Introduction
Heavy metals, including zinc, copper, cadmium, lead,
chromium, mercury and nickel, are widely used in industry (1). Cadmium enters the human body
primarily through food and water. By binding with thiol proteins in
the body, cadmium can inhibit the activity of enzymes (2). Epidemiological data indicate that the
joint pollution of lead and cadmium can increase the incidence and
mortality rates of cardiovascular diseases (3). Experimental results have shown that
joint treatment with low doses of lead and cadmium induces high
blood pressure (4–6), with cadmium having a synergistic
effect on the high blood pressure induced by lead (7,8).
These findings indicate that lead and cadmium have synergistic
effects in toxicity (9,10). Nickel can cause lung cancer in
humans, which is classified by the International Agency for
Research on Cancer as a ‘group 1’ carcinogen (11). Heavy metals can pollute water, air
and soil (12). The most important
route of contamination is water. Heavy metals are usually difficult
to degrade in the environment, which results in them readily
accumulating in plants and aquatic organisms. Heavy metals in
contaminated water accumulate in a stepwise manner in algae,
shellfish and fish, and finally reach the human body through the
food chain (13–16). Therefore, the enrichment of
multiple heavy metals in aquatic products or foods, including
vegetables, is considered to be a health threat in China (17,18).
At present, there have been no investigations on the combined toxic
effects of multiple heavy metals at levels mimicking those
contaminating water or through food exposure.
Ningbo is a city on the eastern coast of China.
Residents in the Ningbo region are accustomed to consuming aquatic
products, particularly marine foods (19), leading to the rapid development of
offshore aquaculture and marine farming in previous years. In the
present study, eight of the most common heavy metals contaminating
the offshore waters off the eastern coast of Ningbo were examined,
including lead, cadmium, mercury, copper, zinc, manganese, nickel
and chromium. These eight metals were selected for preparation as a
mixture, with the proportions matching those found in the region of
contamination, in order to investigate their joint toxicity and
underlying mechanisms of multi-heavy metal exposure.
Materials and methods
Materials
Chemical compounds for the eight heavy metals,
including (CH3COO)2Pb·3H2O,
CdCl2·2.5H2O,
NiCl2·6H2O, MnCl2·4H2O,
ZnSO4·7H2O, CuSO4·5H2O,
K2Cr2O7 and CH3ClHg
(all analytical reagents) were purchased from Sinopharm Chemical
Reagent Beijing Co. Ltd. (Beijing, China). Dulbecco's modified
Eagle's medium (DMEM), heat-inactivated newborn calf serum (NCS),
Tris-EDTA, 0.5% Trypsin-EDTA and propidium iodide (PI) were
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Hoechst 33342, 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCFDA) and dihydroethidium (DHE) were purchased from
Molecular Probes (Eugene, OR, USA). An Annexin V-fluorescein
isothiocyanate (FITC)/PI apoptosis detection kit and apoptosis
assay kit were purchased from Thermo Fisher Scientific, Inc
(Waltham, MA, USA). A luciferase assay system and TPA were
purchased from Promega (Madison, WI, USA). All other antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Preparation of the heavy metal
mixture
The mixture of heavy metals used in the present
study comprised eight heavy metals commonly contaminating the
surface waters in the Ningbo region, including zinc, manganese,
lead, copper, cadmium, mercury, chromium, and nickel. The
proportions of these eight heavy metal ions in the offshore water
were determined according to previous reports (20,21).
The chemical compounds were dissolved in DMEM to prepare the stock
solution (Table I). The final
concentration of the stock solution (total of the eight heavy metal
ions) was 25.233 mg/l. The stock solution was diluted in DMEM at
concentrations of 2.523, 5047, 7.570, 10.093, 12.617, 15.140 and
17.663 mg/l, respectively, to form working solutions of the
multi-heavy metal mixture.
 | Table I.Preparation of the heavy metal
mixture. |
Table I.
Preparation of the heavy metal
mixture.
| Heavy metal | Concentration in
surface water (µg/l)a | Compound used for
heavy metal | Concentration in
stock solution (mg/l) |
|---|
| Cu | 2.05 |
CuSO4·5H2O | 2.05 |
| Pb | 2.5 |
(CH3COO)2Pb·3H2O | 2.5 |
| Cd | 0.15 |
CdCl2·2.5H2O | 0.15 |
| Zn | 13.2 |
ZnSO4·7H2O | 13.2 |
| Hg | 0.033 |
CH3ClHg | 0.033 |
| Cr | 0.3 |
K2Cr2O7 | 0.3 |
| Mn | 5 |
MnCl2·4H2O | 5 |
| Ni | 2 |
Cl2H12NiO6 | 2 |
| Total | 25.233 | N/A | 25.233 |
Cell culture
JB6 cells (a mouse epidermal cell line) were
provided by the National Institute of Occupational Safety and
Health (Morgantown, WV, USA) were cultured in 10% NCS DMEM
supplemented with penicillin-streptomycin (10,000 U/ml penicillin
and 10 mg/ml streptomycin) at 37°C (80% humidified air and 5%
CO2).
Cytotoxicity assay
The toxicity of the multi-heavy metal mixture
towards the JB6 cells was assessed using an MTT assay. Briefly, the
cells were plated in 180 µl DMEM at a density of 0.5×104
cells/well in a 96-well plate. The cells were treated with the
various concentrations of the multi-heavy metal mixture and
cultured for 24 h at 37°C. After 24 h, 20 µl MTT labeling reagent
was added in each well and the plates were incubated for a further
4 h at 37°C. Subsequently, 150 µl solubilization solution was added
to each well and the plate was incubated for 10 min at 37°C. The
optical density of each well was measured at a wavelength of 490 nm
using an ELISA plate reader.
