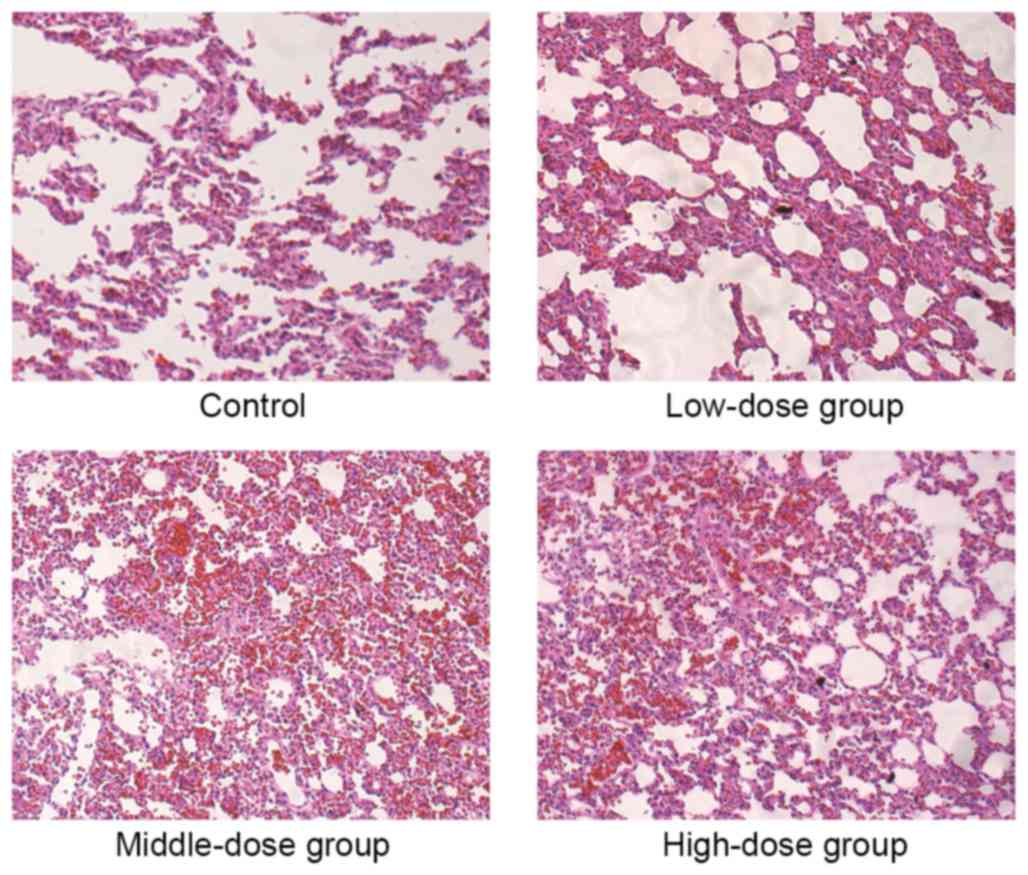
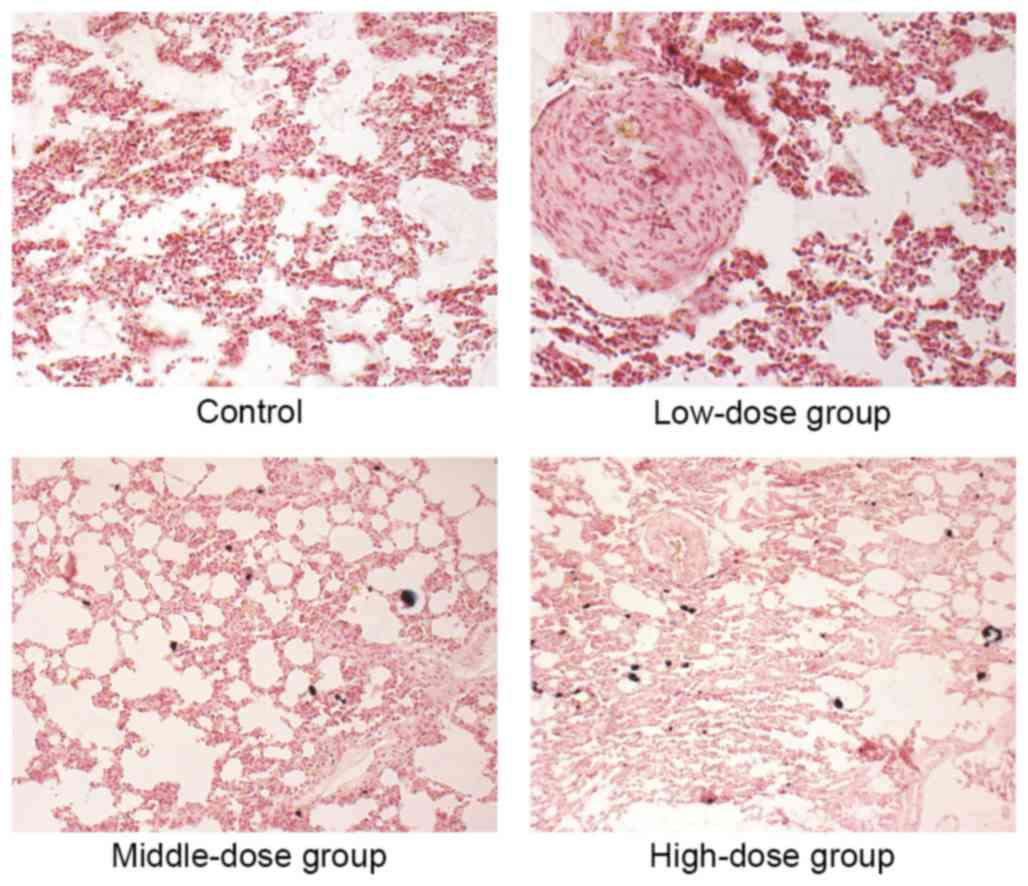
|
1
|
Wu F, Lin GZ and Zhang JX: An overview of
cancer incidence and trend in China. Zhong Guo Ai Zheng. 21:81–83.
