Introduction
Dilated cardiomyopathy (DCM) is characterized by
left ventricular dilation and systolic dysfunction with an ejection
fraction of <50% (1). DCM is
associated with sudden cardiac death and heart failure, resulting
in a significant medical burden due to the high rate of hospital
admissions and the potential requirement for heart transplantation
(2). Despite therapeutic advances,
the prevalence of DCM was 1 in 2,500–3,000 and the 5-year mortality
remained ~20% in 2010 (2,3). Patients with DCM may also develop
diastolic dysfunction and impaired right ventricular function.
Genetic inheritance, myocarditis, alcohol intake and toxic effects
from medications may contribute to the development and progression
of DCM (2).
Cardiomyocyte apoptosis and fibrosis are the major
pathological changes that occur in DCM. Apoptosis is an actively
regulated process that includes DNA fragmentation, membrane
blebbing, cell shrinking and chromatin condensation (4). Fibrosis is the formation of excess
fibrous tissues or scar tissue, which is characterized by the net
accumulation of extracellular matrix proteins in the cardiac
interstitium (5). In the hearts of
patients with DCM, apoptosis of cardiomyocytes leads to the
increase of the intercellular space, where it is filled by
granulation tissue, composed of macrophages, endothelial cells and
fibroblasts (6,7). In addition, cardiac fibroblasts
excessively produce extracellular matrix (ECM), leading to fibrosis
and increasing tissue stiffness.
B-cell lymphoma-2 (Bcl-2) is an important
antiapoptotic protein (8,9). It was isolated from the t(14;18)
chromosomal breakpoint in follicular B-cell lymphoma (10). The Bcl-2 protein has been
demonstrated to bind to Bak, Bax, Bcl-xL, Bcl-xS, mcl-1 and a
variety of other intracellular proteins (11). The relative levels and competing
dimerizations between Bcl-2 and its binding proteins determine the
potential of a cell to undergo apoptosis (12). Overexpression of Bcl-2 in
transgenic models leads to accumulation of cells, due to evasion of
cell death mechanisms (13).
Induction of apoptosis by diverse stimuli, including radiation,
hyperthermia, growth factor withdrawal and multiple classes of
chemotherapeutic agents, is inhibited by Bcl-2 in vitro
(14). The inhibitory effects are
proportional to the level of Bcl-2 expression (14). In addition, an increase in the
expression levels of the Bcl-2 family proteins was observed in the
end stage of heart failure (15).
However, the role of Bcl-2 in DCM remains to be thoroughly
studied.
Micro (mi)RNAs are 22 nucleotides in length and
serve important regulatory roles by targeting messenger RNAs for
cleavage or translational repression via RNA silencing (16). They are the most abundant class of
regulatory molecules for the expression of protein-coding genes in
response to pathological stimuli (17). Genome-wide profiling of miRNAs
demonstrated that 43 miRNAs were differentially expressed during
human heart failure (18). The
level of miRNAs in blood circulation may function as markers for
DCM (19). Specific miRNAs,
including miR-21, may regulate Bcl-2 and mediate fibrosis (20). Therefore, the expression of miRNAs
in cardiac tissues may be associated with the pathogenesis of
DCM.
In the present study, human hearts from three DCM
patients and three healthy donors were collected. Myocardial
fibrosis and apoptosis of the cardiac tissues was evaluated, and
the mRNA and protein expression levels of Bcl-2 were examined. In
addition, the expression of three miRNAs and their family members
(miR-21; miR-29a, miR-29b and miR-29c; miR-133a and miR-133b) were
examined in normal and DCM cardiac tissues. The results of the
present study suggested that Bcl-2 and specific miRNAs are involved
in human DCM.
Materials and methods
Human cardiac tissues
Experiments involving human tissues complied with
the Declaration of Helsinki and were approved by the Shandong
Provincial Qianfoshan Hospital Ethical Review Board (Jinan, China;
no. S074). Human hearts were collected from three patients with
DCM-caused heart failure (46.57±9.06 years) who underwent a heart
transplantation. The normal cardiac tissues were from three adults
(32.0±7.16 years old) with accidental traumatic brain death, who
had no medical evidence of cardiac disease. All patients were
functionally classified according to the New York Heart Association
criteria. The diagnosis of DCM was based on the World Health
Organization/International Society and Federation Cardiology
definition (21). Clinical
history, electrocardiogram, echocardiography, hemodynamic studies
and coronary angiography data were measured for all patients
(Table I). Transmural samples were
taken from near the apex, septum, and left and right ventricle, and
were stored in frozen liquid nitrogen or fixed in 10% buffered
formalin for analysis.
 | Table I.Clinical characteristics of the
patient groups. |
Table I.
Clinical characteristics of the
patient groups.
| Characteristic | Normal group
(n=3) | DCM group
(n=3) |
|---|
| LVEF (%) | 68.12±5.09 |
29.06±5.47a |
| LVEDD (cm) | 2.79±0.84 |
6.98±0.68a |
| CO (l/min) | 5.38±1.13 |
2.07±0.83a |
| PCWP (mmHg) | 9.59±1.98 |
26.13±9.06a |
| NT-ProBNP
(ng/ml) | 109±19.39 |
1709.19±78.97a |
| hs-CRP (mg/l) | 0.61±0.14 |
1709.19±78.97a |
Masson's trichrome staining
Masson's trichrome staining was performed as
previously described (22). The
frozen cardiac tissues were cut into 6 µm transverse sections,
stained with Weigert's iron hematoxylin for 10 min at room
temperature, then washed and stained in 1% ponceau-acetic acid
solution (equal volumes of 0.5% ponceau 2R in 1% acetic acid and
0.5% acid fuchsin in 1% acetic acid) for 5 min at room temperature.
