (Yawatahama, Japan). The eggs were incubated in a
clean bench (CCV-800E-AG; Hitachi Koki Co., Ltd., Tokyo, Japan) at
25°C in the dark for 3–5 days, according to the manufacturer's
protocol. The hatched larvae were maintained at 27°C, humidity
60–90% and fed artificial food (Silkmate 2S; Nosan Corporation,
Yokohama, Japan) for ~3 weeks. The larvae shed their shells four
times, and the fifth-instar larvae were fed antibiotic-free
artificial food (Silkmate; Katakura Industries, Tokyo, Japan) for
24–26 h prior to inoculation.
The bacteria were cultivated at 37°C in 2 ml of LB
medium until the optical density at 600 nm (OD600)
reached 1.0. The bacterial cultures were diluted with 10 mM
phosphate-buffered saline (pH 7.3), containing 0.01% gelatin (PBSG)
to cell densities of 2.4×107 and 2.4×108
cfu/ml, and were maintained for up to 2 h at room temperature until
they were inoculated into the silkworms. The culture filtrates were
prepared by filtering the diluted cultures through a 0.45-µm filter
(Merck Millipore, Tokyo, Japan). A 50-µl aliquot of the diluted
bacterial cell suspension or culture filtrate was injected into the
hemolymph of the silkworms using a 1-ml plastic disposable
tuberculin syringe attached to a 27-gauge needle (Terumo
Corporation, Tokyo, Japan). The quantities of bacteria injected
were estimated by colony forming units (cfu), determined by
inoculation of the diluted bacterial culture suspensions onto LB
agar and counting the number of colonies grown following incubation
for 18 h at 37°C. The inoculated silkworms were maintained in
plastic containers without feeding, and the larval status (dead or
alive) was assessed. The silkworms were considered to be dead when
they showed no reaction to touch.
Construction of the transposon
insertion mutants by conjugation
The transposon insertion mutants were constructed by
conjugation, as described previously (23). Briefly, V. vulnificus
OPU1-Rf was mated with E. coli BW19795 harboring the pUTy69
conjugative suicide plasmid, which contained the mini-Tn5Km2-y67
Km-resistant transposon. The transposon-inserted mutants were
assessed by their growth on Km- and Rf-containing agar medium.
Cloning and sequence analysis of the
attenuated mutant
The locations of the transposon-inserted regions in
the mutant genome were determined by sequencing of the DNA sequence
adjacent to the insertion site, as described previously (23). Briefly, whole genome DNA of
attenuated mutants were extracted and digested with the restriction
enzyme SalI (Nippon Gene Co., Ltd, Tokyo, Japan). The enzyme
digested DNA fragments were ligated into pUC18, followed by
transformation into E. coli DH10B (Thermo Fisher Scientific,
Inc.). The colonies resistant to both Km and Am were selected and
the plasmid DNA that ligated with the fragments containing
transposon insertion sites of genomic DNA was extracted. The
transposon insertion sites were determined by DNA sequencing using
the primer P279 (5′-CTAGGTACCTACAACCTC-3′) which anneal to the
transposon. DNA sequencing was performed with an Applied biosystems
DNA sequencing system and the BigDye terminator cycle sequencing
kit (Thermo Fisher Scientific, Inc.). Sequence homologies were
searched with the BLAST search algorithm at the National Center for
Biotechnology Information (www.ncbi.nlm.nih.gov/)
Results
Lethality of V. vulnificus towards
silkworms
To investigate the applicability of silkworms for
in vivo V. vulnificus infection experiments, the present
study first examined the lethality of V. vulnificus towards
silkworms. The spontaneous Rf-resistant V. vulnificus
OPU1-Rf was selected for injection into silkworms, as the
infectivity and lethality of this strain have been confirmed in
mice, and a mutagenesis method using the mini-Tn5Km2
transposon was established (23).
