Introduction
Osteoporosis is a common disease resulting from bone
resorption by osteoclasts dominating over bone formation by
osteoblasts, which is characterized by decreased bone mineral
density and degraded bone fiber tissue. The disease can result in
electrolyte imbalance and bone fracture, severely affecting quality
of life in elderly patients. Current therapies for osteoporosis,
including bisphosphonates, denosumab and teriparatide (parathyroid
hormone), are associated with the following side effects:
Osteonecrosis (1,2), prolonged union time for fractures
(3), hypocalcaemia (4–6),
headaches, nausea, dizziness, limb pain and transient severe
hypotension (7). Furthermore, the
cathepsin K inhibitor odanacatib, the anti-sclerostin antibody
romosozumab and anti-Dickkopf-related protein 1 antibody exert
their anti-osteoporotic effects via biological pathways, which may
induce undesirable effects with long-term use. Traditional
treatments, such as dietary control and supplementation of calcium
and vitamin D, are generally unsatisfactory.
Gallic acid (GA) and its derivatives are a group of
polyphenol compounds that are well known for their potent
antioxidative (8) and
anti-inflammatory abilities, which affect several biochemical and
pharmacological pathways (9).
However, GA has also been shown to suppress cell proliferation
(10), which may influence its
cell protective effects. Santamaria et al (11) suggested that modifying GA by
introducing a sulfonamide group may enhance its bioactivity and
hydrophobicity, thus allowing it to support cell growth. The
introduction of sulfamethoxazole (SMZ), specifically, may then
promote antibiotic ability and hydrophobicity of GA by displacing
hydrogen atoms on amino para-positions to create different
heterocyclic structures (Fig.
1).
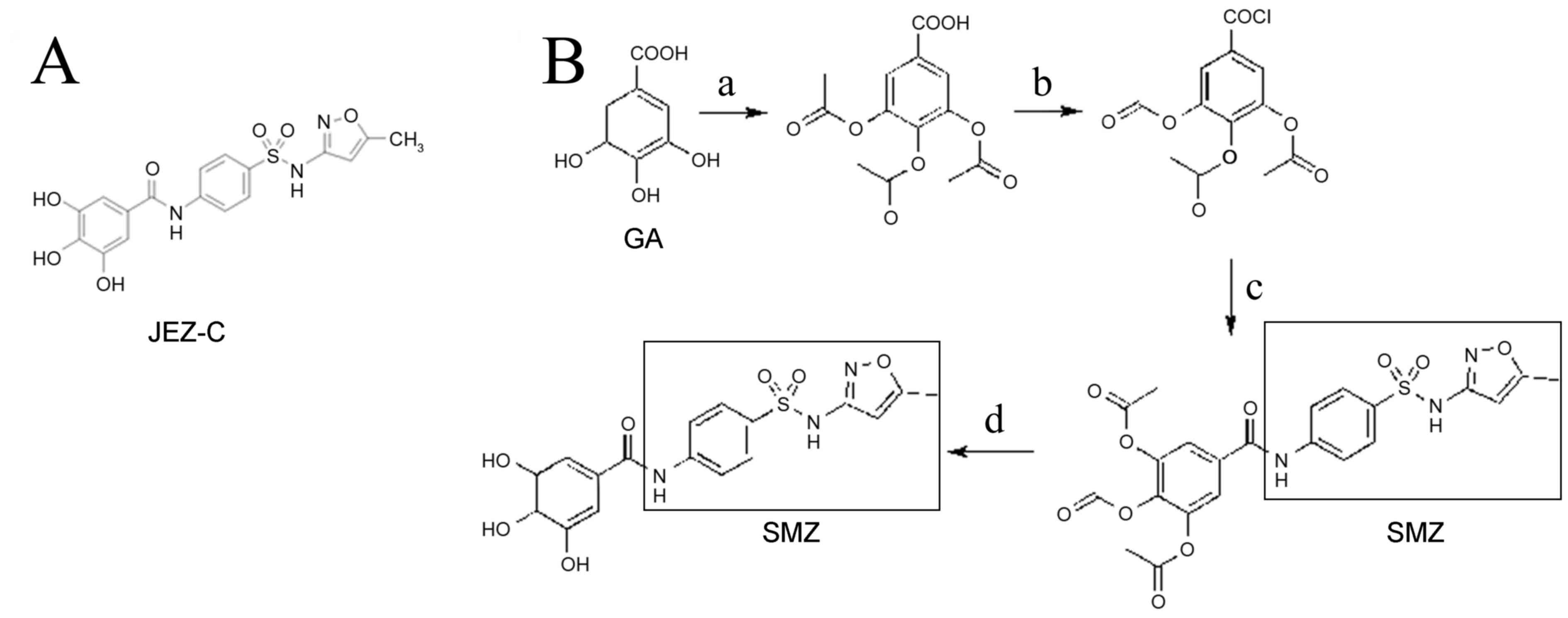 | Figure 1.(A) Chemical structure of JEZ-C. (B)
JEZ-C synthesis route. Reagents and conditions: (a) Acetyl oxide,
oil bath, 120°C; (b) SOCl2, oil bath, 80°C; (c) SMZ,
THF, Pyridine, ice bath; (d) HCl, THF, 60°C. JEZ-C,
3,4,5-trihydroxy-N-{4-[(5-methylisoxazol-3-yl)sulfamoyl]phenyl}benzamide;
SMZ, sulfamethoxazole; THF, tetrahydrofuran. |
Based on the hypothesis that synthetic gallate
compounds and sulfonamides may improve GA multiplication and exert
protective effects toward osteoblasts, the present study
synthesized sulfonamide-based gallates. Subsequently, their
biological effects were evaluated by examining the proliferation,
morphology, viability and extracellular matrix (ECM) synthesis of
osteoblasts, as well as osteoblast-specific gene expression. The
findings of the present study may provide a valuable reference
point for enhancing the proliferative capacity of osteoblasts and
the development of future therapies for the treatment of
osteoporosis.
Materials and methods
Synthesis of JEZ-C
JEZ-C
[3,4,5-trihydroxy-N-{4-[(5-methylisoxazol-3-yl)sulfamoyl]phenyl}benzamide;
Fig. 1A] was prepared from GA and
SMZ, as outlined in Fig. 1B.
Subsequently, an appropriate amount of distilled water was added to
the mixture, and the raw precipitated product was separated by
vacuum filtration. The raw product was then recrystallized in a
tetrahydrofuran-methanol solvent system. Electrospray ionization
mass spectrum (ESI-MS) was detected on a Shimadzu LC-MS 2010A. 1H
and 13C NMR spectra were assessed by using a Bruker Advance III 300
at 400 and 125 MHz, respectively.