Following washing, the sections were incubated with 1%
phosphomolybdic acid for 5 min and counterstained with light green.
The images were captured under a FSX100 microscope (Olympus
Corporation, Tokyo, Japan) and analyzed using Image-Pro Plus
Software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Terminal deoxynucleotidyl-mediated
dUTP nick end labeling (TUNEL) assay
TUNEL (EMD Millipore, Billerica, MA, USA) was used
to detect the apoptosis of cardiac tissues. The frozen tissue
sections were pretreated with 20 µg/ml proteinase K (EMD Millipore)
for 15 min at room temperature, and the endogenous peroxidase
activity was eliminated using 3% hydrogen peroxide in
phosphate-buffered saline (PBS; 5 min at room temperature).
Following washing with equilibration buffer from the kit, the
tissue sections were incubated with the reaction mixture from the
kit for 50 min at 37°C. Following the anti-digoxigenin-peroxidase
reaction for 30 min, the positive cells were visualized by
diaminobenzidine (Dako, Glostrup, Denmark) and the slides were
counterstained with hematoxylin. The number of positively stained
cells and total cells were calculated under a FSX100 microscope
(Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA isolation was performed using the RNeasy Mini
kit (Qiagen GmbH, Hilden, Germany). The cDNA was synthesized with
the High Capacity RNA-to-cDNA kit (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). qPCR was performed
using a ViiA7 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. Reactions were performed at 50°C for 2 min and 95°C
for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. The relative Bcl-2 expression was calculated using GAPDH as an
endogenous internal control. The primer sequences were as follows:
Bcl-2, forward: 5′-ATGGGCTGGGCATAGAGAC-3′ and reverse:
5′-TTGAAGCCATGTGTCCACTC-3′; GAPDH, forward:
5′-AGCTGAACGGGAAGCTCACT-3′ and reverse:
5′-TAGGTCCACCACTGACACGTTG-3′.
Additionally, RT-qPCR was performed with primers
specific for human miR-21 (MIMAT0004494), miR-29a (MIMAT0000086),
miR-29b (MIMAT0000100), miR-29c (MI MAT0000681), miR-133a
(MIMAT0000427) and miR-133b (MIMAT0000770). All primers were
designed by Tiangen Biotech Co., Ltd. (Beijing, China). Each
reaction was repeated in triplicate, and the experiments were
repeated to confirm reproducibility.
Immunohistochemical analysis
Tissue sections were deparaffinized in xylene and
rehydrated through graded alcohol washes, prior to incubating with
1% H2O2 in methanol for 10 min at room
temperature to inhibit endogenous peroxidase activity. Following
washing with PBS, the tissue sections were incubated with a mouse
anti-Bcl-2 antibody (1:100; catalog no. ab692; Abcam, Cambridge,
MA, USA) in a humid chamber at 4°C overnight, followed by
incubation with a biotinylated anti-mouse secondary antibody
(1:200; catalog no. ZDR-5307; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) for 30 min at room
temperature. Diaminobenzidine tetrahydrochloride was used to
visualize the antibody binding, and hematoxylin counterstain was
performed prior to the addition of coverslips. Omission of the
primary antibody resulted in a lack of specific staining,
therefore, serving as the negative control. Specimens were examined
with an FSX100 microscope (Olympus Corporation). A total of 20
images of each section were captured at a magnification of ×20. The
expression of Bcl-2 was analyzed using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc.). The positive expression of
Bcl-2 was determined by the ratio of the positive area to the total
area.
Western blot analysis
Tissue samples were lysed in ice-cold RIPA buffer
[20 mM Tris (pH 7.5), 150 mM NaCl, 50 mM NaF, 1% NP-40, 0.1%
deoxycholate, 0.1% SDS and 1 mM EDTA, supplemented with 1 mM PMSF
and 1 µg/ml leupeptin]. The protein concentration was determined
using Bio-Rad DC Protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). An equal quantity (20 mg) of the samples were
fractionated by SDS-PAGE and transferred to a polyvinylidene
difluoride membrane (GE Healthcare Life Sciences, Chalfont, UK).
The membranes were blocked for 1 h at room temperature with 5%
non-fat dry milk in Tris-buffered saline containing 0.05% Tween,
and were subsequently incubated overnight at 4°C with a polyclonal
rabbit anti-Bcl-2 antibody (1:1,000; catalog no. ab59348; Abcam) or
a rabbit GAPDH polyclonal antibody (1:3,000, catalog no.
10494-1-AP; ProteinTech Group, Inc., Chicago, IL, USA). Following
washing three times, the membranes were incubated with a
horseradish peroxidase-labeled goat anti-rabbit IgG (1:5,000;
catalog no. BA1054; Boster Systems, Inc., Pleasanton, CA, USA), and
the immunoblots were visualized using enhanced chemiluminescence
Western Blotting Luminol reagent (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The intensity of the bands from each protein
under investigation was quantified using ImageJ software version
1.48 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Statistical significance was assessed using paired
Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS software (version, 18.0; SPSS, Inc., Chicago,
IL, USA).