Cell cycle detection
The JB6 cells were seeded onto a 6-well plate
overnight in 2 ml DMEM at a density of 2×105 cells/well
(22). The cells were then treated
with or without the various concentrations of the multi-heavy metal
mixture for 24 h. The cells were harvested using 0.1% trypsin and
fixed overnight in a pre-cooling 70% ethanol at −20°C. The fixed
cells were subsequently centrifuged at 10,000 × g for 30 min at
4°C, resuspended in PBS and stained with 0.02 mg/ml PI for 30 min
in the dark. The population of cells in each phase was then
determined using flow cytometry (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Identification of cell apoptosis
Double staining with Annexin V-FITC (apoptotic cell
marker) and PI (nuclear marker) was used to determine whether the
cell death induced by the multi-heavy metal mixture was apoptotic.
As stated above, the JB6 cells were seeded onto a 6-well plate and
incubated overnight, and were treated with or without the various
concentrations of the multi-heavy metal mixture for 24 h. The cells
were then harvested using 0.1% trypsin and were washed twice with
cold PBS. Annexin V-FITC (5 µl) and PI (1 µl) were added to the
cultures, and the cells were incubated in the dark for 15 min at
25°C. Cell apoptosis was analyzed using flow cytometry (Bio-Rad
Laboratories, Inc.) within 1 h.
ROS detection
H2DCFDA and DHE are used for general ROS
and oxygen radical staining in cells, respectively. In the present
study, the cells (2.0×105 cells in 2 ml DMEM/well) were
seeded in a 6-well plate overnight, following which they were
treated with or without the various concentrations of the
multi-heavy metal mixture. The cells were fixed in 95% ethanol for
5 min and then washed twice with PBS. The cells were then incubated
in PBS in the presence of H2DCFDA (5 µM), DHE (2 µM) and
Hoechst 33342 (3 µM) for 1 h at 37°C. Following incubation, the
cells were washed three times with PBS and images of ROS generation
in cells were captured using fluorescence microscopy (Axiovert
100M; Zeiss GmbH, Jena, Germany).
Luciferase assay
The JB6 cells transfected with the nuclear
transcription factors AP-1 or NF-κB (provided by the National
Institute of Occupational Safety and Health) were used to detect
the luciferase activities of AP-1 or NF-κB. A cell suspension (2
ml; 1×105 cells/ml) was seeded in a 24-well plate in 10%
NCS DMEM and incubated at standard culture conditions (37°C, 80%
humidified air and 5% CO2) for 24 h. The cultures were
then starved in 0.1% NCS DMEM overnight. The cells were treated
with or without the various concentrations of the multi-heavy metal
mixture for 8 h in 10% NCS DMEM. Following two washes with PBS, 120
µl of 1X Promage lysate buffer was added to each well. Following
incubation on ice for 60 min, the lysate was centrifuged at 12,000
× g for 20 min at 4°C to obtain the supernatant. Luciferase
activity was measured using a luciferase assay kit according to the
manufacturer's protocol. TPA (20 nM) was used as a positive control
for the upregulation of AP-1 or NF-κB luciferase activity.
Western blot analysis
The cells (5×104 cells in 1 ml of culture
medium) were plated into a 6-well plate and incubated overnight.
The cultures were treated with or without the various
concentrations of the multi-heavy metal mixture for 8 h. To collect
the cell pellet, the cells were centrifuged at 12,000 × g for 5 min
at 4°C following which the cells were lysed in 100 µl of NP40
lysate supplemented with a protease inhibitor cocktail. Following
incubation on ice for 60 min, the cell suspension was centrifuged
at 12,000 × g for 20 min at 4°C to obtain the supernatant. The
protein concentrations in the supernatants were determined using
the BCA protein quantitative method. Equal quantities of proteins
(50 µg) were denatured for 5 min at 95°C, electrophoresed by 10%
SDS-PAGE, and transferred onto PVDF membranes. After proteins were
transferred, Ponceau S dye was used indicate protein transfer.
Initially, the blots were blocked in 5% milk blocking buffer for 3
h and then incubated overnight at 4°C with the primary antibody.
C-jun and p65 antibodies (Cell signaling Technologies, Inc.,
Danvers, MA, USA; cat. nos. 1694 and 372, respectively) were
diluted in 5% milk blocking buffer at 1:1,000 and β-actin antibody
(Beyotime Institute of Biotechnology, Shanghai, China; cat. no.
AF0003) at 1:10,000. Then, after washing with TBST, the blots were
incubated for 2 h at room temperature with the IgH horseradish
peroxidase-conjugated secondary antibody (Boster Biological
Engineering Co. Ltd., Wuhan, China; cat. no. BA1054) at 1:1,000.
Finally, the chemiluminescence of proteins transferred onto PVDF
membranes were detected with ECL Plus (Asvansta Inc., Menlo Park,
CA, USA). Then the images of the blots were captured using Bio-Rad
image capture system (Bio-Rad Laboratories, Inc.) and Quantity One
v.465 software (Bio-Rad Laboratories, Inc.) was used for band
analysis.
Statistical analysis
The experiments were repeated at least three times
and data are presented as the mean ± standard deviation.
