Introduction
Numerous clinical events, including sepsis, trauma,
transient ischemia and reperfusion, cause acute lung injury (ALI)
(1). Sepsis, as a result of severe
infection, is one of the most common clinical causes of ALI and
leads to a high mortality of 60–80% (2). Although the management of critically
ill patients is improving, the mortality rate remains ~40%, and
survivors often do not return to a normal life (3). In both immunocompromised and
immunocompetent individuals, bacterial pneumonia is a leading cause
of mortality from ALI. During the process, neutrophil infiltration
is the first event, followed by large amount of proinflammatory
cytokines and proteases, including myeloperoxidase (MPO), which are
the main mediators in the causes of serious lung injury. Presently,
only a few pharmacological treatments are available for
lipopolysaccharide (LPS)-induced ALI; the inhibition of
inflammation or the production of antioxidative effects.
Ethyl pyruvate is a stable and simple lipophilic
ester derived from the endogenous metabolite pyruvate and has been
demonstrated to protect against inflammation and to mitigate organ
dysfunction in several animal models of various disorders,
including severe sepsis, burns and acute pancreatitis. Ethyl
pyruvate has also been demonstrated to possess a protective effect
on ALI, probably through the inhibition of the mitogen-activated
protein kinase (MAPK) pathway in lungs (4,5).
However, these results simply reveal the general ameliorative
effect of ethyl pyruvate with no cellular target and therefore,
more data are required to elucidate the underlying mechanism.
Previous studies (6–9) have
demonstrated that neutrophils extensively accumulate in the lung
during the initial stage of ALI, which substantially contributes to
the severity of this disorder in human and animal models by several
mechanisms: i) The release of granule components, including serine
proteases, matrix metalloproteinases and lactoferrin; ii) the
generation of reactive oxygen species; and iii) the formation of
neutrophil extracellular traps, thus making neutrophils a potential
target for ALI attenuation. Different therapeutic approaches have
been developed against neutrophil infiltration, the releasing of
granule components and the induction of apoptosis.
Autophagy is a constitutive regulatory means of
cellular homeostasis involved in diverse physiological and
pathological events (10–15). Autophagy has been demonstrated to
be important for bacterial infection and inflammation (16–18),
for example, autophagy in macrophages is important for the
maturation, activation, polarization and regulation of cytokine
production (19–23). Several mechanisms of
anti-inflammation by autophagy have been proposed, including
clearing the cytoplasm of non-functional organelles (e.g.,
mitochondria), and removing the aggregated inflammasome structure
(16). However, few studies exist
on the regulation of neutrophils by autophagy, and recently it was
demonstrated that autophagy deficiency reduced neutrophil
degranulation (24). Whether ethyl
pyruvate contributes to neutrophil autophagy in ALI remains to be
elucidated.
The present study aimed to confirm the protective
effects of ethyl pyruvate on ALI in vivo using an
LPS-induced mouse model, and to further elucidate the underlying
mechanism. The data demonstrated that neutrophils infiltrated into
airspace during ALI experience increased autophagy, which is
required for granule release, while ethyl pyruvate inhibited
autophagy in neutrophils, and decreased granule release, thus
attenuating lung injury in ALI. This novel mechanism illuminates
the role of ethyl pyruvate in ALI, and provides a basis from which
to develop a novel therapeutic approach with autophagy as a
target.
Materials and methods
Reagents and antibodies
Ethyl pyruvate, lipopolysaccharide and
N-Formyl-Met-Leu-Phe were obtained from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). Antibodies against LC3 (cat. no.
4108S, 1:2,000), Becn1 (cat. no. 3738S, 1:2,000), ATG5 (cat. no.
12994, 1:2,000), and β-actin (cat. no. 4970S, 1:2,000) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
PE-Ly6G [1A8] and APC-Gr1 [RB6-8C5] were purchased from BioLegend,
Inc. (San Diego, CA, USA). ELISA Kits for tumor necrosis factor-α
(TNF-α), interleukin-6 (IL-6) and MPO were purchased from R&D
Systems, Inc. (Minneapolis, MN, USA).
Experimental model
C57BL/6 male mice (n=56; 8 weeks old) used for
preparing ALI models were obtained from the Shanghai Laboratory
Animal Center of the Chinese Academy of Sciences (Shanghai, China),
and maintained under specific pathogen-free conditions at 22°C, 50%
humidity and a 12 h light/dark cycle, with free access to food and
sterile water. The animals were weighed, injected intratracheally
with LPS (5 mg/kg) or vehicle (phosphate-buffered saline; PBS) and
euthanized with CO2 at 2 l/min in a closed box of ~10 l
volume. The concentration of CO2 was gradually increased
to 70% within ~4 min) for 15 min, and mortality was confirmed upon
no response to hind limb pinching at 4 or 24 h following injection.
All experiments were performed in accordance with the guidelines
of, and with the approval of, the Animal Care and Usage Committee
of Xinhua Hospital, Shanghai Jiao Tong University School of
Medicine (Shanghai, China).