Results
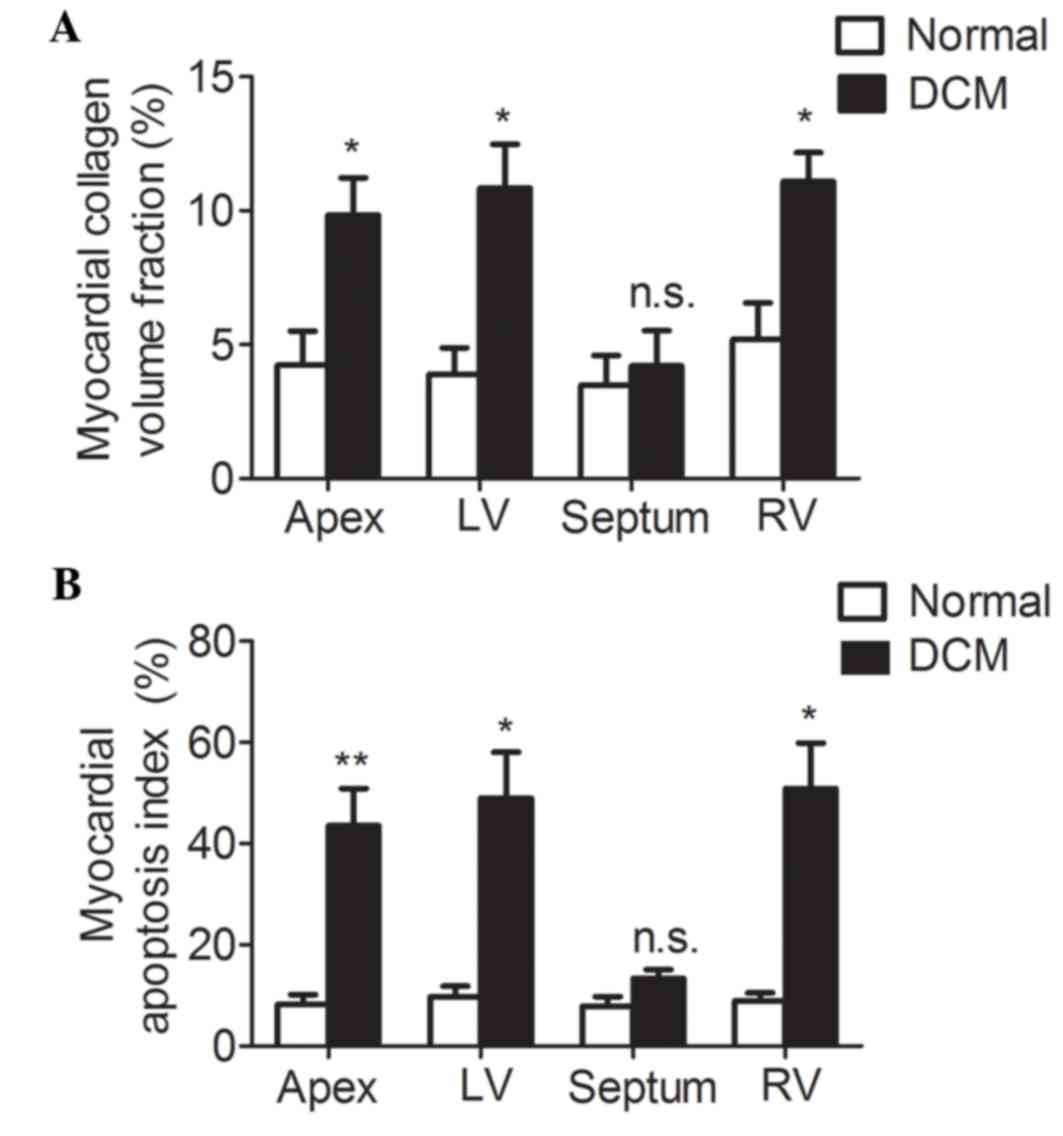
Fibrosis and apoptosis is increased in
cardiac tissues of patients with DCM
Pathological changes in myocardial tissue of DCM
patients were examined using Masson's trichrome staining and TUNEL.
Masson's trichrome staining demonstrated that collagen was
appropriately arranged among cardiomyocytes in the normal group,
while it was increased and disorganized in the DCM group (data not
shown). The size of the nucleus was uniform in the normal
cardiomyocytes, but irregular in the cardiomyocytes from patients
with DCM (data not shown). In the DCM group, the collagen volume
fractions, a morphometric measure of the percentage of interstitial
collagen, were significantly higher when compared with the normal
group in the apex, and the left and right ventricles (Fig. 1A; 4.23±1.28 vs. 9.84±1.39%,
P<0.05; 3.89±0.99 vs. 10.84±1.65%, P<0.05; 5.19±1.36 vs.
11.09±1.07%, P<0.05, respectively); however, were not
significantly increased in the septum (Fig. 1A; 3.48±1.11 vs. 4.21±1.31, P=0.69).
TUNEL was used to label apoptotic cells. The results presented in
the present study indicated a greater TUNEL staining in the tissue
sections of apex, and left and right ventricles of the DCM group
when compared with the normal group (Fig. 1B). The myocardial apoptosis index,
denoted as the percentage of TUNEL positive cells, was
significantly higher in the apex, and the left and right ventricle
of the DCM group when compared with the normal group (Fig. 1B; 8.25±1.93 vs. 43.6±7.31%,
P<0.01; 9.70±2.13 vs. 49.02±9.08%, P<0.05; 8.92±1.56 vs.
50.89±8.99%, P<0.05, respectively). The myocardial apoptosis
index in the septum presented no statistical difference between the
two groups (Fig. 1B; 7.92±1.83 vs.
13.35±1.76%, P=0.09). Therefore, cardiac fibrosis and apoptosis
were significantly increased in apex, and the left and right
ventricle of patients with DCM when compared with the normal
control group.
