Introduction
The incidence of malignant cancer types in China
demonstrates an upward trend, and is increasing at an average rate
of 3–5% annually (1). Melanoma is
a form of superficial tumor, with a high malignancy and difficulty
to treat (2). Currently, no
effective prevention and treatment methods exist (3). The traditional surgical treatment
methods lead to surgical trauma. The magnetic targeted drug
delivery system (MTDDS) has provided a novel avenue for cancer
therapy, and has become a priority of domestic and foreign
pharmaceutical research (4). MTDDS
is a stable system composed of a magnetic substance and drugs,
using a suitable carrier. Under the external magnetic field with a
specific intensity, the drug may be directionally moved,
positioned, concentrated and released in vivo towards the
lesions, thereby exerting its efficacies at the lesion site
(5). The highly specific
characteristic of this system has been previously demonstrated to
significantly reduce the side effects (6).
The present study used self-made angiopoietin-2
(Ang2)-small interfering (si)RNA plasmid-chitosan magnetic
nanoparticles (CMNPs) with the aim of inhibiting the growth of
malignant melanoma. However, its safety remains to be investigated.
Ang2-CMNPs have magnetic iron oxide nanoparticles as their core and
are wrapped in chitosan polysaccharides to adsorb to the Ang2-siRNA
plasmid vector, thus forming the magnetic nanoparticle carrier.
This particle is nano-grade, so its features are making
nanomaterials more readily absorbed in vivo, as well as
entry into the blood circulation (7). A previous study (8) demonstrated that nanoparticles may
penetrate the blood-brain-barrier by passive transferring,
vector-mediation or phagocytosis, thereby exhibiting certain
impacts on the body. Therefore, it is necessary to perform a
comprehensive safety evaluation on nanomaterials. However, no
research institute thus far has performed a full and systematic
evaluation towards the safety of nanomaterials, and the data
concerning the potential toxicity are scarce. The present study
predominantly investigated the acute and chronic toxicity of
Ang2-CMNPs, aiming to examine their safety and provide a
theoretical basis for their application in tumor-targeted
therapy.
Materials and methods
Preparation of CMNPs
A total of 0.15 g of magnetic
Fe3O4 nanoparticles (Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) were dispersed
in 20 ml 1.5% chitosan solution (CS; molecular weight,
1.38×106; 90% deacetylation; Zhejiang Aoxing
Biotechnology Co., Ltd., Hangzhou, China) by ultrasound and
stirring. Subsequently, 80 ml liquid paraffin and petroleum ether
mixture (v/v, 7:5), containing 2 ml Span-80 (all provided by
Sigma-Aldrich, Merck-Millipore, Darmstadt, Germany), was added,
followed by stirring at 40°C for 30 min for sufficient
emulsification. A total of 10 ml 25% glutaraldehyde (Sigma-Aldrich)
solution was slowly added, followed by stirring at 40°C for 30 min.
NaOH (l mol/l) (Sigma-Aldrich) solution was used to adjust the pH
to 9.0, followed by heating at 40°C for 1 h. After thoroughly
washing successively with anhydrous ether, acetone, absolute
ethanol and distilled water, the CMNPs were obtained.
Preparation of Ang2-CMNPs
A total of 1 mg CMNPs was added to 1 ml PBS buffer
(pH 7.4; Sigma-Aldrich), followed by ultrasonic oscillation for 3
min. Subsequently, 2 ml polylysine (diluted in PBS buffer to a
concentration as 0.1 mg/ml; Sigma-Aldrich) was added, followed by
mixing well and incubation at room temperature for 10 min. An
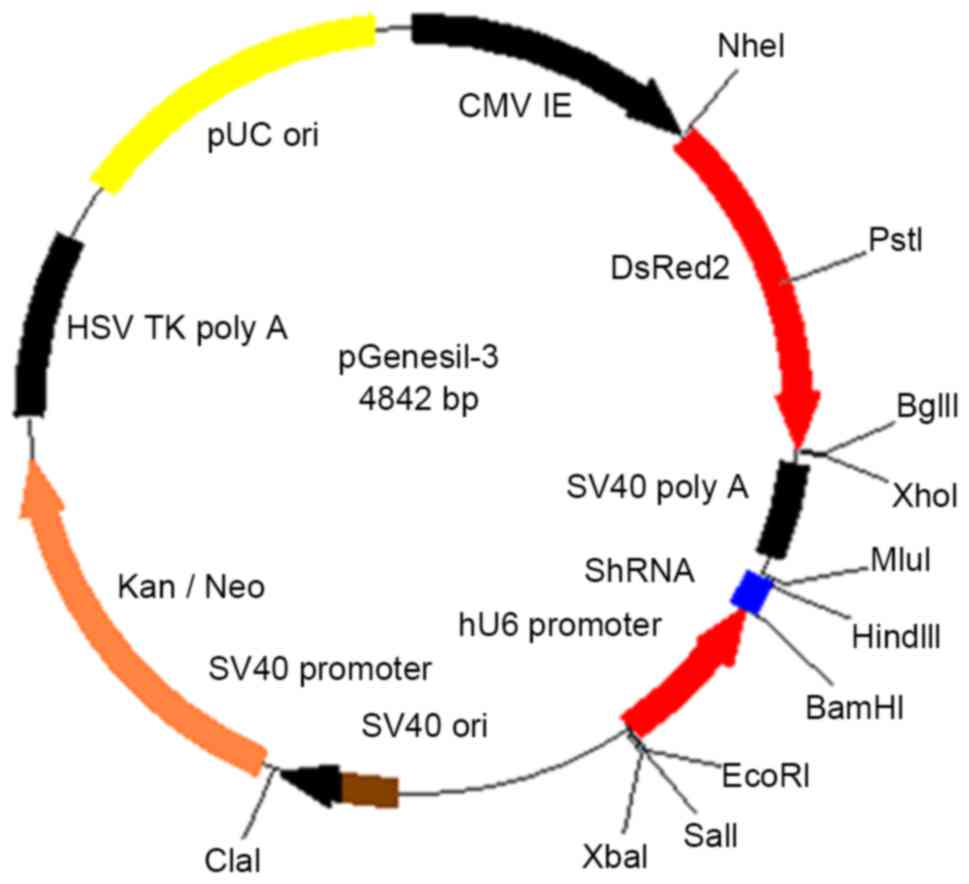
Ang2-siRNA plasmid (Fig. 1; Fuzhou
Maixin Biotechnology Development Co., Ltd.) was mixed with
polylysine-modified CMNPs, to a ratio of 1:1, 1:10, 1:100 or
1:1,000, followed by incubation at room temperature for 1 h.
