Introduction
Serotonin (5-hydroxytryptamine; 5-HT) is an
important neurotransmitter that is expressed in the
gastrointestinal tract, blood platelets and the central nervous
system (CNS) (1). However, >90%
of total body 5-HT is synthesized, stored and secreted in the
enterochromaffin (EC) cells of the intestinal mucosa (2). Following secretion, 5-HT exerts
numerous effects on the cells and tissues of the intestine,
including activation or relaxation of smooth muscle, epithelial
cell secretion, stimulation of sensory neurons and activation of
cholinergic neurons (3–5); however, 5-HT is rapidly removed by
the serotonin uptake transporter in enterocytes and neurons
(6). Furthermore, the various
effects of 5-HT may be mediated by 14 5-HT receptors. Of these, the
important 5-HT3 and 5-HT4 receptors, which
are G protein-coupled receptors expressed by smooth muscle cells,
EC cells and myenteric plexus neurons, are considered to be the
primary pharmacological targets for gastrointestinal disorder
treatment (7,8). This is due to significant alterations
in the 5-HT receptor signaling pathway during inflammatory bowel
disease, irritable bowel syndrome, postinfectious irritable bowel
syndrome and idiopathic constipation (7,9–11).
Several safe and effective chemical drugs for the treatment of
irritable bowel syndrome with constipation are widely used.
Agonists of the 5-HT4 receptor, including tegaserod,
cisapride and benimidazolones, have been developed to treat slow
transit through the promotion of gastric emptying and alleviation
of chronic constipation, whereas 5-HT3 receptor
antagonists, including alosetron and cilansetron, have been used to
treat nausea, emesis and diarrhea through decreased visceral
sensitivity and postprandial motility (12–14).
However, investigators are seeking novel drugs originating from
natural products due to the pharmacological and economical
limitations of chemical drugs, including toxicity, side effects and
high cost.
Various herbal plants exhibiting laxative activity
have been investigated as possible novel therapeutic strategies for
the treatment of constipation to circumvent the side effects of
chemical drugs (15–17). Significant improvements in
intestinal motility, stool number, stool water content and distal
colon thickness have previously been observed in constipated
animals treated with Aloe ferox Mill (18), Aquilaria
sinensis/Aquilaria crasna (15), and Ficus carica (16,19).
Treatment with aqueous extract of Liriope platyphylla
(AEtLP) has also been shown to effectively improve loperamide
(Lop)-induced constipation in Sprague Dawley (SD) rats via
increased stool and urine excretion, villus length, crypt layer,
muscle thickness and lipid droplet secretion (19). This previous study provided the
first evidence that the laxative effects of AEtLP may be closely
correlated with the muscarinic acetylcholine receptors signaling
pathway (19). However, the
primary components responsible for laxative activity and the
underlying mechanisms remain to be elucidated.
The present study was conducted to investigate the
effects of five laxative candidates [diosgenin (DG),
5-hydroxymethylfurfural (5-HMF), adenosine (AD), hydroxypropyl
cellulose (HPC) and uridine (UD)] derived from L.
platyphylla on the 5-HT receptor downstream signaling pathway
in primary rat intestine smooth muscle cells (pRISMCs), intestinal
epithelial cells (IEC)-18 and B35 cells. The results of the present
study provide evidence that UD may be a potential candidate for
5-HT receptor signaling pathway regulation.
Materials and methods
Care and use of laboratory
animals
The animal protocol used in the present study was
reviewed and approved based on ethical procedures and scientific
care by the Pusan National University-Institutional Animal Care and
Use Committee (Miryang, South Korea; Approval Number
PNU-2014-0572). Adult male and female SD rats (age, 8 weeks;
weight, 220–250 g; n=6) purchased from Samtako Inc. (Osan, South
Korea) were handled in the Pusan National University-Laboratory
Animal Resources Center accredited by the Ministry of Food and Drug
Safety (Osong, South Korea; Accredited Unit Number-000231) and the
Association for Assessment and Accreditation of Laboratory Animal
Care International (Frederick, MD, USA; Accredited Unit Number;
001525). Animals were provided with ad libitum access to
water and a standard irradiated chow diet (Samtako Inc.) consisting
of moisture (12.5%), crude protein (25.43%), crude fat (6.06%),
crude fiber (3.9%), crude ash (5.31%), calcium (1.14%) and
phosphorus (0.99%), throughout the feeding study. During the
experiment, all rats were maintained in specific pathogen-free
conditions under a 12-h light/dark cycle at 23±2°C and 50±10%
relative humidity. Infant rats (age, 3 days) were obtained by
breeding male and female SD rats.
Preparation of pRISMCs
pRISMCs were prepared as previously described
(20), with slight modifications
to the treatment duration and enzyme concentrations. The cells were
collected from infant rather than adult rats, since the infant
tissue had greater differentiation and proliferation abilities,
similar to those of the fetus (20,21).
Briefly, 3-day-old rats (n=5) were euthanized using a
CO2 chamber, following which their intestines were
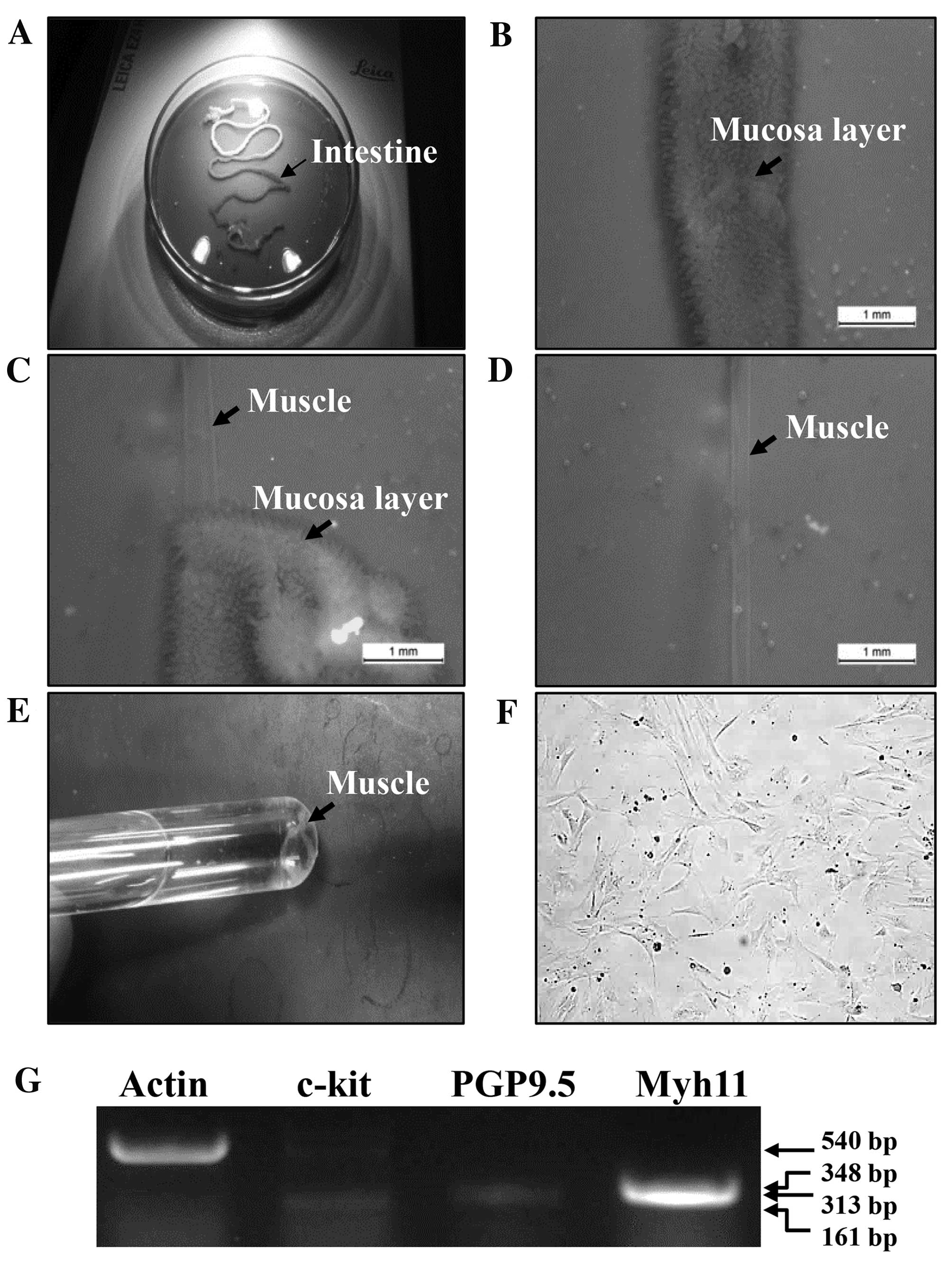
collected (Fig. 1A). The small
intestines from 1 cm below the pyloric ring to the cecum were
removed and opened along the mesenteric border (Fig. 1B). Luminal contents were then
removed by washing with calcium-free Hank's solution [5.36 mmol/l
KCl, 125 mmol/l NaCl, 0.34 mmol/l NaOH, 0.44 mmol/l
Na2HCO3, 10 mmol/l glucose, 2.9 mmol/l
sucrose and 11 mmol/l HEPES (pH 7.4)]. Tissues were pinned to the
base of a silicon-covered Petri dish, following which the mucosa
layers were removed by sharp dissection (Fig. 1C and D). Small tissue strips of the
intestinal muscle (consisting of circular and longitudinal muscles)
were incubated in digestion solution [1 mg/ml collagenase (catalog
no. 4174; Worthington Biochemical Corporation, Lakewood, NJ, USA),
0.5 mg/ml trypsin inhibitor (catalog no. T9128; Sigma-Aldrich, St.