Significant differences were determined using SAS 9.1 software (SAS
Institute, Inc., Cary, NC, USA) or one-way analysis of variance.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
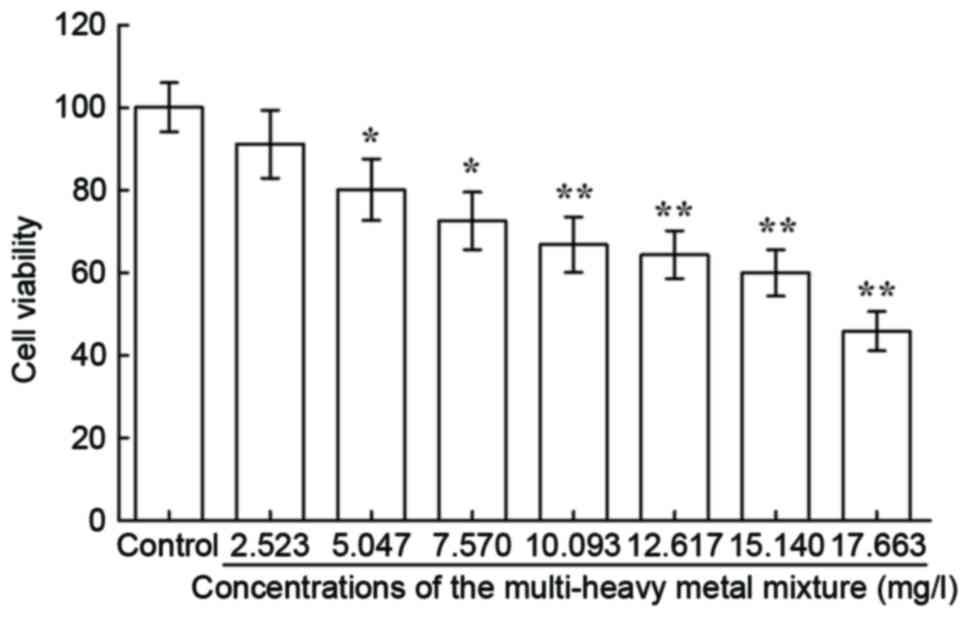
Cytotoxicity
The results of the MTT assay (Fig. 1) and cell morphological observation
under the microscope (Fig. 2)
showed that the multi-heavy metal mixture induced dose-dependent
cytotoxicity in the JB6 cells.
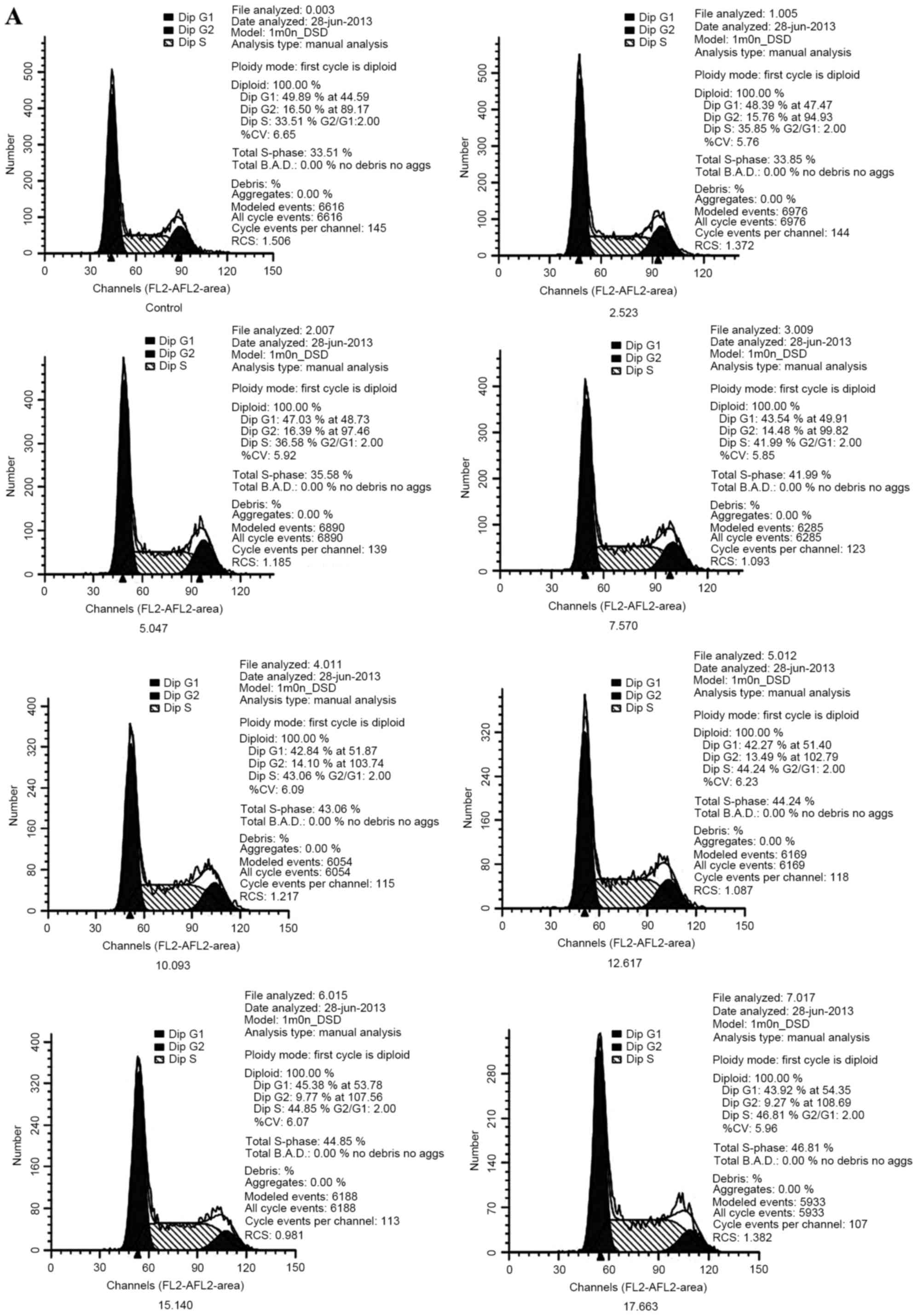
Cell cycle detection
The histograms in Fig.
3A show cell cycle distribution, detected using flow cytometry.
Further analysis indicated that, as the concentration of the
multi-heavy metal mixture increased, the number of cells arrested
at the S phase increased, which was accompanied by a decrease in
the number of cells at the G1 and G2 phases. These results were
found to be dose-dependent (Fig.