Histopathology
Lung samples were fixed in 10% formalin, sectioned
and dehydrated through 70, 80 and 95% alcohol, 45 min each,
followed by 3 changes of 100% alcohol, 1 h each, followed by
embedding in paraffin wax. Tissue blocks were sectioned to 5 mm,
transferred to glass slides and stained with hematoxylin and eosin.
Morphological examinations were performed using light microscopy
and images were captured.
Cell culture and transfection
The murine myeloid cell line, 32Dcl3 (CRL-11346),
was obtained from American Type Culture Collection (Manassas, VA,
USA), and cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum. The 32Dcl3 cells were transfected with Lipofectamine
3000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol.
Determination of cytokines and MPO in
murine bronchoalveolar lavage fluid (BALF) by ELISA
TNF-α, IL-6, and MPO in murine BALF were determined
by sandwich ELISA (R&D Systems, Inc.), according to the
manufacturer's protocol.
Immunoblotting
Immunoblotting was performed as described previously
(17). Briefly, cells with or
without treatment were collected and lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Following brief vortexing and
rotation, cell lysates were separated by SDS-PAGE and transferred
onto PVDF membranes. The membranes were blocked with 5% non-fat
milk in PBS for 30 min at room temperature and were subsequently
incubated with appropriate antibody in PBS with 0.5% non-fat milk
for 2 h at room temperature. Following washing in PBS/Tween-20, the
membranes were incubated for 1 h with horseradish
peroxidase-conjugated secondary antibody. Bands were detected with
enhanced chemiluminescence plus detection reagents (Amersham
Pharmacia Biotech, Piscataway, NJ, USA).
Autophagy analyses
Autophagy was analyzed by immunoblotting or
fluorescence microscopy, as described previously (25). Briefly, cell lysates were
immunoblotted with rabbit monoclonal anti-microtubule-associated
protein 1A/1B-light chain 3 (LC3) antibody (Cell Signaling
Technology, Inc.; cat. no. 4108S) at 1:2,000 dilution, followed by
incubation with HRP-linked anti-rabbit secondary antibody (cat. no.
7074S; Cell Signaling Technology, Inc.) to monitor LC3-II generated
during the formation of autophagosomes.
Pulmonary leukocyte isolation
Animals were euthanized with 70% CO2
following approved protocols, and cells from individual mice were
collected for further experiments. BAL was collected, and the cells
were dispersed by repetitive suction through a 10 ml syringe and
centrifuged at 400 × g for 10 min at room temperature. Pellets were
resuspended in 1 ml sterile double-distilled H2O to lyse
red blood cells and centrifuged, as before. The pellets were
resuspended in 5 ml complete medium or PBS.
Flow cytometric analysis
BALF cells and lung leukocytes were assessed using
flow cytometry. BAL cells (50,000 cells) in 100 µl flow assay
buffer were incubated with PE-Ly6G [1A8] (#127607) and APC-Gr1
[RB6-8C5] (#108411) (Biolegend, San Diego, CA, USA). Cells were
washed again, resuspended in 3% paraformaldehyde and analyzed using
a FACS Caliber flow cytometer (BD Bioscience, San Jose, CA,
USA).
Statistics
Two-tailed Student's t-test or one-way analysis of
variance were used for statistical analyses with GraphPad Prism 6
(GraphPad, San Diego, CA, USA). Quantitated data from at least 3