Louis, MO, USA), 1 mg/ml bovine serum albumin (catalog no. A2153;
Sigma-Aldrich)] at 37°C for 30 min (Fig. 1E). Following centrifugation at
1,000 × g, 23–25°C for 10 min, pRISMCs were seeded into culture
plates containing Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and grown in
a 37°C humidified incubator under 5% CO2 (Fig. 1F). pRISMCs were limited to passage
5 for cell culture studies to avoid significant genetic drift.
Contamination of the pRISMC population was assessed
by reverse transcription-polymerase chain reaction (RT-PCR)
analysis, performed as described previously (22) with slight modifications to the
total RNA concentration. Total RNA was purified by removing media
from pRISMCs and homogenizing the cells in RNAzol (Tel-Test Inc.,
Friendswood, TX, USA). The isolated RNA was then measured by UV
spectroscopy and 5 µg total RNA was used to synthesize cDNA. Oligo
dT primers (500 ng; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) were annealed at 70°C for 10 min, and
deoxyadenosine, deoxycytidine, deoxyguanosine and deoxythymidine
triphosphates were added with 200 units of 200 U/µl Superscript II
reverse transcriptase (catalog no. 18064–014; Invitrogen; Thermo
Fisher Scientific, Inc.). Subsequently, 10 pmol sense and antisense
primers (Macrogen, Inc., Seoul, Korea) were added, and the reaction
mixture was subjected to 25 cycles of amplification using a PCR
Core kit (Roche Diagnostics, Basel, Switzerland). Amplification was
conducted in a Perkin-Elmer Thermal Cycler using the following
cycling conditions: An initial denaturation step of 7 min at 94°C
was performed, followed by 25 cycles of 30 sec at 94°C, 30 sec at
62°C and 45 sec at 72°C, and a final extension step of 7 min at
72°C. The primer sequences for target gene expression
identification were as follows: Sense, 5′-GCCTG CCGAA ATGTA
TGACG-3′ and antisense, 5′-GGTTC TCTGG GTTGG GGT-3′ for c-kit (an
interstitial cell of cajal marker); sense, 5′-TACTT CATGA AGCAG
ACCAT CG-3′ and antisense, 5′-CTGCA GCAGA GAGTC CTCTG AACTG-3′ for
protein gene product 9.5 (PGP9.5; a neuronal cell marker); sense,
5′-GCAAC TGAGC AATGA GCTGG TCAC-3′ and antisense, 5′-CTGCT CCTTG
TACTG CTCCA CCATC-3′ for myosin, heavy chain 11 (Myh11; a
myosin-smooth muscle cell marker); and sense, 5′-TGG AAT CCT GTG
GCA TCC ATG AAA C-3′ and antisense, 5′-TAA AAC GCA GCT CAG TAA CAG
TCC G-3′ for β-actin. The experiment was repeated three times and
all samples were analyzed in triplicate. The final PCR products
were separated on a 1.2% agarose gel and were visualized by
ethidium bromide staining. Of the three markers, only high
expression levels of Myh11 were detected (Fig. 1G).
Preparation of five candidates
As listed in Table
I, five laxative candidates (DG, 5-HMF, AD, HPC, UD) were
selected from various compounds and substances derived from the
roots of L. platyphylla as previously described (23–30).
Candidates were selected according to the following criteria: i)
Compounds and substances associated with the metabolic function of
the intestines; ii) compounds and substances that stimulate
intestinal cells; and iii) compounds and substances that have
similar structures to laxative materials reported in previous
studies (23–30). The root samples of L.
platyphylla were collected from plantations in the Miryang area
of South Korea between 2009 and 2010, and were identified by Dr Cha
Shin Woo at the Herbal Crop Research Division, National Institute
of Horticultural & Herbal Science (Eumseong, South Korea).
Voucher specimens (ref. WPC-11-010) were deposited at the
Functional Materials Bank of the Wellbeing RIS Center of Pusan
National University. DG, 5-HMF, AD, HPC and UD were purchased from
Sigma-Aldrich, whereas two commercial drugs, prucalopride (PCP;
Resolor) and bisacodyl mixture (BS; Bicogreen), were acquired from
Janssen Korea Ltd. (Seoul, South Korea) and Kolon Pharmaceuticals,
Inc. (Gwachon, South Korea), respectively.
 | Table I.Candidates derived from L.
platyphylla to investigate the laxative effects on the
5-hydroxytryptamine receptor signaling pathway. |
Table I.
Candidates derived from L.
platyphylla to investigate the laxative effects on the
5-hydroxytryptamine receptor signaling pathway.
| Candidate | Medicinal effect
(ref.) |
|---|
| Diosgenin | Anti-diabetic
effect (21) |
|
| Anti-allergic
effect (22) |
|
| Anti-inflammatory
effect (23) |
|
5-Hydroxymethylfurfural | Wound healing
effect (24) |
| Adenosine | Improves hemolytic
disease (25) |
|
| Improves
hematopoietic malignancy (26) |
| Hydroxypropyl
cellulose | Improves
cholesterol concentration (27) |
| Uridine | Sterol-lowering
effect (28) |
The roots of L. platyphylla were weighed and
then ground with a Hanil mixer (HMF-3100S; Hanil Electronics Co.,
Ltd., Seoul, South Korea). L. platyphylla powder (~1 g) was
sonicated in 10 ml distilled water for 1 h, followed by
centrifugation for 10 min at 2,500 × g, 23–25°C. The supernatant
was transferred to a 30 ml volumetric flask. This procedure was
repeated three times and respective supernatants were combined. The
final volume was adjusted to 30 ml with distilled water. Prior to
use all samples were filtered through 0.45-µm nylon membrane
filters.
High performance liquid chromatography
(HPLC) analysis
The one-dimensional HPLC system (Agilent 1100;
Agilent Technologies, Inc., Santa Clara, CA, USA) consisted of a
quaternary pump, an auto-sampler, a degasser, an automatic
thermostatic column compartment and a diode array detector.
Chromatographic conditions for AD, HPC and UD analysis were as
follows: A Phenomenex Luna C18 column (150×4.6 mm internal
diameter; 5 mm particle size; Phenomenex, Torrance, CA, USA) was
used; gradient elution was performed with (A) 0.025% formic acid in
water and (B) acetonitrile (0–10 min, 0–5% B; 10–20 min, 5% B;
20–30 min, 5–15% B; 30–40 min, 15% B; 40–50 min, 15–100% B; 50–55
min, 100% B; and 55–60 min, 100–0% B); the flow rate was 0.5
ml/min; and the column temperature was 30°C. For DD and 5-HMF
analysis, gradient elution was performed with (A) deionized water
and (B) acetonitrile (0–25 min, 30–90% B; and 25–40 min, 90% B). A
flow rate of 1.0 ml/min was used. The flow rate and pressure were
maintained at 1.53 ml/min and 35±2 psi, respectively. The
wavelength was set at 254 nm and the output signal of the detector
was recorded using Clarity™ Chromatography Software version 6.0
(DataApex, Prague, Czech Republic).
