Introduction
The incidence of colorectal carcinoma in the United
States is among the highest in the world, affecting ~52/100,000
individuals, and the incidence of colorectal cancer in India is
among the lowest, affecting ~7/100,000 individuals, suggesting that
lifestyle factors may contribute to the development of the disease
(1). Previous epidemiological and
dietary intervention studies have suggested that diet-derived
flavonoids may have a beneficial contribution to cancer therapy,
primarily due to their pro-apoptotic or anti-angiogenic activities
(2–4). Quercetin (also termed 3,3′, 4′,
5,7-pentahydroxyflavone) is a ubiquitous flavonoid found in various
fruits, vegetables, nuts and red wine. Its antitumor effects have
been confirmed in various cancer cells, including leukemia, breast,
ovarian, colon, cervical, prostate and lymphoma (5–8).
Different molecular mechanisms underlying the antitumor activity of
quercetin have been identified, including upregulation of cell
cycle inhibitors, downregulation of oncogene expression and the
inhibition of glycolysis (5,9–12).
However, the precise target for quercetin and its mechanisms of
action remain to be elucidated.
The constitutive photomorphogenesis 9 (COP9)
signalosome (CSN), is an evolutionarily conserved multiprotein
complex that is present in all eukaryotes. It consists of eight
subunits termed CSN1-CSN8 (13).
Previous studies have identified that CSN6 of the COP9 complex is
crucial for proteasome-mediated protein degradation, as it
regulates E3 ligases, including MDM2 proto-oncogene and COP1
(14,15). Notably, CSN6 overexpression has
been identified in various types of cancer, including glioblastoma,
breast cancer, myeloma and leukemia (16). It has been determined that the
CSN6-MDM2-p53 signaling axis is important for cell proliferation
and has antiapoptotic effects (14). Previous studies have demonstrated
that CSN6 prevents MDM2 autoubiquitination at lysine 364, which
results in the stabilization of MDM2 and the degradation of p53
(14,17,18).
A previous study revealed that the HER2-Akt signaling axis is
associated with CSN6 regulation and that Akt acts as a positive
regulator of CSN6 (19). A recent
study determined that CSN6 promotes carcinogenesis by positively
regulating v-myc avian myelocytomatosis viral oncogene homolog
(Myc) stability. Additionally, CSN6 overexpression was positively
correlated with Myc protein expression. Additionally, the gene
expression signature of Myc target genes have been identified in
human breast and pancreatic cancer (20). A previous study also determined
that CSN6 overexpression may be is as high as 40% in colon
adenocarcinoma (20). The
overexpression of Myc may be 70–80% in colorectal cancer (21); however, the function of the
Akt-CSN6-Myc signaling axis remains to be elucidated in colorectal
cancer.
CSN6 has been identified as a potential novel
therapeutic agent in cancer treatment and has thus been widely
previously investigated (16). The
present study determined that quercetin may reduce the protein
expression levels of CSN6 in HT-29 colon cancer cells and that it
may be one of the important targets for quercetin-induced apoptosis
in HT-29 cells. The present study determined that quercetin may
reduce cell viability and induce apoptosis of HT-29 cells by
mediating the phosphorylation of Akt and increasing CSN6 protein
degradation, which also affected the expression levels of Myc, p53,
B-cell lymphoma 2 (Bcl-2) and Bcl-2 associated X protein (Bax),
indicating that quercetin-induced apoptosis of HT-29 cells may
involve the Akt-CSN6-Myc signaling axis.
Materials and methods
Chemicals, reagents and growth
media
RPMI-1640 and 10% fetal bovine serum (FBS) were
purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Antibodies against p53 (cat. no. 9282), Bax (cat. no. 2772),
Bcl-2 (cat. no. 2872), caspase-3 (cat. no. 9665) and β-actin (cat.
no. 4970s) were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). The antibody against Myc (cat. no. NB600-336)
was purchased from Novus Biologicals (Littleton, CO, USA) and
antibody against CSN6 (cat. no. LS-C174568) was acquired from
LifeSpan BioSciences Inc. (Seattle, WA, USA). The enhanced
chemiluminescence kits used for the visualization of the proteins
were purchased from GE Healthcare Life Sciences (Chalfont, UK).
Quercetin, DMSO and MTT were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). All other reagents were purchased
from Beyotime Institute of Biotechnology, Inc. (Jiangsu, China).
Quercetin was dissolved in DMSO to a concentration of 100 µM.
Further dilutions were performed in cell culture media.
Cell line and culture
The HT-29 human colorectal cancer cell line was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). HT-29 cells were cultured in
RPMI-1640 containing 10% FBS and were maintained at 37°C in a
humidified incubator in an atmosphere of 5% CO2.
Cell viability assay
Cell viability was determined using an MTT assay.
HT-29 cells were cultured until the log-phase and were subsequently
seeded into a 96-well plate at a density of 1.0×104 cells/well
overnight prior to treatment with different concentrations of
quercetin (12.5, 25, 50, 100 and 200 µM) or DMSO. Following an
incubation of 24, 48 or 72 h, the cells were then incubated with
medium containing MTT for 4 h and the formazan crystals were
dissolved with 150 µl DMSO. The plates were incubated on a shaker
for 15 min at room temperature. The absorbance was measured at 490
nm using a microplate reader. The drug dose at which the cell
viability was reduced by 50% (IC50) at 48 h of treatment was
quantified. The experiments were repeated in triplicate.