independent experiments were shown as the mean ± standard error of
the mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
Accumulated neutrophils in BALF from
LPS-induced ALI in mice
Sepsis is one of the most common clinical causes of
ALI/acute respiratory distress syndrome (ARDS), so in order to
further research on the mechanism underlying the acute lung injury,
an LPS-induced lung injury model mouse was established. LPS, a
component of the cell wall of Gram-negative bacteria, can induce
severe inflammatory responses, and intratracheal administration of
LPS has gained wide acceptance as a clinically relevant model of
ALI/ARDS in mice. During the development of ALI, changes in
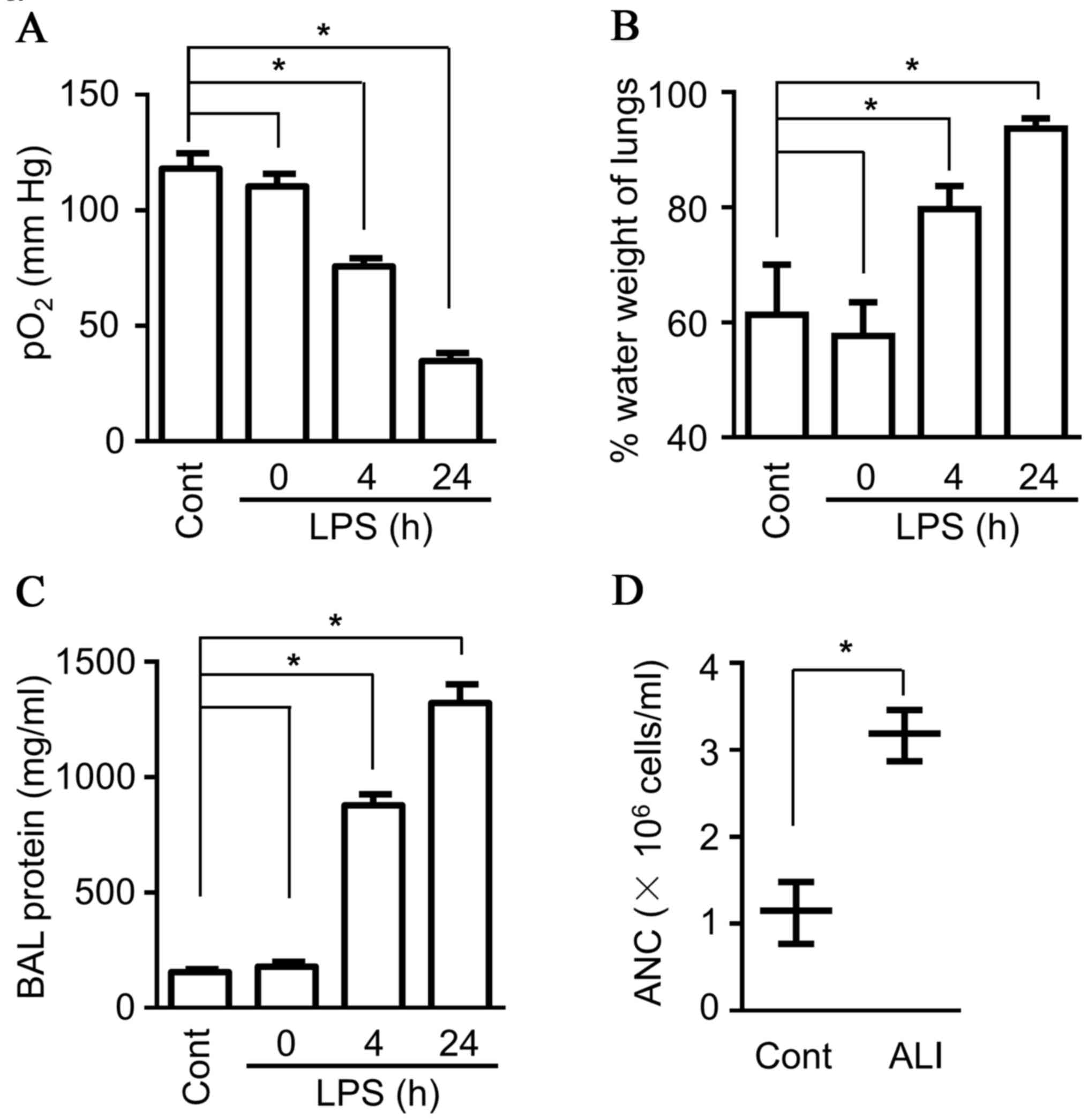
breathing patterns are observed, accompanied by lower blood
oxygenation (Fig. 1A; known
indicators of ALI) (26,27). In addition, more intravascular
content leakage was observed due to dysfunction of the capillary
walls, including increased lung water content (Fig. 1B), as well as more protein and more
blood granulocytes present in BALF (Fig. 1C and D). Flow cytometry analysis
was performed to characterize the neutrophils more accurately, and
the data demonstrated considerably more Gr1+Ly6G+ cells
(neutrophils) in BALF (Fig. 1E).
As the first line of defense for the host, neutrophils contain
diverse proteinases, including lactoferrin, serine protease and
MPO, which can be rapidly released upon activation, and they also
produce multiple pro-inflammatory cytokines, both contributing to
tissue injury, including ALI (28). The present study detected the
production of MPO in BALF, and the data indicated a much higher
level of MPO in BALF compared with sham control mice (Fig. 1D). Therefore, the ALI mouse model
demonstrated characteristic features of ALI, including high protein
levels and neutrophil infiltration in BALF.
Protective effects of ethyl pyruvate
on ALI
Ethyl pyruvate, a stable and simple lipophilic ester
that derives from the endogenous metabolite pyruvate, has been
demonstrated to protect against inflammation and to reduce organ
dysfunction in several animal models of clinical illnesses,
including burn injuries, severe sepsis and acute pancreatitis.
Ethyl pyruvate has been demonstrated to exhibit a protective effect
in ALI, probably through inhibition of the MAPK pathway in lungs.
However, allowing for the multiple mechanisms of anti-inflammation,
ethyl pyruvate contributes to protective effects against ALI
through other pathways, remains to be elucidated. Following the
application of ethyl pyruvate in ALI, an attenuation of ALI was
observed, based on the inflammatory cell infiltration detected in
H&E staining (Fig. 2A), and
decreased production of cytokines, including TNF-α (Fig. 2B), and IL-6 (Fig. 2C) in BALF from ALI mice compared
with the saline group at 4 and 24 h (Fig. 3A). The production of MPO, which is
mainly released from neutrophils, was also detected in BALF, and
the results indicated a significant decrease of MPO production in
BALF (Fig. 2D). However, the
infiltration of neutrophils in BALF exhibited no marked inhibition
by ethyl pyruvate (Fig. 2E),
suggesting that there exists other mechanisms for MPO release by
neutrophils. Thus, the data suggested that ethyl pyruvate inhibits
degranulation and attenuates ALI.