Ang2-CMNPs were transfected into human malignant melanoma cells.
Following transfection, the cells were observed using fluorescence
microscopy and the transfection efficiency was calculated by cell
counting.
Acute toxicity assay
A total of 60 Kunming mice (30 male and 30 female;
weight, 18–22 g, specific-pathogen-free grade) were purchased from
Shanghai Experimental Animal Center, [Shanghai, China; license
number SCXK (Min) 2012–0001]. The animals were house under the
following conditions: Temperature, 25±1°C; humidity, 50–60%; 12:12
light:dark cycles. The animal experiments were approved by the
Institutional Animals Ethics Committee of Fujian Medical University
(Fuzhou, China). The mice were randomly divided into six groups (10
mice/group; male/female ratio, 1:1). The mice were fasted with free
access to water for the 12 h prior to the experiment. Ang2-CMNPs
were divided into five different dose groups. The doses in each
were as follows: 91.6, 152.8, 254.6, 424.2 and 707.0 mg·kg-1·d-1
(groups 1–5, respectively). A total of 0.4 ml Ang2-CMNPs in
suspension with normal saline was injected into the mice via the
tail vein, while the control group was injected with an equal
volume of normal saline. Diet and body weight changes of each group
were observed for 14 consecutive days, as well as whether any mice
died during experimentation. Following experimentation, the mice
were sacrificed by cervical dislocation and two mice from each
group were randomly selected for dissection to observe the color
and shape of the gross specimen. The major organs, including heart,
liver, spleen, lungs and kidneys were performed underwent
pathological examinations. Hematoxylin and eosin (H&E)
staining, and Prussian blue staining (Fuzhou Maixin Biotechnology
Development Co., Ltd., Fuzhou, China) were performed, according to
the manufacturer's protocols, to observe the conditions of edema,
deformation, necrosis and iron-particle deposition in cells.
Chronic toxicity assay
A total of 40 Sprague-Dawley rats (20 male and 20
female; weight, 90–100 g; specific-pathogen-free grade) were
purchased from Shanghai Experimental Animal Center [Shanghai,
China; license number SCXK (Min) 2012–0001]. The rats were randomly
divided into low-, middle- and high-dose groups, and a control
group (10 rats/group; male/female ratio, 1:1) and allowed free
access to food and water. The control group was injected with
saline, while the low-, middle- and high-dose groups were injected
with 1 ml Ang2-CMNP suspension (35.35, 70.70 and 353.50
mg·kg-1·d-1, respectively) via the tail vein. The injection was
performed for 14 consecutive days. Following the initiation of
medication, the response of the rats was observed and they were
weighed once every 3 days to monitor weight changes. On day 15, the
medication was stopped and the observation was continued for 7
days. On day 21, the rats were sacrificed by cervical dislocation,
and the blood was sampled from the eyeball for routine blood tests
(red cell count, white cell count, hemoglobin content, blood
platelet count) and biochemical tests (blood urea nitrogen,
albumin, creatinine-2, alkaline phosphatase, g-glutamyltransferase,
alanine aminotransferase, aspartate aminotransferase, total
bilirubin-1, total protein). Two rats from each group were selected
for dissection to observe the color and shape of the gross
specimen. As aforementioned for the acute assay, the major organs,
including the heart, liver, spleen, lungs and kidneys, underwent
pathological examination, and the organ co-efficient (organ wet
weight/body weight; 60 mice) was calculated. H&E staining and
Prussian blue staining were performed to observe the conditions of
edema, deformation, necrosis and iron-particle deposition in cells,
and the chronic toxicity of the particles.