JEZ-C has the following properties: White powder,
mp: 217–218°C, yield 63%, MS-ESI: 404.0[M-H]−,
1H-NMR (400 MHz, DMSO-d6) δ 11.33 (s, 1H,
-SO2-NH), 10.29 (s, 1H, -CO-NH), 7.95–7.77 (m, 4H,
4xAr-H), 6.94 (s, 2H, 2xAr-H), 6.13 (s, 1H, isoxazol-H), 2.28 (s,
3H, -CH3). 13C-NMR (125 MHz, DMSO-d6)
δ170.30, 166.09, 157.64, 145.60, 144.06, 137.37, 132.96, 127.83,
124.31, 119.79, 107.47, 95.43, 12.10.
Primary osteoblast separation and
culture
The study was approved by the ethics committee of
Guangxi Medical University (Nanning, China; Protocol Number:
20141008A). A total of 6 newborn Sprague-Dawley rats (3–7 days old;
3 male, 3 female) were used in the present study. Specific Pathogen
Free Sprague-Dawley rats were purchased from the Animal Resources
Center of Guangxi Medical University (Nanning, China). Animals were
housed in temperature a temperature controlled environment at 24°C,
with a 12 h light/dark cycle, and were provided with food and water
ad libitum. After delivery, neonatal rats were carefully placed as
close to the dams as possible. Following sacrifice by cervical
dislocation, osteoblasts were acquired from neonatal rat parietal
bones by enzymatic digestion with 0.25% trypsin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) for 30 min at
37°C, followed by 2 mg/ml collagenase type I (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in serum-free alpha-modified
Eagle's medium (α-MEM; Gibco; Thermo Fisher Scientific, Inc.) for 4
h at 37°C. The cells were resuspended following centrifugation at
800 × g for 5 min in α-MEM basal culture medium containing 20%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% antibiotic mixture (100 U/ml penicillin, 100 U/ml streptomycin).
The culture medium was changed every 2 days, and culture conditions
in a humidified incubator (Thermo Fisher Scientific, Inc.) were
maintained at 5% CO2 and 37°C. Cells were used for
analysis upon reaching 80–90% confluence.
JEZ-C treatment
JEZ-C was dissolved in dimethylsulfoxide (DMSO;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and diluted in
PBS to obtain a final concentration of 6.25 mg/ml. The final
concentration of DMSO was less than 0.1% (v/v) in all experiments.
The stock solution was stored at 4°C for one week and diluted with
culture medium immediately before the experiment. Cells were
treated with the obtained JEZ-C at various concentrations (0 µg/ml
as control, 6.25×10-3, 6.25×10-2 and 6.25×10-1 µg/ml).
Cell viability assay
Osteoblast cell viability was determined by staining
samples with fluorescein diacetate (FDA; Genway Biotech, Inc., San
Diego, CA, USA) and propidium iodide (PI; Sigma-Aldrich; Merck
Millipore) at 2, 4 and 6 days. Briefly, FDA and PI stock solutions
were added to the cells at final concentrations of 2 µmol/l and 2
µg/l, respectively; the cells were then incubated in the dark for 5
min at 37°C. Images were captured under a laser scanning confocal
microscope (Nikon A1; Nikon Corporation, Tokyo, Japan).
Cell proliferation assay
To investigate the dose-dependent effects of JEZ-C
on osteoblast proliferation, an MTT assay was used to determine the
amount of cells in the samples. Cells were initially digested with
0.25% trypsin/EDTA, resuspended in culture medium, and seeded into
24-well plates at 5×103 cells/well density. After a 24-h culture at
37°C, the culture medium was replaced with various concentrations
of JEZ-C (0, 6.25×10-3, 6.25×10-2 and 6.25×10-1 µg/ml). Assays were
performed at 2, 4 and 6 days; 1 ml 0.5 mg/ml MTT (Sigma-Aldrich;
Merck Millipore) was added to each well and the samples were
incubated in an atmosphere containing 5% CO2 (Forma™
Series II Water-Jacketed incubator; Thermo Fisher Scientific, Inc.)
at 37°C for 4 h. The formed formazan crystals were then dissolved
in 1 ml dimethyl sulfoxide. After thoroughly and evenly mixing the
samples, 200 µl was randomly extracted from three parallel wells at
each JEZ-C concentration and transferred to a 96-well plate. Sample
absorbance values were measured at 570 nm using a microplate reader
(Multiskan™ GO Microplate Spectrophotometer; Thermo Fisher
Scientific, Inc.). Results are presented as optical density
absorbance values.
Cell morphological analysis
Samples in the three experimental groups were
permeabilized with 0.5% Triton X-100 (Sigma-Aldrich; Merck
Millipore) after being cultured for 2, 4 and 6 days. Subsequently,
cells were incubated with 1% bovine serum albumin (BSA; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) as a blocking buffer for 30
min at 37°C. Cells were then stained for 30 min at room temperature
with rhodamine phalloidin (Invitrogen; Thermo Fisher Scientific,
Inc.), followed by Hoechst 33258 (Beyotime Institute of
Biotechnoloy, Haimen, China) for 5 min to visualize nuclei. All
imaging was performed with a scanning confocal microscope.
Alkaline phosphatase (ALP) staining
and activity assay
To determine the effects of JEZ-C on osteoblasts and
to allow for subsequent staining, cells were seeded at a density of
1×104 on to coverslips of 24-well plate and cultured in
media with various JEZ-C concentrations. After 2, 4 and 6 days of
culturing, the cells were washed with PBS and stained using an ALP
staining kit (Nanjing Jiancheng Bioengineering Research Institute,
Nanjing, China) according to the manufacturer's protocol. Staining
was observed and images were captured with an inverted phase
contrast microscope (Olympus Corporation, Tokyo, Japan) and imaging
software for microscopy (cellSens Dimension 1.14; Olympus
Corporation).
A second cell sample was lysed with 200 µl
Radioimmunoprecipitation Assay Lysis Buffer (Beyotime Institute of
Biotechnology) and phenylmethanesulfonyl fluoride to a final
concentration of 1 mM for ALP activity analysis. Total protein
concentration (mg/ml) and ALP activity (units/ml) were measured
with an improved bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) and ALP reagent kit (cat. no. A059-2;
Nanjing Jiancheng Bioengineering Research Institute), respectively,
according to the manufacturers' protocols. ALP levels were
standardized to total protein content. All samples were examined in
triplicate.