3B; Table II).
 | Table II.Effects of the multi-heavy metal
mixture on the cell cycle of JB6 cells. |
Table II.
Effects of the multi-heavy metal
mixture on the cell cycle of JB6 cells.
|
| Phase of cell cycle
(%) |
|---|
|
|
|
|---|
| Concentration of
mixture (mg/l) | G1 | S | G2 |
|---|
| 0 (control) | 48.74±0.76 | 35.20±1.49 | 16.05±1.00 |
| 2.523 | 47.76±0.81 | 36.39±2.18 | 15.86±1.45 |
| 5.047 | 47.49±1.39 | 36.79±0.82 | 15.73±0.57 |
| 7.570 |
43.34±0.68b |
42.17±0.65b |
14.49±0.03a |
| 10.093 |
41.66±1.21b |
44.04±0.96b |
14.30±0.65a |
| 12.617 |
41.65±0.88b |
44.91±0.95b |
13.44±0.07a |
| 15.140 |
39.23±0.22a |
51.21±0.50b |
9.57±0.28b |
| 17.663 |
39.09±0.71b |
51.53±0.79b |
9.38±0.16b |
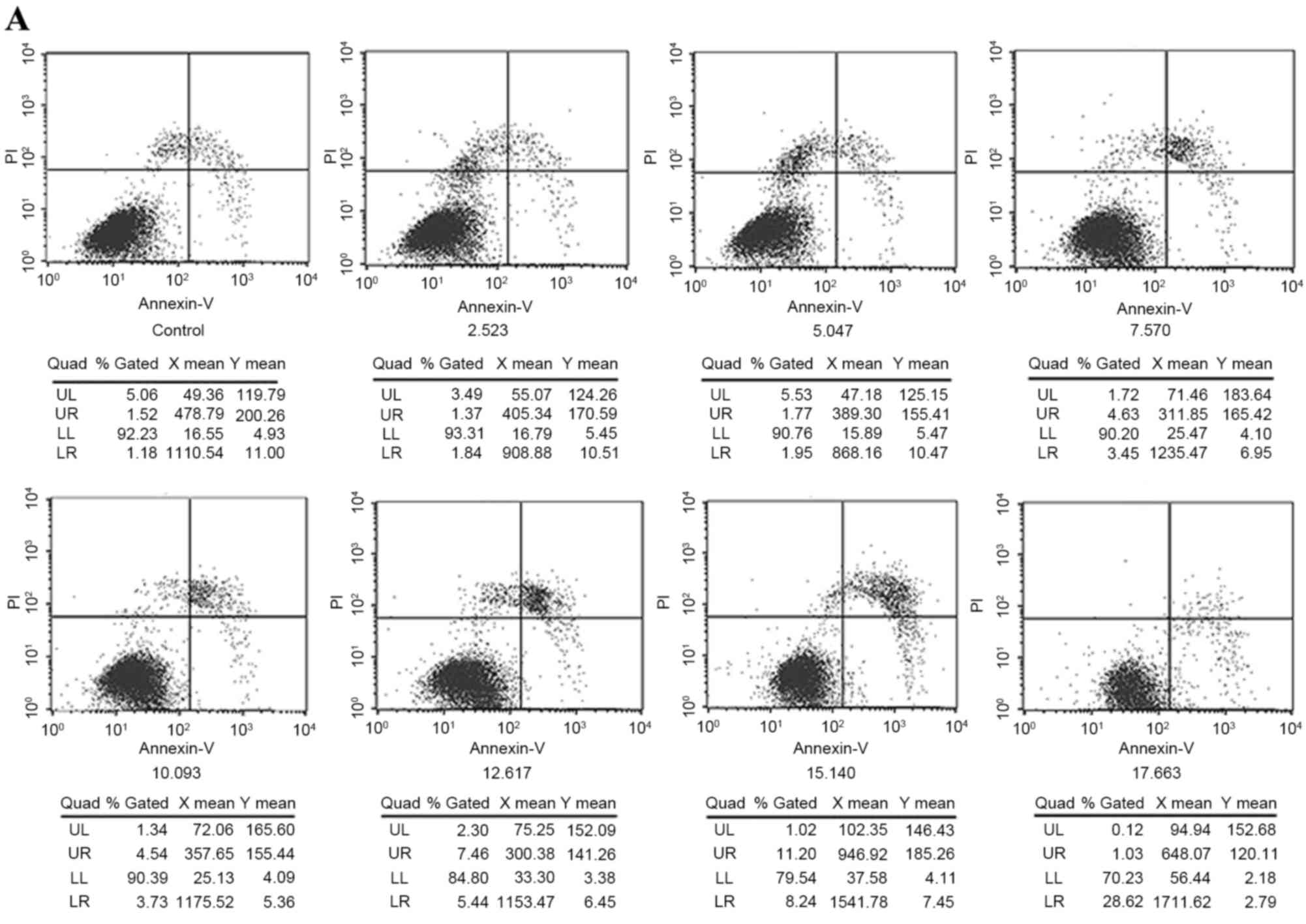
Cell apoptosis induction
Following treatment with or without different
concentrations of the multi-heavy metal mixture for 24 h, double
staining with Annexin V-FITC and PI (Fig. 4A) showed that the multi-heavy metal
mixture induced JB6 cell apoptosis in a dose-dependent manner
(Fig. 4B).