Determination of UD and AD
concentration
Individual stock solutions of UD and AD were
prepared at a concentration of 0.5 mg ml-1 in distilled water. The
quantification was performed using seven levels of external
standards. The ranges obtained were 0.5–50 µg ml-1 depending on the
concentration of each stock solution. Table II presents the calibration data
and calculated limit of detection. The linearity of the method was
evaluated by analyzing a series of standard UD and AD. Each of the
six working UD and AD solutions (10 µl) containing 0.5–50 µg was
subjected to HPLC. The elution was performed as previously
described and standard calibration curves were obtained by plotting
the concentration of UD and AD vs. peak area. The calibration range
was chosen to reflect UD and AD concentrations in watermelon
samples. The range included concentrations from the lower limit of
detection (LOD) and limit of quantification (LOQ).
 | Table II.Contents of AD and UD in roots sample
of L. platyphylla. |
Table II.
Contents of AD and UD in roots sample
of L. platyphylla.
| Compound | Regression
equation | Correlation
coefficient | Linear range
(µg/l) | Limit of detection
(µg/l) | Limit of
quantification (µg/l) | Content (µg/g dry
weight) |
|---|
| AD | y=33.02x-2.35 | 0.999994 | 0.5–50.0 | 0.224 | 0.738 | 78±2 |
| UD | y=23.79x+0.34 | 0.999999 | 0.5–50.0 | 0.062 | 0.205 | 172±2 |
Treatment schedule
IEC-18, an epithelial cell line derived from the
ileum of rat intestines, and B35, a neuroblastoma cell line derived
from tumors of the neonatal rat CNS, were purchased from the Korean
Cell Line Bank (Seoul, South Korea). The cells were cultured in a
humidified incubator at 37°C under 5% CO2 in Eagle's
minimal essential medium with Earle's balanced salt solution
(MEM/EBSS; catalog no. SH30024.01; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 2 mM glutamine, 100 U/ml penicillin and 100
µg/ml streptomycin.
To prepare the total cell lysate, the three cell
types were seeded at a density of 1×107 cells/10 ml in
100-mm diameter culture dishes, and cultured with 20 µM Lop
(Sigma-Aldrich) for 12 h in a 37°C incubator. The Lop-containing
culture media was then removed, and cells of each group were
treated with 25 µM DG, 100 µM 5-HMF, 100 µM AD, 100 µM UD, 100
µg/ml of HPC, 100 µg/ml of AEtLP, 1 µg/ml of PCP or 5 µg/ml BS,
whereas the vehicle control group received the same volume of
dH2O, for a further 12 h. The untreated group did not
receive any treatment during the experimental period. Subsequently,
cells harvested from 100-mm diameter culture dishes were
homogenized with 1% Nonidet P-40 in 150 mM NaCl, 10 mM Tris HCl (pH
7.5) and 1 mM EDTA, then supplemented with a protein inhibitor
mixture (Roche Diagnostics, Basel, Switzerland). Lysates were
stored at −70°C until use.
Western blot analysis
Total proteins were extracted from pRISMC, IEC-18
and B35 cells treated with the five candidates using Pro-Prep
Protein Extraction Solution (Intron Biotechnology, Inc., Seongnam,
Korea). Following centrifugation at 11,000 × g, 4°C for 5 min, the
protein concentrations were determined using a SMARTTM
Bicinchoninic Acid Protein assay kit (Thermo Fisher Scientific,
Inc.). Proteins (30 µg) were separated by 4–20% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis for 3 h, following which
the resolved proteins were transferred to nitrocellulose membranes
for 2 h at 40 V. Membranes were blocked with 10% skim milk in
phosphate-buffered saline (PBS) for 1 h, and incubated with the
primary antibodies, rabbit anti-G protein a subunit (1:1,000;
catalog no. ab58916; Gα; Abcam, Cambridge, UK), rabbit anti-protein
kinase C (1:1,000; catalog no. 2056; PKC; Cell Signaling Technology
Inc., Danvers, MA, USA), rabbit anti-phosphorylated PKC (1:1,000;
catalog no. 9371; p-PKC; Cell Signaling Technology Inc.) and rabbit
anti-β-actin (1:2,000; catalog no. A2066; Sigma-Aldrich) overnight
at 4°C. Membranes were washed with washing buffer (137 mM NaCl, 2.7
mM KCl, 10 mM Na2HPO4, 2 mM
KH2PO4 and 0.05% Tween 20) and incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000;
catalog no. 81–6120; Thermo Fisher Scientific, Inc.) at room
temperature for 2 h. Finally, the blots were developed using
Chemiluminescence Reagent Plus kit (Pfizer Inc., Gladstone, NJ,
USA). The signal image for each protein was acquired using the
digital camera (1.92 MP resolution) of the FluorChem®
FC2 Imaging system (Alpha Innotech Corporation, San Leandro, CA,
USA) and their density was semi-quantified using AlphaView Program
version 3.2.2 (Cell Biosciences, Inc., Santa Clara, CA). Total
protein levels of three samples from each group were analyzed in
three separate western blot analyses.
Analysis of inositol triphosphate
(IP3) concentration
The concentration of IP3 in the three cell types was
determined using a rat IP3 enzyme-linked immunosorbent assay kit
(Cusabio Biotech Co., Ltd., Wuhan, China) according to the
manufacturer's protocol. Following treatment with the five
candidates, cells (2×107) were harvested and homogenized in
ice-cold phosphate-buffered saline (pH 7.2–7.4) with a glass
homogenizer (Sigma-Aldrich). Total cell lysates were prepared with
a glass homogenizer and PBS, and centrifuged at 5,000 × g for 5 min
at room temperature, following which the supernatant was collected
for analysis. An anti-IP3 detection antibody was added and samples
were incubated at 37°C for 60 min, followed by the addition of
substrate solution for 15 min at 37°C. The reaction was terminated
by the addition of stop solution and the plates were read at an
absorbance of 450 nm using a VersaMax Plate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software version 10.10 (SPSS, Inc. Chicago, IL, USA). One-way
analysis of variance, followed by Tukey's post hoc test, was
performed to identify significant differences between the vehicle
and candidate-treated groups, or between the untreated and
Lop-treated groups. All values are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of five candidates in
L. platyphylla
The presence of the five candidates in L.
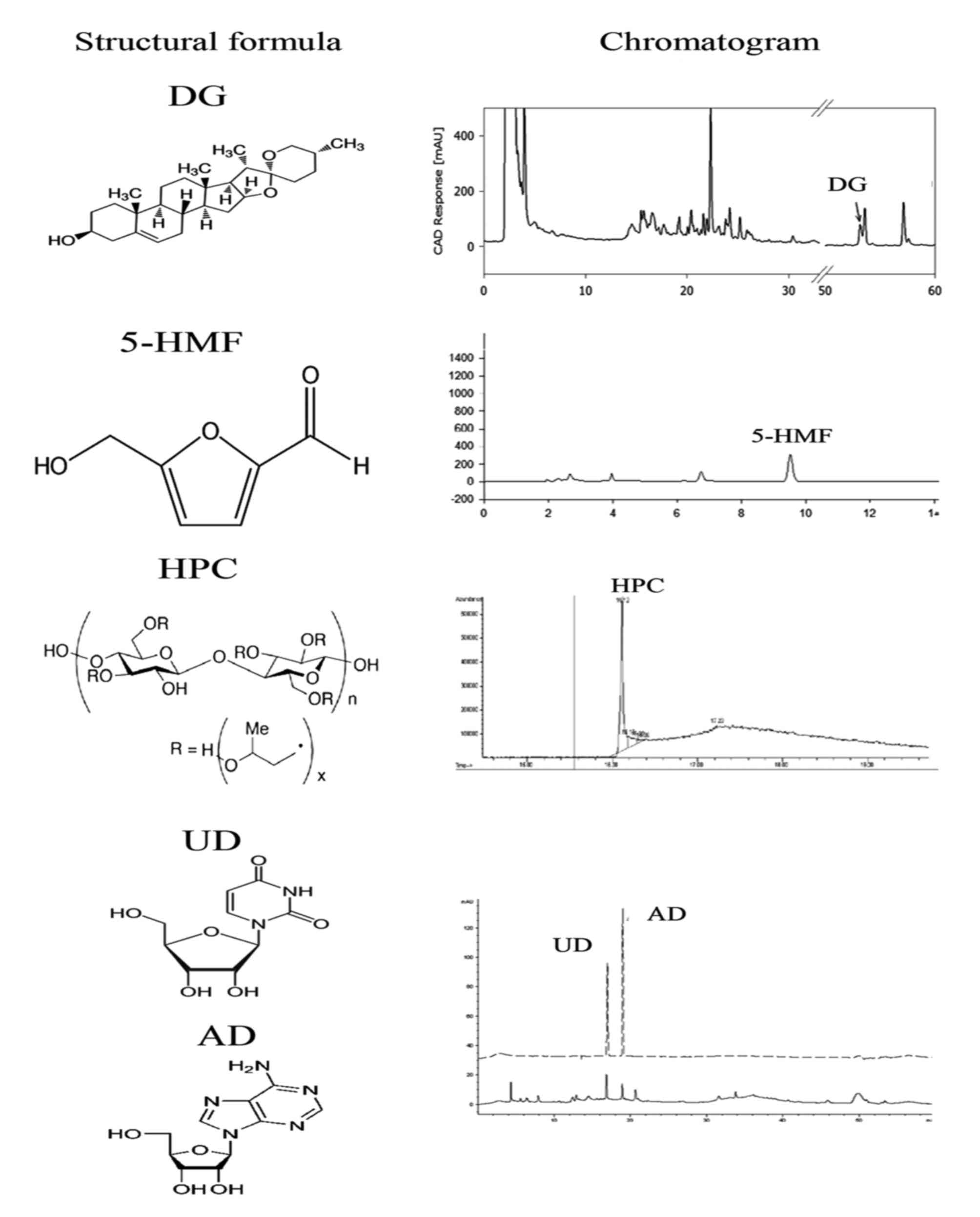
platyphylla was confirmed using HPLC analysis. As presented in
Fig. 2, DG, 5-HMF, AD, HPC and UD
were detected in the HPLC chromatogram of L. platyphylla
under the optimal conditions, although their concentrations and
detection times varied. Of the candidates, HPC had the highest
peak, and UD the lowest.