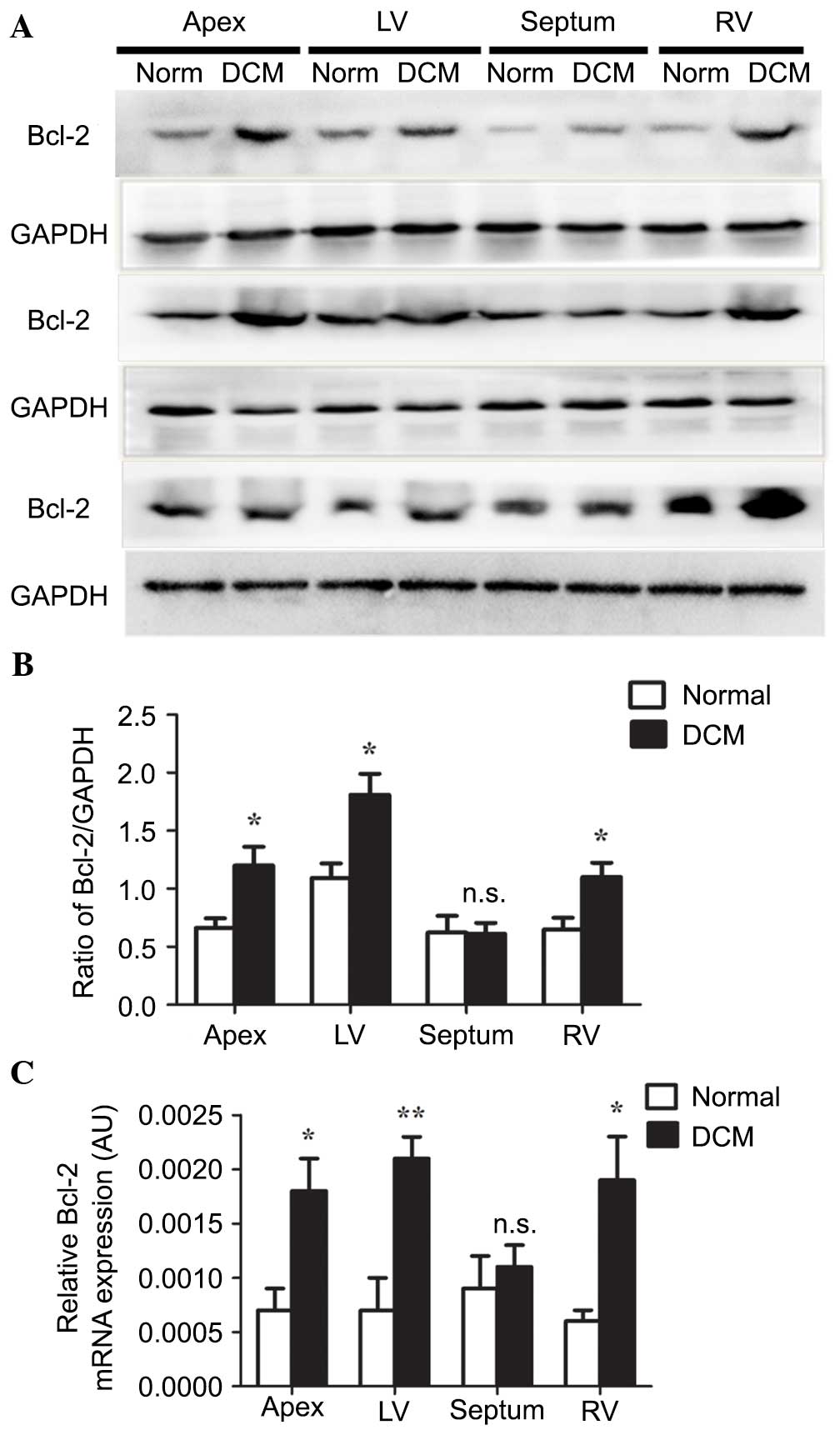
Expression of Bcl-2 is upregulated in
cardiac tissues from patients with DCM
Bcl-2 is an inner mitochondrial membrane protein
that blocks programmed cell death. The present study analyzed the
expression levels of Bcl-2 in cardiac tissue from human samples.
Western blot analysis demonstrated that Bcl-2 was significantly
increased in the apex, and the left and right ventricle of DCM
samples when compared with the normal group (Fig. 2A), which was confirmed by
densitometry analysis (Fig. 2B;
0.6623±0.0824 vs. 1.1991±0.1623, P<0.05; 1.0925±0.1262 vs.
1.8084±0.1831, P<0.05; 0.6459±0.1029 vs. 1.0994±0.1243,
P<0.05, respectively). The mRNA expression of Bcl-2 was
determined by RT-qPCR. This indicated that Bcl-2 was significantly
upregulated in the apex, and the left and right ventricle of the
patients with DCM compared with the normal group (Fig. 2C; 0.0007±0.0002 vs. 0.0018±0.0003,
P<0.05; 0.0007±0.0003 vs. 0.0021±0.0002, P<0.01;
0.0006±0.0001 vs. 0.0019±0.0004, P<0.05, respectively). No
significant difference was observed in either the mRNA or protein
expression level of Bcl-2 in the septum (Fig. 2B, P=0.95; Fig. 2C, P=0.60, respectively).