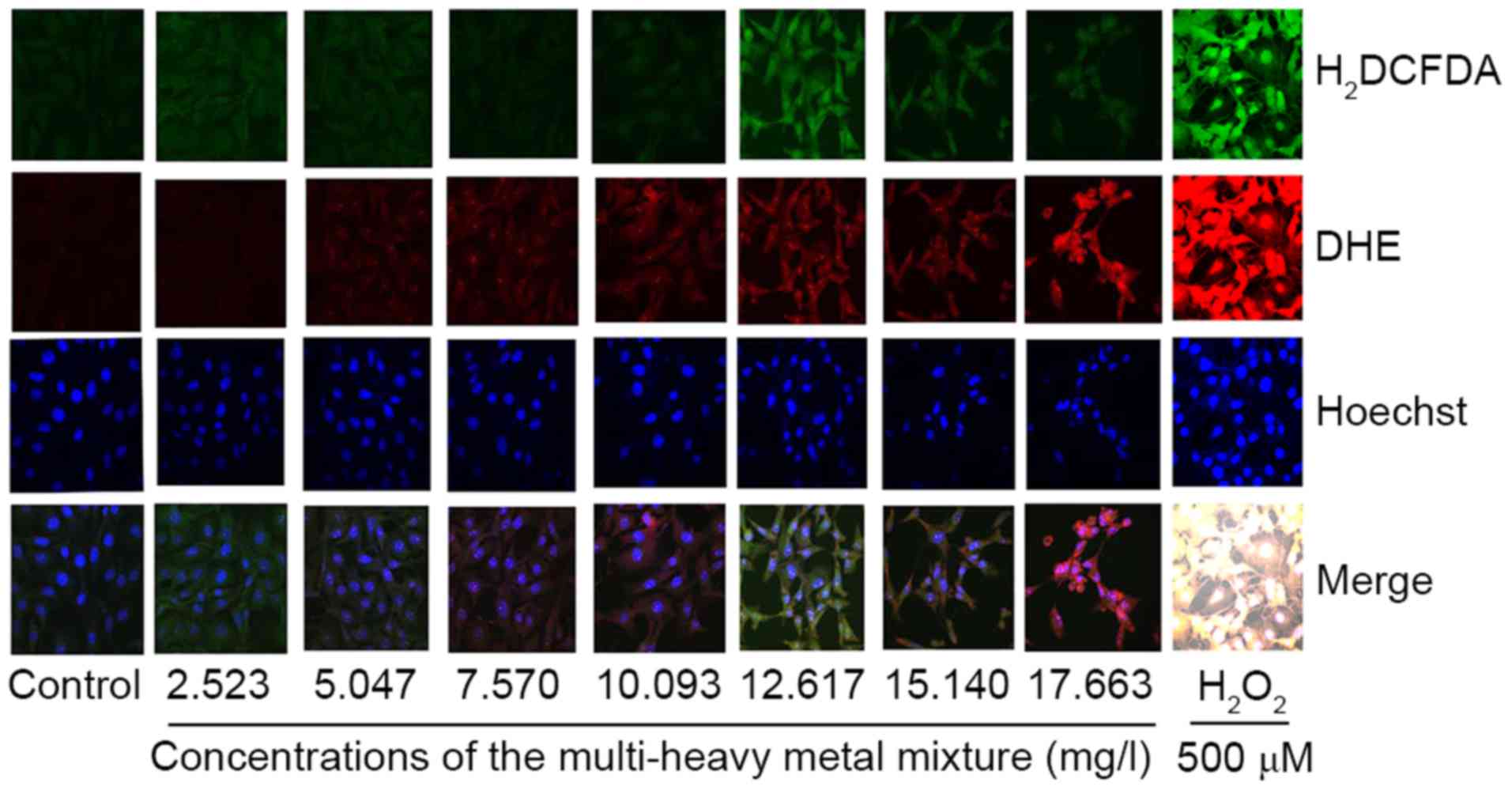
ROS generation
The results of the present study showed that higher
doses of the multi-heavy metal mixture induced significant ROS
generation (Fig. 5).
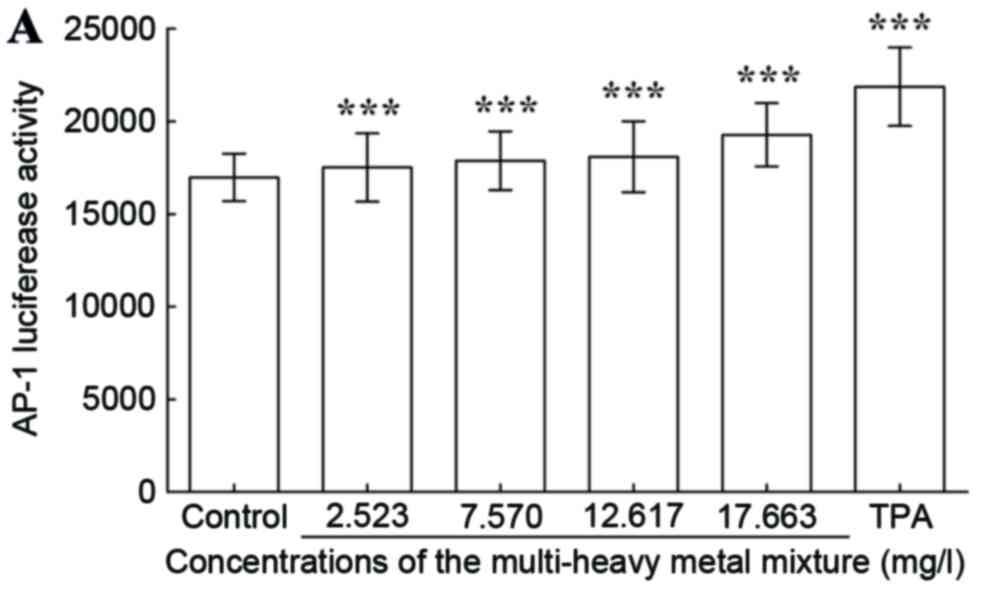
Luciferase activities of AP-1 and NF-κB. The results
demonstrated that the multi-heavy metal mixture induced
upregulation in the luciferase activities of AP-1 (Fig. 6A) and NF-κB (Fig. 6B) in a dose-dependent manner.