Ultrastructures observed by
transmission electron microscopy (TEM)
Following treatment with quercetin, the cells were
washed with PBS, collected by centrifugation (1,500 × g,
4°C, 5 min) and fixed in 2.5% electron microscopy-grade
glutaraldehyde. Next, they were rinsed with 0.1 M PBS, fixed in 1%
osmium tetroxide, dehydrated through a graded series of ethanol and
processed for Epon epoxy embedding. Ultra-thin sections (60 nm)
stained with uranyl acetate and lead citrate and were observed
using a JEM-1230 electron microscope.
Apoptosis and cell cycle analysis by
flow cytometry
Control and quercetin-treated cells were collected,
washed twice with ice-cold PBS and resuspended in 500 µl binding
buffer (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Next, 5
µl annexin V-fluorescein isothiocyanate (FITC) and 5 µl propidium
iodide (PI) were added and the cells were incubated for 15 min at
room temperature in the dark. FITC and PI staining was analyzed to
determine the apoptotic rate. The percentage of total apoptotic
cells was calculated by adding the percentages of early apoptotic
gated cells (annexin-V+) and late apoptotic gated cells
(annexin-V+/PI+)
For cell cycle analysis, the cells were fixed in 70%
ethanol at 4°C for a minimum of 4 h and washed twice with ice-cold
PBS. Subsequently, 100 µl RNase A was added and the cells were
incubated in a 37°C water bath for 30 min. Following incubation,
the cells were stained with 400 µl PI for 30 min in dark
conditions. The assays were performed in triplicate using a FACSort
flow cytometer and quantified using BD CellQuest™ Pro software (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
The protein expression levels of Akt, phosphorylated
(p-)Akt, CSN6, Myc, Bax, Bcl-2, caspase-3 cleaved caspase-3 and p53
in HT-29 cells were determined using western blotting. Briefly, a
cell lysis solution was prepared using an extraction reagents kit
(Fermentas; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. A 50 µg sample of protein was separated by
10% SDS-PAGE and was transferred onto nitrocellulose membranes
(Merck Millipore). The membranes were blocked with 5% not-fat dry
milk for 2 h at room temperature and were then incubated with the
appropriate primary antibodies in a shaker overnight at 4°C.
Subsequently, the membranes were washed 3 times at room temperature
with washing buffer (1X TBS T: 10 mM Tris-HCl, pH 8.0, 150 mM NaCl,
0.05% Tween 20) for 10 min and then incubated with secondary
antibodies (1:1,000; cat. no. G130321; Hangzhou HuaAn Biotechnology
Co., Ltd., Hangzhou, China) for 2 h at room temperature. β-actin
was used as a loading control. Enhanced chemiluminescence was used
to visualize the proteins with SuperSignal West Pico
Chemiluminescent substrate (Thermo Fisher Scientific, Inc.) on a
Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Densitometry was performed using 170–9600
Quantity One® 1-D software (Bio-Rad Laboratories,
Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following treatment with quercetin, the total RNA
was extracted from the cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) under RNase-free conditions and
reverse transcription was performed in a 20 µl reaction with 200 ng
total RNA using a two-step reverse-transcription reaction kit
(Takara Biotechnology Co., Ltd., Dalian, China). The RT-qPCR was
performed on an Applied Biosystems 7500 Real-time PCR system using
a SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.) in Axygen
96-well reaction plates.
The primers used in the present study were obtained
from Sangon Biotech Co., Ltd. (Shanghai, China) and their sequences
were as follows: CSN6 (NM_486571), forward (F)
5′-AGAGGCCACAATGCTGTTTG-3′ and reverse (R)
5′-CGTGGTCTACACCAATGCGTT-3′; GAPDH (NM_002046), F:
5′-TGGCACCCAGCACAATGAA-3′ and R: 5′-CTAAGTCATAGTCCGCCTAGA-3′. GAPDH
was used as a housekeeping gene and internal control. The data was
analyzed using the 2−∆∆Cq method (22).
Retroviral constructs and
transfection
The complete codon sequence of CSN6 (NM_474971) was
amplified using Platinum Taq DNA Polymerase high fidelity
(Invitrogen; Thermo Fisher Scientific, Inc.) and the following
primers: F 5′-GACTCGAGATGGCGGCGGCGGCGGCGGCGGCTGCAGCTA-3′ and R
5′-GAGAATTCTCAGAAAAAGAGCCCGCGCATTCTCCTGCCGA-3′. The PCR product was
cloned into XhoI and EcoRI sites on the retroviral
vector MSCV MIGR1 (provided by Professor Duonan Yu, University of
Pennsylvania, Philadelphia, PA, USA). Sequence fidelity was
confirmed using DNA sequencing by Sangon Biotech Co., Ltd. HT-29
cells were seeded into 6-well plates (1.0×105 cells/well) overnight
and transfected with the recombinant retroviral expression plasmid
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The cells were
visualized under a fluorescence microscope (Nikon Corporation,
Tokyo, Japan) to detect transfection efficiency and were then
treated with quercetin for an additional 48 h.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Each experiment was repeated at least three times.