Effects of the five laxative
candidates on the 5-HT receptor signaling pathway in intestinal
muscle cells
To examine the effects of the five laxative
candidates on the 5-HT receptor signaling pathway, the expression
levels of Gα and the phosphorylation levels of PKC were measured in
Lop-pretreated pRISMCs following treatment with the five laxative
candidates (Fig. 3A). The protein
expression levels of Gα were 140% greater in the Lop+vehicle group
compared with the untreated group. However, Gα protein expression
levels decreased to varying extents in the majority of treatment
groups. Similar levels were detected in the Lop+AEtLP and Lop+PCP
treatment groups, which were used as positive controls, whereas the
lowest expression levels of Gα were measured in the Lop+5-HMF
treatment group (Fig. 3B). The
phosphorylation levels of PKC differed from Gα expression levels.
Phosphorylation levels were 430% greater in the Lop+vehicle
treatment group compared with the untreated group. However, levels
were rapidly recovered in the positive control Lop+AEtLP, Lop+PCP
and Lop+BS treatment groups. Furthermore, a marked decrease in PKC
phosphorylation was observed in the Lop+DG and Lop+AD treatment
groups, whereas partial recovery was observed in the Lop+5-HMF
treatment group. However, the level of PKC protein phosphorylation
was not decreased in the Lop+HPC and Lop+UD treatment groups
(Fig. 3C). Taken together, these
results suggest that DG, 5-HMF and AD may stimulate the complete or
partial recovery of Gα expression and PKC phosphorylation levels
induced by Lop treatment in smooth muscle cells of rat
intestine.
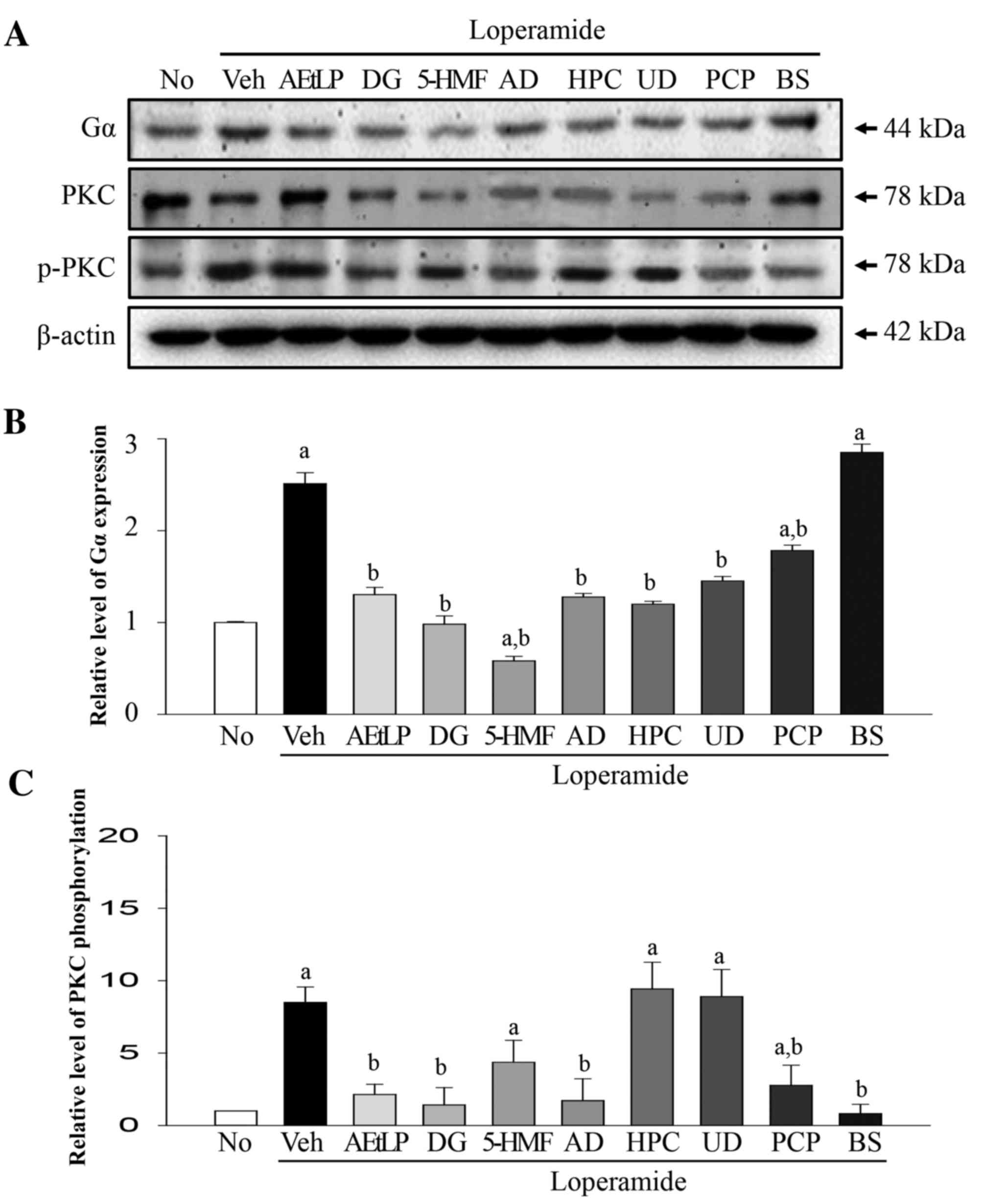 | Figure 3.Alteration of Gα expression and PKC
phosphorylation levels in pRISMC. (A) Western blot analysis was
performed on total protein extracted from Lop-pretreated pRISMCs
following treatment with the five laxative candidates. The levels
of Gα expression and PKC phosphorylation were detected using
specific antibodies. The β-actin protein expression level served as
an endogenous control. The band intensity of the three proteins was
determined by densitometry and the level of (B) Gα expression and
(C) PKC phosphorylation was calculated relative to β-actin. Data
are presented as the mean ± standard deviation of three replicates.
aP<0.05 vs. untreated group; bP<0.05
vs. Lop+vehicle treatment group. Gα, G protein α; PKC, protein
kinase C; pRISMCs, primary rat intestine smooth muscle cells; Lop,
loperamide; No, untreated; Veh, vehicle; AEtLP, aqueous extract of
Liriope platyphylla; DG, diosgenin; 5-HMF,
5-hydroxymethylfurfural; AD, adenosine; HPC, hydroxypropyl
cellulose; UD, uridine; PCP, prucalopride; BS, bisacodyl. |
Effects of the five laxative
candidates on the 5-HT receptor signaling pathway in epithelial
cells
To determine whether the effect on the 5-HT receptor
signaling pathway observed in pRISMCs occurred in epithelial cells,
the previous experiment was repeated in IEC-18 cells (Fig. 4A). Increased Gα expression levels
(75%) were detected in the Lop+vehicle treatment group compared
with the untreated group (Fig.