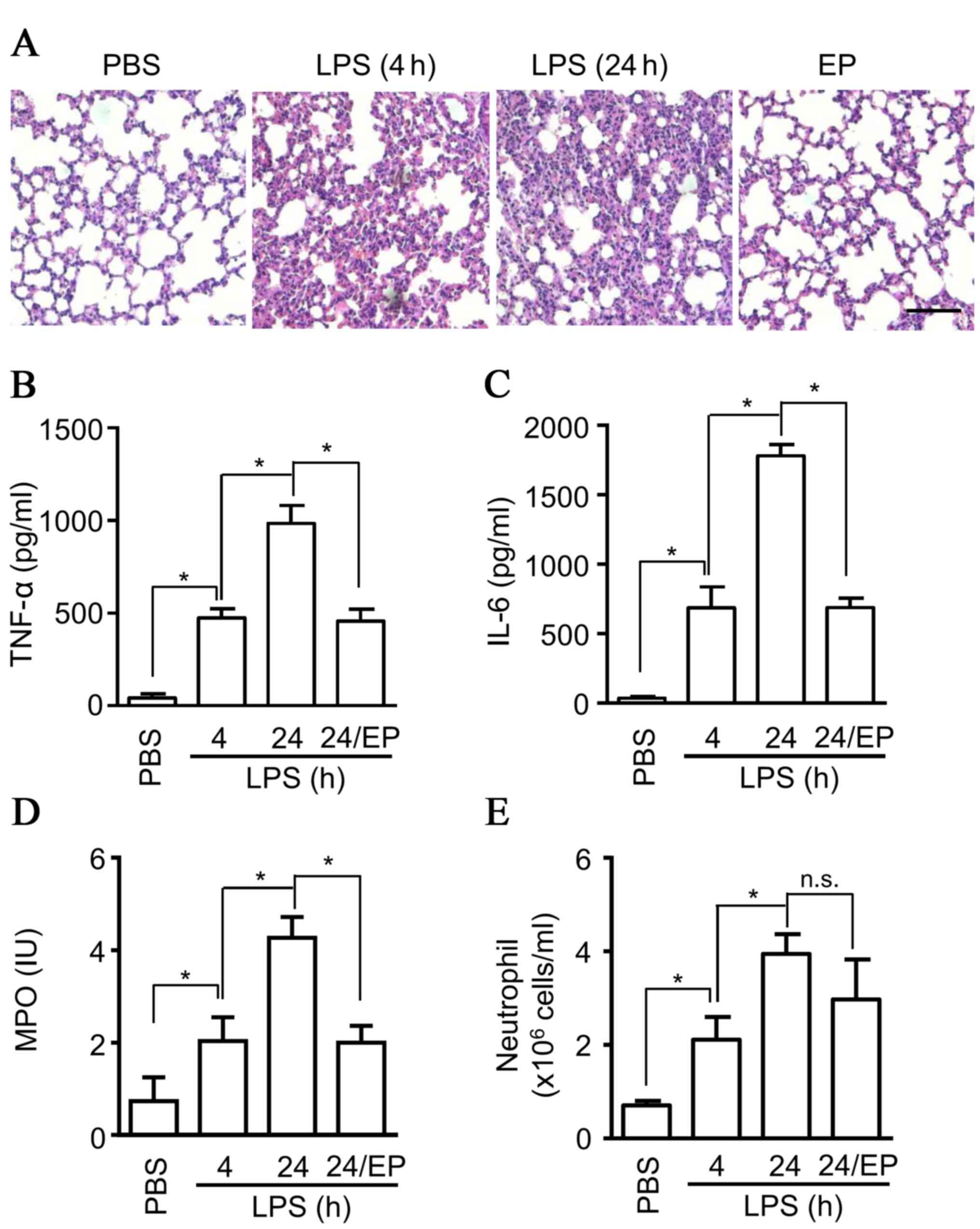 | Figure 2.Protective effects of ethyl pyruvate
on ALI. Eight-week old C57BL/6 male mice (6 mice per group) were
administrated with ethyl pyruvate (80 mg/ml) or saline, then
injected intratracheally with LPS (5 mg/kgs) at 4 or 24 h. (A)
Lungs samples were fixed, embedded in paraffin wax, sectioned at 5
µm and stained with hematoxylin and eosin. The images are
representative of three experiments (scale bar, 20 µm). BALF was
collected from the mice and (B) TNF-α, (C) IL-6, and (D) MPO were
measured by ELISA. (E) The cells collected from BALF were stained
with Gr1 and Ly6G+ antibody, and were subsequently
counted using flow cytometry. The data are presented as the mean ±
standard deviation of three independent experiments (*P<0.05).