Statistical comparisons of >2 groups were performed using a
one-way analysis of variance, followed by a Bonferroni post-hoc
test. All statistical analyses were performed using SPSS version
18.0 statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Quercetin reduces cell viability of
HT-29 cells
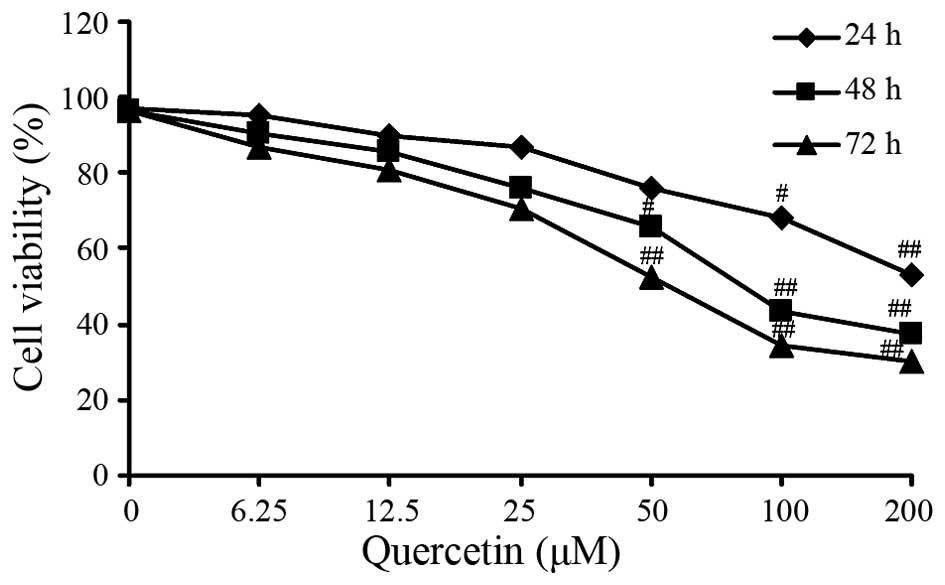
The MTT assay revealed that HT-29 cell viability was
decreased in a dose-dependent manner with increasing concentration
of quercetin. Dose-dependent inhibition of cell viability was also
observed in the HT-29 cells. The IC50 value for treatment for 48 h
was determined as 81.65±0.49 µM quercetin (Fig. 1). Therefore, it was determined that
quercetin exerted an negative activity against viability of
colorectal cancer cells.
Quercetin induces apoptosis in HT-29
cells
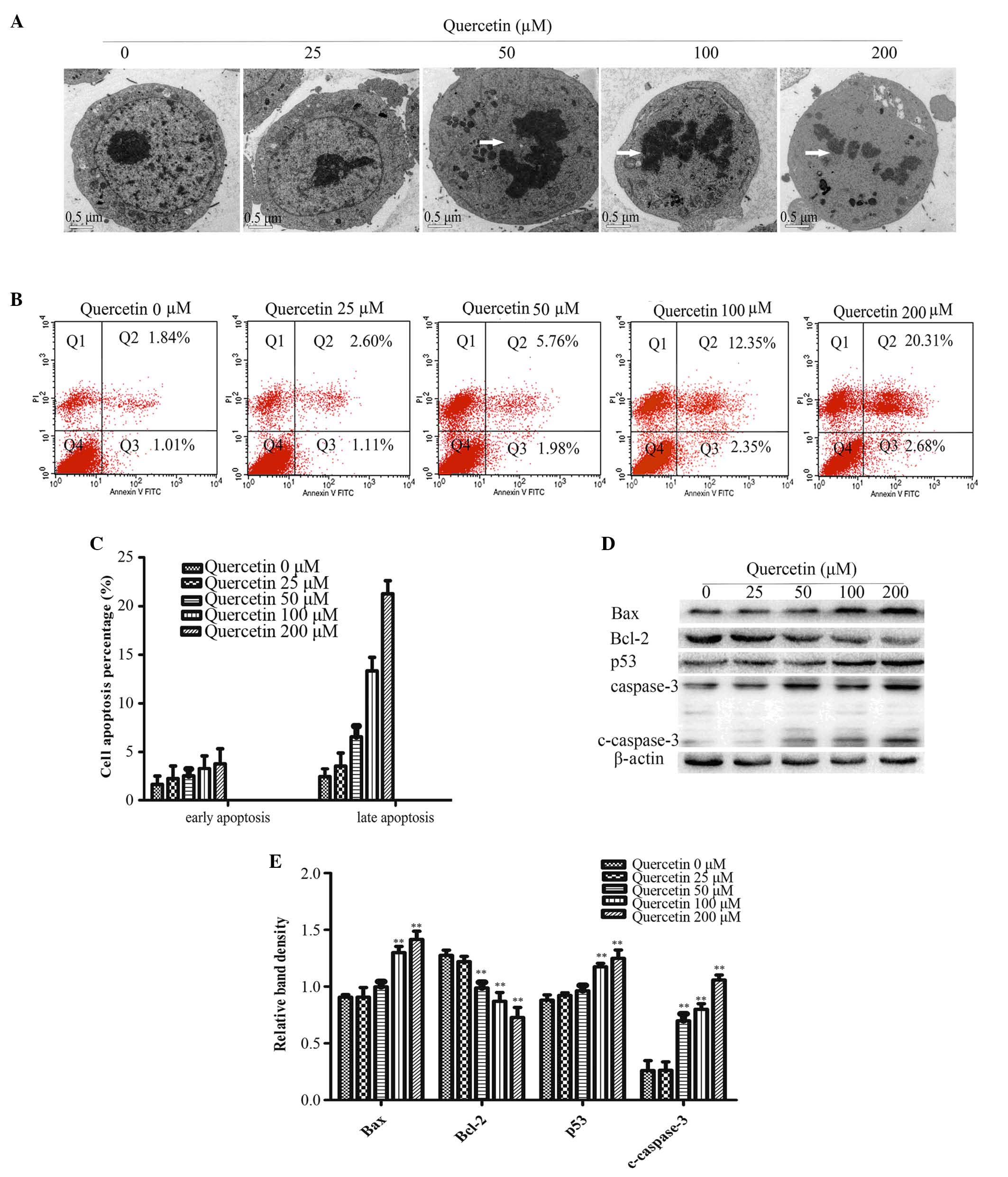
As presented in Fig.