4B). In the Lop+AEtLP and Lop+PCP groups, this level increased
by 12.6 and 8% compared with the Lop+vehicle treatment group. A
significant decrease (P=0.042) was detected only following
treatment with three (DG, 5-HMF and UD) out of the five
candidates.
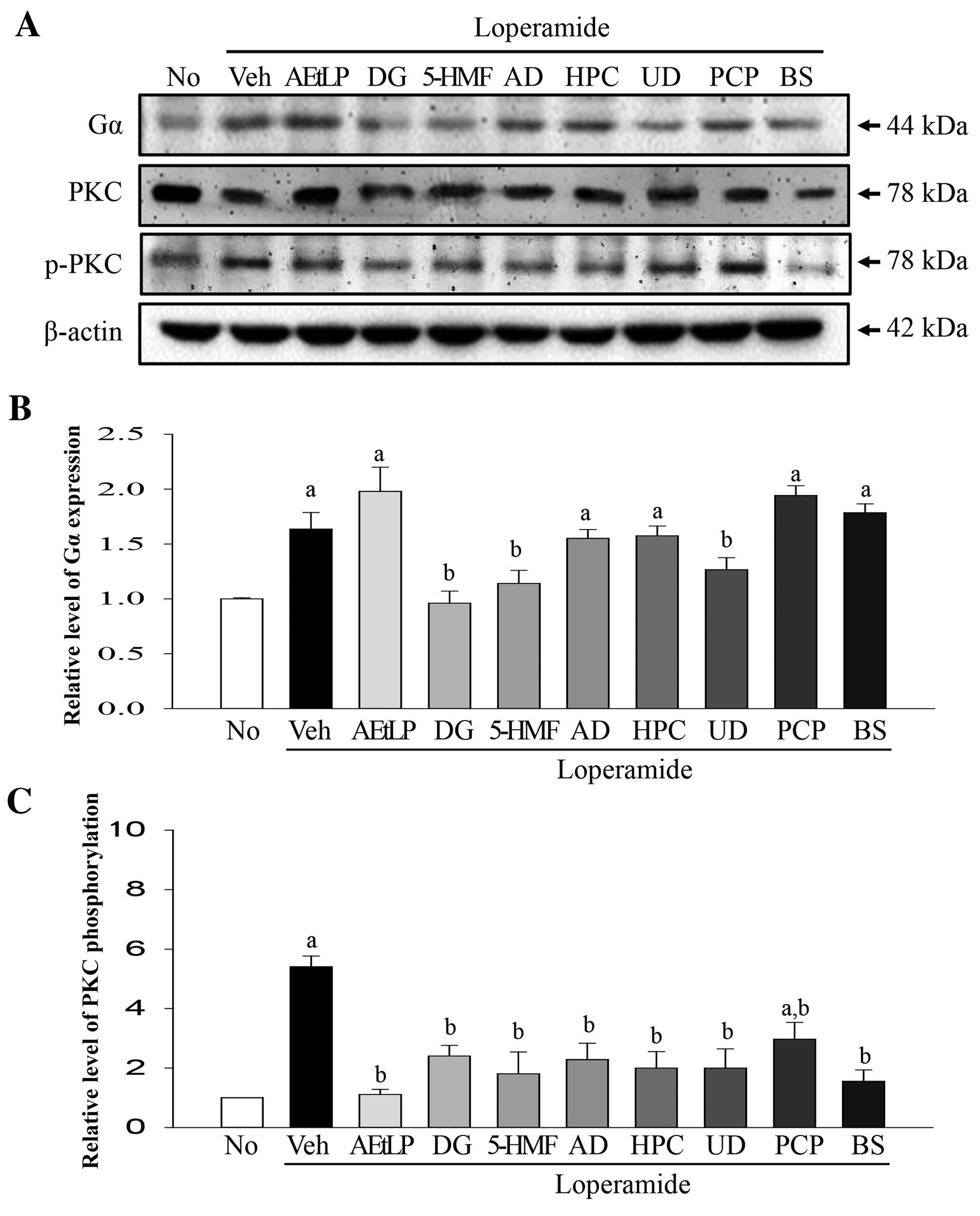 | Figure 4.Alteration of Gα expression and PKC
phosphorylation levels in IEC-18 cells. (A) Western blot analysis
was performed on total protein extracted from the Lop-pretreated
IEC-18 cells following treatment with the five laxative candidates.
The levels of Gα expression and PKC phosphorylation were detected
with specific antibodies. The β-actin protein expression level
served as an endogenous control. The band intensity of the three
proteins was determined by densitometry and the level of (B) Gα
expression and (C) PKC phosphorylation was calculated relative to
β-actin. Data are presented as the mean ± standard deviation of
three replicates. aP<0.05 vs. untreated group;
bP<0.05 vs. Lop+vehicle treatment group. Gα, G
protein α; PKC, protein kinase C; IEC-18, intestinal epithelial
cells 18; Lop, loperamide; No, untreated; Veh, vehicle; AEtLP,
aqueous extract of Liriope platyphylla; DG, diosgenin;
5-HMF, 5-hydroxymethylfurfural; AD, adenosine; HPC, hydroxypropyl
cellulose; UD, uridine; PCP, prucalopride; BS, bisacodyl. |
The phosphorylation levels of PKC differed from Gα
expression levels (Fig. 4C).
Phosphorylation levels increased 358% in the Lop+vehicle treatment
group compared with the untreated group. However, Lop+AEtLP and
Lop+BS treatment induced complete recovery of PKC phosphorylation
levels, whereas Lop+PCP treatment induced a significant decrease of
56.4% (P=0.016). Taken together, these results indicate that all
five candidates may mimic the effects of AEtLP and BS in intestinal
epithelial cells.
Effect of the five laxative candidates
on the 5-HT receptor signaling pathway in neuronal cells
To determine whether the five laxative candidates
affect the 5-HT receptor signaling pathway in neuronal cells, the
expression levels of Gα and phosphorylation levels of PKC were
measured in Lop-pretreated B35 cells following treatment with the
five laxative candidates (Fig.
5A). The Gα expression levels were reduced by 49% in the
Lop+vehicle treatment group compared with the untreated group
(Fig. 5B). These levels were
further decreased by 83–95% in the Lop+AEtLP, Lop+PCP and Lop+BS
positive control treatment groups. Following treatment with the
five candidates, the expression levels of Gα increased in the
Lop+5-HMF and Lop+HPC treatment groups compared with the
Lop+vehicle treatment group. The Gα expression levels remained
constant in the three remaining groups (DG, AD and UD). PKC
phosphorylation levels did not correspond with Gα expression levels
(Fig. 5C). Phosphorylation levels
were not altered in the Lop+vehicle and Lop+AEtLP treatment groups;
however, they were increased by 120% in the Lop+PCP and BS
treatment groups. The Lop+UD treatment group also exhibited
increased PKC phosphorylation levels; however, a significant
decrease (P=0.032) was detected in the DG, 5-HMF and AD treatment
groups. Taken together, the above results indicated that only UD
may mimic the effect of PCP and BS on PKC phosphorylation levels in
B35 neuroblastoma cells.