ALI, acute lung injury; LPS, lipopolysaccharide; BALF,
bronchoalveolar lavage fluid; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6; MPO, myeloperoxidase; PBS, phosphate-buffered
saline; n.s., non-significant. |
Enhanced autophagy in neutrophils in
ALI mice model
Autophagy is a constitutive regulatory means of
cellular homeostasis involved in diverse physiological and
pathological events (10–15), and has been demonstrated to possess
a pivotal role in neutrophil-mediated inflammation, thus the
present study detected autophagy in neutrophils from BALF.
Autophagy induction was assessed by monitoring LC3, as LC3 I
conjugated to phosphatidylethanolamine (LC3 II), which links to
autophagosome-membrane following the induction of autophagy.
Following preparation from BAL fluid from mice, neutrophils were
lysed and analyzed by immunoblotting for LC3-II. LC3-II levels in
neutrophils from mice with ALI were increased significantly
compared with the control mice, while ethyl pyruvate reduced the
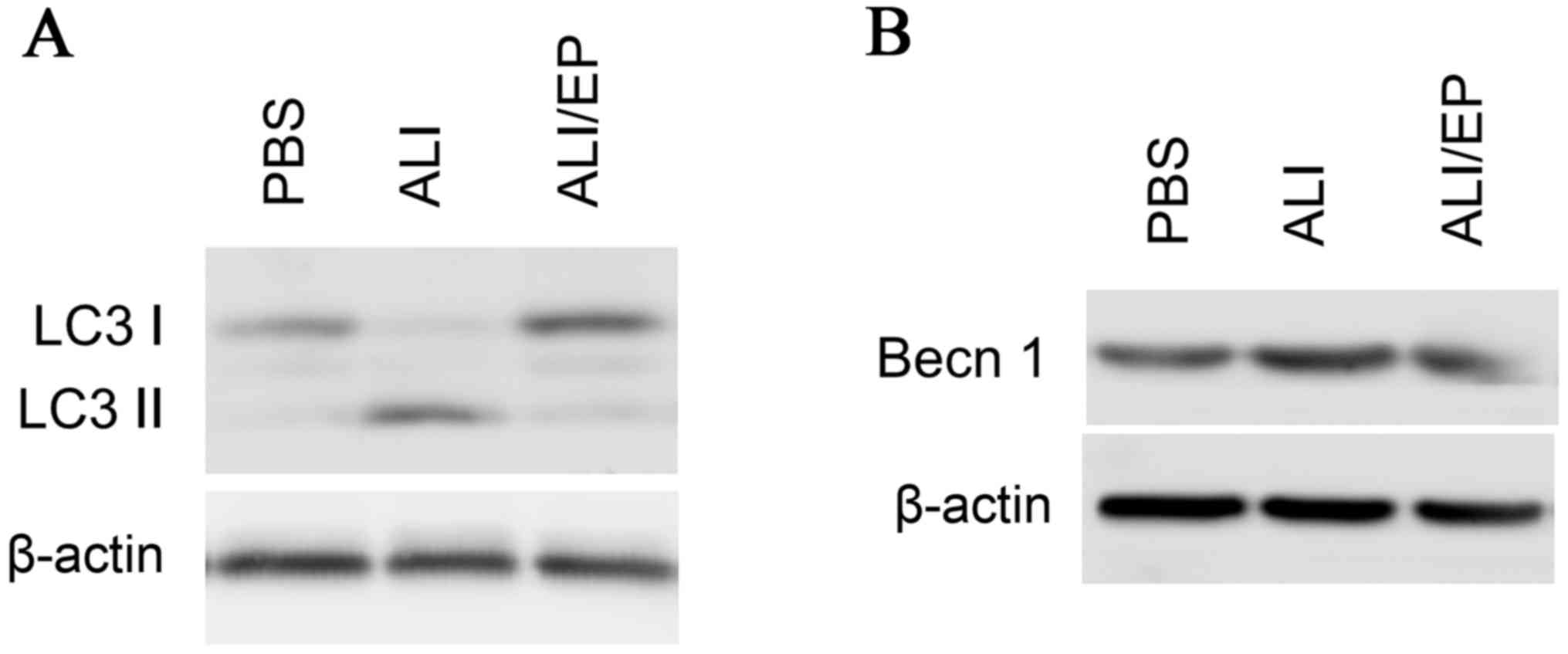
autophagy (Fig. 3A). The
expression levels of Beclin 1, a key regulator in autophagy, were
monitored and the similar results to LC3 II were obtained (Fig. 3B). No significant differences were
observed in the neutrophil amount present in BALF between the ALI
group and the EP-treated group, while the cytokines, including
TNF-α and IL-6, and particularly MPO, which are mainly released
from neutrophils, are much higher in the BALF from ALI mice
compared with the BALF from the EP-treated group. It was assumed
that autophagy is important for neutrophil activation and
degranulation, and ethyl pyruvate exerts protective effect in ALI
by inhibiting neutrophil autophagy.