2A, ultrastructures in HT-29 cells were observed by TEM 48 h
after quercetin treatment. The cells in the control group had
intact organelles, with normal nuclei and nucleolus chromatin.
However, upon treatment with 50, 100 or 200 µM quercetin, cell
shrinkage, chromatin condensation and nuclear collapse were
observed (Fig. 2A).
Flow cytometry analysis revealed an increase in the
apoptotic rate in treatment groups with higher quercetin
concentration compared with the control group (Fig. 2B and C).
Cleaved-caspase-3 is a key factor required for
apoptosis and is the active form of pro-caspase-3. Immunoblotting
analysis determined that cleaved-caspase-3 was significantly higher
in the 50, 100 and 200 µM quercetin treatment groups compared with
the control group (0 µM quercetin; P<0.01; Fig. 2D and E).
Members of the Bcl-2 family are crucial for the
regulation of apoptosis. Therefore, the present study used western
blot analysis to determine the protein expression levels of Bax and
Bcl-2. Bcl-2 expression decreased and Bax expression increased with
increasing concentrations of quercetin compared with the control
group (Fig. 2D and E). The protein
expression of p53 was significantly increased compared with the
control group (P<0.01; Fig.
2D)
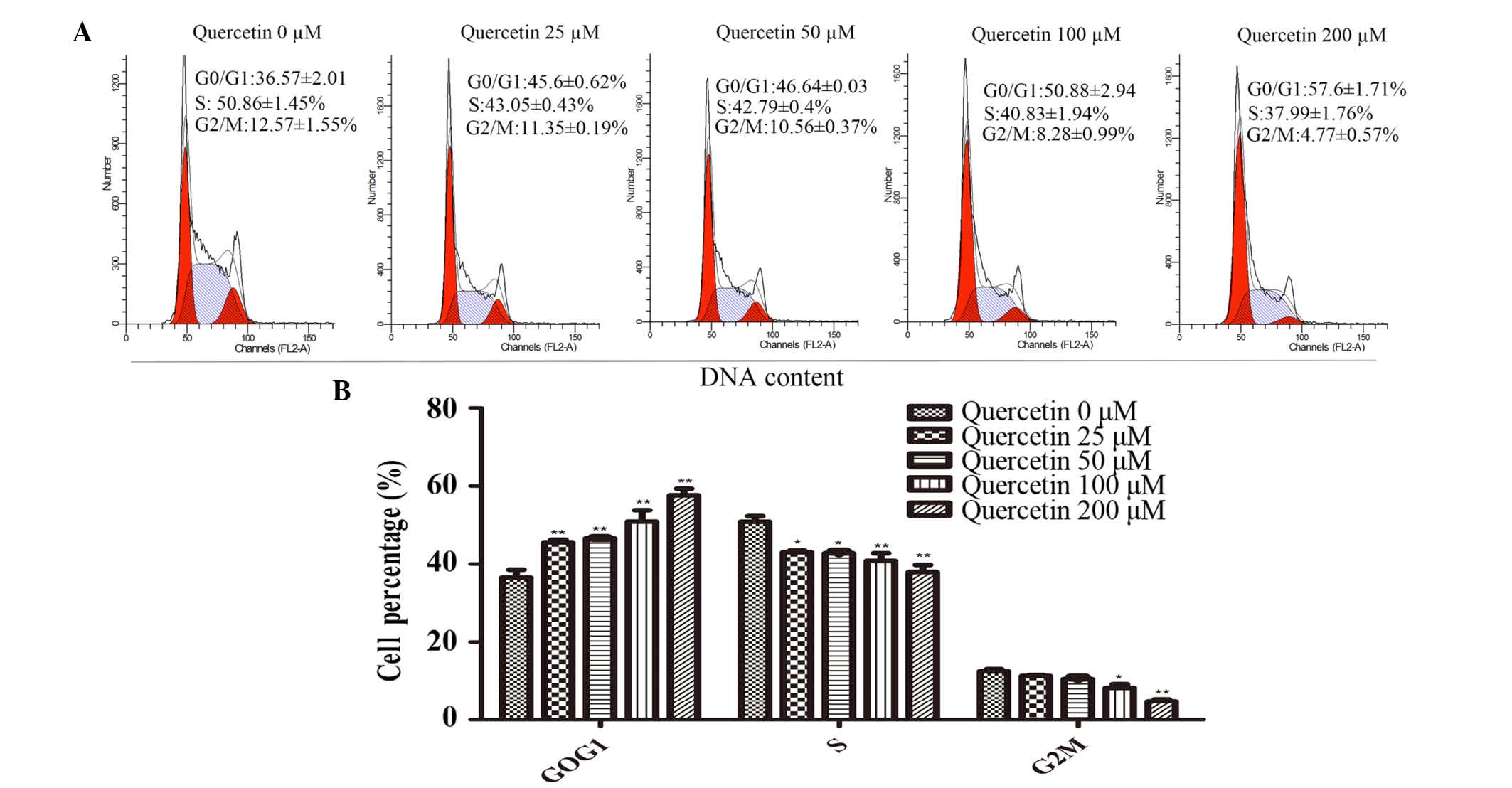
Effect of quercetin on the cycle
progression of HT-29 cells
In order to determine whether the
proliferation-inhibiting effect of quercetin on HT-29 cells was a
result of cell-cycle arrest, cell-cycle analysis was performed
using flow cytometry (Fig. 3A).
The proportion of cells in the G0/G1 phase of the cell cycle was
significantly increased in the treatment groups exposed to
quercetin compared with the control group (P<0.01; Fig 3B). The number of cells in the S and
G2/M phases in the quercetin treatment groups were significantly
decreased (P<0.05; Fig. 2B).
