Introduction
Asparagus cochinchinesis (A.
cochinchinesis), a perennial herb belonging to the Liliaceae
family, is widely distributed in China, Japan and Korea, and has
been used as a traditional medicine in those countries for
thousands of years (1). The root
of A. cochinchinesis has long been considered a therapeutic
drug due to its anti-inflammatory, diuretic, antiseptic,
antitussive, antibacterial, nervine, sialogogue, antipyretic and
stomachic effects, and is also administered in combination with
other herbs as a medicine to treat lung disease, immune
system-associated diseases and aging (1,2).
The root of A. cochinchinesis contains 19
amino acids, polysaccharides, and >20 multi-functional
compounds. These functional compounds include β-sitosterol
(3), daucosterol (4), n-ethatriacontanoic acid (5), palmitic acid (6), 9-heptacosylene (7), smilagenin (8), diosgenin (9), sarsasapogenin-3-O-β-D-glucoside
imidacloprid (10), 5-methoxy
methyl furfural, yame sapogenin, diosgenin-3-O-β-D imidacloprid
glycosides (11,12), aspacochioside D (13), iso-agatharesinoside (14) and seven steroidal saponins
(15). In addition, the
polysaccharide composition of A. cochinchinesis roots is
reported to have several therapeutic properties, including
antioxidant and anti-aging properties (16–18),
antibacterial-inflammatory effects (16), antitumor effects (19–21),
blood sugar reducing activity (22) and improvement of cough (23,24).
Previous studies have reported the anti-inflammatory
activity of A. cochinchinesis extract. Secretion of the
pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α), in
lipopolysaccharide (LPS)-and substance P-stimulated mouse
astrocytes was significantly inhibited by A. cochinchinesis
extract (25). Aspacochinosides N,
O and P extracted from ethanol-treated A. cochinchinesis
decreased the nitric oxide (NO) concentration in LPS-stimulated
BV-2 microglial cells (26).
Furthermore, ethanol extract from A. cochinchinesis
decreased the degree of ectopic edema, ear thickness, cytokine
secretion TNF-α and interleukin (IL)-1β and myeloperoxidase
activity in a skin inflammation-induced mouse model treated with
12-O-tetradecanoyl-phorbol-13-acetate, all of which are considered
indicators of skin inflammation progression (27). A crude aqueous extract of A.
cochinchinensis effectively inhibited TNF-α-induced
cytotoxicity (21), increased the
spleen index and superoxide dismutase (SOD) activity and decreased
malondialdehyde (MDA) in mice (1).
A recent study reported the inhibitory effects of A.
cochinchinensis in allergic asthma-associated airway
remodeling. The standardized herbal formula PM014, which includes
the roots of A. cochinchinensis, efficiently inhibited the
number of total cells, eosinophils, neutrophils, macrophages and
lymphocytes in the bronchoalveolar lavage fluid of cockroach
allergen-induced mice (28).
Meanwhile, LPS used to induce an inflammatory response, is
recognized by toll-like receptors (TLRs) distributed on the
membrane of macrophages. The engagement of the TLR results in a
potent inflammatory response characterized by the release of
inflammatory cytokines, NO production and cell cycle regulation
through the activation of the MAPK signaling pathway. Therefore,
these markers and pathways may be considered as key factors for the
analysis of the inflammatory response, to identify novel drugs with
anti-inflammatory properties (29). However, the underlying mechanism by
which A. cochinchinensis exerts its anti-inflammatory
effects in macrophages has not yet been clearly identified, even
though the induction of inflammatory lung diseases including
asthma, cystic fibrosis, emphysema, and chronic obstructive
pulmonary disorder have been implicated in the activation of
macrophages.
The present study investigated the fundamental
mechanisms responsible for anti-inflammatory activities of ethyl
acetate extract from A. cochinchinesis root (EaEAC) in
LPS-induced RAW264.7 microphage cells.