Fresh V. vulnificus OPU1-Rf cultures with an
OD600 of 1.0 were diluted with PBSG solution to cell
densities of 2.4×107 and 2.4×108 cfu/ml, and
were maintained for up to 2 h at room temperature until they were
inoculated into the silkworms. A 50-µl aliquot of the diluted
culture specimen was injected into the hemolymph of the silkworms
from a syringe through the dorsal surface, in a manner similar to
that of infection into the human blood stream. The movement of the
silkworms reduced immediately following the injection, however, the
animals behaved normally at ~1 h. This blunting of silkworm
behavior was also observed when the animals were injected with the
diluent (PBSG) alone. These findings may have been associated with
the injection stimulus, an example being a decrease in body
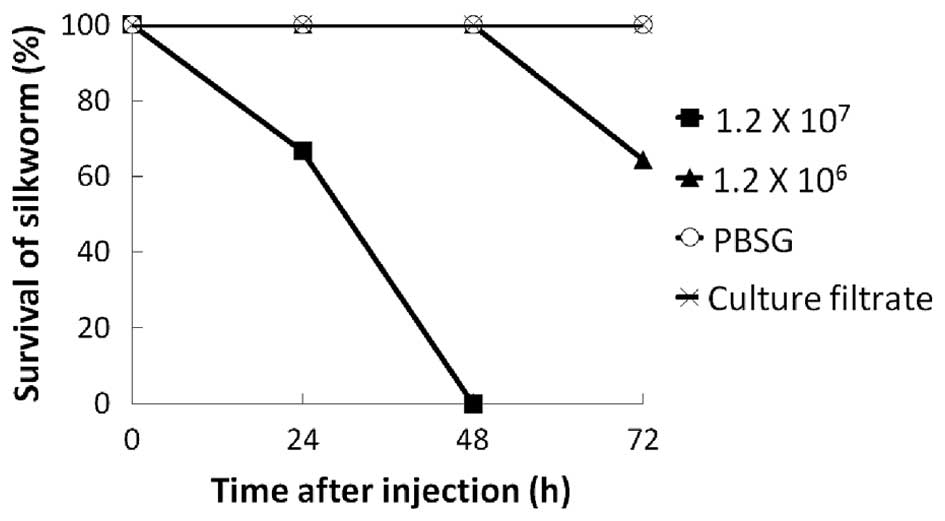
temperature caused by the injected solution. As shown in Fig. 1, the silkworms injected with V.
vulnificus OPU1-Rf began to die at 24 h. By contrast, the
silkworms injected with PBSG or culture filtrate remained alive at
72 h. In the deceased silkworms, the points of injection turned
black in the initial hours, and these black spots gradually spread
throughout the entire body. All silkworms injected with
1.2×107 cfu/silkworm died within 48 h. A reduced dose of
1.2×106 cfu/silkworm extended survival rates, and 60% of
the worms were alive at 72 h (Fig.
1). Thus, the virulence of V. vulnificus towards
silkworms may be dose-dependent at these doses, although only two
doses were examined in the present study. These results indicated
that V. vulnificus is life-threatening to silkworms, and
that silkworms can be used for investigating the virulence of V.
vulnificus. As the injection of 1.2×107 cfu/silkworm
led to the death of all silkworms within 48 h, 107
cfu/silkworm was used in the subsequent experiments.
Screening of the V.
vulnificus-attenuated mutants through the assessment of silkworm
lethality
As the silkworms were found to be sensitive to V.
vulnificus infection, the present study attempted to use a
silkworm infection model to identify the pathogenic genes of V.
vulnificus against silkworms. The attenuated mutants, which had
lost pathogenicity to silkworms, were screened from the 1,016
transposon insertion mutants.
The transposon insertion mutants were cultured for
4–6 h at 37°C with agitation. Culture fluid with an
OD600 of 1.0 was diluted with PBSG, and a 50-µl aliquot
was injected into the hemolymph of silkworms. Subsequently,
~3.3×107±2.9×107 cfu of the organism were
injected into each silkworm, and silkworm status (dead or alive)
was monitored for 5 days. In the process of identifying less
virulent mutants, which did not lead to silkworm death within 5
days, 78 candidates were obtained (primary screening). To reduce
experimental error, a second inoculation of the first candidate
mutant was performed. Of the 78 candidates, 16 mutants did not lead
to silkworm death within 5 days of injection (secondary screening).