Therefore, quercetin inhibited the proliferation of HT-29 cells via
G0/G1 phase arrest (Fig. 3A and
B).
Akt-CSN6-Myc signaling axis mediates
quercetin cytotoxicity
To determine the effect of CSN6 on quercetin-induced
apoptosis of HT-29 cells, the recombinant retrovirus plasmid
MIGR1-CSN6, containing the full-length human CSN6 gene, was
constructed. HT-29 cells were transfected with MIGR1-CSN6 and an
empty plasmid (MIGR1, used as a control), and were separately
selected by flow cytometry for GFP+ cells. A transduction of ~100%
was achieved (Fig. 4A and B). To
determine whether the Akt-CSN6-Myc signaling axis was involved in
quercetin-induced apoptosis of HT-29 cells, the levels of Akt,
p-Akt, CSN6, Myc and p53 were examined using western blotting. The
protein expression levels of p-Akt-Thr308, CSN6 and Myc were
significantly reduced in the quercetin treatment groups compared
with the control group (P<0.01; Fig. 4C and D). Additionally, the mRNA
expression of CSN6 was determined using RT-qPCR. No significant
difference was identified in terms of CSN6 mRNA expression when
quercetin treatment groups were compared with the control group
(Fig. 4E). The western blot
analysis demonstrated that the protein expression levels of
cleaved-caspase 3, p53 and Bax were also downregulated, while Myc
and Bcl-2 were upregulated compared with cells treated with 50 µM
quercetin alone and empty plasmid controls (Fig. 4F and G). The MTT assay revealed
that the overexpression of CSN6 reduced the effect of quercetin on
cell viability compared with the empty plasmid MIGR1 (Fig. 4H).
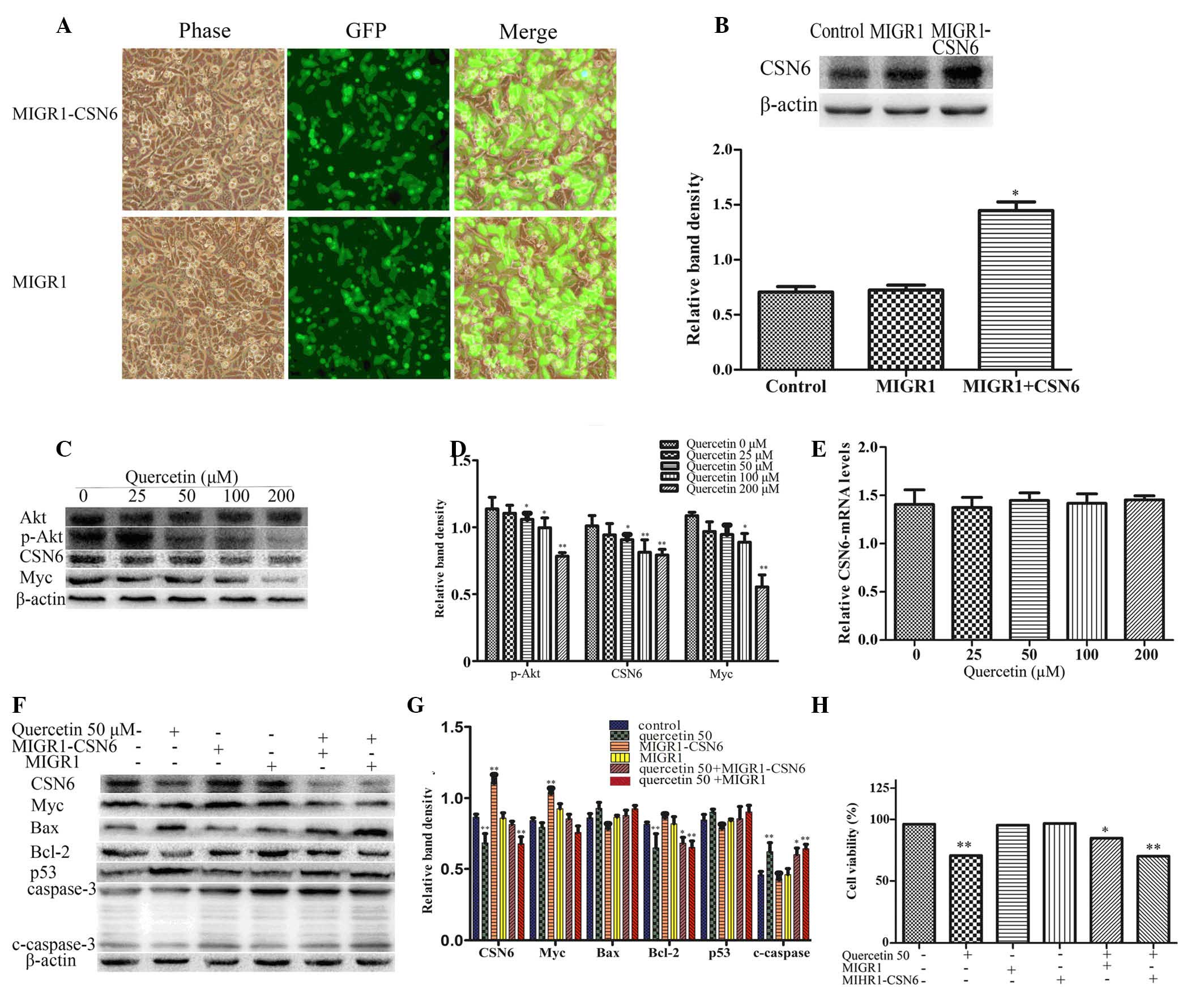 | Figure 4.Quercetin-induced apoptosis involves
the Akt-CSN6-Myc signaling pathway in HT-29 cells. (A)
Overexpression of CSN6 was performed by transfection with the
recombinant retroviral expression plasmid and the cells were
selected by flow cytometry for GFP+ cells; ~100% transduction
efficiency was achieved. (B) Expression levels of CSN6 were
analyzed by western blotting in HT-29 cells transfected with
control (empty) or CSN6 vectors. The data are expressed as the mean
± standard deviation (n=3; *P<0.05 vs. control). (C and D)
Western blot analysis of the protein expression levels of p-Akt,
CSN6 and Myc in HT-29 cells treated with different concentrations
of quercetin for 48 h. The data are expressed as the mean ±
standard deviation (n=3; *P<0.05, **P<0.01 vs. control). (E)
The mRNA expression levels of CSN6 were examined by reverse
transcription-quantitative polymerase chain reaction using total
mRNA of HT-29 cells and compared with the control. (F and G)
Western blotting was used to determine protein expression levels of
CSN6, Myc, Bax, Bcl-2, p53 and caspase 3 in the CSN6 overexpression
cell line and control (empty) cell line, which remained untreated
or exposed to 50 µM quercetin for 48 h. The data are expressed as
the mean ± standard deviation (n=3; *P<0.05, **P<0.01 vs.