Alizarin red staining
The mineralization of osteoblast ECMs was determined
using Alizarin red staining. After 2, 4 and 6 days of culturing,
the cells were washed with distilled water and fixed in ice-cold
70% (v/v) ethanol for 1 h at 4°C. The cells were then placed on
coverslips and rinsed twice with deionized water [Tiangen Biotech
(Beijing) Co., Ltd. Beijing, China], prior to staining with
Alizarin red S (Sigma-Aldrich; Merck Millipore) solution (40 mM, pH
4.2) for 10 min at room temperature. Dyestuff was prepared in
Tris-HCl (Sigma-Aldrich; Merck Millipore) buffer solution and
adjusted to the target pH, then excess dye was gently removed with
running water. Calcification deposits, typically stained red, were
examined under an optical microscope (Nikon Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR assays were performed to measure osteogenic
gene expression in cells cultured in 24-well plates with various
concentrations of JEZ-C (0, 6.25×10-3, 6.25×10-2 and 6.25×10-1
µg/ml). Total RNA was extracted with an RNA isolation kit (Tiangen
Biotechnology, Beijing, China) according to the manufacturer's
instructions on days 2, 4 and 6. Subsequently, 300 ng total RNA was
reverse transcribed to cDNA with an RT system (Promega Corporation,
Madison, WI, USA). The total qPCR was performed using SYBR Green
master mix (BioTeke Corporation, Beijing, China) and thermocycling
conditions as follows: 95°C for 10 min, then 40 cycles of 95°C for
15s and 60°C for 1 min. Details regarding the primers are listed in
Table I. Marker gene expressions
were analyzed by the 2-ΔΔCT method (12), using β-actin as the normalizing
control. Each sample was repeated three times for each gene.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| β-actin |
5-CCCATCTATGAGGGTTACGC-3′ |
5-TTTAATGTCACGCACGATTTC-3′ |
| RUNX2 |
5′-TGTCATGGCGGGTAACGATG-3′ |
5′-CCCTAAATCACTGAGGCGGT-3′ |
| BSP |
5′-CAATCTGTGCCACTCACTGC-3′ |
5′-TGCCCTGAACTGGAAATCGTT-3′ |
| OCN |
5′-ACACTCCTCGCCCTATTGGC-3′ |
5′-CCATTGATACAGGTAGCGCCT-3′ |
| COL1A1 |
5′-GTTCAGCTTTGTGGACCTCCG-3′ |
5′-GCAGTTCTTGGTCTCGTCAC-3′ |
Immunohistochemical assay
After 2, 4 and 6 days of culturing, COL1
immunohistochemical staining was performed according to the
manufacturer's protocol. Briefly, cells at density of 1×104 on
coverslips of 24-well plate were washed with PBS, rinsed with 0.01%
Triton X-100, washed again thoroughly in PBS and treated with 3%
hydrogen peroxide (Wuhan Boster Biological Technology, Ltd., Wuhan,
China). The cells were then washed once more in PBS, and blocked
with 3% BSA. After incubation with a 1:200 dilution of the COL1
primary antibody (cat. no. BA0325; Wuhan Boster Biological
Technology, Ltd.) at 4°C for 12 h, and the secondary antibody (cat.
no. SP-0023; Zymed Technologines, Thermo Fisher Scientific, Inc.)
at 37°C for 20 min, biotin-labeled horseradish peroxidase was added
to the cells at 37°C for 20 min. Eventually, After color was
developed using a 3′-3′-diaminobenzidine tetrahydrochloride (DAB)
kit (Wuhan Boster Biological Technology, Ltd.) for 3 min and
nucleus were stained with hematoxylin for 1 min, the coverslips
were finally air-dried and sealed with neutral resin. Cells were
observed and photographed with an inverted phase contrast
microscope (Olympus Corporation) and imaging software for
microscopy (cellSens Dimension 1.14; Olympus Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation.
All data were evaluated by one-way analysis of variance followed by
least significant difference post-hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell viability
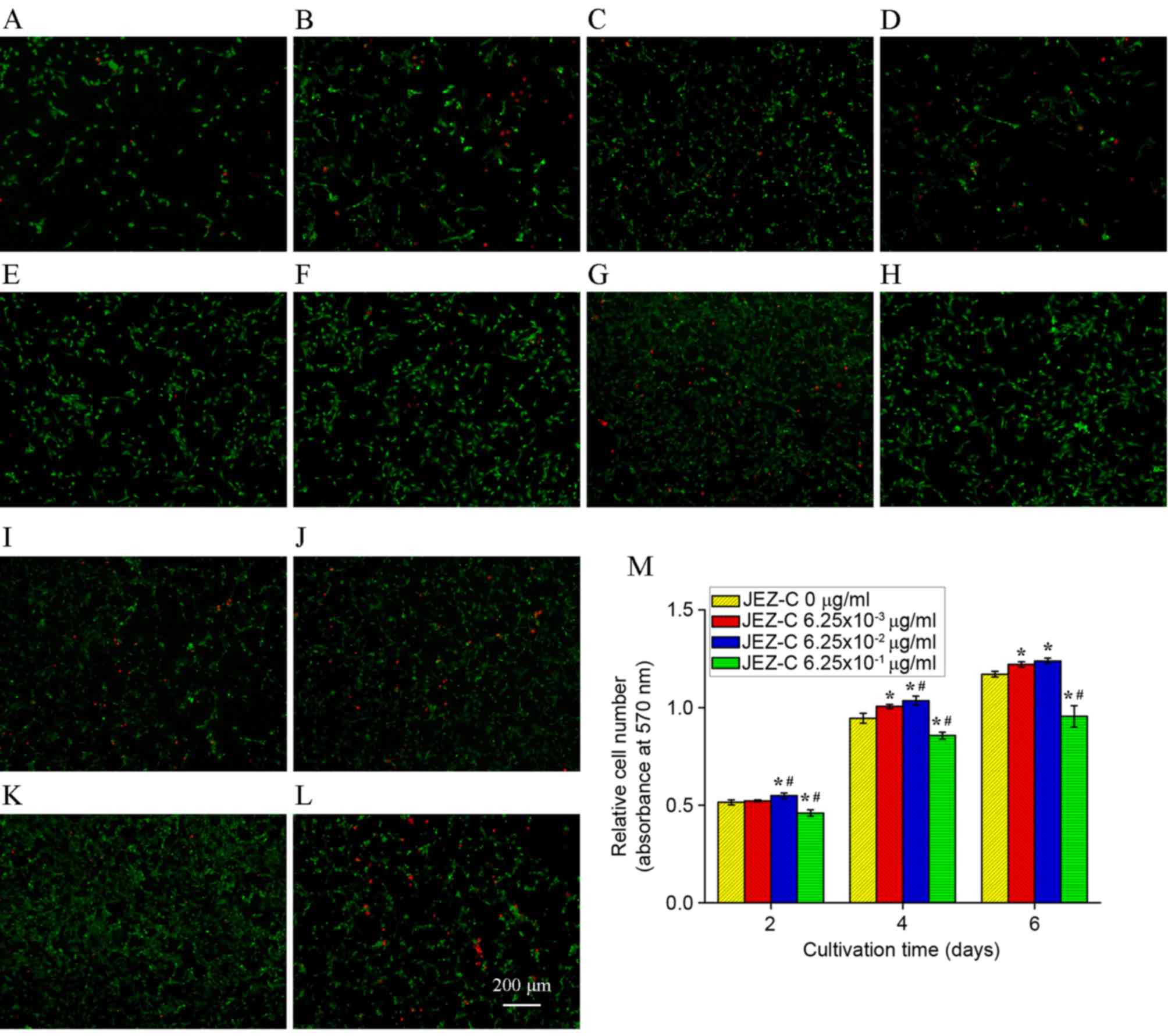
FDA/PI staining was used to detect cell viability,
as presented in Fig. 2A-L. JEZ-C
had a marked effect on osteoblast cell viability. FDA/PI staining
demonstrated that live cells in JEZ-C groups were more abundant
compared with in the control group.