Materials and methods
Preparation of EaEAC
Roots of A. cochinchinensis were collected
from plantations in the Go-Chang county of North Jeolla (Korea) and
were dried in a drying machine (model FD5510S-FD5520S;
IlShinBioBase Co., Dongducheon, Korea) at 60°C. Voucher specimens
of A. cochinchinensis (WPC-14-003) were deposited in the
Functional Materials Bank of the PNU-Wellbeing RIS Center at Pusan
National University (Pusan, Korea). Dry roots of A.
cochinchinensis were reduced to powder using a pulverizer
(model MF-3100S; Hanil Electric Group Co., Ltd., Seoul, Korea),
followed by extraction of EaEAC at 50°C for 24 h in a fixed liquor
ratio (solid powder of A. cochinchinensis/ethyl acetate
solvent ratio, 1:10) using circulating extraction equipment
(SHWB-30/45, Woori Science Instrument Co., Pocheon, Korea). Extract
solutions were subsequently passed through a 0.4 µm filter, and
concentrated by vacuum evaporation and lyophilized using
circulating extraction equipment (IKA Labortechnik, Staufen im
Breisgau, Germany). Finally, the EaEAC powder was dissolved in
dimethyl sulfoxide (DMSO; Duchefa Biochemie B.V., Haarlem,
Netherlands) to 1 mg/ml, then further diluted to the required
concentration.
Free radical scavenging activity
The scavenging activity of
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was measured as
previously described (30).
Briefly, each 100 µl sample in eight different concentrations of
EaEAC (250–2,000 µg/ml) was mixed with 100 µl DPPH (0.1 mM;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) in 95% ethanol
solution or 100 µl 95% ethanol solution, then incubated for 30 min
at room temperature. The absorbance of the reaction mixture was
measured at 517 nm using a VersaMax plate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA). The DPPH radical scavenging
activity of the EaEAC was expressed as the percent decrease in
absorbance relative to the control. The half maximal inhibitory
concentration (IC50) is defined as the concentration of
substrate that causes a 50% loss in DPPH activity.
Cell culture
The RAW264.7 cell line used in the present study is
an Abelson murine leukemia virus-transformed macrophage cell line,
provided by the Korean Cell Line Bank (Seoul, Korea). RAW264.7
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; cat. no. S001-01; Welgene, Gyeongsan,
Korea), L-glutamine, penicillin, and streptomycin (Thermo Fisher
Scientific, Inc.) in a humidified incubator at 37°C with 5%
CO2 and 95% air.
Cell viability assay
Cell viability was determined using the tetrazolium
compound 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT; Sigma-Aldrich; Merck Millipore). To determine cell
viability, RAW264.7 cells were seeded at a density of
5×104 cells/0.2 ml and cultured for 12 h in a 37°C
incubator. When the cells reached 70–80% confluence, they were
either untreated (NC group), treated with vehicle (DMSO), or
pretreated with 100 or 200 µg/ml EaEAC dissolved in DMSO for 2 h.
Cells were subsequently incubated for 24 h with 1 µg/ml LPS
(derived from Escherichia coli, serotype 055:B5;
Sigma-Aldrich; Merck Millipore), then the supernatants were
discarded and 0.2 ml fresh DMEM and 50 µl MTT solution [2 mg/ml in
phosphate-buffered saline (PBS)] was added to each well. Cells were
incubated at 37°C for a further 4 h. Formazan precipitate was
dissolved in DMSO and the absorbance at 570 nm was read directly in
the wells using a VersaMax plate reader (Molecular Devices, LLC).
The morphological features of RAW264.7 cells in each treated group
were also observed using an inverted light microscope [Leica
Microsystems (Schweiz) AG, Heerbrugg, Switzerland].
Measurement of NO concentration
NO accumulation was measured in the cell culture
medium using Griess reagent [1% sulfanilamide, 5% phosphoric acid,
0.1% N-(1-naphthyl) ethylenediamine dihydrochloride; Sigma-Aldrich;
Merck Millipore] as described previously (31). Briefly, RAW264.7 cells were treated
with EaEAC (100 and 200 µg/ml) for 2 h followed by LPS (1 µg/ml)
for 24 hr. Following the collection of the supernatant, each sample
(100 µl) was mixed with the same volume of Griess reagent and
incubated at room temperature for 10 min. The absorbance was read
at 540 nm using a VersaMax microplate reader (Molecular Devices,
Sunnyvale, CA, USA).
Analysis of intracellular ROS
level
ROS levels in cells were measured by staining with
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich,
Merck Millipore), a cell permeable and nonfluorescent agent that
can be deacetylated by intracellular esterases to nonfluorescent
DCFH, and is converted to highly fluorescent
2′,7′-dichlorofluorescein (DCF) in the presence of intracellular
ROS. When the RAW264.7 cells reached 70–80% confluence, they were
either untreated, treated with vehicle, or pretreated with 100 or
200 µg/ml EaEAC dissolved in DMSO for 2 h. Cells were subsequently
incubated for 24 h with 1 µg/ml LPS. Cells were then incubated with
25 µM DCFH-DA for 30 min at 37°C, before they were washed twice
with PBS. Green fluorescence was visualized at ×200 and ×400
magnification using an Eclipse TX100 fluorescent microscope (Nikon
Corporation, Tokyo, Japan).