To confirm the avirulent properties of the secondary screened
candidates, each of the 16 secondary screened candidates was
injected into five silkworms. The candidates, in which all five
injected silkworms survived for 5 days, were selected as attenuated
mutants. Of the 16 mutants, 15 were not selected as an attenuated
mutant as the silkworms died during the observation period. The
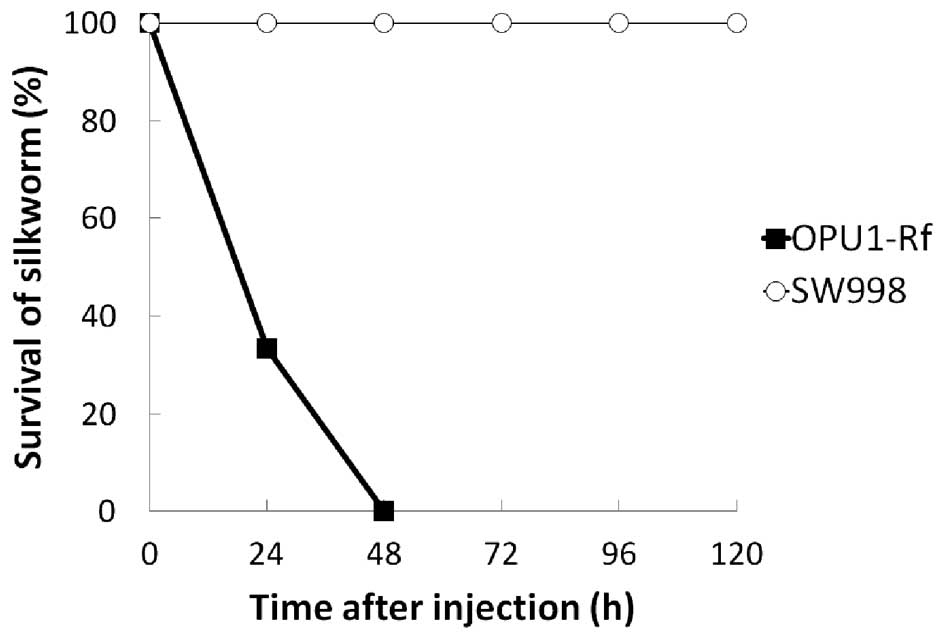
SW998 mutant was found to be avirulent to all five silkworms
(Fig. 2).
Transposon insertion sites in the
attenuated mutant
As SW998 was avirulent in the silkworms (Fig. 2), the virulence-associated gene was
expected to have been disrupted by the transposon insertion in
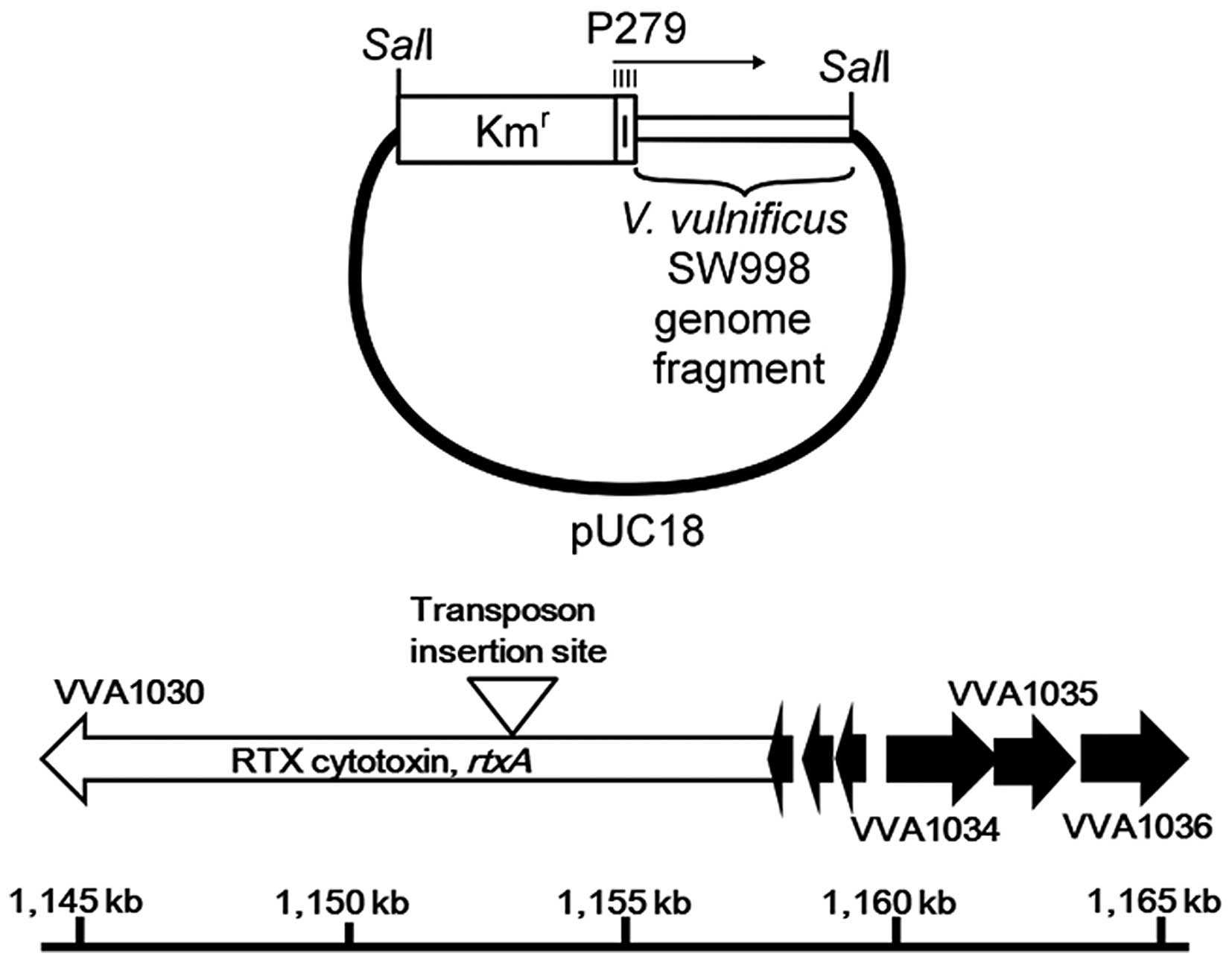
SW998. In order to locate the transposon-inserted gene in the SW998
genome, recombinant plasmid DNA which contained the transposon
insertion site was prepared as aforementioned and the DNA sequences
adjacent to the transposon insertion sites were determined
(Fig. 3). The nucleotide sequences
of the cloned V. vulnificus DNA fragment adjacent to the
transposon insertion site showed a high level of homology to the
V. vulnificus rtxA gene, which has been reported as a V.
vulnificus cytotoxic factor (24).
Discussion
Certain in vivo experiments designed to
investigate the precise mechanisms of microbial infections are
difficult to replace using in vitro experiments or models.
Mice are often used to investigate V. vulnificus infection;
however, using a larger number of animals may not always be
economically or ethically feasible. Random transposon insertion
mutagenesis and subsequent screening of attenuated mutants have
been applied to identify virulence genes in several bacterial
pathogens (25). Thousands of mice
are required to isolate attenuated mutants. Animal experiments
using mammals are required; however, to obtain satisfactory
results, the sacrifice of a large number of animals may be
required. In vivo infection models using invertebrates
instead of mammals have been successfully applied in pathogenic
microbe investigations, including Staphylococcus aureus
virulence genes identified using a silkworm infection model
(18). The purpose of the present
study was to examine whether the silkworm infection model can be
applied to investigate the virulence of V. vulnificus.