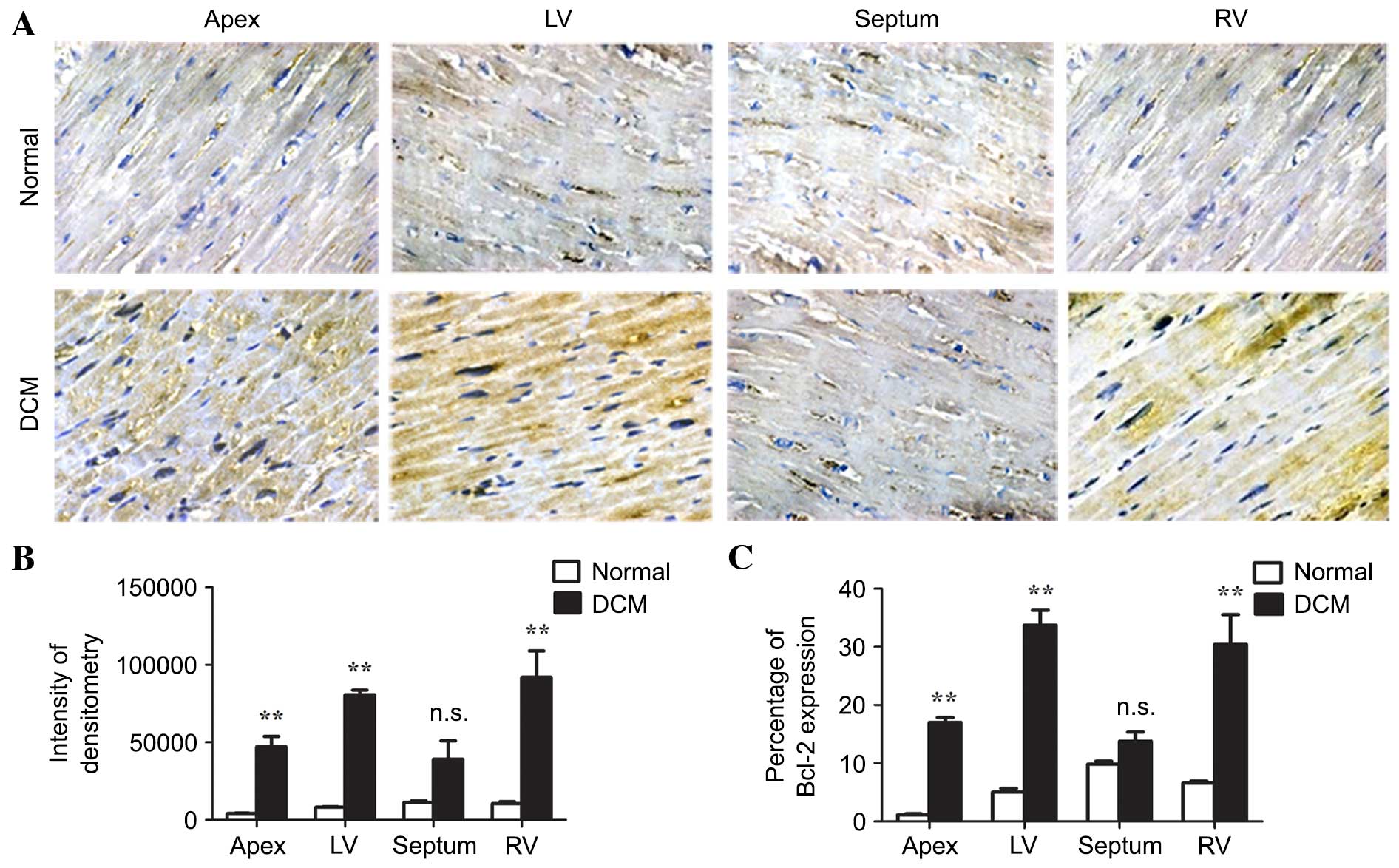
Immunohistochemistry of Bcl-2 was performed on the cardiac tissues
(Fig. 3A) and the staining was
quantified by the intensity of cytoplasmic staining and percentage
of expression. Each analysis indicated that the expression of Bcl-2
was increased in the apex, and the left and right ventricle
(P<0.01), however, not in the septum (P=0.08, P=0.07,
respectively) of the DCM cardiac tissues (Fig. 3B and C). These results indicated
that Bcl-2 was upregulated in the majority of regions of the
ventricles, with the exception except the septum.
Expression of miRNAs is differentially
regulated in cardiac tissues from patients with DCM
The expression levels of miR-21, and members of the
miR-29 and miR-133 families were examined in the apex, septum, and
left and right ventricles using RT-qPCR. miR-21 was significantly
upregulated in the apex, and the left and right ventricles of
patients with DCM when compared with the normal group (Fig. 4A; P<0.05, P<0.01, P<0.05,
respectively). In these locations, the expression levels of
miR-29a, miR-29b, miR-29c, miR-133a and miR-133b were significantly
downregulated in comparison with the normal group (Fig. 4B-F; miR-29a: P<0.05, P<0.01,
P<0.01, respectively; miR-29b: P<0.05, P<0.05, P<0.05,
respectively; miR-29c: P<0.05, P<0.05, P<0.05,
respectively; miR-133a: P<0.05, P<0.01, P<0.05,
respectively; miR-133b: P<0.01, P<0.01, P<0.05,
respectively). In the septum, no significant difference was
identified for the expression of all miRNAs between normal and DCM
groups (Fig. 4A-F; miR-21, P=0.86;
miR-29a, P=0.09; miR-29b, P=0.59; miR-29c, P=0.55; miR-133a,
P=0.99; miR-133b, P=0.96). Thus, the expression of miRNAs is
differentially regulated in DCM.