Cell proliferation
Cells were subjected to MTT assay after being
cultured with three different concentrations of JEZ-C. The three
sample groups produced quite diverse results, as presented in
Fig. 2M. Osteoblast numbers
increased over time, and cells cultured with 6.25×10-3 and
6.25×10-2 µg/ml grew significantly faster than other groups
(P<0.05; Fig. 2M). These
results were in accordance with the cell viability assay findings,
thus suggesting that JEZ-C exerted a positive effect on osteoblast
growth. Among all groups, JEZ-C at a concentration of 6.25×10-2
µg/ml had the most marked effect.
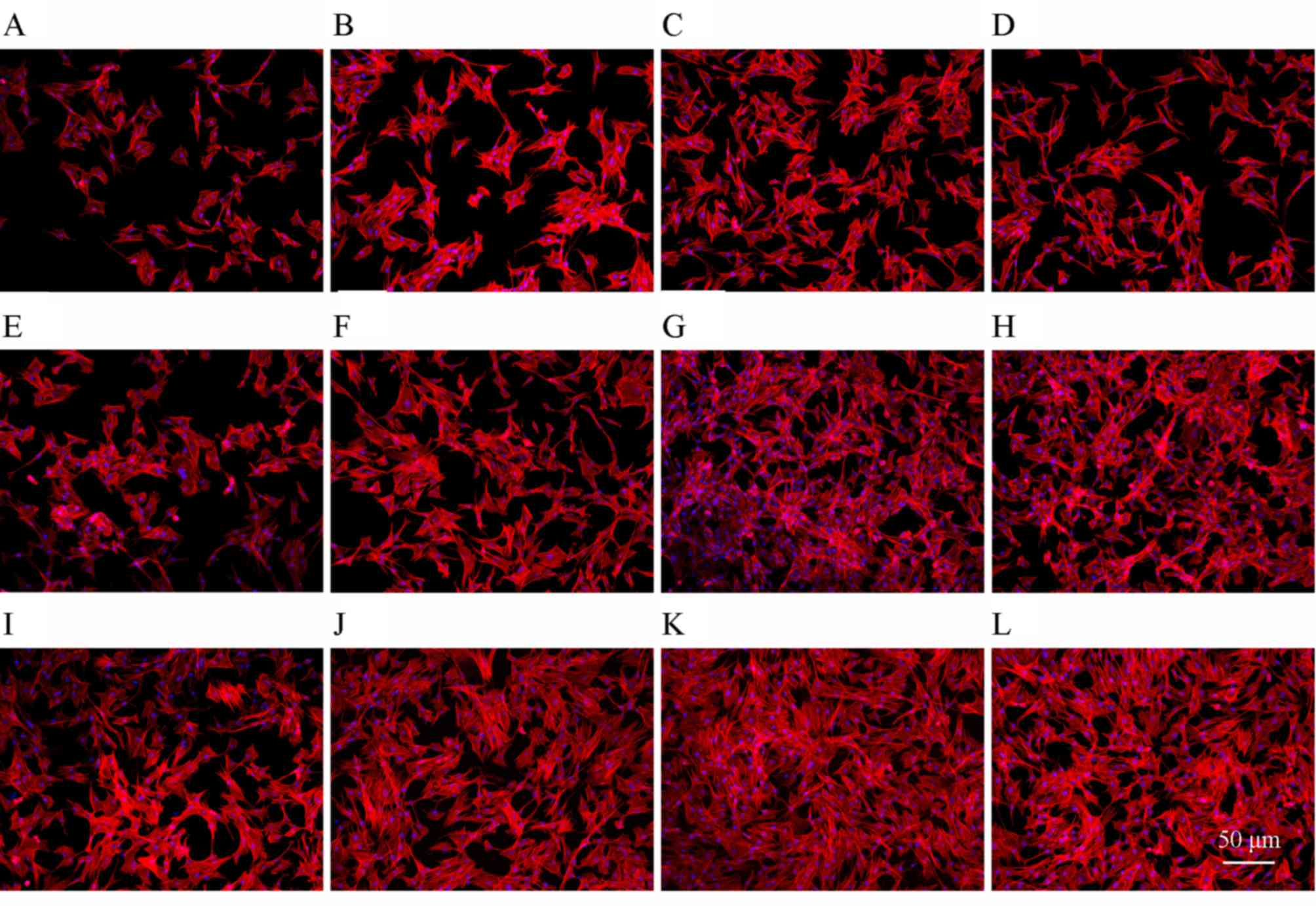
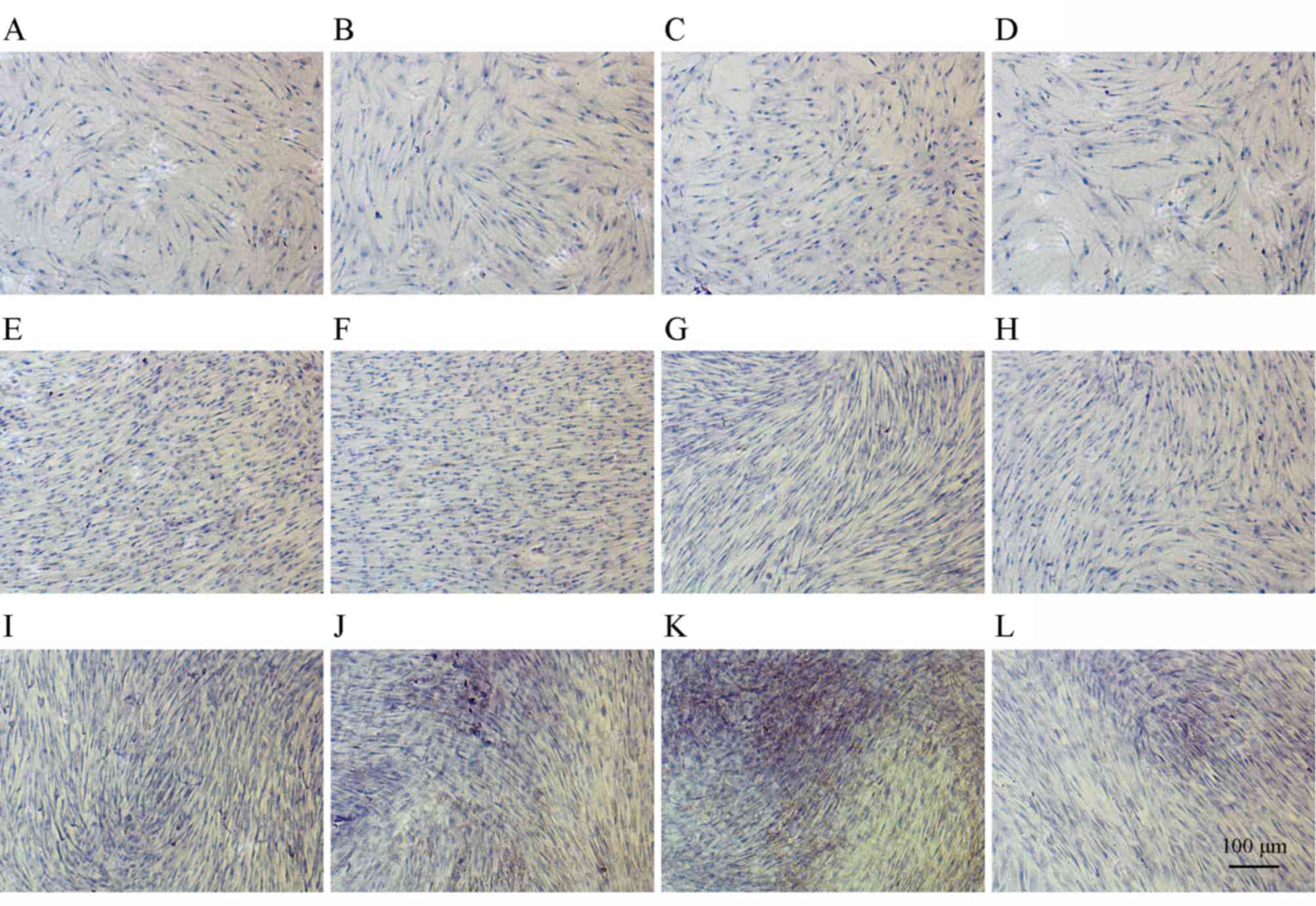
Cell morphology
The actin filaments in osteoblasts were stained with
rhodamine phalloidin/Hoechst 33258, as presented in Fig. 3A-L. Cells treated with JEZ-C grew
in clumps distributed with dense ECMs, whereas in the control group