Expression of cell signaling
proteins
The results of the western blot analysis showed that
the multi-heavy metal mixture induced an upregulation in cell
signaling protein expressions of C-jun and p65 (Fig. 7).
Discussion
In the past 30 years, China has experienced a
rapidly developing economy, which has led to serious environmental
pollution. Among the different types of pollution, heavy metal
pollution has become one of the major environmental issues
(23). In China, coastal areas are
under increased threat due to heavy metal contamination caused by
an increase in urbanization and industrialization, compared with
inland areas. Evidence indicates that the heavy metals present in
certain seafood from coastal areas may exceed the safety limit,
caused by heavy metal contamination in the surface water (24). The contamination of heavy metals in
the water in the coastal area is considered to be a threat to
marine farming and human health in China. The toxicities of single
heavy metals, including lead and nickel, have been well documented
(25). However, in daily life,
individuals are usually exposed to a contamination by multiple
heavy metals simultaneously from water (26), the air or food consumption. To
investigate the joint toxicity and the underlying mechanisms of
multi-heavy metal exposure, the present study selected eight heavy
metals commonly found contaminating the offshore waters of the
Ningbo region, and prepared a mixture with the proportions of each
contaminant matching those in the region.
In China, although different regions have different
spectrums in heavy metal contamination, Zn, Pb, Cu and Cd remain
the dominant contaminations whether in the water or soil (27), which is similar to the
contamination spectrum of the offshore water in the Ningbo region
(Table II). Although heavy
metals, including Zn and Cu, are essential elements in the human
body, exposure to high doses can also be harmful to human health,
particularly when combined with other toxic heavy metals.
Detecting levels of cytotoxicity provides an
economical method for evaluating the toxicity of compounds. In the
present study, it was found that the mixture of eight common heavy
metals induced cytotoxicity in the JB6 cells in a dose-dependent
manner. The cause underlying the cytotoxic effect of heavy metals
remains to be fully elucidated, however, it has been suggested that
their toxicity, at least in part, may be due to the formation of
hydroxyl radicals, which can lead to lipid, protein or DNA damage
(28). In the present study, it
was found that the multi-heavy metal mixture induced significant
ROS generation and cell apoptosis. Exposure to the mixture exposure
also arrested the JB6 cells at the S phase, indicated by an
increase in the proportion of cells in the S phase from 35.2% in
the control to 51.53% (17.663 mg/l), and a decrease in the
proportion of cells in the G2 phase from 16.05% in the control to
9.35% (17.663 mg/l). Evidence shows that different heavy metals can
exert different effects on the cell cycle. A significant increase
of intracellular Zn content in MDAMB231 cells results in G1 and
G2/M cell cycle arrest, and cell apoptosis (29). Manganese chloride induces G0/G1
phase arrest and apoptosis in cultured rat astrocytes (30). Sub-lethal exposure of Swiss albino
mice to copper, as copper chloride, induces an increase in G0/G1
cell numbers in the thymus and spleen (31). Cadmium can induce a delay in the
transition between the G1 and S phases of the cell cycle, and slows
progression through the S phase (28). Nickel (II) modulates cellular
responses through effectors involved in G2/M arrest and apoptosis
regulatory pathways (32,33). Rodríguez-Sastre et al
(34) evaluated the capability of
a mixture of 2 µM NaAsO2, 2 µM CdCl2 and 5 µM
Pb(C2H3O2)2·3H2O
at relevant epidemiological concentrations to induce cell
transformation processes. The results revealed a decrease in cell
viability and an increase in ROS, accompanied by G1 cell cycle
arrest. Costa et al (35)
found that 1–6 µM of CdCl2, HgCl2,
CoCl2, CuSO4, NiCl2,
ZnCl2 and PbSO4 slowed cell growth and
selectively arrested cells in the S phase. It was concluded that
the potency of the metal compounds in arresting cells in the S
phase was associated with their chemical reactivity and uptake into
cells. Specifically, the S phase arrest produced by the metals
examined was consistent with their genotoxic or carcinogenic
activities; as such activity indicated a selective interaction with
DNA metabolism. In the present study, H2DCFDA and DHE
were used for the staining of general ROS and oxygen radicals
produced in JB6 cells, respectively (36). It was found that treatment of cells
with the multi-heavy metal mixture induced significant ROS
generation followed by apoptosis of the JB6 cells. It is known that
ROS can damage DNA (37). DNA
damage can cause S phase arrest in the cell and switch on cell
apoptotic pathways. The detailed mechanism underlying the effects
of the multi-heavy metal mixture on inducing the combined toxic
effects of S phase arrest in the cell requires further
investigation.
The AP-1 transcription factor family consists of the
subfamilies, C-jun, C-fos, activating transcription factor and Jun
dimerization partners. AP-1 family members can induce physiological
or pathological responses under certain stimulation, including
stress, radiation or growth signals, and are involved in processes,
including cell proliferation, differentiation and transformation,
which are important in tumor formation, metastasis and invasion.
The activation of AP-1 can directly regulate the expression of
several cancer genes. C-jun, as one of the subunits of the AP-1
transcriptional regulatory protein family, can react to a variety
of stimuli (38). An increase of
C-jun can increase the risk of cancer (39,40).
The NF-κB transcription factor is a particularly broad inducible
transcription factor (41,42). Similar to AP-1, NF-κB is also
important in immunity, inflammation, cell survival, proliferation,
differentiation and apoptosis (43–45).
p65 is a subunit of NF-κB and the activation of p65 is associated
with carcinogenesis (46–48). In the present study, it was found
that the multi-heavy metal mixture induced an upregulation in the
luciferase activities of AP-1 and NF-κB, and the protein levels of
C-jun and p65 in a dose-dependent manner. These results suggested
that multi-heavy metal exposure at a high concentration for a
prolonged duration may be carcinogenetic.