Statistical analysis
All statistical analysis were performed using SPSS
software (version, 17.0; SPSS, Inc., Chicago, IL, USA). The data
are presented as the mean ± standard deviation. Comparisons between
two groups were performed using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Confirmation of suitable quality ratio
of Ang2/CMNPs
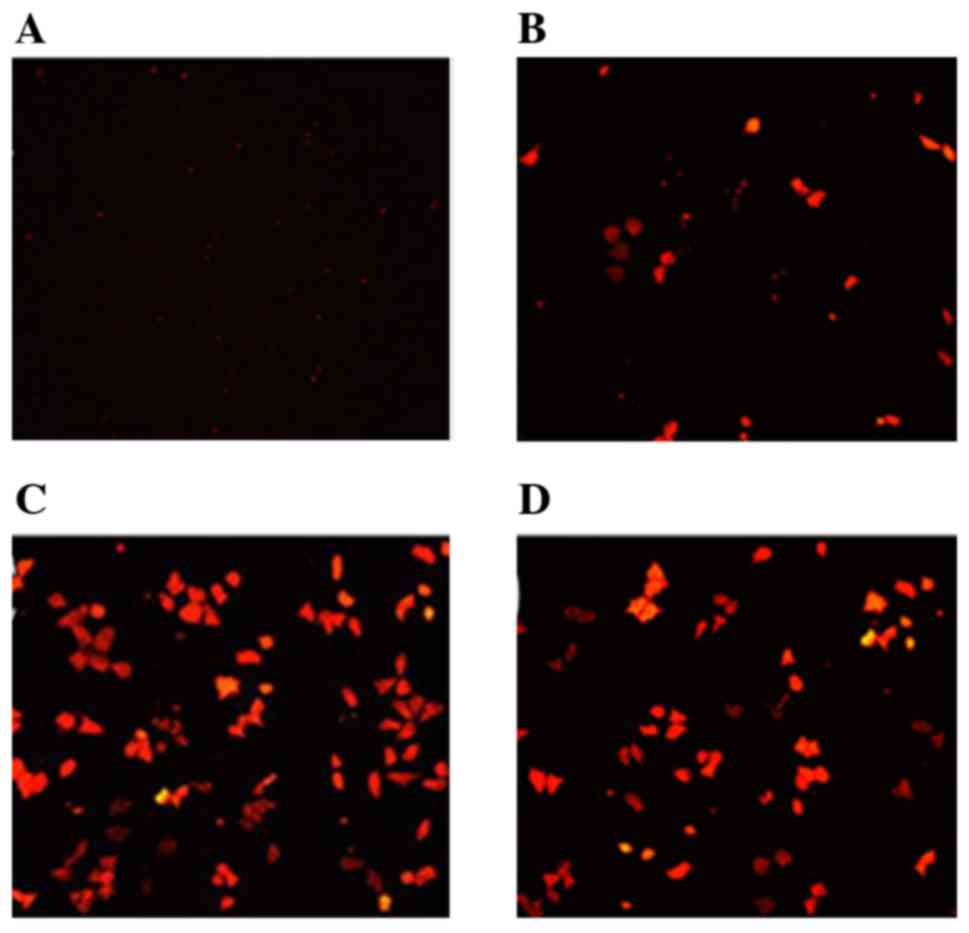
Fluorescence microscopy revealed that when the
quality ratio of Ang2/CMNPs ranged between 1:1 to 1:1,000, the red
fluorescence of transfected human malignant melanoma cells
gradually increased (Fig. 2). When
the ratio was 1:100, the red fluorescence was the most intense. The
transfection efficiency of Ang2-CMNPs towards malignant melanoma
cells is shown in Table I. A ratio
of 1:100 was determined to be the appropriate quality ratio of
Ang2/CMNPs for subsequent experiments.
 | Table I.Transfection efficiency of Ang2-CMNPs
into human malignant melanoma cells. |
Table I.
Transfection efficiency of Ang2-CMNPs
into human malignant melanoma cells.
| Quality
ratioa | Cell
numberb | Cell
numberc | Transfection
efficiency (%) |
|---|
| 1:1 | 0 | 118 | 0 |
| 1:10 | 10 | 107 | 9.35 |
| 1:100 | 63 | 103 | 61.17 |
| 1:1,000 | 35 | 84 | 41.67 |
Results of the acute toxicity
assay
Following administration of Ang2-CMNPs, the mice in
the 254.6, 424.2 and 707 mg·kg-1·d-1 groups exhibited short-term
staggering, reduced activity and accelerated breathing. The 91.6,
152.8 mg·kg-1·d-1 and control groups exhibited no abnormality. No
mouse in any group died during experimentation. The body weight
gain in each treatment group demonstrated no significant difference