there were fewer cells and actin filaments. This effect was
particularly evident on day 6 with a concentration of 6.25×10-2
µg/ml JEZ-C (Fig. 3K) compared
with 0 µg/ml (Fig. 3I).
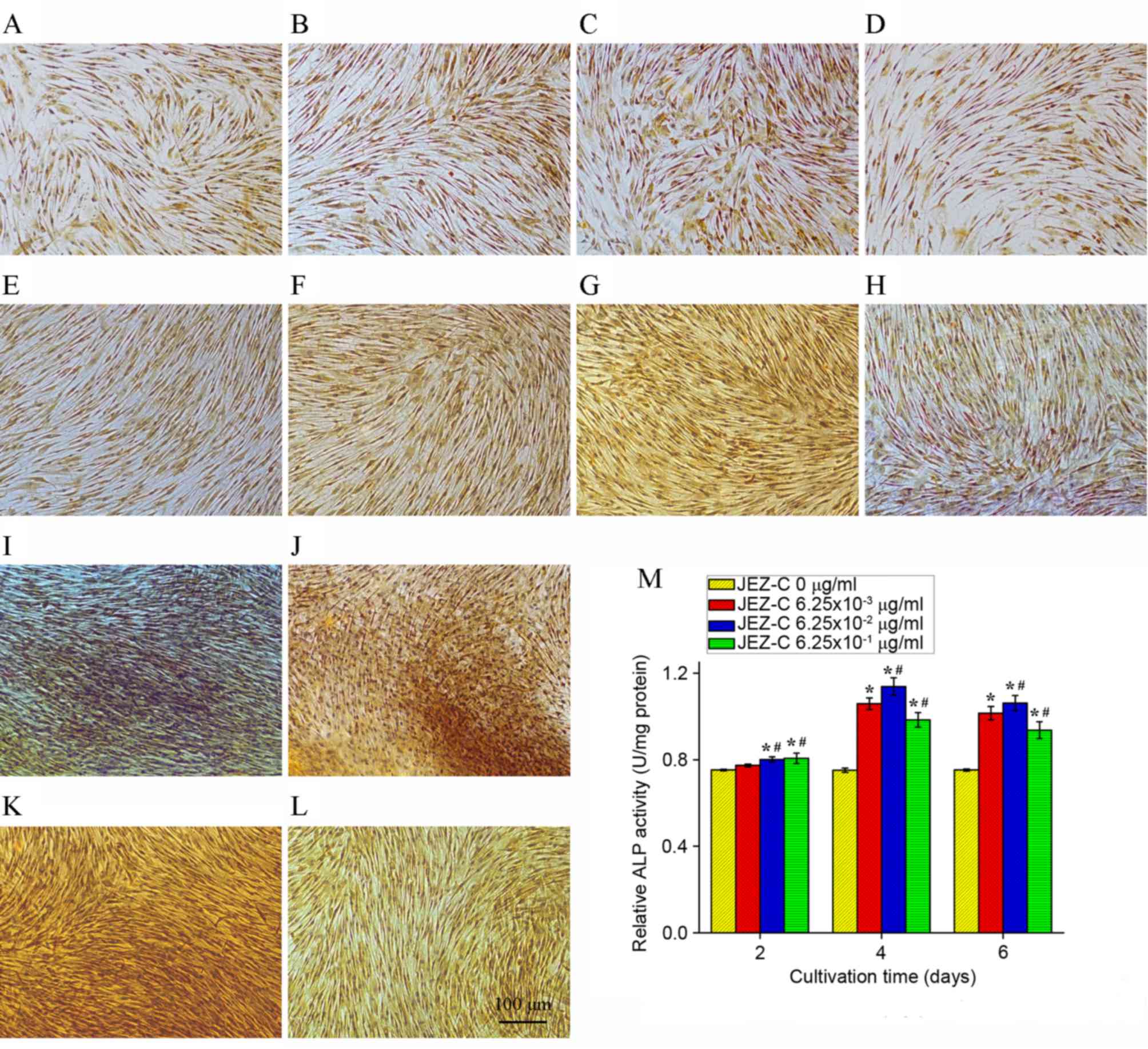
ALP staining and activity
Osteoblasts secrete ALP, which likely causes enough
inorganic pyrophosphate downregulation to provide sufficient local
phosphate (13) for mineralization
(14). ALP activity is commonly
considered a marker of osteogenesis, and is assumed to represent
the degree of osteogenic differentiation. The ALP activity assay
and staining results are presented in Fig. 4. After being cultured for 4 days,
JEZ-C groups exhibited much higher ALP activity compared with the
control group. In particular, the 6.25×10-2 µg/ml JEZ-C group
exhibited the highest ALP levels. ALP activity continuously
increased over the course of the experiment from day 2 to 4 and
slightly decreased from day 4 to 6 (Fig. 4M).