In the present study, silkworms were demonstrated to
be sensitive to the V. vulnificus; inoculation of this
bacterium into the hemolymph of animals led to death (Fig. 1). Kaito et al (26) reported that several bacterial
species, including S. aureus, Streptococcus pyogenes,
Pseudomonas aeruginosa and V. cholerae, which are
human pathogens, can also infect and induce death in silkworms when
injected into the blood stream of silkworms, whereas non-pathogenic
laboratory strains of E. coli cannot. Although there is no
direct evidence to confirm whether the death of silkworms is caused
by the same factors as mammals, silkworms may recognize virulence
factors of pathogens as they have the Toll and immune deficiency
pathways, which are homologous to the mammalian Toll-like receptor
and tumor necrosis factor receptor signaling pathways, respectively
(27). Using the silkworm
infection model, the SW998 attenuated mutant strain was obtained in
the present study, in which the rtxA gene was disrupted by a
transposon insertion. The rtxA gene is a member of the
rtx gene cluster in the V. vulnificus genome. RtxA
has been suggested to be a toxin essential for V. vulnificus
virulence, which has been confirmed by experimental infection of a
mouse model and in an in vitro tissue culture model
(24). In the present study, the
injection of culture filtrate did not induce silkworm death
(Fig. 1), which suggested that the
expression of RtxA increased following interaction of the pathogen
with host cells, as reported previously (28). Therefore, the bacteria secreted
RtxA in the silkworm in vivo and RtxA may be toxic to
silkworms. Complete removal of the genes from the genome and
lethality assessment are required to determine whether RtxA is
actually toxic to silkworms.
In conclusion, the silkworms examined in the present
study died when inoculated experimentally with V. vulnificus
and the rtxA gene was identified successfully using this
infection model. It is expected this model may be useful for
investigating V. vulnificus virulence factors and assist in
developing effective therapies to protect against V.
vulnificus infection.
Acknowledgements
The authors would like to thank Dr Toru Takahashi
(Okayama Prefectural University, Okayama, Japan) for their advice
during preparation of the manuscript. The present study was
supported in part by JSPS Kakenhi (grant no. 22590400) and the
Grant for Special Scientific Research of Okayama Prefectural
University, 2012.
References
|
1
|
de Araujo MR, Aquino C, Scaramal E, Ciola
CS, Schettino G and Machado MC: Vibrio vulnificus infection in São
Paulo, Brazil: Case report and literature review. Braz J Infect
Dis. 11:302–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vinh DC, Mubareka S, Fatoye B, Plourde P
and Orr P: Vibrio vulnificus septicemia after handling Tilapia
species fish: A Canadian case report and review. Can J Infect Dis
Med Microbiol. 17:129–132. 2006.PubMed/NCBI
|
|
3
|
Horseman MA and Surani S: A comprehensive
review of Vibrio vulnificus: An important cause of severe sepsis
and skin and soft-tissue infection. Int J Infect Dis. 15:e157–e166.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hlady WG and Klontz KC: The epidemiology
of Vibrio infections in Florida, 1981–1993. J Infect Dis.
173:1176–1183. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyasaka J, Yahiro S, Arahira Y, Tokunaga
H, Katsuki K and Hara-Kudo Y: Isolation of Vibrio parahaemolyticus
and Vibrio vulnificus from wild aquatic birds in Japan. Epidemiol
Infect. 134:780–785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osaka K, Komatsuzaki M, Takahashi H,
Sakano S and Okabe N: Vibrio vulnificus septicaemia in Japan: An
estimated number of infections and physicians' knowledge of the
syndrome. Epidemiol Infect. 132:993–996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Enteric Disease Surveillance, .
Centers for Disease Control and Prevention. Atlanta, Georgia, USA:
COVIS Annual Summary; 2012, https://www.cdc.gov/ncezid/dfwed/pdfs/covis-annual-report-2012-508c.pdf
|
|
8
|
Fouz B, Roig FJ and Amaro C: Phenotypic
and genotypic characterization of a new fish-virulent Vibrio
vulnificus serovar that lacks potential to infect humans.
Microbiology. 153:1926–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bier N, Bechlars S, Diescher S, Klein F,
Hauk G, Duty O, Strauch E and Dieckmann R: Genotypic diversity and
virulence characteristics of clinical and environmental Vibrio
vulnificus isolates from the Baltic Sea region. Appl Environ
Microbiol. 79:3570–3581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreger A..Lockwood D: Detection of
extracellular toxin(s) produced by Vibrio vulnificus. Infect.