2012.(In Chinese).
|
|
2
|
Venza M, Visalli M, Beninati C, De Gaetano
GV, Teti D and Venza I: Cellular mechanisms of oxidative stress and
action in melanoma. Oxid Med Cell Longev. 2015:4817822015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schlaak M, Kreuzberg N, Mauch C and
Kurschat P: Personalized therapy concepts for malignant melanoma.
Internist (Berl). 54:188–193. 2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Unger E, Porter T, Lindner J and Grayburn
P: Cardiovascular drug delivery with ultrasound and microbubbles.
Adv Drug Deliv Rev. 72:110–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sehrig F Zeinali, Majidi S, Nikzamir N,
Nikzamir N, Nikzamir M and Akbarzadeh A: Magnetic nanoparticles as
potential candidates for biomedical and biological applications.
Artif Cells Nanomed Biotechnol. 44:918–927. 2016.PubMed/NCBI
|
|
6
|
Ishii T, Okahata Y and Sato T: Mechanism
of cell transfection with plasmid/chitosan complexes. Biochim
Biophys Acta. 1514:51–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoet PH, Brüske-Hohlfeld I and Salata OV:
Nanoparticles-known and unknown health risk. Nanobiotechnology.
2:122004. View Article : Google Scholar
|
|
8
|
Nel A, Xia T, Mädler L and Li N: Toxic
potential of materials at the nanolevel. Science. 311:622–627.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Unsoy G, Khodadust R, Yalcin S, Mutlu P
and Gunduz U: Synthesis of Doxorubicin loaded magnetic chitosan
nanoparticles for pH responsive targeted drug delivery. Eur J Pharm
Sci. 62:243–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng XH, Qian X, Mao H, Wang AY, Chen ZG,
Nie S and Shin DM: Targeted magnetic iron oxide nanoparticles for
tumor imaging and therapy. Int J Nanomedicine. 3:311–321.
2008.PubMed/NCBI
|
|
11
|
Shi Z, Guo R, Li W and Zhang Y, Xue W,
Tang Y and Zhang Y: Nanoparticles of deoxycholic acid, polyethylene
glycol and folic acid-modified chitosan for targeted delivery of
doxorubicin. J Mater Sci Mater Med. 25:723–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Wang M, Zheng M, Guo Q, Wang Y,
Wang H, Xie X, Huang F and Gong R: Folate mediated self-assembled
phytosterol-alginate nanoparticles for targeted intracellular
anticancer drug delivery. Colloids Surf B Biointerfaces. 129:63–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen JM, Xu L, Lu Y, Cao HM, Xu ZG, Chen T
and Zhang HX: Chitosan-based luminescent/magnetic hybrid nanogels
for insulin delivery, cell imaging and antidiabetic research of
dietary supplements. Int J Pharm. 427:400–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng M, Li H, Luo Z, Kong J, Wan Y, Zheng
L, Zhang Q, Niu H, Vermorken A, Van De Ven W, et al: Dextran-coated
superparamagnetic nanoparticles as potential cancer drug carriers
in vivo. Nanoscale. 7:11155–11162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadighian S, Hosseini-Monfared H,
Rostamizadeh K and Hamidi M: pH-triggered magnetic-chitosan
nanogels (MCNs) for doxorubicin delivery: Physically vs. Chemically
cross linking approach. Adv Pharm Bull. 5:115–120. 2015.PubMed/NCBI
|
|
16
|
Yu B, Tai HC, Xue W, Lee LJ and Lee RJ:
Receptor-targeted nanocarriers for therapeutic delivery to cancer.
Mol Membr Biol. 27:286–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torchilin VP: Passive and active drug
targeting: Drug delivery to tumors as an example. Handb Exp
Pharmacol. 3-53:2010. View Article : Google Scholar
|
|
18
|
Mansouri S, Lavigne P, Corsi K, Benderdour
M, Beaumont E and Fernandes JC: Chitosan-DNA nanoparticles as
non-viral vectors in gene therapy: Strategies to improve
transfection efficacy. Eur J Pharm Biopharm. 57:1–8. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sionkowska A, Wisniewski M, Skopinska J,
Kennedy CJ and Wess TJ: Molecular interactions in collagen and
chitosan blends. Biomaterials. 25:795–801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghadi A, Mahjoub S, Tabandeh F and
Talebnia F: Synthesis and optimization of chitosan nanoparticles:
Potential applications in nanomedicine and biomedical engineering.
Caspian J Intern Med. 5:156–161. 2014.PubMed/NCBI
|
|
21
|
Chang YC and Chen DH: Preparation and
adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic
nanoparticles for removal of Cu(II) ions. J Colloid Interface Sci.
283:446–451. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thorat ND, Otari SV, Patil RM, Bohara RA,
Yadav HM, Koli VB, Chaurasia AK and Ningthoujam RS: Synthesis,
characterization and biocompatibility of chitosan functionalized
super paramagnetic nanoparticles for heat activated curing of
cancer cells. Dalton Trans. 43:17343–17351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Rorke S, Keeney M and Pandit A:
Non-viral polyplexes: Scaffold mediated delivery for gene therapy.
Prog Polym Sci. 35:441–458. 2010. View Article : Google Scholar
|