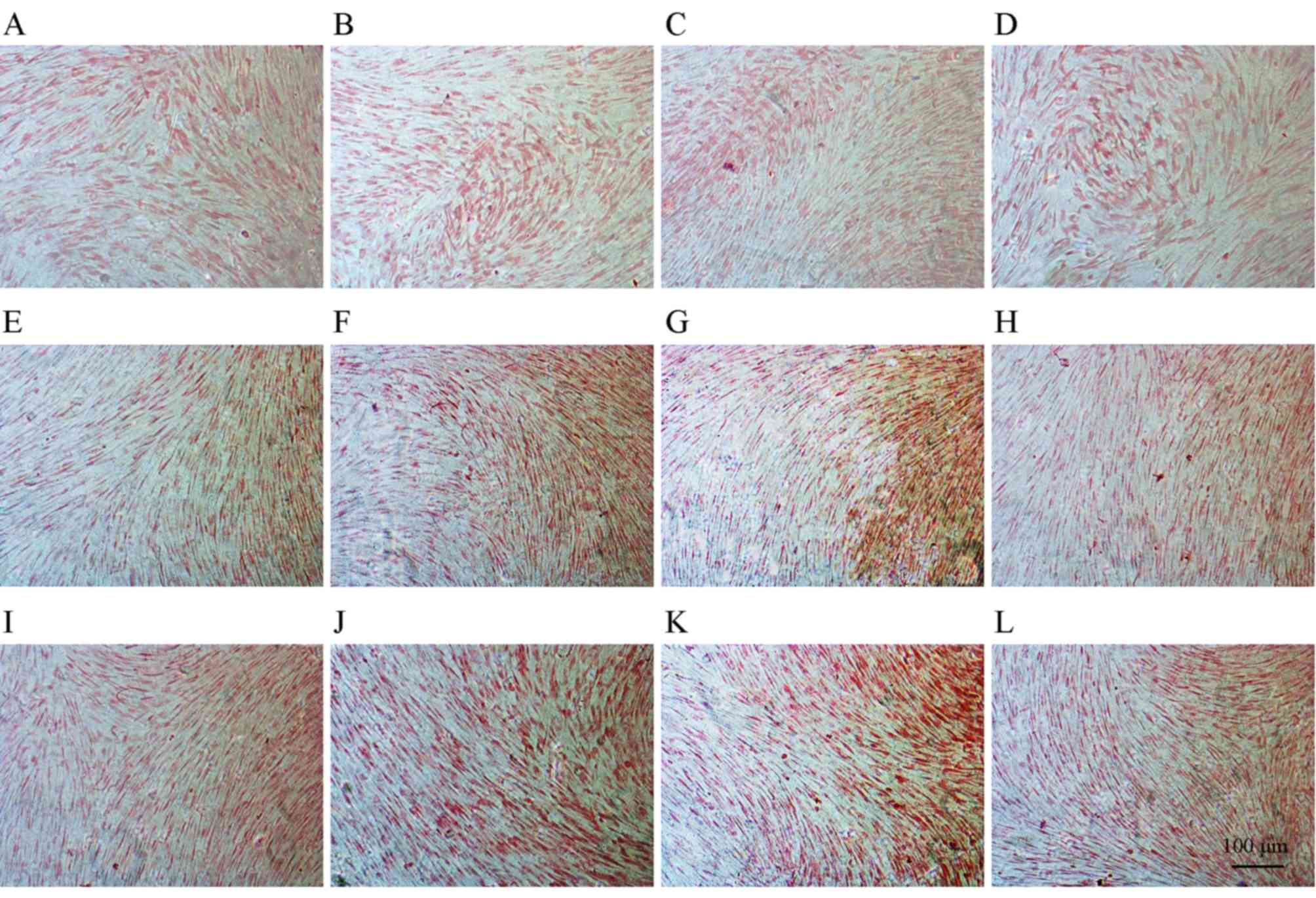
Alizarin red staining
The calcium content of each sample was determined by
Alizarin red staining. The staining indicated that bone-like
nodules formed in all groups in a time-dependent manner (Fig. 5), and that JEZ-C enhanced
mineralization. In particular, treatment with JEZ-C at a
concentration of 6.25×10−2 µg/ml resulted in the most
enhanced levels of mineralization. Mineralization was not entirely
complete in any sample by day 6.