untreated control). (H) Cell viability was examined in a cell line
overexpressing CSN6 and control (empty) cell line treated with or
without 50 µM quercetin for 48 h, *P<0.05 and **P<0.01 vs.
untreated control. MIGR1, plasmid; CSN6, COP9 signalosome subunit
6; Akt, Akt serine/threonine kinase 1; p-Akt, phosphorylated-Akt;
Myc, v-myc avian myelocytomatosis viral oncogene homolog; Bcl2, B
cell leukemia/lymphoma 2; Bax, Bcl2 associated X; c-caspase 3,
cleaved-caspase 3. |
Discussion
Epidemiological evidence has revealed that cancer
incidence may be significantly modulated by an increased dietary
intake of flavonoids through increased consumption of fruits and
vegetables (23). Flavonoids are
one of the largest groups of naturally occurring phenols, including
flavones, flavanols, isoflavones, flavonols, flavanones and
flavanonols (24). These dietary
antioxidants have been identified to exert significant antitumor
effects and have been extensively investigated (4,25,26).
The present study investigated the effect of
quercetin, which is one of the frequently researched flavonoids
(27), due to its positive effect
on the growth inhibition and the induction of apoptosis in HT-29
cells. The anti-proliferative effects of quercetin were initially
assessed following incubation with different concentrations of
quercetin for 24, 48 and 72 h. The cell viability of HT-29 cells
was significantly inhibited in a time-and dose-dependent manner.
Following a 24 h incubation, the inhibitory effect of quercetin on
HT-29 cell viability was not evident at low concentrations of
quercetin. However, following a 48 h incubation, significant
inhibition of cell growth was observed at 50, 100 and 200 µM
quercetin (Fig. 1).
Quercetin induces pro-apoptotic signaling pathways,
which lead to cell death (5,8,9). In
the present study, TEM observation of the quercetin-treated HT-29
cells revealed chromatin condensation, nuclear collapse and
apoptotic body formation in cells treated with 50, 100 and 200 µM
quercetin (Fig. 2A). Apoptotic
cell death was also quantified by determining the percentage of
early apoptotic gated cells (Annexin-V+) and late
apoptotic gated cells (Annexin-V+/PI+). The
results revealed an increase in the percentage of apoptotic cells
in quercetin treatment groups in a dose-dependent manner (Fig. 2B and C). Caspase-3 is a key factor
in apoptosis execution and cleaved caspase-3 is an activated form
of caspase-3. Both the mitochondria-initiated intrinsic apoptotic
pathway and the death receptor-triggered extrinsic apoptotic
pathway may lead to caspase-3 activation. Therefore, cleaved
caspase-3 protein expression levels were evaluated using western
blotting, the result revealed that the expression levels of cleaved
caspase-3 were significantly increased following quercetin
treatment (Fig. 2D and E). Bcl-2
and Bax have also been identified as key proteins for controlling
the release of cytochrome c and other pro-apoptotic factors
from the mitochondria, which leads to subsequent caspase activation
and apoptotic cell death (28).
The present study determined that quercetin treatment significantly
decreased the expression of Bcl-2, while increasing the expression
of Bax (Fig. 2D and E). This
indicated that quercetin induced apoptosis via the regulation of
the expression levels of Bcl-2 family proteins.
In order to determine whether this quercetin-induced
inhibition of cell viability was due to cell cycle arrest, PI
staining was performed and revealed that quercetin treatment
significantly increased cell cycle arrest in the G0/G1 phase of the
cell cycle, and that the number of cells in the S and the G2/M
phase was reduced (Fig. 3A and B).
This result was consistent with the findings of Kim et al
(29). The immunoblot analysis
revealed that the expression levels of p53 increased and those of
Myc decreased following treatment with quercetin for 48 h (Figs. 2D and 4C). The upregulation of p53 proteins led
to an inhibition of growth and proliferation, involved with the G1
and G2/M phase arrest in cancer cells (30–32).
A previous study reported that the downregulation of Myc-associated
genes was involved in cell cycle arrest in acute myeloid leukemia
(33). The cell cycle arrest of
nasopharyngeal carcinoma cells also involved the inhibition of the
c-Myc signaling pathway (34).