Western blotting
When the RAW264.7 cells reached 70–80% confluence,
they were either untreated, treated with vehicle, or pretreated
with 100 or 200 µg/ml EaEAC dissolved in DMSO for 2 h. Cells were
subsequently incubated for 24 h with 1 µg/ml LPS. Total protein for
western blotting was extracted from RAW264.7 cells using Pro-Prep
Protein Extraction Solution (iNtRON Biotechnology, Seongnam,
Korea), centrifuged at 11,000 × g for 5 min, and quantified using a
SMART bicinchoninic acid assay kit (Thermo Fisher Scientific,
Inc.). Proteins were separated by 4–20% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis for 2 h (30 µg per gel),
then resolved proteins were transferred to nitrocellulose membranes
for 2 h at 40 V. After the blocking with 3–5% skim milk in TNT
solution (137 mM NaCl, 2.7 mM KCl, 10 mM
Na2HPO4 and 0.05% Tween-20) and incubating at
room temperature for 1 h, the membranes were incubated overnight at
4°C with the following primary antibodies: Anti-SAPK/JNK (cat. no.
9252; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-phosphorylated (p-) SAPK/JNK (Thr183/Tyr185; cat.
no. 9251; dilution, 1:1,000; Cell Signaling Technology, Inc.),
anti-ERK1 (K-23; cat. no. sc-94; dilution, 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-p-ERK1/2
(Thr202/Tyr204; cat. no. 9101; dilution, 1:1,000; Cell Signaling
Technology, Inc.), anti-p38 mitogen-activated protein kinase (MAPK;
cat. no. 9212; dilution, 1:1,000; Cell Signaling Technology, Inc.),
anti-p-p38 MAPK (Thr180/Tyr182; cat. no. 9211; dilution, 1:1,000;
Cell Signaling Technology, Inc.) and anti-β-actin (cat. no. A5316;
dilution, 1:3,000; Sigma-Aldrich; Merck Millipore). Membranes were
then washed with TNT solution and incubated with 1:1,000 diluted
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat.
no. 81–6120; Zymed; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Blots were developed using Amersham ECL Select
Western Blotting detection reagent (GE Healthcare Life Sciences,
Chalfont, UK). Protein bands were visualized using the
FluorChem® FC2 Imaging system (Alpha Innotech
Corporation, San Leandro, CA, USA) and the band density was
semi-quantified using the AlphaView Program (version, 3.2.2; Cell
Biosciences, Inc., Palo Alto, CA, USA). The target protein
expression levels of three samples from each group were analyzed in
three separate western blot analyses.
Enzyme-linked immunosorbent assay
(ELISA) for IL-6
The concentration of IL-6 secreted from RAW264.7
cells was determined using an IL-6 ELISA kit (cat. no. 431304;
Biolegend, Inc., San Diego, CA, USA) according to the
manufacturer's protocols. Briefly, RAW264.7 cells were treated with
two different concentrations of EaEAC (100 and 200 µg/ml) for 2 h,
followed by 1 µg/ml of LPS for 24 h. Cell supernatant was collected
and 100 ml serial dilutions of the standard or the supernatant were
added to a 96-well plate coated with anti-IL-6 antibody, then
incubated for 2 h at room temperature. Following five washes with
wash solution (PBS, 0.05% Tween-20, pH 7.4), 100 µl avidin-HRP
solution was added to each well, and the plates incubated at 37°C
for 2 h. Following five washes, 100 µl substrate solution was added
and the plate was incubated at 37°C for a further 30 min. The
reaction was terminated by the addition of 100 µl stop solution (2N
H2SO4) and the absorbance at 450 nm was
determined with a VersaMax plate reader (Molecular Devices,
LLC).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis for cytokine gene
expression
The relative expression of inducible nitric oxide
synthase (iNOS), cyclooxygenase-2 (COX-2), TNF-α, IL-1β, IL-6 and
IL-10 mRNAs were measured by RT-qPCR as previously described
(32). Total RNA was purified by
removing media from each cultured sample and homogenizing the cells
in RNAbee (cat. no. CS104; Tel-Test Inc., Friendswood, TX, USA).