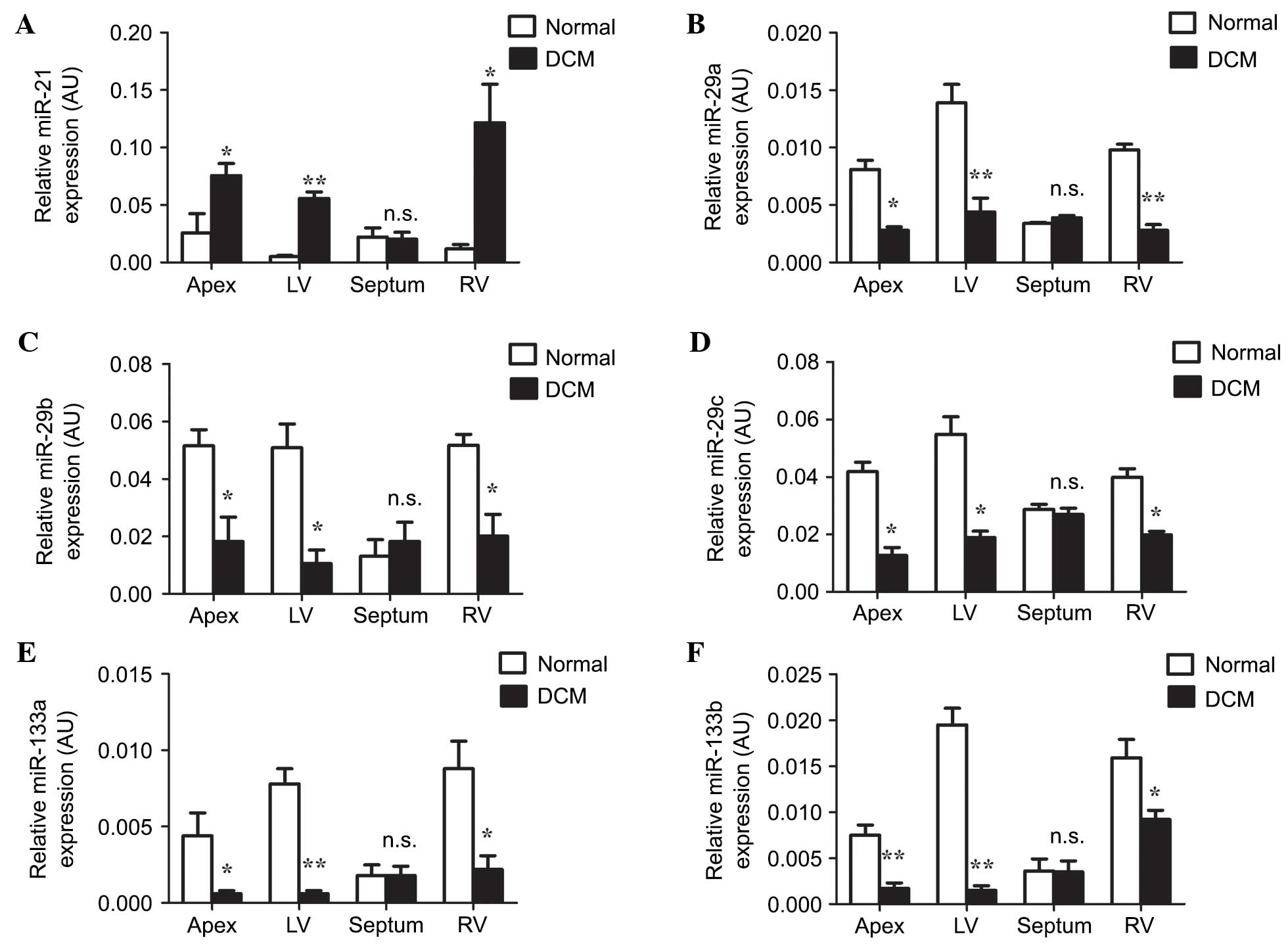 | Figure 4.Reverse transcription-quantitative
polymerase chain reaction analysis of microRNA expression in human
cardiac tissues. The relative expression levels of (A) miR-21, (B)
miR-29a, (C) miR-29b, (D) miR-29c, (E) miR-133a and (F) miR-133b
were determined. The data are presented as the mean ± standard
error of the mean (n=3; n.s., non-significant; *P<0.05;
**P<0.01 compared with the Normal group). LV, left ventricle;
RV, right ventricle; RT-qPCR, quantitative reverse transcriptase
polymerase chain reaction; AU, arbitrary units; DCM, dilated
cardiomyopathy; miR, microRNA. |
Discussion
DCM is associated with significant morbidity and
premature mortality (23). The
mechanisms of DCM pathogenesis remain unclear. In the present
study, fibrosis and apoptosis in cardiac tissues of healthy donors
and patients with DCM were examined. It was demonstrated that the
expression of Bcl-2 was upregulated in the apex, and the left and
right ventricles of the heart in patients with DCM. Furthermore,
these results demonstrated that miRNAs are differentially regulated
in the cardiac tissues of DCM patients, which are associated with
the expression of Bcl-2. Taken together, the present study
suggested that dysregulation of Bcl-2 and specific miRNAs may
involve in the pathogenesis of DCM.
Cardiovascular fibrosis alters myocardial stiffness
and leads to ventricular dysfunction (5). Biopsy studies have demonstrated that
DCM is associated with diffuse myocardial fibrosis in the
ventricles (24). Unverferth et
al (25) observed extensive
left ventricle tissue fibrosis in patients with DCM. In the present
study, it was identified that fibrosis, which is calculated by
collagen content, significantly increased in the apex, and the left
and right ventricles of patients with DCM when compared with the
normal group. In these locations of the heart, an elevated number
of TUNEL-positive cells were observed in the tissues from patients
with DCM. Based on the observation of dead myocytes and replacement
fibrosis, myocardial cell loss was likely attributed to tissue
necrosis. However, apoptosis also occurs in human hearts under
various pathological conditions, including acute myocardial
infarction, arrhythmogenic right ventricular dysplasia and
restenosis (4,26,27).
These results support that apoptosis serves a critical role in DCM.
No significant change was demonstrated in fibrosis and apoptosis in
the septum, suggesting that septum may be affected in a later stage
compared with the ventricles and apex.