Additionally, quercetin has been considered a
powerful modulator of several cellular signaling pathways,
including the phosphatidylinositol-3-kinase (PI3K)-mediated
signaling pathway, which important for quercetin-repressed tumors
(35). Akt is a downstream target
of PI3K and regulates cell survival through the phosphorylation of
downstream substrates that control apoptosis either directly or
indirectly. Previous studies have revealed that oncogenic
activation through Akt may act as an antiapoptotic signal via the
rapid destabilization of p53 (36,37).
A previous study revealed that quercetin inhibited lymphoma by
downregulating the PI3K-Akt-p53 signaling pathway (38); however, the mechanism by which Akt
regulates p53 remains to be elucidated. Previous studies determined
that the MDM2-p53 signaling axis may be regulated by CSN6 (14,39),
and a subsequent study revealed that the HER2-Akt axis was
associated with CSN6 regulation and that Akt is a positive
regulator of CSN6 (19). A recent
study also demonstrated that CSN6 contributed to carcinogenesis by
positive regulation of Myc stability (20). The present study aimed to determine
the importance of the Akt-CSN6-Myc signaling axis in
quercetin-induced apoptosis of HT-29 cells. The immunoblot analysis
revealed that the expression of p-Akt-Thr308 and CSN6 decreased in
quercetin treatment groups. The expression of direct or indirect
CSN6 target genes, including Myc and Bcl-2 decreased, whereas p53
and Bax increased in HT-29 cells treated with quercetin (Figs. 2D and 4C). In order to determine the effect of
CSN6 on quercetin-induced apoptosis, HT-29 cells were transfected
with plasmid MIGR1-CSN6 or an empty MIGR1 plasmid and then treated
with 50 µM quercetin for 48 h. The MTT assay revealed that the
overexpression of CSN6 reduced the effect of quercetin on cell
viability compared with the empty MIGR1 plasmid (Fig. 4H). Additionally, the western blot
analysis determined that the protein expression levels of
cleaved-caspase 3, p53 and Bax were downregulated, whereas the
levels of Myc and Bcl-2 were upregulated in the CSN6 overexpression
group compared with the control group where cells were treated with
quercetin and transfected with an empty plasmid (Fig. 4F and G), indicating that
quercetin-induced apoptosis involves the Akt-CSN6-Myc signaling
axis in HT-29 cells.
In conclusion, the present study demonstrated that
quercetin inhibited cell viability, induced apoptosis and led to
cell-cycle arrest in HT-29 cells. The protein expression levels of
p-Akt-Thr308 and CSN6 were significantly downregulated following
quercetin treatment. Additionally, the expression levels of genes
downstream of CSN6, including Myc and Bcl-2 were reduced and the
levels of p53 and Bax were increased following treatment with
quercetin. The overexpression of CSN6; however, reduced the effect
of quercetin treatment on HT-29 cells. Therefore, it is possible
that the Akt-CSN6-Myc signaling axis may be a potential target for
novel treatment strategies of colorectal cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81403232,
81274141 and 81450051), the Natural Science Foundation of Jiangsu
Province of China (grant nos. BK2012686 and SBK2014021480) and the
Bureau of Traditional Chinese Medicine, Science and Technology
Project of Jiangsu Province (grant no. YB2015183).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirpara KV, Aggarwal P, Mukherjee AJ,
Joshi N and Burman AC: Quercetin and its derivatives: Synthesis,
pharmacological uses with special emphasis on anti-tumor properties
and prodrug with enhanced bio-availability. Anticancer Agents Med
Chem. 9:138–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan WF, Lin LP, Li MH, Zhang YX, Tong YG,
Xiao D and Ding J: Quercetin, a dietary-derived flavonoid,
possesses antiangiogenic potential. Eur J Pharmacol. 459:255–262.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prasad S, Phromnoi K, Yadav VR, Chaturvedi
MM and Aggarwal BB: Targeting inflammatory pathways by flavonoids
for prevention and treatment of cancer. Planta Med. 76:1044–1063.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murakami A, Ashida H and Terao J:
Multitargeted cancer prevention by quercetin. Cancer Lett.
269:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawahara T, Kawaguchi-Ihara N, Okuhashi Y,
Itoh M, Nara N and Tohda S: Cyclopamine and quercetin suppress the
growth of leukemia and lymphoma cells. Anticancer Res.
29:4629–4632. 2009.PubMed/NCBI
|
|
7
|
Luo H, Jiang BH, King SM and Chen YC:
Inhibition of cell growth and VEGF expression in ovarian cancer
cells by flavonoids. Nutr Cancer. 60:800–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vidya PR, Senthil MR, Maitreyi S,
Ramalingam K, Karunagaran D and Nagini S: The flavonoid quercetin
induces cell cycle arrest and mitochondria-mediated apoptosis in
human cervical cancer (HeLa) cells through p53 induction and
NF-kappaB inhibition. Eur J Pharmacol. 649:84–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suolinna EM, Buchsbaum RN and Racker E:
The effect of flavonoids on aerobic glycolysis and growth of tumor
cells. Cancer Res. 35:1865–1872. 1975.PubMed/NCBI
|
|
10
|
Gibellini L, Pinti M, Nasi M, Montagna JP,
De Biasi S, Roat E, Bertoncelli L, Cooper EL and Cossarizza A:
Quercetin and cancer chemoprevention. Evid Based Complement
Alternat Med. 2011:5913562011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walker EH, Pacold ME, Perisic O, Stephens
L, Hawkins PT, Wymann MP and Williams RL: Structural determinants
of phosphoinositide 3-kinase inhibition by wortmannin, LY294002,
quercetin, myricetin and staurosporine. Mol Cell. 6:909–919. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Triantafyllou A, Mylonis I, Simos G,
Bonanou S and Tsakalof A: Flavonoids induce HIF-1alpha but impair
its nuclear accumulation and activity. Free Radic Biol Med.