In conclusion, the joint toxicities of the mixture
of eight heavy metals included intercellular ROS generation,
apoptosis and cell cycle arrest at the S phase. The AP-1 and NF-κB
transcription factors, and the protein levels of C-jun and p65 were
upregulated, suggesting carcinogenesis as a result of the
multi-heavy metal exposure. Intercellular ROS generation may be an
important effect induced by the joint toxicity of the multi-heavy
metal mixture. To the best of our knowledge, this is the first
study demonstrating the joint toxicities of an eight heavy metal
mixture, which comprised of eight heavy metals commonly
contaminating the surface waters in the Ningbo region. These
results will be of benefit for elucidating the combined toxicity
and the underlying mechanisms of multi-heavy metal pollution. In
addition, the result may also be useful as a reference when
examining the toxicity of multi-heavy metal pollutants in aquatic
products in further studies.
Acknowledgements
The authors would like to thank Ms. Linda Bowman
(National Institute of Occupational Safety and Health, Morgantown,
WV, USA) for her assistance in manuscript preparation and the KC
Wong Magna Fund in Ningbo University, Hong Kong, for their support.
This study was partly supported by the National Nature Science
Foundation of China (grant no. 81273111), the Ningbo Scientific
Project (grant no. 2012C5019), the Science Technology Department of
Zhejiang Province (grant nos. 2015C33148 and 2015C37117) and the
Scientific Innovation Team Project of Ningbo (grant no.
2011B82014).
References
|
1
|
Xu X, Li Y and Wang Y and Wang Y:
Assessment of toxic interactions of heavy metals in multi-component
mixtures using sea urchin embryo-larval bioassay. Toxicol In Vitro.
25:294–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie LH and Xu ZR: The toxicity of heavy
metal Cadmium to animals and humans. Acta Agriculture
Zhejiangensis. 15:376–381. 2003.(In Chinese).
|
|
3
|
Neuberger JS, Mulhall M, Pomatto MC,
Sheverbush J and Hassanein RS: Health problems in Galena, Kansas: A
heavy metal mining Superfund site. Sci Total Environ. 94:261–272.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans DH and Weingarten K: The effect of
cadmium and other metals on vascular smooth muscle of the dogfish
shark, Squalus acanthias. Toxicology. 61:275–281. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holécyová A and Török J: The effect of
cadmium on neuromuscular transmission in rabbit blood vessels.
Bratisl Lek Listy. 91:839–843. 1990.PubMed/NCBI
|
|
6
|
Ozdem S and Oğütman Ç: The effects of
short-term nifedipine treatment on responsiveness of aortic rings
of cadmium-hypertensive rats. Clin Exp Hypertens. 21:423–440. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perry H and Erlanger MW: Pressor effects
of chronically feeding cadmium and lead together. Trace substances
in environmental health. 12:268–275. 1978.
|
|
8
|
Perry HM Jr, Erlanger MW and Perry EF:
Effect of a second metal on cadmium-induced hypertension. Arch
Environ Health. 38:80–85. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skoczyńska A, Wróbel J and Andrzejak R:
Lead-cadmium interaction effect on the responsiveness of rat
mesenteric vessels to norepinephrine and angiotensin II.
Toxicology. 162:157–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lock K and Janssen C: Mixture toxicity of
zinc, cadmium, copper, and lead to the potworm Enchytraeus albidus.
Ecotoxicol Environ Saf. 52:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Shi X, Castranova V and Ding M:
Occupational toxicology of nickel and nickel compounds. J Environ
Pathol Toxicol Oncol. 28:177–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma R Kumar, Agrawal M and Marshall F:
Heavy metal contamination of soil and vegetables in suburban areas
of Varanasi, India. Ecotoxicol Environ Saf. 66:258–266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uluturhan E and Kucuksezgin F: Heavy metal
contaminants in Red Pandora (Pagellus erythrinus) tissues from the
Eastern Aegean Sea, Turkey. Water Res. 41:1185–1192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGeer JC, Szebedinszky C, McDonald DG and
Wood CM: Effects of chronic sublethal exposure to waterborne Cu, Cd
or Zn in rainbow trout. 1: Iono-regulatory disturbance and
metabolic costs. Aquat Toxicol. 50:231–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones I, Kille P and Sweeney G: Cadmium
delays growth hormone expression during rainbow trout development.
J Fish Biol. 59:1015–1022. 2005. View Article : Google Scholar
|
|
16
|
Almeida J, Diniz Y, Marques S, Faine LA,
Ribas BO, Burneiko RC and Novelli EL: The use of the oxidative
stress responses as biomarkers in Nile tilapia (Oreochromis
niloticus) exposed to in vivo cadmium contamination. Environ Int.
27:673–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang CZ, Zhang LJ, Rong JR, Yu AF and Qi
M: Survey and evaluation of heavy metal content in fresh aquatic
product in Ningbo. Chinese Journal of Health Laboratory Technology.
10:1866–1867. 2007.(In Chinese).
|
|
18
|
Farombi E, Adelowo O and Ajimoko Y:
Biomarkers of oxidative stress and heavy metal levels as indicators
of environmental pollution in African cat fish (Clarias gariepinus)
from Nigeria Ogun River. Int J Environ Res Public Health.
4:158–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai YT: Study on ecological environment
and health quality of aquatic products in sansha bay. J Fuj Fish.
49–55. 2004.
|
|
20
|
Zhongjie Y, Feijin K, Jianping W and
Jikang S: Assessment on heavy metal contents and environment in
tidal shellfish cultured area of Ningbo. Mar Envir Sci. 30:508–511.