Ethyl pyruvate inhibits autophagy in
neutrophils
To clarify the effects of autophagy on neutrophil
degranulation, Atg5, a key regulator for autophagy, was knocked
down with small interfering RNA in the neutrophil line, 32Dcl3
(Fig. 4A). Both LPS (100 ng/ml)
and N-Formyl-Met-Leu-Phe (fMLP) (200 nM) strongly induced
neutrophil degranulation, as presented in Fig. 4B. However, the knockdown of Atg5
abolished the effect of LPS and fMLP on MPO release from
neutrophils (Fig. 4D), emphasizing
the essential role of autophagy for degranulation of neutrophils.
32Dcl3 cells were subsequently treated with LPS (100 ng/ml) and
fMLP (200 nM) for 14 h in the absence and presence of ethyl
pyruvate, and the results demonstrated that ethyl pyruvate
inhibited the induction of autophagy in 32Dcl3 cells (Fig. 4C). In addition, MPO release and
TNF-α production by neutrophils were also decreased in the presence
of ethyl pyruvate (Fig. 4D and E),
consistent with the results of ATG5-knockdown treatment and
confirming the role of autophagy in MPO release and TNF-α
production by neutrophils. Together, the data illustrated the
protective effect of ethyl pyruvate on ALI by inhibiting
degranulation through blocking autophagy of neutrophils, unveiling
a novel mechanism, which may lead to a novel therapeutic strategy
for ALI.
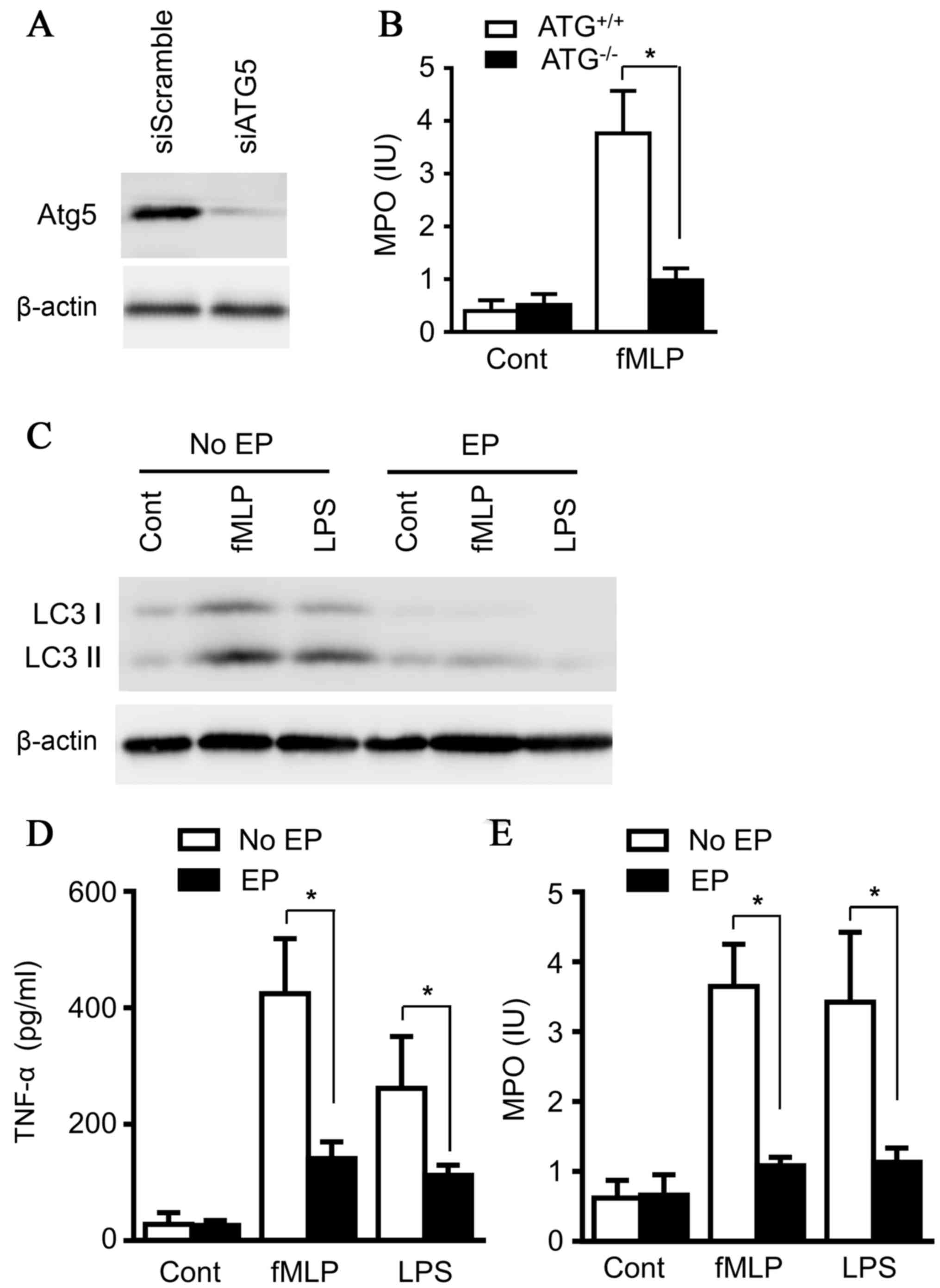 | Figure 4.Ethyl pyruvate inhibits autophagy in
neutrophils. (A) Murine myeloid cells, 32Dcl, 3 were transiently
transfected with siScramble or siATG5 overnight, and the cells were
lysed. Lysates were analyzed by immunoblotting with ATG5 antibody.