44:657–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng XW, Dubiel W, Wei N, Hofmann K and
Mundt K: Unified nomenclature for the COP9 signalosome and its
subunits: An essential regulator of development. Trends Genet.
16:2892000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao RY, Yeung SC, Chen J, Iwakuma T, Su
CH, Chen B, Qu C, Zhang F, Chen YT, Lin YL, et al: Subunit 6 of the
COP9 signalosome promotes tumorigenesis in mice through
stabilization of MDM2 and is upregulated in human cancers. J Clin
Invest. 121:851–865. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi HH, Gully C, Su CH, Velazquez-Torres
G, Chou PC, Tseng C, Zhao R, Phan L, Shaiken T, Chen J, et al: COP9
signalosome subunit 6 stabilizes COP1, which functions as an E3
ubiquitin ligase for 14-3-3sigma. Oncogene. 30:4791–4801. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee MH, Zhao R, Phan L and Yeung SC: Roles
of COP9 signalosome in cancer. Cell cycle. 10:3057–3066. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang S, Jensen JP, Ludwig RL, Vousden KH
and Weissman AM: Mdm2 is a ring finger-dependent ubiquitin protein
ligase for itself and p53. J Biol Chem. 275:8945–8951. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inuzuka H, Fukushima H, Shaik S and Wei W:
Novel insights into the molecular mechanisms governing Mdm2
ubiquitination and destruction. Oncotarget. 1:685–690. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue Y, Chen J, Choi HH, Phan L, Chou PC,
Zhao R, Yang H, Santiago J, Liu M, Yeung GE, et al: HER2-Akt
signaling in regulating COP9 signalsome subunit 6 and p53. Cell
cycle. 11:4181–4190. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Shin JH, Zhao R, Phan L, Wang H,
Xue Y, Post SM, Ho Choi H, Chen JS, Wang E, et al: CSN6 drives
carcinogenesis by positively regulating Myc stability. Nat Commun.
5:53842014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sikora K, Chan S, Evan G, Gabra H, Markham
N, Stewart J and Watson J: c-myc oncogene expression in colorectal
cancer. Cancer. 59:1289–1295. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harborne JB and Williams CA: Advances in
flavonoid research since 1992. Phytochemistry. 55:481–504. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirpara KV, Aggarwal P, Mukherjee AJ,
Joshi N and Burman AC: Quercetin and its derivatives: Synthesis,
pharmacological uses with special emphasis on anti-tumor properties
and prodrug with enhanced bio-availability. Anti-Cancer Agents Med
Chem. 9:138–161. 2009. View Article : Google Scholar
|
|
26
|
Zhao D, Beiring B and Glorius F:
Ruthenium-NHC-catalyzed asymmetric hydrogenation of flavones and
chromones: General access to enantiomerically enriched flavanones,
flavanols, chromanones and chromanols. Angew Chem Int Ed Engl.
52:8454–8458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veeriah S, Kautenburger T, Habermann N,
Sauer J, Dietrich H, Will F and Pool-Zobel BL: Apple flavonoids
inhibit growth of HT29 human colon cancer cells and modulate
expression of genes involved in the biotransformation of
xenobiotics. Mol Carcinog. 45:164–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Kim SK, Kim BS, Lee SH, Park YS,
Park BK, Kim SJ, Kim J, Choi C, Kim JS, et al: Apoptotic effect of
quercetin on HT-29 colon cancer cells via the AMPK signaling
pathway. J Agric. Food Chem. 58:8643–8650. 2010. View Article : Google Scholar
|
|
30
|
Jin Q, Feng L, Behrens C, Bekele BN,
Wistuba II, Hong WK and Lee HY: Implication of AMP-activated
protein kinase and Akt-regulated survivin in lung cancer
chemopreventive activities of deguelin. Cancer Res. 67:11630–11639.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su RY, Chao Y, Chen TY, Huang DY and Lin
WW: 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL-and
TNF(alpha)-induced cytotoxicity in colon cancer cells through
AMP-activated protein kinase signaling. Mol Cancer Ther.
6:1562–1571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang JT, Ha J, Park IJ, Lee SK, Baik HW,
Kim YM and Park OJ: Apoptotic effect of EGCG in HT-29 colon cancer
cells via AMPK signal pathway. Cancer Lett. 247:115–121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eriksson A, Kalushkova A, Jarvius M,
Hilhorst R, Rickardson L, Kultima HG, de Wijn R, Hovestad L,
Fryknäs M, Öberg F, et al: AKN-028 induces cell cycle arrest,
downregulation of Myc associated genes and dose dependent reduction
of tyrosine kinase activity in acute myeloid leukemia. Biochem
Pharmacol. 87:284–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu ZY, Sun J, Zhu XF, Yang D and Zeng YX:
ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells
by suppressing the c-Myc signaling pathway. J Transl Med. 7:742009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR-and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moll UM and Petrenko O: The MDM2-p53
interaction. Mol Cancer Research. 1:1001–1008. 2003.
|
|
37
|
Mayo LD and Dormer DB: The PTEN, Mdm2, p53
tumor suppressor-oncoprotein network. Trends Biochem Sci.
27:462–467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maurya AK and Vinayak M: Quercetin
regresses dalton's lymphoma growth via suppression of PI3K/AKT
signaling leading to upregulation of p53 and decrease in energy
metabolism. Nutr Cancer. 67:354–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B and
Hung MC: HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2
phosphorylation. Nat Cell Biol. 3:973–982. 2001. View Article : Google Scholar : PubMed/NCBI
|