Bcl-2 is one of the primary regulators of apoptosis
and the Bcl-2 proto-oncogene suppresses apoptosis (28). Using immunochemistry, western
blotting and RT-qPCR, significant increases in the expression of
Bcl-2 were observed in the apex, and the left and right ventricles
in patients with DCM compared with the normal donors. These
locations also demonstrated intensive fibrosis and apoptosis,
suggesting that upregulation of Bcl-2 may be associated with
cardiac fibrosis and apoptosis. Upregulation of this antiapoptotic
protein raises the apoptotic threshold, which increases apoptosis
resistance under cellular abnormalities. Although the detailed
mechanisms of Bcl-2 protein function remain to be fully understood,
it binds to proapoptotic proteins and inhibits channels crossing
mitochondria (29). Since
apoptosis is responsible for the progression of heart failure and
the chronic loss of ventricular function, the increased expression
of Bcl-2 may implicate the presence of a compensatory mechanism in
patients with DCM.
miRNAs are endogenous, non-coding, 21–23 nucleotide
RNAs that regulate gene expression by binding to the 3′untranslated
region (3′UTR) of the target gene mRNAs to repress translation or
induce mRNA cleavage. Increasing evidence suggests that certain
miRNAs contribute to cardiac fibrosis (30). The present study identified that
six miRNAs (miR-21; miR-29a, miR-29b and miR-29c; miR-133a and
miR-133b) were significantly associated with the pathogenesis of
DCM. miR-21 has been increasingly drawing the interests of
cardiovascular research. Thum et al (31) demonstrated that upregulated
expression of miR-21 enhances the signal transduction of the
EPK-MAPK pathway, thereby leading to the proliferation of
fibroblasts and cardiac fibrosis. In addition, silencing of the
miR-21 gene inhibits the transduction of EPK-MAPK signals, thus
inhibiting the fibrosis and improving cardiac functions (31). In a mouse model of
ischemia-reperfusion, miR-21 regulates MMP-2 expression via a PTEN
pathway during cardiac fibrosis and remodeling (32). This previous study revealed that
the expression of miR-21 was higher in highly fibrotic sections of
DCM tissue, which is consistent with its role in fibrosis.
miR-29 and miR-133 are also positive regulators of
fibrosis (32–35). The miR-29 family of miRNAs has been
demonstrated to target gene transcripts that encode ECM proteins
involved in fibrotic responses, including different collagen
isoforms (COL1A1, COL1A2, and COL3A1), fibrillin-1 and elastin
(33). Downregulation of miR-29
induces the expression of collagens, whereas overexpression of
miR-29 in fibroblasts reduces collagen expression (33). miR-29 acts as a positive regulator
of fibrosis and represents a potential therapeutic target.
Additionally, miR-133 is a novel molecular player in the heart.
Decreased miR-133 in myocytes induces an upregulation of TGF-β1 and
TGF-βRII proteins, which leads to enhanced collagen production and
fibrosis generation (34). In
addition, miR-133 positively regulates the β1AR pathway at multiple
levels, which causes pathological cardiac hypertrophy and fibrosis
(35,36). In the present study, the expression
levels of miR-29 and miR-133 family members were lower in DCM
tissues compared with in the corresponding locations in normal
heart tissue. This suggested that mechanisms of negative regulation
on fibrosis through miRNAs may exist during the progression of
DCM.
In conclusion, extensive myocardial fibrosis and
apoptosis in human DCM cardiac tissues was observed. The expression
of the apoptosis regulator Bcl-2 was increased in the apex, and the
left and right ventricles in patients with DCM compared with that
in normal controls. In these locations, miR-21 was upregulated,
while members of miR-29 family and miR-133 family were
downregulated. The present study suggested that miRNAs are
differentially regulated during cardiac fibrosis, thus they may
represent a novel class of therapeutic targets for DCM.
Acknowledgements
The present study was supported by grants from The
Natural Science Foundation of Shandong Province (nos. Z2006C10 and
ZR2013HM028) and The National Natural Science Foundation of China
(no. 81370269). The authors are grateful for the support from
Shandong Taishan Scholarship awarded to Ju Liu.
References
|
1
|
Burkett EL and Hershberger RE: Clinical
and genetic issues in familial dilated cardiomyopathy. J Am Coll
Cardiol. 45:969–981. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jefferies JL and Towbin JA: Dilated
cardiomyopathy. Lancet. 375:752–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Felker GM, Thompson RE, Hare JM, Hruban
RH, Clemetson DE, Howard DL, Baughman KL and Kasper EK: Underlying
causes and long-term survival in patients with initially
unexplained cardiomyopathy. N Engl J Med. 342:1077–1084. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isner JM, Kearney M, Bortman S and Passeri
J: Apoptosis in human atherosclerosis and restenosis. Circulation.
91:2703–2711. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weber KT, Brilla CG and Janicki JS:
Myocardial fibrosis: Functional significance and regulatory
factors. Cardiovasc Res. 27:341–348. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agocha A, Lee HW and Eghbali-Webb M:
Hypoxia regulates basal and induced DNA synthesis and collagen type
I production in human cardiac fibroblasts: Effects of transforming
growth factor-beta1, thyroid hormone, angiotensin II and basic
fibroblast growth factor. J Mol Cell Cardiol. 29:2233–2244. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Napoli P, Taccardi AA, Grilli A, Felaco
M, Balbone A, Angelucci D, Gallina S, Calafiore AM, De Caterina R
and Barsotti A: Left ventricular wall stress as a direct correlate
of cardiomyocyte apoptosis in patients with severe dilated
cardiomyopathy. Am Heart J. 146:1105–1111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsipis A, Athanassiadou AM, Athanassiadou
P, Kavantzas N, Agrogiannis G and Patsouris E: Apoptosis-related
factors p53, bcl-2 and the defects of force transmission in dilated
cardiomyopathy. Pathol Res Pract. 206:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cleary ML and Sklar J: Nucleotide sequence
of a t(14;18) chromosomal breakpoint in follicular lymphoma and
demonstration of a breakpoint-cluster region near a
transcriptionally active locus on chromosome 18. Proc Natl Acad Sci
USA. 82:7439–7443. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato T, Hanada M, Bodrug S, Irie S, Iwama
N, Boise LH, Thompson CB, Golemis E, Fong L and Wang HG:
Interactions among members of the Bcl-2 protein family analyzed
with a yeast two-hybrid system. Proc Natl Acad Sci USA.