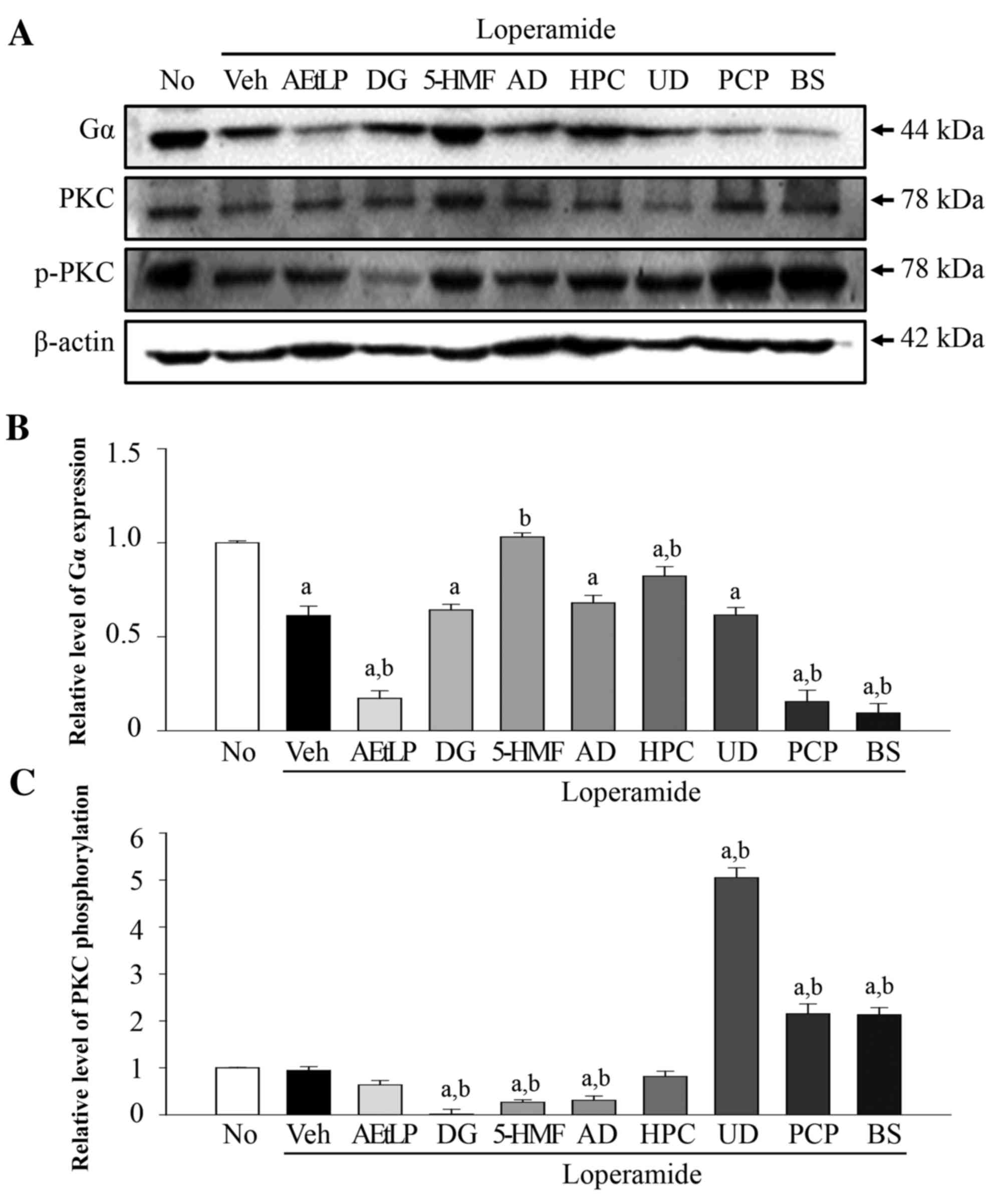 | Figure 5.Alteration of Gα expression and PKC
phosphorylation levels in B35 cells. (A) Western blot analysis was
performed on total protein extracted from the Lop-pretreated B35
cells following treatment with the five laxative candidates. The
levels of Gα expression and PKC phosphorylation were detected with
specific antibodies. The β-actin protein expression level served as
an endogenous control. The band intensity of the three proteins was
determined using densitometry and the level of (B) Gα expression
and (C) PKC phosphorylation was calculated relative to β-actin.
Data are presented as the mean ± standard deviation of three
replicates. aP<0.05 vs. untreated group;
bP<0.05 vs. Lop+vehicle treatment group. Gα, G
protein α; PKC, protein kinase C; Lop, loperamide; No, untreated;
Veh, vehicle; AEtLP, aqueous extract of Liriope platyphylla;
DG, diosgenin; 5-HMF, 5-hydroxymethylfurfural; AD, adenosine; HPC,
hydroxypropyl cellulose; UD, uridine; PCP, prucalopride; BS,
bisacodyl. |
Effects of the five laxative
candidates on IP3 concentration
IP3 concentrations in pRISMCs, IEC-18 and B35 cells
co-treated with Lop and the five candidates were examined to
confirm the results of the 5-HT receptor signaling pathway
analysis. Although IP3 was detected in pRISMCs, IEC-18 and B35
cells, a significant alteration (P=0.017) during Lop treatment was
detected only in pRISMCs (Fig.
6A). IEC-18 (Fig. 6B) and B35
(Fig. 6C) cells did not exhibit
any significant alterations (P=0.028) in IP3 concentrations. In
pRISMCs, the relative levels of IP3 were decreased by 21% in the
Lop+vehicle treatment group compared with the untreated group.
However, these levels recovered following AEtLP, PCP and BS
treatment. In addition, IP3 levels were increased in all five
candidate-treated groups, although to a lesser extent than AEtLP
and PCP. The greatest increase (223%) was detected in the Lop+UD
treatment group. These results demonstrate that UD induces a marked
increase of IP3 concentration in smooth muscle cells.
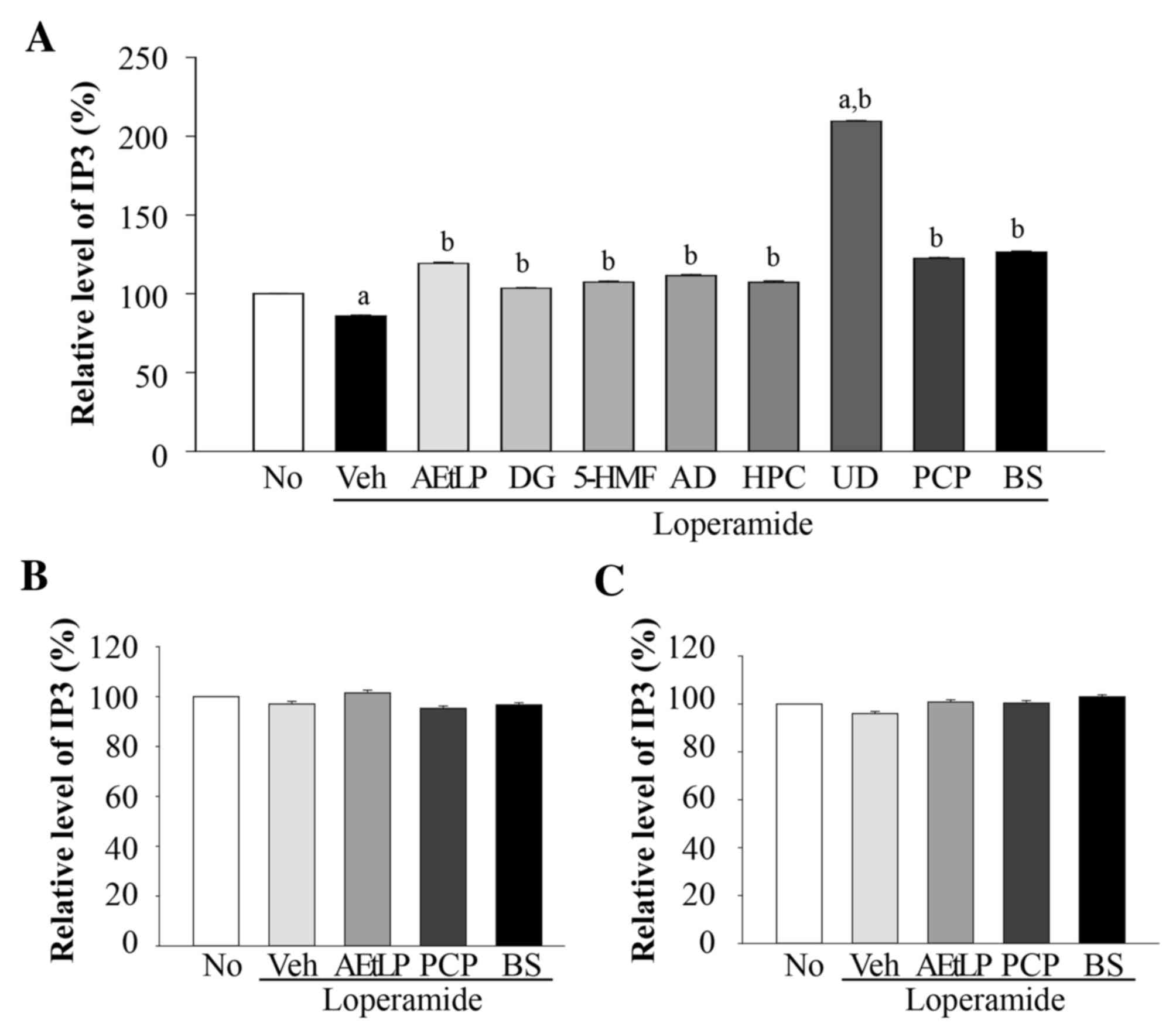 | Figure 6.Alteration of IP3 concentration in
pRISMCs, IEC-18 and B35 cells. Following treatment with Lop for 12
h, the three cell types were incubated with the five candidates.