when compared with the control group (data not shown).
The naked eye observation indicated that the
anterior left lung lobe of the mice in the 254.6
mg·kg−1·d−1 group was dark red in color and
each lung lobe of the mice in the 424.2 and 707
mg·kg−1·d−1 groups were identical color. The
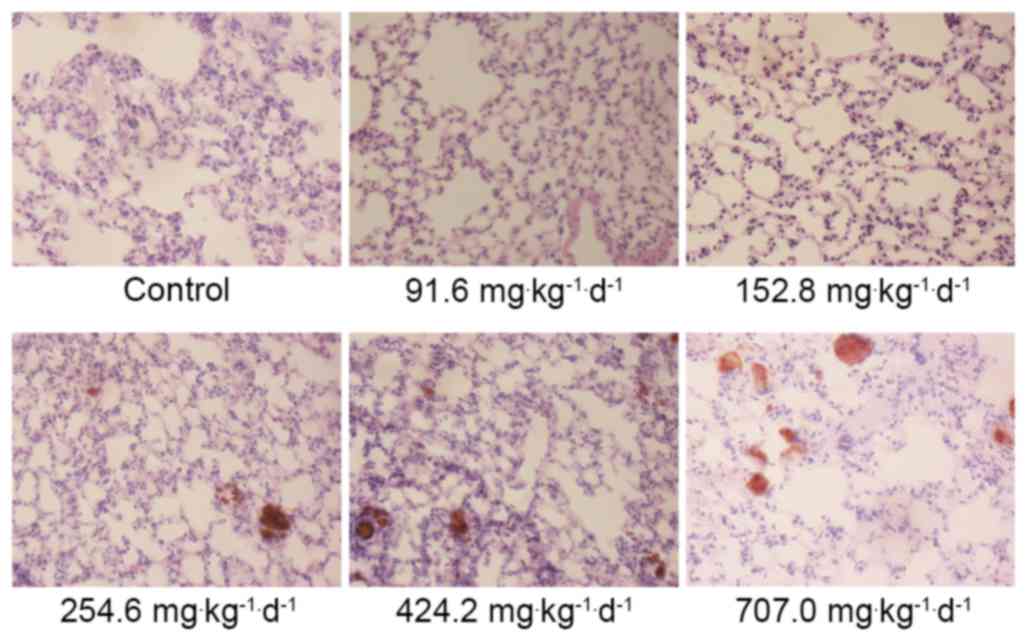
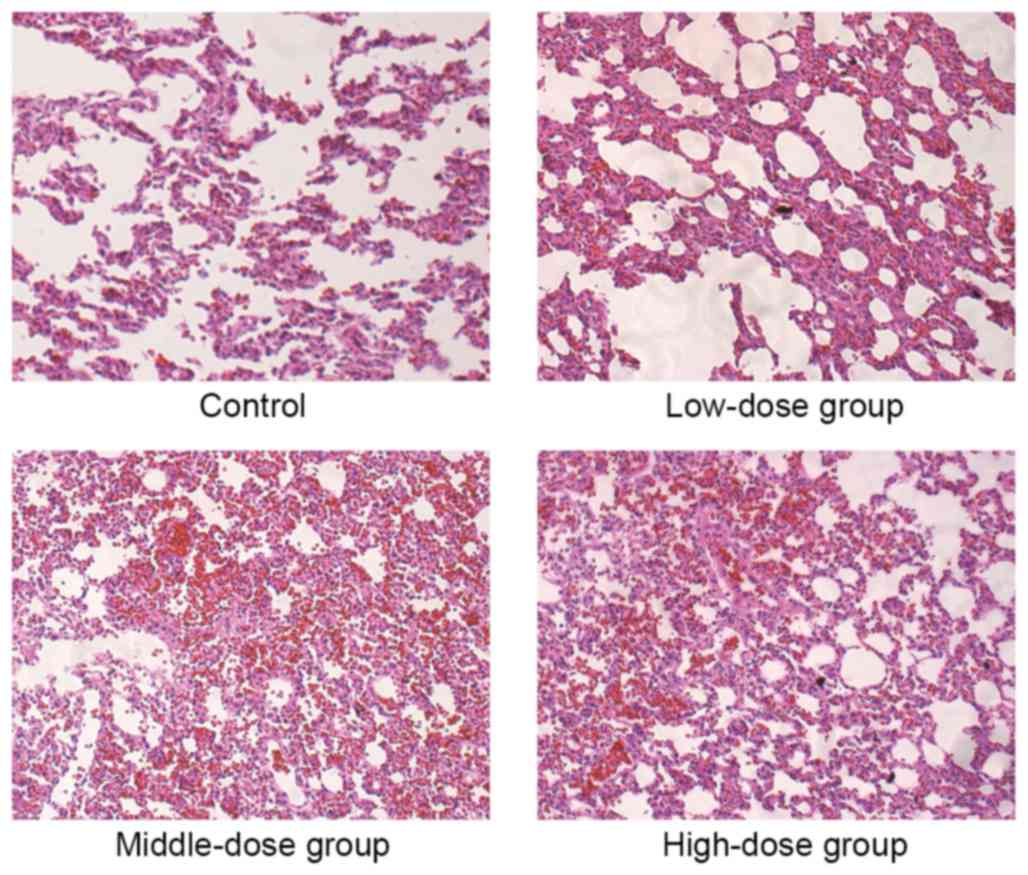
H&E staining revealed that the alveolar wall of the mice in the
254.6, 424.2 and 707.0 mg·kg−1·d−1 groups
exhibited telangiectasia, with red blood cells appearing inside the
alveolar cavity, presenting signs of pulmonary congestion (Fig. 3). The Prussian blue staining
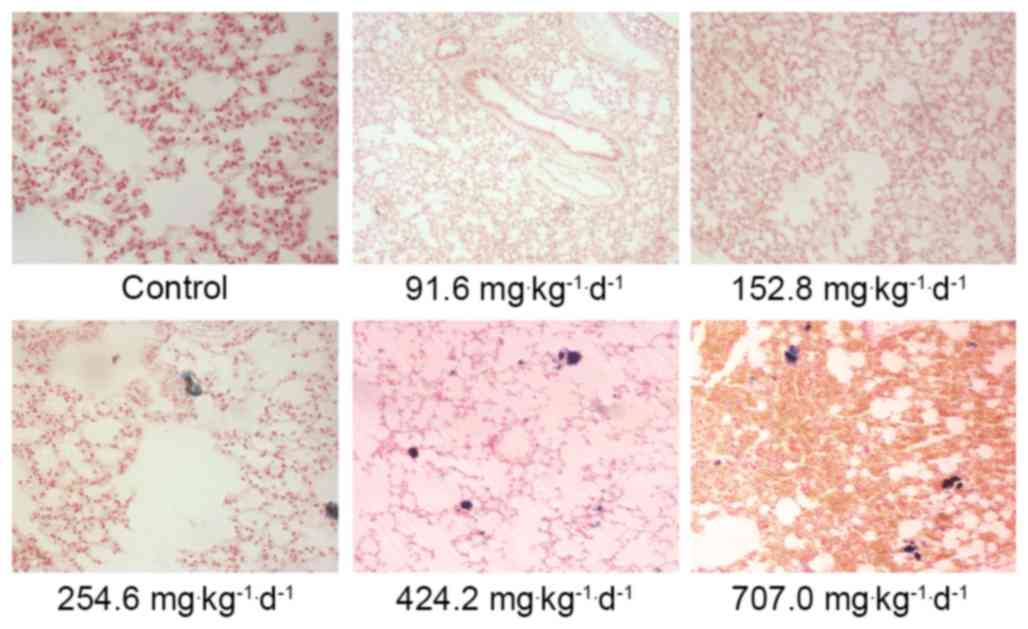
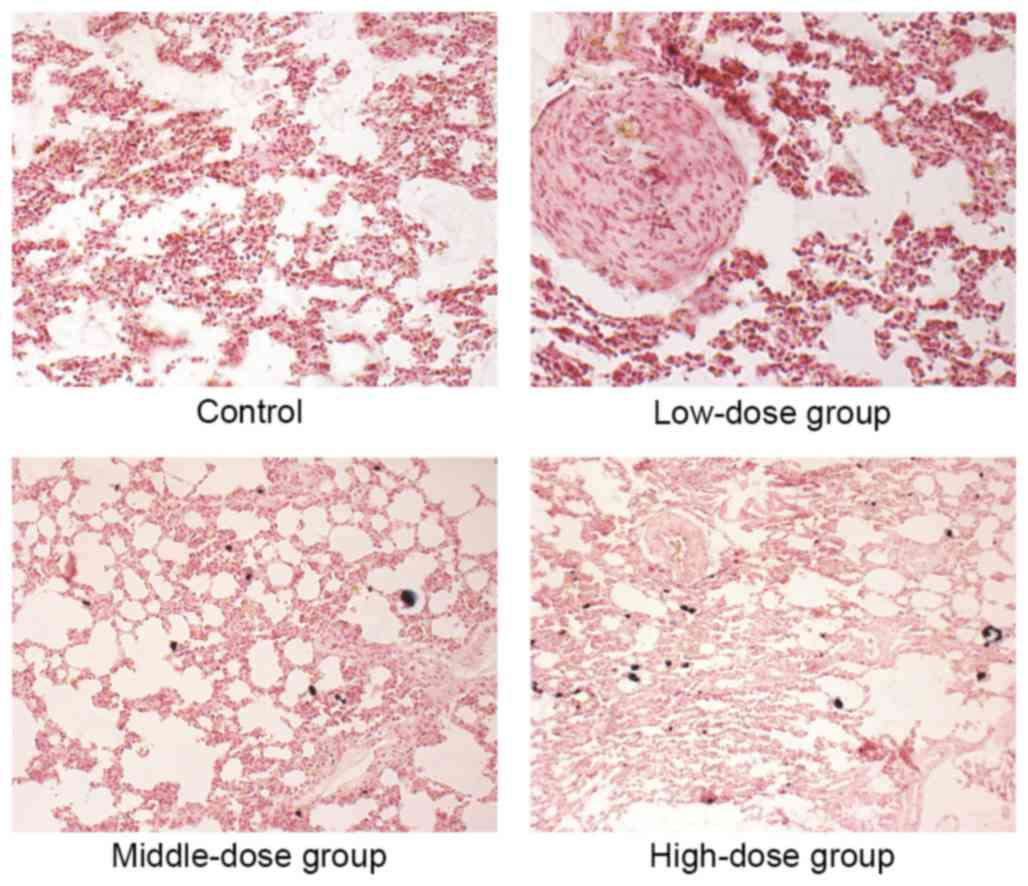
demonstrated that the lungs in the 254.6, 424.2 and 707
mg·kg−1·d−1 groups were deposited with blue
aggregated particles. The lung phagocytes exhibited phagocytosis
targeting these particles and the surrounding areas exhibited
inflammatory reactions (Fig. 4).
The other assessed organs exhibited no abnormality in each group.
The control group and the low-dose groups demonstrated no obvious
abnormalities.
Results of chronic toxicity assay
Following administration of Ang2-CMNPs, all rats in
each group exhibited normal drinking and eating activity, and no
animal died throughout the experimental period. The changes in body
weight between each treatment group and the control group
demonstrated no significant difference (F♀=0.85,
F♂=0.72, P>0.05).
As presented in Table
II, the lung/body ratio of treatment groups was significantly
higher compared with the control group of the same gender
(F♀=38.58, F♂=12.56, P<0.05). With the increasing dose, the