(B) Murine myeloid cells, 32Dcl3, transiently transfected with
siScramble or siATG5 overnight, were treated with fMLP (200 nM) or
LPS (100 ng/ml) for 14 h, and supernatants from cultured
neutrophils were collected and subjected to ELISA for detecting
MPO. (C) Murine myeloid cells, 32Dcl3, were treated with fMLP (200
nM) or LPS (100 ng/ml) for 14 h in the absence or presence of EP.
Supernatants were collected and the cells were lysed. Lysates were
analyzed by immunoblotting with LC3 antibody. Images presented here
were representatives of three experiments. Supernatants were
subjected to ELISA for (D) TNF-α and (E) MPO. Images presented here
were representatives of three experiments. The data are presented
as the mean ± standard deviation of three independent experiments
(*P<0.05). ATG5, autophagy related 5; fMLP,
N-Formyl-Met-Leu-Phe; LPS, lipopolysaccharide; MPO,
myeloperoxidase; EP, ethyl pyruvate, LC3, microtubule-associated
protein 1A/1B-light chain 3; TNF-α, tumor necrosis factor-α. |
Discussion
Neutrophils contribute to ALI through a number of
mechanisms, including the generation of ROS due to excessive
activation, the formation of neutrophil extracellular traps, the
production of proinflammatory cytokines and the release of granule
components. The evidence indicates that the biological alteration
of neutrophils is involved in the initiation, development and
resolution of ALI. Thus, therapy concentrated on inhibiting
neutrophil activation and degranulation may provide one promising
approach for ALI. The data from the present study demonstrated that
neutrophils infiltrated into airspace during ALI experience
increased autophagy, which is required for granule release, while
ethyl pyruvate inhibited autophagy in neutrophils, and decreased
granule release from neutrophils, thus attenuating lung injury in
ALI. This novel mechanism clarifies the roles of ethyl pyruvate in
ALI, and also provides a basis to develop a novel therapeutic
approach, with autophagy as its target.
Ethyl pyruvate has been administrated in various
animal models to ameliorate organ injury, including lung and
hepatic injury (4,5,29).
It is widely accepted that ethyl pyruvate has an anti-inflammatory
effect and decreases the production of cytokines, including TNF-α,
IL-6 and high mobility group box 1 protein, thus reducing
mortality. However, the cellular targets in these previous studies
are elusive (30). In the present
study, based on decreased MPO release, it was hypothesized that the
neutrophil can be the cell type affected. Yet no significant
difference in neutrophil infiltration between the control group and
the ethyl pyruvate-treated group was observed, suggesting some
other mechanism to regulate neutrophil biology. It was noticed that
the results of the present study are inconsistent with the previous
study by Kung et al (5).
These inconsistences may be the result of different species of
animal used and different methods, as Kung et al (5) used rats to construct their ALI model
and counted all inflammatory cells instead of neutrophils.
As autophagy has been reported to serve an important
role in neutrophil degranulation, autophagy in neutrophils isolated
from BALF was identified and increased autophagy was observed,
while ethyl pyruvate prevented the increase. In order to explore
the underlying mechanism, 32Dcl3, a neutrophil cell line, was
treated with LPS and enhanced autophagy and MPO release was
observed, while the presence of ethyl pyruvate prevented the
increase of autophagy. When Atg5 was knocked down, MPO release upon
LPS stimulation was reduced, providing more evidence on the
essential role of autophagy in neutrophil degranulation. Thus,
using various methods, the present study emphasized the
contribution of ethyl pyruvate in alleviating lung injury by
dampening neutrophil autophagy.
In summary, the results revealed a novel mechanism
of ethyl pyruvate in the alleviation of ALI by inhibiting autophagy
in neutrophils and in turn dampening granule release; the results
may also aid the identification of a potential therapeutic approach
for ALI.
Acknowledgements
The present study was supported by the Shanghai
Municipal Education Commission for Scientific Research in
Outstanding Young Teachers of Universities in Shanghai, the
National Health and Family Planning Commission of the People's
Republic of China for 2013–2014 National Clinical Key Specialty
Construction Project, and the Science and Technology Commission of
Shanghai Municipality (grant no. 13ZR1426500).
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
BAL
|
branchoalveolar lavage
|
|
fMLP
|
N-Formyl-Met-Leu-Phe
|
|
IL-6
|
interleukin-6
|
|
LPS
|
lipopolysaccharide
|
|
MPO
|
myeloperoxidase
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Tsushima K, King LS, Aggarwal NR, De
Gorordo A, D'Alessio FR and Kubo K: Acute lung injury review.