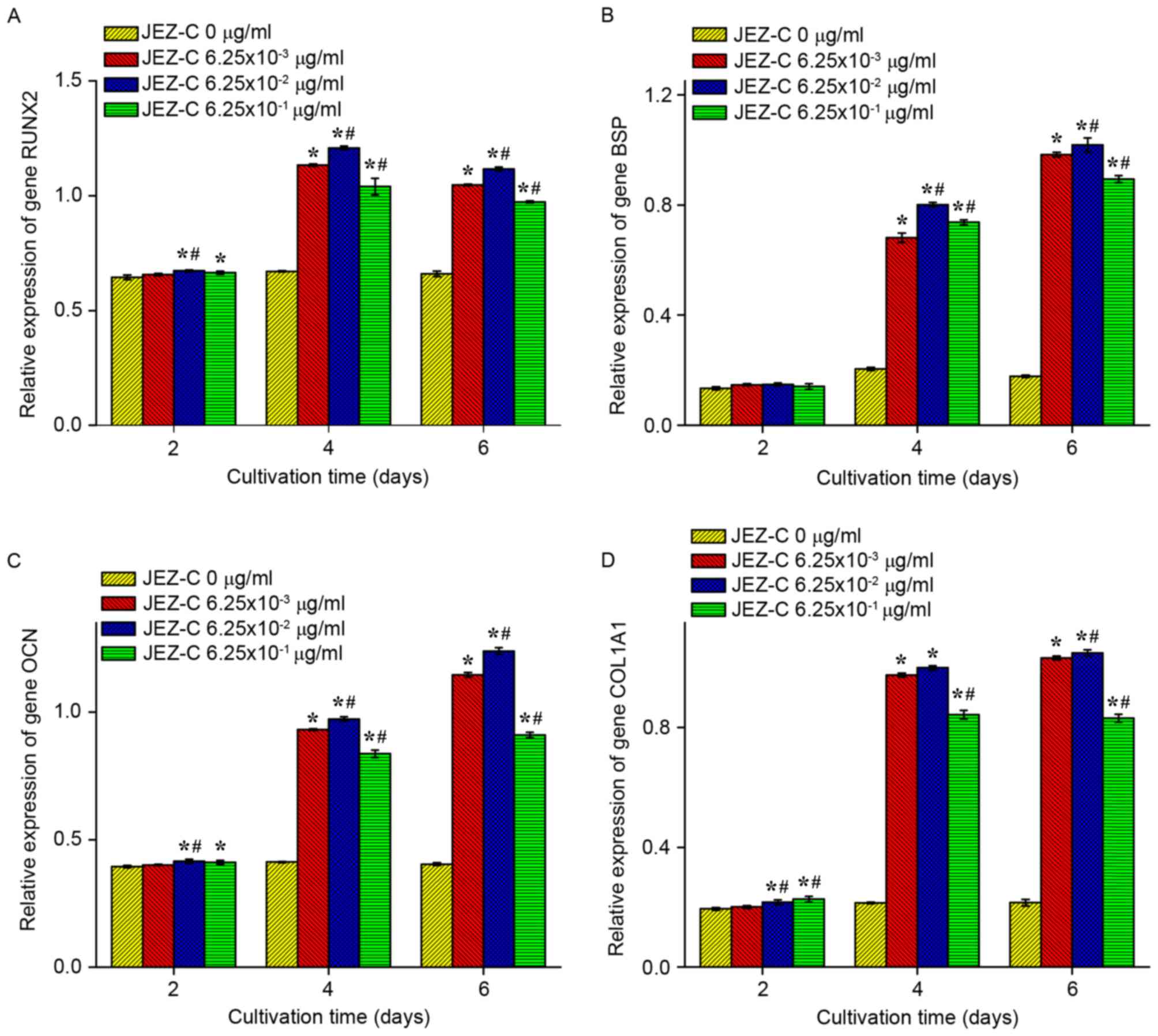
RT-qPCR
The expression levels of the following genes: RUNX2,
BSP, OCN and COL1A1, were used to validate the pro-osteogenic
effects of JEZ-C on osteoblasts, as presented in Fig. 6. The expression levels of these
genes were significantly upregulated in samples treated with JEZ-C
(P<0.05), particularly at a dose of 6.25×10-2 µg/ml. The results
further indicated that the effects of JEZ-C on osteogenic
differentiation were dose-dependent between 0 and 6.25×10-2 µg/ml;
however, for all genes detected, compared with the 6.25×10-2 µg/ml
JEZ-C group, treatment with 6.25×10-1 µg/ml JEZ-C resulted in a
slight downregulation. RUNX2 and COL1A1 gene expression peaked at
day 4, whereas the other genes increased continuously throughout
the culture period.
Immunohistochemical analysis
Immunohistochemical staining indicated that COL1 is
expressed strongly in accordance with bone formation. As presented
in Fig. 7A-L, secretion of COL1
increased over time and JEZ-C up-regulated the level. High staining
confirmed that JEZ-C enhances osteoblast mineralization, most
effectively at a concentration of 6.25×10-2 µg/ml (Fig. 7C, G and K).
Discussion
The present study synthesized JEZ-C by coupling
sulfonamide groups with GA; subsequently, its effects on osteoblast
growth at various concentrations were investigated. The results
indicated that JEZ-C is able to increase osteoblast growth, as
evidenced by rapid cell proliferation compared with in the control
group (Fig. 2M). Furthermore,
JEZ-C markedly promoted ALP secretion in cultured osteoblasts, as
indicated by a biochemical assay (Fig.
4M). As an early marker of osteoblast growth, ALP is involved
in matrix mineralization and organization (15,16).
Throughout the culture period, the present study observed a
progressive upregulation in ALP activity from day 2 to 4 in all
groups, which is indicative of ECM development preceding full
mineralization. ALP activity decreases once osteoblasts are fully
mature (17); therefore, it may be
hypothesized that ALP activity decreases with prolonged culture
time, whereas the continuously increasing ALP detected in the
present study marks the growth stage.
The present study demonstrated that JEZ-C
upregulates the gene expression of RUNX2, BSP, OCN and COL1A1
(Fig. 6). As opposed to BSP and
OCN, which exhibited continual upregulation, RUNX2 and COL1A1
expression increased gradually until it peaked at day 4 and
subsequently declined. RUNX2, which is a critical regulator for
osteoblast development and maturation, is essential in the early
stages of bone calcification (18); furthermore, RUNX2 affects the
expression of COL1A1, BSP and OCN to a certain degree (19). COL1A1, which is another marker of
mature osteoblasts, influences cellular matrix production in
osteoblasts (20). The results of
the present study suggested that JEZ-C accelerates osteoblast
growth and stimulates ECM secretion by regulating COL1, the key
activator of the osteoblast-specific enhancer, which is further
evidenced by the continuous upregulation of other genes, including
BSP and OCN. In the control group, the genes were expressed at
lower levels, thus indicating that JEZ-C exerts potent regulatory
effects on gene expression. The results of an MTT assay also
indicated that JEZ-C promotes cell proliferation in a
dose-dependent manner. In particular, treatment with JEZ-C at a
concentration of 6.25×10−2 µg/ml markedly promoted
osteoblast proliferation compared with the other concentrations
(Fig. 2).
GA and its derivatives have been reported to
suppress cell growth. Epigallocatechin-3-gallate, one such GA
derivative, has been reported to inhibit the degradation of human
cartilage proteoglycan and type II collagen, and to selectively
inhibit a disintegrin and metalloproteinase with thrombospondin
motifs ADAMTS-1, ADAMTS-4 and ADAMTS-5 (21). Sulfonamides also exhibit slight
cytotoxicity in human keratinocytes and rat hepatocytes (22), and inhibitory effects on cell wall
synthesis (23). In the present
study, JEZ-C, a novel derivative of GA, was able to effectively
support osteoblast growth and phenotypic maintenance, indicating
that appropriately modifying GA with sulfonamides may improve its
pharmacological effects. The present study demonstrated that the
JEZ-C concentrations most effective for enhancing osteoblast
proliferation ranged between 6.25×10−3 and
6.25×10−1 µg/ml, among which 6.25×10−2 µg/ml
was considered the optimal concentration.