lung/body ratio also increased accordingly. The co-efficient of the
other organs in each treatment group exhibited no significant
difference when compared with the control group (P>0.05).
 | Table II.Effects of Ang2-CMNPs on organ
co-efficiencies of male rats (%). |
Table II.
Effects of Ang2-CMNPs on organ
co-efficiencies of male rats (%).
| Organ
co-efficiency | Control | Low-dose | Middle-dose | High-dose |
|---|
| ♂Heart/body | 0.52±0.08 | 0.52±0.13 | 0.46±0.04 | 0.47±0.07 |
| ♂Liver/body | 5.73±0.48 | 5.21±0.05 | 5.09±0.17 | 4.75±0.37 |
| ♂Spleen/body | 0.32±0.03 | 0.44±0.03 | 0.38±0.03 | 0.49±0.13 |
| ♂Lung/body | 0.57±0.06 |
0.74±0.05a |
0.81±0.03a |
0.84±0.00a |
| ♂Kidney/body | 0.97±0.15 | 1.04±0.18 | 1.03±0.06 | 0.92±0.00 |
| ♀Heart/body | 0.54±0.10 | 0.50±0.01 | 0.49±0.04 | 0.45±0.05 |
| ♀Liver/body | 5.14±0.21 | 5.13±0.67 | 4.86±0.46 | 5.01±0.38 |
| ♀Spleen/body | 0.41±0.03 | 0.43±0.03 | 0.49±0.04 | 0.35±0.09 |
| ♀Lung/body | 0.57±0.03 |
0.77±0.03a |
0.79±0.04a |
0.88±0.01a |
| ♀Kidney/body | 1.04±0.11 | 1.01±0.07 | 0.94±0.04 | 0.97±0.08 |
The routine blood test demonstrated that the total
number of white blood cells in the treatment groups were
significantly higher compared with the control group of the same
gender (F♀=93.37, F♂=80.04, P<0.05). The remaining indexes
revealed no significant differences between the different groups
(Table III). The blood
biochemical assay indicated that there was no significant
difference in blood biochemistry indexes between each treatment
group and control group of the same gender (Table IV).
 | Table III.Effects of Ang2-CMNPs on blood
routine indexes of rats. |
Table III.