The IP3 concentration in the total cell lysate of (A) pRISMCs, (B)
IEC-18 and (C) B35 cells was measured using an enzyme-linked
immunosorbent assay kit that detected 5 to 1000 pg/ml IP3. Data are
presented as the mean ± standard deviation of three replicates.
aP<0.05 vs. untreated group; bP<0.05
vs. Lop+vehicle treatment group. IP3, inositol triphosphate;
pRISMC, primary rat intestine smooth muscle cells; IEC-18,
intestinal epithelial cells 18; Lop, loperamide; No, untreated;
Veh, vehicle; AEtLP, aqueous extract of Liriope platyphylla;
DG, diosgenin; 5-HMF, 5-hydroxymethylfurfural; AD, adenosine; HPC,
hydroxypropyl cellulose; UD, uridine; PCP, prucalopride; BS,
bisacodyl. |
Quantification of AD and UD
UD was selected as the candidate with the greatest
potential as a novel laxative based on the Gα expression, PKC
phosphorylation and IP3 concentration levels data; therefore, the
concentration of UD was quantified. In addition, AD was quantified
as a control. The linearity for AD and UD analysis was assessed
using six standard solutions (each injected in triplicate) in a
0.5–50.0 µg ml-1 concentration range. The six-point calibration
curves were observed to be linear as least squares regression gave
a correlation coefficient of 0.9999 (Table II). LOD and LOQ were defined as a
signal-to-noise ratio of 3 and 10, respectively; LOD ranged from
0.205 to 0.738 µg ml-1 and LOQ from 0.062 to 0.224 µg ml-1.
Comparing retention times with those of authentic standards, UD and
AD were revealed to be 172 and 78 µg/g dry weight, respectively
(Table II).
Discussion
Constipation is a heterogeneous disease associated
with several symptoms, including infrequent bowel movements,
difficult fecal passage and a sensation of incomplete defecation
(31,32). In accordance with an increase in
the incidence of constipation, attempts have been made to identify
novel drugs from various natural resources. In an effort to purify
and examine novel drugs for the treatment of constipation, the
effects of five laxative candidates originating from L.
platyphylla were examined in three major cell types present in
the transverse colon. The results of the present study demonstrated
the potential of UD to mimic the effects of PCP and AEtLP in
pRISMCs, ICE-180 and B35 cells, although animal studies are
necessary to clarify the laxative effects on chronic constipation.
The present study investigated Gα, PKC and IP3 within the
downstream signaling pathway of the 5-HT receptor as their
regulation may closely associate with movement through the
gastrointestinal tract.
DG is a steroid sapogenin produced by saponin
hydrolysis during treatment with acids, strong bases or enzymes
(33). In addition, DG may be
extracted from the tubers of Dioscorea esculenta,
Angelica gigas and Trigonella foenum-graecum
(34), and exerts biological
activity against various metabolic diseases, including
dyslipidemia, obesity, diabetes, cholestasis and cancer (33,35).
A limited number of studies have been conducted to investigate the
effects of DG on PKC activation in the 5-HT receptor signaling
pathway; however, a previous study revealed significantly increased
PKC phosphorylation induced by DG administration in type I diabetic
rats (35). In the present study,
the phosphorylation levels of PKC were decreased in smooth muscle
cells, epithelial cells and neuronal cells following Lop + DG
treatment. These differences may be due to various factors,
including Lop cotreatment and experimental conditions.
Extracellular nucleotide triphosphates (NTPs),
including adenosine triphosphate (ATP) and uridine triphosphate
(UTP), regulate various physiological actions in numerous tissues
and cell types (36). In the
airways of the lung, these compounds activate Cl−
secretion from cystic fibrosis (CF) and non-CF airway epithelia,
and regulate goblet cell-mediated mucin release (37,38).
This process is mediated by P2Y or P2 U receptors, which are G
protein-coupled receptors that activate the phospholipase C (PLC)
signaling cascade (39,40). PLC results in calcium release, PKC
activation and phosphatidylinositol 4,5-bisphosphate depletion
through cleavage into diacylglycerol and IP3 (40). Although numerous studies have
reported the function of extracellular NTPs as regulatory
molecules, their role in the 5-HT receptor signaling pathway
associated with constipation has not been investigated. However, it
has previously been suggested that ATP and UTP are associated with
the improvement of constipation since their activity stimulates
mucin release from goblet cells (38). In the present study, AD in muscle
cells and epithelial cells induced effects on PKC phosphorylation
that mimicked PCP and BS, while UD was effective in epithelial
cells and neuronal cells. These results provide the first evidence
that AD and UD may improve Lop-induced constipation via regulation
of the 5-HT receptor signaling pathway.
Of the various insoluble dietary fibers, including
psyllium, glucomannan and chitosan, cellulose facilitates the
movement of waste through the human digestive tract and prevents
chronic constipation (41). Oral
administration of 5 mg/day cellulose significantly increased fecal
excretion in Lop-induced constipated rats (41), whereas methylcellulose treatment
increased the frequency of bowel movements and the ease of fecal
passage (42). To the best of our
knowledge, no studies have reported an association between HPC and
chronic constipation, although it is widely used to treat
insufficient tear production, and as food additives and
disintegrants. In the present study, the effect of HPC treatment on
Gα expression and PKC phosphorylation levels was examined in three
cell types present in the transverse colon, and the results
revealed that HPC mimicked the effect of AEtLP on Gα expression in
pRISMCs and IEC-18 as well as PKC phosphorylation in IEC-18 and B35
cells.
In conclusion, the present study investigated the
effects of five laxative candidates derived from L.
platyphylla on the 5-HT receptor signaling pathway to select a
potential novel laxative. Laxative candidates were selected on the
basis of reversing the effects of Lop and mimicking the activity of
current therapeutic agents or AEtLP in at least two cell types.
Analysis of the Gα expression levels in the three cell types
revealed three laxative candidates (DG, 5-HMF and UD), whereas
results from PKC phosphorylation in the three cell types suggested
three laxative candidates (DG, AD and UD). The additional analysis
of IP3 concentration indicated only one strong candidate (UD).
Therefore, the results of the present study suggested that UD may
be considered as a potential laxative in the treatment of chronic
constipation, although it did not exactly mimic the effects of
AEtLP, PCP and BS. Further studies are required to investigate the
therapeutic effects of these laxatives in a Lop-induced
constipation model based on the measurement of the number of feces,
histological analysis and the expression of associated
proteins.
Acknowledgements
The authors would like to thank Miss. Jin Hyang
Hwang, the animal technician, for directing the Animal Facility and
Care at the Laboratory Animal Resources Center of Pusan National
University (Miryang, Korea), and Professor Byung Joo Kim (Pusan
National University School of Korean Medicine, Yangsan, Korea) for
technical support in the collection of primary smooth muscle cells
from the rat intestines. The present study was supported by the
Basic Science Research Program through the National Research
Foundation of Korea funded by the Ministry of Education (grant no.
2014R1A1A2058360).
References
|
1
|
Young SN: How to increase serotonin in the
human brain without drugs. J Psychiatry Neurosci. 32:394–399.
2007.PubMed/NCBI
|
|
2
|
Ahn J and Ehrenpreis ED: Emerging
treatments for irritable bowel syndrome. Expert Opin pharmacother.
3:9–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bulbring E and Crema A: The release of
5-hydroxytryptamine in relation to pressure exerted on the
intestinal mucosa. J Physiol. 146:18–28. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gershon MD: Serotonin: Its role and
receptors in enteric neurotransmission. Adv Exp Med Biol.
294:221–230. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baker DE: Rationale for using serotonergic
agents to treat irritable bowel syndrome. Am J Health Syst Pharm.