Immun. 33:583–590. 1981.
|
|
11
|
Miyoshi S, Oh EG, Hirata K and Shinoda S:
Exocellular toxic factors produced by Vibrio vulnificus. J Toxicol
Rev. 12:253–288. 1993.
|
|
12
|
Powell JL, Wright AC, Wasserman SS, Hone
DM and Morris JG Jr: Release of tumor necrosis factor alpha in
response to Vibrio vulnificus capsular polysaccharide in vivo and
in vitro models. Infect Immun. 65:3713–3718. 1997.PubMed/NCBI
|
|
13
|
Jeong HG, Oh MH, Kim BS, Lee MY, Han HJ
and Choi SH: The capability of catabolic utilization of
N-acetylneuraminic acid, a sialic acid, is essential for Vibrio
vulnificus pathogenesis. Infect Immun. 77:3209–3217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hor LI, Chang YK, Chang CC, Lei HY and Ou
JT: Mechanism of high susceptibility of iron-overloaded mouse to
Vibrio vulnificus infection. Microbiol Immunol. 44:871–878. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bae T, Banger AK, Wallace A, Glass EM,
Aslund F, Schneewind O and Missiakas DM: Staphylococcus aureus
virulence genes identified by bursa aurealis mutagenesis and
nematode killing. Proc Natl Acad Sci USA. 101:12312–12317. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seed KD and Dennis JJ: Development of
Galleria mellonella as an alternative infection model for the
Burkholderia cepacia complex. Infect Immun. 76:1267–1275. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mylonakis E and Aballay A: Worms and flies
as genetically tractable animal models to study host-pathogen
interactions. Infect Immun. 73:3833–3841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaito C, Kurokawa K, Matsumoto Y, Terao Y,
Kawabata S, Hamada S and Sekimizu K: Silkworm pathogenic bacteria
infection model for identification of novel virulence genes. Mol
Microbiol. 56:934–944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Metcalf WW, Jiang W and Wanner BL: Use of
the rep technique for allele replacement to construct new
Escherichia coli hosts for maintenance of R6K gamma origin plasmids
at different copy numbers. Gene. 138:1–7. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hensel M, Shea JE, Gleeson C, Jones MD,
Dalton E and Holden DW: Simultaneous identification of bacterial
virulence genes by negative selection. Science. 269:400–403. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sambrook J and Russell DW: Molecular
Cloning: A laboratory manual. Cold Spring Harbor Laboratory Press,
Cold Spring Harbor; New York: 3. Appendix 2.2. 2001
|
|
22
|
Hamamoto H, Kurokawa K, Kaito C, Kamura K,
Razanajatovo I Manitra, Kusuhara H, Santa T and Sekimizu K:
Quantitative evaluation of the therapeutic effects of antibiotics
using silkworms infected with human pathogenic microorganisms.
Antimicrob Agents Chemother. 48:774–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto M, Kashimoto T, Tong P, Xiao J,
Sugiyama M, Inoue M, Matsunaga R, Hosohara K, Nakata K, Yokota K,
et al: Signature-tagged mutagenesis of Vibrio vulnificus. J Vet Med
Sci. 77:823–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Kim MW, Kim BS, Kim SM, Lee BC,
Kim TS and Choi SH: Identification and characterization of the
Vibrio vulnificus rtxA essential for cytotoxicity in vitro and
virulence in mice. J Microbiol. 45:146–152. 2007.PubMed/NCBI
|
|
25
|
Saenz HL and Dehio C: Signature-tagged
mutagenesis: Technical advances in a negative selection method for
virulence gene identification. Curr Opin Microbiol. 8:612–619.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaito C, Akimitsu N, Watanabe H and
Sekimizu K: Silkworm larvae as an animal model of bacterial
infection pathogenic to humans. Microb Pathog. 32:183–190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka H and Yamakawa M: Regulation of the
innate immune responses in the silkworm, Bombyx mori. ISJ. 8:59–69.
2011.
|
|
28
|
Kim YR, Lee SE, Kook H, Yeom JA, Na HS,
Kim SY, Chung SS, Choy HE and Rhee JH: Vibrio vulnificus RTX toxin
kills host cells only after contact of the bacteria with host
cells. Cell Microbiol. 10:848–862. 2008. View Article : Google Scholar : PubMed/NCBI
|