Effects of Ang2-CMNPs on blood
routine indexes of rats.
| Group | Red cell count
(1012 cells/l) | White cell count
(109 cells/l) | Hemoglobin content
(g/l) | Blood platelet
count (109 cells/l) |
|---|
| ♂Control | 6.78±0.58 | 3.45±0.26 | 133.60±4.32 | 480.00±30.33 |
| ♂Low-dose | 6.81±0.31 |
5.05±0.35a | 133.00±5.18 | 492.00±25.61 |
| ♂Middle-dose | 6.79±0.32 |
5.58±0.38a | 138.00±5.66 | 478.00±38.68 |
| ♂High-dose | 6.66±0.28 |
6.99±0.31a | 139.20±5.04 | 484.00±37.20 |
| ♀Control | 6.73±0.39 | 3.48±0.27 | 130.80±6.31 | 464.00±31.37 |
| ♀Low-dose | 6.69±0.34 |
5.06±0.26a | 133.20±3.06 | 478.00±41.18 |
| ♀Middle-dose | 6.80±0.35 |
5.46±0.44a | 136.40±7.03 | 478.00±44.00 |
| ♀High-dose | 6.62±0.29 |
7.15±0.23a | 136.40±4.45 | 466.00±48.83 |
 | Table IV.Effects of Ang2-CMNPs on blood
biochemistry indexes of rats. |
Table IV.
Effects of Ang2-CMNPs on blood
biochemistry indexes of rats.
| Group | BUN (mM) | ALB (g/l) | ECRE-2 (µM) | ALP (U/l) | GGT (U/l) | ALT(U/l) | AST (U/l) | TBIL-2
(µmol/l) | TP (g/l) |
|---|
| ♂Control | 3.66±0.27 | 32.48±0.34 | 17.60±0.49 | 319.20±22.96 | 12.40±2.73 | 43.40±5.24 | 117.40±12.08 | 1.14±0.27 | 48.60±1.21 |
| ♂Low-dose | 3.68±0.28 | 31.40±1.02 | 17.60±1.02 | 326.60±27.55 | 10.80±1.60 | 40.00±1.79 | 119.40±14.01 | 1.04±0.23 | 47.34±0.77 |
| ♂Middle-dose | 3.46±0.30 | 32.00±1.33 | 17.40±1.36 | 312.20±23.94 | 13.80±2.32 | 41.00±2.76 | 112.60±12.13 | 1.36±0.29 | 47.84±0.77 |
| ♂High-dose | 3.92±0.29 | 30.60±0.26 | 31.14±0.74 | 327.40±36.95 | 12.80±2.23 | 40.40±3.61 | 115.80±10.34 | 1.30±0.19 | 47.46±0.38 |
| ♀Control | 3.78±0.48 | 33.02±1.41 | 19.40±1.96 | 240.20±32.49 | 8.63±2.97 | 38.80±4.75 | 135.40±8.91 | 0.98±0.16 | 49.62±2.07 |
| ♀Low-dose | 3.54±0.46 | 31.36±1.75 | 19.40±1.02 | 233.60±19.47 | 7.60±1.62 | 39.60±7.71 | 122.60±15.93 | 1.12±0.13 | 48.66±2.61 |
| ♀Middle-dose | 4.22±0.74 | 31.64±0.83 | 19.20±0.75 | 225.00±34.44 | 8.00±2.45 | 45.00±2.45 | 126.60±15.17 | 1.20±0.13 | 51.20±2.40 |
| ♀High-dose | 3.96±0.26 | 31.32±2.12 | 19.40±0.49 | 228.80±20.51 | 8.40±2.15 | 41.20±4.83 | 120.80±29.69 | 1.14±0.21 | 49.80±2.93 |
The H&E staining revealed that the alveolar wall
of the middle- and high-dose group was thickened and exhibited mild
fibrosis, and red blood cells leaked from the alveolar cavity
(Fig. 5). The Prussian blue
staining demonstrated that blue particles in the middle- and
high-dose group were scattered inside the lungs, that the particles
aggregated in lungs and were engulfed by pulmonary phagocytes, and
inflammatory reactions existed around the lungs (Fig. 6). The control group and the
low-dose group demonstrated no abnormalities. The remaining organs
exhibited no obvious abnormality.
Discussion
Nano-targeted drug delivery systems can overcome the
limitation of traditional chemotherapies, and therefore, have
become a novel and prosperous tumor treatment method. In MTDDS, the
magnetic nanoparticles may be used as an ideal carrier for direct
targeted accumulation into the tumor tissues, thus selectively
releasing the therapeutic molecules (9). MTDDS predominantly consists of three
parts: i) Magnetic material; ii) the frame material; and iii) the
drug. The most representative magnetic material at present is
Fe304 powder, which can not only provide
magnetism for the targeting drugs, but can also load the drugs for
targeted therapy (10). The drugs
used in MTDDS currently include adriamycin (11), folic acid (12), cisplatin and insulin (13). The frame material is the part
connecting the magnetic material and the drug, including glucans
(14) and chitosan (15). The specific targeting
characteristics of the magnetic nano-carrier include active and
passive targeting (16). Active
targeting vectors have a higher specificity compared with passive
targeting vectors, and include the ligand or antibody coupling of
the targeted cells (17), or are
directly aggregated in the targeted tissues under the influence of
an external magnetic field to achieve its targeting property.