91:9238–9242. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oltvai ZN and Korsmeyer SJ: Checkpoints of
dueling dimers foil death wishes. Cell. 79:189–192. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin XM, Oltvai ZN and Korsmeyer SJ: BH1
and BH2 domains of Bcl-2 are required for inhibition of apoptosis
and heterodimerization with Bax. Nature. 369:321–323. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen SJ, Wang JL, Chen JH and Huang RN:
Possible involvement of glutathione and p53 in trichloroethylene-
and perchloroethylene-induced lipid peroxidation and apoptosis in
human lung cancer cells. Free Radic Biol Med. 33:464–472. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Latif N, Khan MA, Birks E, O'Farrell A,
Westbrook J, Dunn MJ and Yacoub MH: Upregulation of the Bcl-2
family of proteins in end stage heart failure. J Am Coll Cardiol.
35:1769–1777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voinnet O: RNA silencing: Small RNAs as
ubiquitous regulators of gene expression. Curr Opin Plant Biol.
5:444–451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hammond SM: Dicing and slicing: The core
machinery of the RNA interference pathway. FEBS Lett.
579:5822–5829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikeda S, Kong SW, Lu J, Bisping E, Zhang
H, Allen PD, Golub TR, Pieske B and Pu WT: Altered microRNA
expression in human heart disease. Physiol Genomics. 31:367–373.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nair N, Kumar S, Gongora E and Gupta S:
Circulating miRNA as novel markers for diastolic dysfunction. Mol
Cell Biochem. 376:33–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong S, Ma W, Hao B, Hu F, Yan L, Yan X,
Wang Y, Chen Z and Wang Z: microRNA-21 promotes cardiac fibrosis
and development of heart failure with preserved left ventricular
ejection fraction by up-regulating Bcl-2. Int J Clin Exp Pathol.
7:565–574. 2014.PubMed/NCBI
|
|
21
|
Richardson P, McKenna W, Bristow M, Maisch
B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I,
et al: Report of the 1995 world health organization/international
society and federation of cardiology task force on the definition
and classification of cardiomyopathies. Circulation. 93:841–842.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang WB, Du QJ, Li H, Sun AJ, Qiu ZH, Wu
CN, Zhao G, Gong H, Hu K, Zou YZ and Ge JB: The therapeutic effect
of rosuvastatin on cardiac remodelling from hypertrophy to fibrosis
during the end-stage hypertension in rats. J Cell Mol Med.
16:2227–2237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adams KF Jr, Dunlap SH, Sueta CA, Clarke
SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L and Koch G:
Relation between gender, etiology and survival in patients with
symptomatic heart failure. J Am Coll Cardiol. 28:1781–1788. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Y, Peters DC, Dokhan B and Manning WJ:
Shorter difference between myocardium and blood optimal inversion
time suggests diffuse fibrosis in dilated cardiomyopathy. J Magn
Reson Imaging. 30:967–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Unverferth DV, Baker PB, Swift SE, Chaffee
R, Fetters JK, Uretsky BF, Thompson ME and Leier CV: Extent of
myocardial fibrosis and cellular hypertrophy in dilated
cardiomyopathy. Am J Cardiol. 57:816–820. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saraste A, Pulkki K, Kallajoki M,
Henriksen K, Parvinen M and Voipio-Pulkki LM: Apoptosis in human
acute myocardial infarction. Circulation. 95:320–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mallat Z, Tedgui A, Fontaliran F, Frank R,
Durigon M and Fontaine G: Evidence of apoptosis in arrhythmogenic
right ventricular dysplasia. N Engl J Med. 335:1190–1196. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boise LH, González-García M, Postema CE,
Ding L, Lindsten T, Turka LA, Mao X, Nuñez G and Thompson CB:
bcl-x, a bcl-2-related gene that functions as a dominant regulator
of apoptotic cell death. Cell. 74:597–608. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antonsson B, Conti F, Ciavatta A,
Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod
JJ, Mazzei G, et al: Inhibition of Bax channel-forming activity by
Bcl-2. Science. 277:370–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roy S, Khanna S, Hussain SR, Biswas S,
Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ and Sen CK: MicroRNA
expression in response to murine myocardial infarction: miR-21
regulates fibroblast metalloprotease-2 via phosphatase and tensin
homologue. Cardiovasc Res. 82:21–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA.
103:18255–18260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai
B, Wang N, Li X, Feng T, Hong Y and Yang B: Downregulation of
miR-133 and miR-590 contributes to nicotine-induced atrial
remodelling in canines. Cardiovasc Res. 83:465–472. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Engelhardt S, Hein L, Wiesmann F and Lohse
MJ: Progressive hypertrophy and heart failure in beta1-adrenergic
receptor transgenic mice. Proc Natl Acad Sci USA. 96:7059–7064.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bisognano JD, Weinberger HD, Bohlmeyer TJ,
Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC,
Wu SC, et al: Myocardial-directed overexpression of the human
beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol.
32:817–830. 2000. View Article : Google Scholar : PubMed/NCBI
|