62:700–711; quiz 712–713. 2005.PubMed/NCBI
|
|
6
|
Sikander A, Rana SV and Prasad KK: Role of
serotonin in gastrointestinal motility and irritable bowel
syndrome. Clin Chim Acta. 403:47–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costedio MM, Hyman N and Mawe GM:
Serotonin and its role in colonic function and in gastrointestinal
disorders. Dis Colon Rectum. 50:376–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crowell MD: Role of serotonin in the
pathophysiology of the irritable bowel syndrome. Br J Pharmacol.
141:1285–1293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Salhy M, Danielsson A, Stenling R and
Grimelius L: Colonic endocrine cells in inflammatory bowel disease.
J Intern Med. 242:413–419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lincoln J, Crowe R, Kamm MA, Burnstock G
and Lennard-Jones JE: Serotonin and 5-hydroxyindol acetic acid are
increased in the sigmoid colon in severe idiopathic constipation.
Gastroenterol. 98:1219–1225. 1990. View Article : Google Scholar
|
|
11
|
Zhao R, Baig MK, Wexner SD, Chen W, Singh
JJ, Nogueras JJ and Woodhouse S: Enterochromaffin and serotonin
cells are abnormal for patients with colonic inertia. Dis Colon
Rectum. 43:858–863. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Callahan MJ: Irritable bowel syndrome
neuropharmacology. A review of approved and investigational
compounds. J Clin Gastroenterol. 35:(1 Suppl). S58–S67. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamm MA, Müller-Lissner S, Talley NJ, Tack
J, Boeckxstaens G, Minushkin ON, Kalinin A, Dzieniszewski J, Haeck
P, Fordham F, et al: Tegaserod for the treatment of chronic
constipation: A randomized, double-blind, placebo-controlled
multinational study. Am J Gastroenterol. 100:362–372. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
von der Ohe MR, Hanson RB and Camilleri M:
Serotonergic mediation of postprandial colonic tonic and phasic
responses in humans. Gut. 35:536–541. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kakino M, Izuta H, Ito T, Tsuruma K, Araki
Y, Shimazawa M, Oyama M, Iinuma M and Hara H: Agarwood induced
laxative effects via acetylcholine receptors on loperamide-induced
constipation in mice. Biosci Biotechnol Biochem. 74:1550–1555.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS,
Li B, Lee GH, Sung MS, Ha KC, Back HI, et al: Effects of Ficus
carica paste on loperamide-induced constipation in rats. Food Chem
Toxicol. 50:895–902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Méité S, Bahi C, Yéo D, Datté JY, Djaman
JA and N'guessan DJ: Laxative activities of Mareya micrantha
(Benth.) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats.
BMC Complement Altern Med. 10:72010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wintola OA, Sunmonu TO and Afolayan AJ:
The effect of Aloe ferox Mill. in the treatmentet of
loperamide-induced constipation in Wistar rats. BMC Gastroenterol.
10:952010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT and
Hwang DY: Aqueous extracts of Liriope platyphylla induced
significant laxative effects on loperamide-induced constipation of
SD rats. BMC Complement Altern Med. 13:3332013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim BJ: Shengmaisan refulates pacemaker
potentials in interstitial cells of cajal in mice. J
Pharmacopuncture. 16:36–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thyberg J, Hedin U, Sjölund M, Palmberg L
and Bottger BA: Regulation of differentiated properties and
proliferation of arterial smooth muscle cells. Arteriosclerosis.
10:966–990. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parajuli SP, Choi S, Lee J, Kim YD, Park
CG, Kim MY, Kim HI, Yeum CH and Jun JY: The inhibitory effects of
hydrogen sulfide on pacemaker activity of interstitial cells of
cajal from mouse small intestine. Korean J Physiol Pharmacol.
14:83–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pari L, Monisha P and Jalaludeen A
Mohamed: Beneficial role of diosgenin on oxidative stress in aorta
of streptozotocin induced diabetic rats. Eur J Pharmacol.
691:143–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CH, Ku CY and Jan TR: Diosgenin
attenuates allergen-induced intestinal inflammation and IgE
production in a murine model of food allergy. Planta Med.
75:1300–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada T, Hoshino M, Hayakawa T, Ohhara H,
Yamada H, Nakazawa T, Inagaki T, Iida M, Ogasawara T, Uchida A, et
al: Dietary diosgenin attenuates subacute intestinal inflammation
associated with indomethacin in rats. Am J Physiol. 273:G355–G364.
1997.PubMed/NCBI
|
|
26
|
Black CT, Hennessey PJ, Ford EG and
Andrassy RJ: Protein glyosylation and collagen metabolism in normal
and diabetic rats. J Surg Res. 47:200–202. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicolau CT, Teitel P, Bratu V, Xenakis A
and Butoianu E: Favorable therapeutic effect of adenosine
monophosphate (AMP) in a case of compensated chronic hemolytic
disease due to insufficiency of erythrocytic energetic metabolism.
Med Interna (Bucur). 17:423–430. 1965.(In Romanian). PubMed/NCBI
|
|
28
|
Tsukamoto H: Extracellular adenosine is a
therapeutic target for limiting graft-versus-host disease and
enhancing the graft-versus-tumor effect against hematopoietic
malignancy. Yakugaku Zasshi. 134:1021–1027. 2014.(In Japanese).
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krishnaiah YS, Kumar MS, Raju V, Lakshmi M
and Rama B: Penetration-enhancing effect of ethanolic solution of
menthol on transdermal permeation of ondansetron hydrochloride
across rat epidermis. Drug Deliv. 15:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oswald S, Giessmann T, Luetjohann D,
Wegner D, Rosskopf D, Weitschies W and Siegmund W: Disposition and
sterol-lowering effect of ezetimibe are influenced by single-dose
coadministration of rifampin, an inhibitor of multidrug transport
proteins. Clin Pharmacol Ther. 80:477–485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herz MJ, Kahan E, Zalevski S, Aframian R,
Kuznitz D and Reichman S: Constipation: A different entity for
patients and doctors. Fam Pract. 13:156–159. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson WG, Longstreth GF, Drossman DA,
Heaton KW, Irvine EJ and Müller-Lissner SA: Functional bowel
disorders and functional abdominal pain. Gut. 45:(Suppl 2).
II43–II47. 1999.PubMed/NCBI
|
|
33
|
Raju J and Rao CV: Diosgenin, a steroid
saponin constituent of Yams and Fenugreek: Emerging evidence for
applications in medicineBioactive Compounds in Phytomedicine.
Rasooli I: InTech; Rijeka: pp. 125–142. 2012
|
|
34
|
Taylor WG, Elder JL, Chang PR and Richards
KW: Microdetermination of diosgenin from fenugreek (Trigonella
foenum-graecum) seeds. J Agric Food Chem. 48:5206–5210. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato K, Fujita S and Iemitsu M: Acute
administration of diosgenin or dioscorea improves hyperglycemia
with increases muscular steroidogenesis in STZ-induced type 1
diabetic rats. J Steroid Biochem Mol Biol. 143:152–159. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barke AJ and Julius D: Signaling by
extracellular nucleotides. Annu Rev Cell Dev Biol. 12:519–541.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knowler MR, Clarke LL and Boucher RC:
Activation by extracellular nucleotides of chloride secretion in
the airway epithelia of patients with cystic fibrosis. N Engl J
Med. 325:533–538. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lethem MI, Dowell ML, Van Scott M,
Yankaskas JR, Egan T, Boucher RC and Davis CW: Nucleotide
regulation of goblet cells in human airway epithelial explants:
Normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol.
9:315–322. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ralevic V and Burnstock G: Receptors for
purines and pyrimidines. Pharmacol Rev. 50:413–492. 1998.PubMed/NCBI
|
|
40
|
Falkengurger BH, Dickson EJ and Hille B:
Quantitative properties and receptor reserve of the DAG and PKC
branch of G(q)-coupled receptor signaling. J Gen Physiol.
141:537–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shimotoyodome A, Meguro S, Hase T,
Tokimitsu I and Sakata T: Sulfated polysaccharides, but not
cellulose, increase colonic mucus in rats with loperamide-induce
constipation. Dig Dis Sci. 46:1482–1489. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Snape WJ Jr: The effect of methylcellulose
on symptoms of constipation. Clin Ther. 11:572–579. 1989.PubMed/NCBI
|