Iron is one of the essential trace elements, and
human tissues store and transport it using hemoglobin, transferrin,
ferritin and transferrin hemosiderin. Excessive iron can be
discharged via urine and sweat. As a gene carrier, chitosan is a
material that has received great attention in recent years
(18). It is a cationic basic
polysaccharide polymer, and is widely used in the food and
pharmaceutical industry for its good compatibilities (19). As a natural polysaccharide, it is
of high safety. In previous years, chitosan nanoparticles have
undergone vast developments in novel drug-sustained release systems
for their high specificity, sensitivity, improved bioavailability
and low toxicity (20). The
present study applied self-made chitosan magnetic nanoparticles
that use Fe3O4 as the core to connect
Ang2-siRNA plasmids in the formation of Ang2-CMNPs. When chitosan
is bound with magnetic Fe3O4 nanoparticles,
the size and structure is altered (21). Therefore, the nature and intensity
of biological effects produced by these nanoparticles to the body
will also change, thus generating certain impact on the body.
Acute toxicity is an important index to evaluate the
safety of drugs. In the present study, the acute toxicity assay
revealed that the mice in groups 3–5 exhibited short-term
staggering, reduced activities and accelerated breathing, as well
as transient reduction of eating; however all mice recovered
normally following experimentation. The weight gains in the
treatment groups revealed no significant difference when compared
with the control group, and no mouse in any group died, indicating
that acute toxicity was low. Following sacrificing and dissection
of the mice, it was revealed that the lungs in groups 3–5 exhibited
uneven dark red coloring. H&E staining revealed acute pulmonary
congestion and Prussian blue staining demonstrated blue particles
scattering inside the lungs. These particles were aggregated inside
the lungs and phagocytized by lung phagocytes. The control group
and the low-dose group demonstrated no obvious abnormalities. This
indicated that low-dose Ang2-CMNPs caused no obvious acute toxicity
in the mice.
In the chronic toxicity assay, the rats in all
groups exhibited normal feeding and drinking activity following
injection of Ang2-CMNPs and no animal died. No significant impact
was observed on weight gain and hepatoenteric functions. Following
the sacrifice and dissection of the rats, the middle- and high-dose
group exhibited uneven dark red coloring in the lungs. H&E
staining revealed chronic pulmonary congestion and pulmonary
inflammation, whilst Prussian blue staining demonstrated more
particles aggregated inside the lungs; the control group and the
low-dose group exhibited no such abnormality. Therefore, it can be
presumed that the non-toxic dose of Ang2-CMNPs was >35.35
mg·kg−1·d−1. As for the organ co-efficients,
the lung/body ratios in the high-, middle- and low-dose group were
significantly higher compared with the control group, and the blood
routine revealed that the total number of white blood cells in each
treatment group was significantly higher compared with the control
group. Therefore, it can be suggested that the target organ for the
Ang2-CMNP-induced toxicity is predominantly the lungs; the repeated
stimulation of particles to the lungs will induce chronic lung
inflammation and mild fibrosis.
The surface functionalization, colloidal stability
and biocompatibility of the magnetic nanoparticles are crucial for
their biomedical applications (22), and the toxicity of the
nanoparticles is often closely associated with their sizes. When
the particle diameter is <100 nm, the toxicity is predominantly
decided by the nature of the particles or the surface-attached
materials, but when the particle size is larger, death can often be
caused by the embolism of capillaries and small blood vessels by
the particles. In the present study, the results demonstrated that
Ang2-CMNPs predominantly caused damage in the lungs. Since the
specific surface areas of nanoparticles are significantly increased
with the decreased particle size, so is the particle surface
energy. Therefore, they would easily mutually aggregate at a high
concentration. It can be presumed that the reason for
Ang2-CMNP-induced lung damage is that, when a large number of
microparticles enter the circulation of the mice, they will firstly
reach the lungs. The aggregation of the particles will then block
the pulmonary vessels, thus leading to the observed acute pulmonary
congestion. In the chronic toxicity assay, the long-term repeated
injection of the particles via the tail vein led to chronic
pulmonary congestion in rats, as well as simultaneous pulmonary
inflammation and partial fibrosis. Since the unaggregated particles
are small in size, they can escape phagocytosis by the liver and
spleen, so no particle deposition was observed around the liver and
spleen. The low-dose group presented no organ damage, so it can be
speculated that, in a certain concentration range, CMNPs will be
safe and not cause side effects towards the body. This non-toxic
dose was determined as >35.35 mg·kg−1·d−1.
Based on the conversion method of equivalent dose co-efficient, the
non-toxic dose in humans should be >222.71
mg·kg−1·d−1 for 14 day, overall a total of
3,117.87 mg·kg−1, which is significantly higher compared
with the quantity required clinically. Therefore, this dose is
relatively safe. Increasing the dispersion degree of the particles
may reduce the aggregation at higher concentrations, thus reducing
the toxicity.
One of the major challenges faced by gene therapy is
the selection of vector system in gene therapy (23). The results of the present study
demonstrated that, Ang2-CMNPs have good security, so they can be
considered as a vector for targeted gene therapy. Furthermore, the
study has also laid a foundation for the in vivo targeting
intervention experiments towards the angiogenesis and tumor growth
of transplanted malignant melanoma in nude mice in the future.