In conclusion, the present study demonstrated that
JEZ-C effectively promotes osteoblast proliferation, increases the
secretion and ECM synthesis of osteoblasts, and maintains cell
phenotype. Osteogenic-related genes, including ALP, RUNX2, COL1A1,
BSP and OCN, were upregulated after JEZ-C treatment. Treatment with
JEZ-C at 6.25×10−2 µg/ml proved the most favorable of
all doses tested. These results suggested that JEZ-C is a useful
pro-osteogenic agent, and may be considered an attractive potential
cell-based therapy for the treatment of osteoporosis.
Acknowledgements
The present study was financially supported by the
National Key Research and Development Program of China (grant no.
2016YFB0700804), the Guangxi Scientific Research and Technological
Development Foundation (grant no. Guikegong 1598013-15) and the
Guangxi Science Fund for Distinguished Young Scholars (grant no.
2014GXNSFGA118006).
References
|
1
|
Gavrić M, Antić S, Jelovac DB, Zarev AI,
Petrović MB, Golubović M and Antunović M: Osteonecrosis of the jaw
as a serious adverse effect of bisphosphonate therapy and its
indistinct etiopathogenesis. Vojnosanit Pregl. 71:772–776. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
You Tm, Lee KH, Lee SH and Park W:
Denosumab-related osteonecrosis of the jaw: A case report and
management based on pharmacokinetics. Oral Surg Oral Med Oral
Pathol Oral Radiol. 120:548–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molvik H and Khan W: Bisphosphonates and
their influence on fracture healing: A systematic review.
Osteoporos Int. 26:1251–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yerram P, Kansagra S and Abdelghany O:
Incidence of hypocalcemia in patients receiving denosumab for
prevention of skeletal-related events in bone metastasis. J Oncol
Pharm Pract. Jan 31–2016.(Epub ahead of print).
|
|
5
|
Blackley S, Anderson K and Berg J: A case
of denosumab-induced hypocalcaemia in a patient with non-metastatic
prostate cancer and renal impairment. J R Coll Physicians Edinb.
45:133–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Setsu N, Kobayashi E, Asano N, Yasui N,
Kawamoto H, Kawai A and Horiuchi K: Severe hypercalcemia following
denosumab treatment in a juvenile patient. J Bone Miner Metab.
34:118–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enishi T, Uemura H, Katoh S, Inatsugi M,
Minato S, Inatsugi K, Inatsugi M, Sato N and Siryo K: Transient
severe hypotension with once-weekly subcutaneous injection of
teriparatide in osteoporotic patient: A case report and insight for
the drug interaction between hypotensive agents and teriparatide. J
Med Invest. 62:93–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sapkota K, Park SE, Kim JE, Kim S, Choi
HS, Chun HS and Kim SJ: Antioxidant and antimelanogenic properties
of chestnut flower extract. Biosci Biotechnol Biochem.
74:1527–1533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiang CY, Hseu YC, Chang YC, Kumar KJ, Ho
TY and Yang HL: Toona sinensis and its major bioactive compound
gallic acid inhibit LPS-induced inflammation in nuclear
factor-kappaB transgenic mice as evaluated by in vivo
bioluminescence imaging. Food Chem. 136:426–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou TT, Lin MC, Wu CH, Lin WL and Wang CJ:
Gallic acid attenuates oleic acid-induced proliferation of vascular
smooth muscle cell through regulation of AMPK-eNOS-FAS signaling.
Curr Med Chem. 20:3944–3953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santamaria S, Nuti E, Cercignani G,
Marinelli L, La Pietra V, Novellino E and Rossello A: N-O-isopropyl
sulfonamido-based hydroxamates: Kinetic characterisation of a
series of MMP-12/MMP-13 dual target inhibitors. Biochem Pharmacol.
84:813–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Wen F, He F, Liu L and Yang G: In
vitro and in vivo evaluation of the osteogenic ability of implant
surfaces with a local delivery of simvastatin. Int J Oral
Maxillofac Implants. 29:211–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Douglas TE, Skwarczynska A, Modrzejewska
Z, Balcaen L, Schaubroeck D, Lycke S, Vanhaecke F, Vandenabeele P,
Dubruel P, Jansen JA and Leeuwenburgh SC: Acceleration of gelation
and promotion of mineralization of chitosan hydrogels by alkaline
phosphatase. Int J Biol Macromol. 56:122–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walter MS, Frank MJ, Satué M, Monjo M,
Rønold HJ, Lyngstadaas SP and Haugen HJ: Bioactive implant surface
with electrochemically bound doxycycline promotes bone formation
markers in vitro and in vivo. Dent Mater. 30:200–214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piattelli A, Scarano A, Corigliano M and
Piattelli M: Effects of alkaline phosphatase on bone healing around
plasma-sprayed titanium implants: A pilot study in rabbits.
Biomaterials. 17:1443–1449. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okamura H, Yoshida K, Yang D and Haneji T:
Protein phosphatase 2A Cα regulates osteoblast differentiation and
the expressions of bone sialoprotein and osteocalcin via osterix
transcription factor. J Cell Physiol. 228:1031–1037. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koromila T, Baniwal SK, Song YS, Martin A,
Xiong J and Frenkel B: Glucocorticoids antagonize RUNX2 during
osteoblast differentiation in cultures of ST2 pluripotent
mesenchymal cells. J Cell Biochem. 115:27–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsubara T, Kida K, Yamaguchi A, Hata K,
Ichida F, Meguro H, Aburatani H, Nishimura R and Yoneda T: BMP2
regulates Osterix through Msx2 and Runx2 during osteoblast
differentiation. J Biol Chem. 283:29119–29125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Chen J, Tao J, Jiang Y, Hu C,
Huang L, Ji J and Ouyang HW: The use of type 1 collagen scaffold
containing stromal cell-derived factor-1 to create a matrix
environment conducive to partial-thickness cartilage defects
repair. Biomaterials. 34:713–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adcocks C, Collin P and Buttle DJ:
Catechins from green tea (Camellia sinensis) inhibit bovine and
human cartilage proteoglycan and type II collagen degradation in
vitro. J Nutr. 132:341–346. 2002.PubMed/NCBI
|
|
22
|
Patoux-Pibouin M, Hirel B, Chesne C,
Watier E, Chevrant-Breton J and Guillouzo A: Cytotoxicity testing
of beta-lactam antibiotics, non-steroidal anti-inflammatory drugs
and sulfonamides in primary human keratinocyte cultures. Toxicol In
Vitro. 9:493–497. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baxter BK, DiDone L, Ogu D, Schor S and
Krysan DJ: Identification, in vitro activity and mode of action of
phosphoinositide-dependent-1 kinase inhibitors as antifungal
molecules. ACS Chem Biol. 6:502–510. 2011. View Article : Google Scholar : PubMed/NCBI
|