2011.
|
|
21
|
Wang J, Qin Y, Sun Y, Wu J and Cheng X:
The distribution and source of heavy metals in an important
aquaculture sea area Xiangshan Bay. Mar Fish. 27:225–231. 2005.
|
|
22
|
Lü L, Liu X, Wang C, Hu F, Wang J and
Huang H: Dissociation of E-cadherin/β-catenin complex by MG132 and
bortezomib enhances CDDP induced cell death in oral cancer SCC-25
cells. Toxicol In Vitro. 29:1965–1976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hui H, Qian J and Kavan P: A study of
heavy metal pollution in China: Current status, pollution-control
policies and countermeasures. Sustainability. 6:5820–5838. 2014.
View Article : Google Scholar
|
|
24
|
Wang SL, Xu XR, Sun YX, Liu JL and Li HB:
Heavy metal pollution in coastal areas of South China: A review.
Mar Pollut Bull. 76:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ip CC, Li XD, Zhang G, Wong CS and Zhang
WL: Heavy metal and Pb isotopic compositions of aquatic organisms
in the Pearl River Estuary, South China. Environ Pollut.
138:494–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morcillo P, Cordero H, Meseguer J, Esteban
MÁ and Cuesta A: Toxicological in vitro effects of heavy metals on
gilthead seabream (Sparus aurata L.) head-kidney leucocytes.
Toxicol In Vitro. 30:412–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S: Heavy metal pollution in the water
environment and countermeasures in China. Journal of Zhangzhou
Vocational College. 54:2002.(In Chinese).
|
|
28
|
Yen JL, Su NY and Kaiser P: The yeast
ubiquitin ligase SCFMet30 regulates heavy metal response. Mol Biol
Cell. 16:1872–1882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang YH, Li KJ, Mao L, Hu X, Zhao WJ, Hu
A, Lian HZ and Zheng WJ: Effects of exogenous zinc on cell cycle,
apoptosis and viability of MDAMB231, HepG2 and 293 T cells. Biol
Trace Elem Res. 154:418–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng Y, Xu D, Xu B, Xu Z, Tian Y, Feng W,
Liu W and Yang H: G0/G1 phase arrest and apoptosis induced by
manganese chloride on cultured rat astrocytes and protective
effects of riluzole. Biol Trace Elem Res. 144:832–842. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitra S, Keswani T, Dey M, Bhattacharya S,
Sarkar S, Goswami S, Ghosh N, Dutta A and Bhattacharyya A:
Copper-induced immunotoxicity involves cell cycle arrest and cell
death in the spleen and thymus. Toxicology. 293:78–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiao YH, Lee SH and Kasprzak KS: Cell
cycle arrest, apoptosis and p53 expression in nickel(II)
acetate-treated Chinese hamster ovary cells. Carcinogenesis.
19:1203–1207. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Lima EM, Kanunfre CC, De Andrade LF,
Granato D and Rosso ND: Cytotoxic effect of inositol hexaphosphate
and its Ni(II) complex on human acute leukemia Jurkat T cells.
Toxicol In Vitro. 29:2081–2088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodriguez-Sastre MA, Rojas E and Valverde
M: Assessing the impact of As-Cd-Pb metal mixture on cell
transformation by two-stage Balb/c 3T3 cell assay. Mutagenesis.
29:251–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Costa M, Cantoni O, de Mars M and
Swartzendruber DE: Toxic metals produce an S-phase-specific cell
cycle block. Res Commun Chem Pathol Pharmacol. 38:405–419.
1982.PubMed/NCBI
|
|
36
|
Zhao J, Bowman L, Magaye R, Leonard SS,
Castranova V and Ding M: Apoptosis induced by tungsten
carbide-cobalt nanoparticles in JB6 cells involves ROS generation
through both extrinsic and intrinsic apoptosis pathways. Int J
Oncol. 42:1349–1359. 2013.PubMed/NCBI
|
|
37
|
Zhu Y, Gu YX, Mo JJ, Shi JY, Qiao SC and
Lai HC: N-acetyl cysteine protects human oral keratinocytes from
Bis-GMA-induced apoptosis and cell cycle arrest by inhibiting
reactive oxygen species-mediated mitochondrial dysfunction and the
PI3K/Akt pathway. Toxicol In Vitro. 29:2089–2101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Angel P and Karin M: The role of Jun, Fos
and the AP-1 complex in cell-proliferation and transformation.
Biochim Biophys Acta. 1072:129–157. 1991.PubMed/NCBI
|
|
39
|
Lee W, Mitchell P and Tjian R: Purified
transcription factor AP-1 interacts with TPA-inducible enhancer
elements. Cell. 49:741–752. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karin M, Liu Zg and Zandi E: AP-1 function
and regulation. Curr Opin Cell Biol. 9:240–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Medzhitov R: Origin and physiological
roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bhakar AL, Tannis LL, Zeindler C, Russo
MP, Jobin C, Park DS, MacPherson S and Barker PA: Constitutive
nuclear factor-kappaB activity is required for central neuron
survival. J Neurosci. 22:8466–8475. 2002.PubMed/NCBI
|
|
45
|
Nawata R, Yujiri T, Nakamura Y, Ariyoshi
K, Takahashi T, Sato Y, Oka Y and Tanizawa Y: MEK kinase 1 mediates
the antiapoptotic effect of the Bcr-Abl oncogene through NF-kappaB
activation. Oncogene. 22:7774–7780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang L, Ye SY, Ulrich B, Chen XS, Tian YJ
and Zhang XB: The relationship of NF-κB p65 protein expression with
Helicobacter species infection and carcinoma of esophagus in
humans. Chinese Journal of Microbiology and Immunology. 27:234–239.
2007.(In Chinese).
|
|
47
|
Wang W, Luo HS and Yu BP: Expression of
NF-κB and c-myc protein in gastric carcinogenesis. Cancer Research
on Prevention and Treatment. 29:285–287. 2002.(In Chinese).
|
|
48
|
Wang Q: The study on the expression of p65
in the thyroid cancer and its correlation with cell cycle. China
Practical Medical. 2:26–28. 2007.(In Chinese).
|