Intern Med. 48:621–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui T, Miksa M, Wu R, Komura H, Zhou M,
Dong W, Wang Z, Higuchi S, Chaung W, Blau SA, et al: Milk fat
globule epidermal growth factor 8 attenuates acute lung injury in
mice after intestinal ischemia and reperfusion. Am J Respir Crit
Care Med. 181:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abraham E, Carmody A, Shenkar R and
Arcaroli J: Neutrophils as early immunologic effectors in
hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 279:L1137–L1145. 2000.PubMed/NCBI
|
|
4
|
Shang GH, Lin DJ, Xiao W, Jia CQ, Li Y,
Wang AH and Dong L: Ethyl pyruvate reduces mortality in an
endotoxin-induced severe acute lung injury mouse model. Respir Res.
10:912009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kung CW, Lee YM, Cheng PY, Peng YJ and Yen
MH: Ethyl pyruvate reduces acute lung injury via regulation of iNOS
and HO-1 expression in endotoxemic rats. J Surg Res. 167:e323–e331.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matute-Bello G, Frevert CW and Martin TR:
Animal models of acute lung injury. Am J Physiol Lung Cell Mol
Physiol. 295:L379–L399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matthay MA and Howard JP: Progress in
modelling acute lung injury in a pre-clinical mouse model. Eur
Respir J. 39:1062–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chopra M, Reuben JS and Sharma AC: Acute
lung injury:Apoptosis and signaling mechanisms. Exp Biol Med
(Maywood). 234:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinkmann V and Zychlinsky A: Neutrophil
extracellular traps: Is immunity the second function of chromatin?
J Cell Biol. 198:773–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh R and Cuervo AM: Autophagy in the
cellular energetic balance. Cell Metab. 13:495–504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cuervo AM: Cell biology. Autophagy's top
chef. Science. 332:1392–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shanware NP, Bray K and Abraham RT: The
PI3K, metabolic, and autophagy networks: Interactive partners in
cellular health and disease. Annu Rev Pharmacol Toxicol. 53:89–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel KK and Stappenbeck TS: Autophagy and
intestinal homeostasis. Annu Rev Physiol. 75:241–262. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. New Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Netea-Maier RT, Plantinga TS, Van De
Veerdonk FL, Smit JW and Netea MG: Modulation of inflammation by
autophagy: Consequences for human disease. Autophagy. 12:245–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu C, Feng K, Zhao X, Huang S, Cheng Y,
Qian L, Wang Y, Sun H, Jin M, Chuang TH and Zhang Y: Regulation of
autophagy by E3 ubiquitin ligase RNF216 through BECN1
ubiquitination. Autophagy. 10:2239–2250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Jagannath C, Liu XD, Sharafkhaneh A,
Kolodziejska KE and Eissa NT: Toll-like receptor 4 is a sensor for
autophagy associated with innate immunity. Immunity. 27:135–144.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nathan C: Secretory products of
macrophages: Twenty-five years on. J Clin Invest. 122:1189–1190.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrovski G, Ayna G, Majai G, Hodrea J,
Benko S, Mádi A and Fésüs L: Phagocytosis of cells dying through
autophagy induces inflammasome activation and IL-1β release in
human macrophages. Autophagy. 7:321–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auffray C, Sieweke MH and Geissmann F:
Blood monocytes: Development, heterogeneity and relationship with
dendritic cells. Annu Rev Immunol. 27:669–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhattacharya A, Wei Q, Shin JN, Fattah
Abdel E, Bonilla DL, Xiang Q and Eissa NT: Autophagy is required
for neutrophil-mediated inflammation. Cell Rep. 12:1731–1739. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu C, Liu J, Hsu LC, Luo Y, Xiang R and
Chuang TH: Functional interaction of heat shock protein 90 and
Beclin 1 modulates Toll-like receptor-mediated autophagy. FASEB J.
25:2700–2710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wysocki M, Cracco C, Teixeira A, Mercat A,
Diehl JL, Lefort Y, Derenne JP and Similowski T: Reduced breathing
variability as a predictor of unsuccessful patient separation from
mechanical ventilation. Crit Care Med. 34:2076–2083. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strait RT, Hicks W, Barasa N, Mahler A,
Khodoun M, Köhl J, Stringer K, Witte D, Van Rooijen N, Susskind BM
and Finkelman FD: MHC class I-specific antibody binding to
nonhematopoietic cells drives complement activation to induce
transfusion-related acute lung injury in mice. J Exp Med.
208:2525–2544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aimone JB, Li Y, Lee SW, Clemenson GD,
Deng W and Gage FH: Regulation and function of adult neurogenesis:
From genes to cognition. Physiol Rev. 94:991–1026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen M, Lu J, Dai W, Wang F, Xu L, Chen K,
He L, Cheng P, Zhang Y, Wang C, et al: Ethyl pyruvate ameliorates
hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway
of apoptosis and autophagy. Mediators Inflamm. 2013:4615362013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davé SH, Tilstra JS, Matsuoka K, Li F,
DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT and Plevy
SE: Ethyl pyruvate decreases HMGB1 release and ameliorates murine
colitis. J Leukoc Biol. 86:633–643. 2009. View Article : Google Scholar : PubMed/NCBI
|