Acknowledgements
The present study was supported by the Foundation of
National Key Clinical Specialty Discipline Construction Program,
Scientific Research Foundation of National Health Planning
Scientific Research Foundation-Joint Research Projects of Fujian
Provincial Health and Education (no. WKJ-FJ-03), Projects of Fujian
Provincial Natural Science Foundation (no. 2012J01125) and Youth
Scientific Research Subject of Fujian Provincial Health and Family
Planning Commission (no. 2015-1-45).
References
|
1
|
Wu F, Lin GZ and Zhang JX: An overview of
cancer incidence and trend in China. Zhong Guo Ai Zheng. 21:81–83.
2012.(In Chinese).
|
|
2
|
Venza M, Visalli M, Beninati C, De Gaetano
GV, Teti D and Venza I: Cellular mechanisms of oxidative stress and
action in melanoma. Oxid Med Cell Longev. 2015:4817822015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schlaak M, Kreuzberg N, Mauch C and
Kurschat P: Personalized therapy concepts for malignant melanoma.
Internist (Berl). 54:188–193. 2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Unger E, Porter T, Lindner J and Grayburn
P: Cardiovascular drug delivery with ultrasound and microbubbles.
Adv Drug Deliv Rev. 72:110–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sehrig F Zeinali, Majidi S, Nikzamir N,
Nikzamir N, Nikzamir M and Akbarzadeh A: Magnetic nanoparticles as
potential candidates for biomedical and biological applications.
Artif Cells Nanomed Biotechnol. 44:918–927. 2016.PubMed/NCBI
|
|
6
|
Ishii T, Okahata Y and Sato T: Mechanism
of cell transfection with plasmid/chitosan complexes. Biochim
Biophys Acta. 1514:51–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoet PH, Brüske-Hohlfeld I and Salata OV:
Nanoparticles-known and unknown health risk. Nanobiotechnology.
2:122004. View Article : Google Scholar
|
|
8
|
Nel A, Xia T, Mädler L and Li N: Toxic
potential of materials at the nanolevel. Science. 311:622–627.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Unsoy G, Khodadust R, Yalcin S, Mutlu P
and Gunduz U: Synthesis of Doxorubicin loaded magnetic chitosan
nanoparticles for pH responsive targeted drug delivery. Eur J Pharm
Sci. 62:243–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng XH, Qian X, Mao H, Wang AY, Chen ZG,
Nie S and Shin DM: Targeted magnetic iron oxide nanoparticles for
tumor imaging and therapy. Int J Nanomedicine. 3:311–321.
2008.PubMed/NCBI
|
|
11
|
Shi Z, Guo R, Li W and Zhang Y, Xue W,
Tang Y and Zhang Y: Nanoparticles of deoxycholic acid, polyethylene
glycol and folic acid-modified chitosan for targeted delivery of
doxorubicin. J Mater Sci Mater Med. 25:723–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Wang M, Zheng M, Guo Q, Wang Y,
Wang H, Xie X, Huang F and Gong R: Folate mediated self-assembled
phytosterol-alginate nanoparticles for targeted intracellular
anticancer drug delivery. Colloids Surf B Biointerfaces. 129:63–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen JM, Xu L, Lu Y, Cao HM, Xu ZG, Chen T
and Zhang HX: Chitosan-based luminescent/magnetic hybrid nanogels
for insulin delivery, cell imaging and antidiabetic research of
dietary supplements. Int J Pharm. 427:400–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng M, Li H, Luo Z, Kong J, Wan Y, Zheng
L, Zhang Q, Niu H, Vermorken A, Van De Ven W, et al: Dextran-coated
superparamagnetic nanoparticles as potential cancer drug carriers
in vivo. Nanoscale. 7:11155–11162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadighian S, Hosseini-Monfared H,
Rostamizadeh K and Hamidi M: pH-triggered magnetic-chitosan
nanogels (MCNs) for doxorubicin delivery: Physically vs. Chemically
cross linking approach. Adv Pharm Bull. 5:115–120. 2015.PubMed/NCBI
|
|
16
|
Yu B, Tai HC, Xue W, Lee LJ and Lee RJ:
Receptor-targeted nanocarriers for therapeutic delivery to cancer.
Mol Membr Biol. 27:286–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torchilin VP: Passive and active drug
targeting: Drug delivery to tumors as an example. Handb Exp
Pharmacol. 3-53:2010. View Article : Google Scholar
|
|
18
|
Mansouri S, Lavigne P, Corsi K, Benderdour
M, Beaumont E and Fernandes JC: Chitosan-DNA nanoparticles as
non-viral vectors in gene therapy: Strategies to improve
transfection efficacy. Eur J Pharm Biopharm. 57:1–8. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sionkowska A, Wisniewski M, Skopinska J,
Kennedy CJ and Wess TJ: Molecular interactions in collagen and
chitosan blends. Biomaterials. 25:795–801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghadi A, Mahjoub S, Tabandeh F and
Talebnia F: Synthesis and optimization of chitosan nanoparticles:
Potential applications in nanomedicine and biomedical engineering.
Caspian J Intern Med. 5:156–161. 2014.PubMed/NCBI
|
|
21
|
Chang YC and Chen DH: Preparation and
adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic
nanoparticles for removal of Cu(II) ions. J Colloid Interface Sci.
283:446–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thorat ND, Otari SV, Patil RM, Bohara RA,
Yadav HM, Koli VB, Chaurasia AK and Ningthoujam RS: Synthesis,
characterization and biocompatibility of chitosan functionalized
super paramagnetic nanoparticles for heat activated curing of
cancer cells. Dalton Trans. 43:17343–17351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Rorke S, Keeney M and Pandit A:
Non-viral polyplexes: Scaffold mediated delivery for gene therapy.
Prog Polym Sci. 35:441–458. 2010. View Article : Google Scholar
|