The isolated RNA concentration was then determined using a NanoDrop
spectrophotometer (BioSpec-Nano, Shimadzu Scientific Instruments,
Columbia, MD, USA). Total RNA (5 µg) was used to synthesize cDNA in
a reaction mixture consisting of oligo (dT) primers (500 ng;
Invitrogen; Thermo Fisher Scientific, Inc.), 10 mM deoxyadenosine,
deoxycytidine, deoxyguanosine and deoxythymidine triphosphates, 0.1
M dithiothreitol, 5X reaction buffer and 1 µl Superscript II
reverse transcriptase (200 U/µl; cat. no. 18064–014; Invitrogen;
Thermo Fisher Scientific, Inc.). Thermal cycling conditions for
reverse transcription consisted of 70°C for 10 min followed by 42°C
for 50 min. RNA was removed from cDNA samples using 3.2 U/µl RNase
H (cat. no. 18021071; Invitrogen; Thermo Fisher Scientific Inc.) at
37°C for 20 min. In order to amplify target genes, 2.5 µl cDNA, 10
pmol specific sense and antisense primers (Macrogene Co., Seoul,
South Korea), 5 U/µl Taq polymerase (0.2 µl; PCR Core kit; Roche
Diagnostics, Basel, Switzerland), 2 mM deoxynucleotide triphosphate
(2.5 µl), and 10X reaction buffer containing 15 mM MgCl2
(2.5 µl) were combined. A thermal cycler (Perkin-Elmer, Inc.,
Waltham, MA, USA) was used to amplify target genes using the
following parameters: A pre-denaturation step of 7 min at 94°C,
followed by 25–32 cycles of 30 sec at 94°C, 30 sec at 62°C, and 45
sec at 72°C and a final post-elongation step of 7 min at 72°C. The
primer sequences for target gene expression identification were as
follows: iNOS, sense 5′-CACTTGGAGTTCACCCAGT-3′ and anti-sense,
5′-ACCACTCGTACTTGGGATGC-3′; COX-2, sense
5′-CAGGTCATTGGTGGAGAGGTGTATC-3′ and anti-sense,
5′-CCAGGAGGATGGAGTTGTTGTAGAG-3′; TNF-α, sense
5′-CCTGTAGCCCACGTCGTAGC-3′ and anti-sense,
5′-TTGACCTCAGCGCTGACTTG-3′; IL-1β, sense
5′-GCACATCAACAAGAGCTTCAGGCAG-3′ and anti-sense,
5′-GCTGCTTGTGAGGTGCTGATGTAC-3′; IL-10, sense
5′-CCAAGCCTTATCGGAAATGA-3′ and anti-sense,
5′-TTTTCACAGGGGAGAAATCG-3′; IL-6, sense 5′-TTGGGACTGATGTTGTTGACA-3′
and anti-sense, 5′-TCATCGCTGTTGATACAATCAGA-3′. The final PCR
products were separated on 1% agarose gel and then visualized by
ethidium bromide staining. The band signal for each sample was
visualized using the AE-9000 E-Graph gel documentation system (ATTO
Corporation, Tokyo, Japan) and the band density was quantified
using Image Saver 5 software (version 5.0; ATTO Corporation). The
experiment was repeated three times and all samples were
PCR-analyzed in triplicate.
Cell cycle assay
The cell cycle was measured using a Muse™ Cell Cycle
kit (cat. no. MCH100106; EMD Millipore, Billerica, MA, USA)
according to the manufacturer's instructions. Briefly, RAW264.7
cells were divided into 100 mm2 dishes
(2.5×106 cells/dish), then pretreated with 100 or 200
µg/ml of EaEAC for 2 h. Following treatment with 1 µg/ml of LPS for
24 h, cells from subset groups were harvested by centrifugation at
3,000 × g for 5 min, then fixed with 70% ethanol at −20°C for 3 h.
The fixed cells were washed with 1X PBS, then added to 200 µl Cell
Cycle Reagent. Following incubation at 37°C in a CO2
incubator for 30 min, cell cycles were analyzed using a
Muse® Cell Analyzer with the Muse 1.3.1 analysis program
(EMD Millipore).
Statistical analysis
Statistical tests were performed using SPSS version
10.10 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
was used to identify significant differences between NC- and
LPS-treated groups. Differences in the responses of the Vehicle +
LPS treated group and EaEAC + LPS treated groups were evaluated
using a post hoc Tukey's test. All values are reported as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
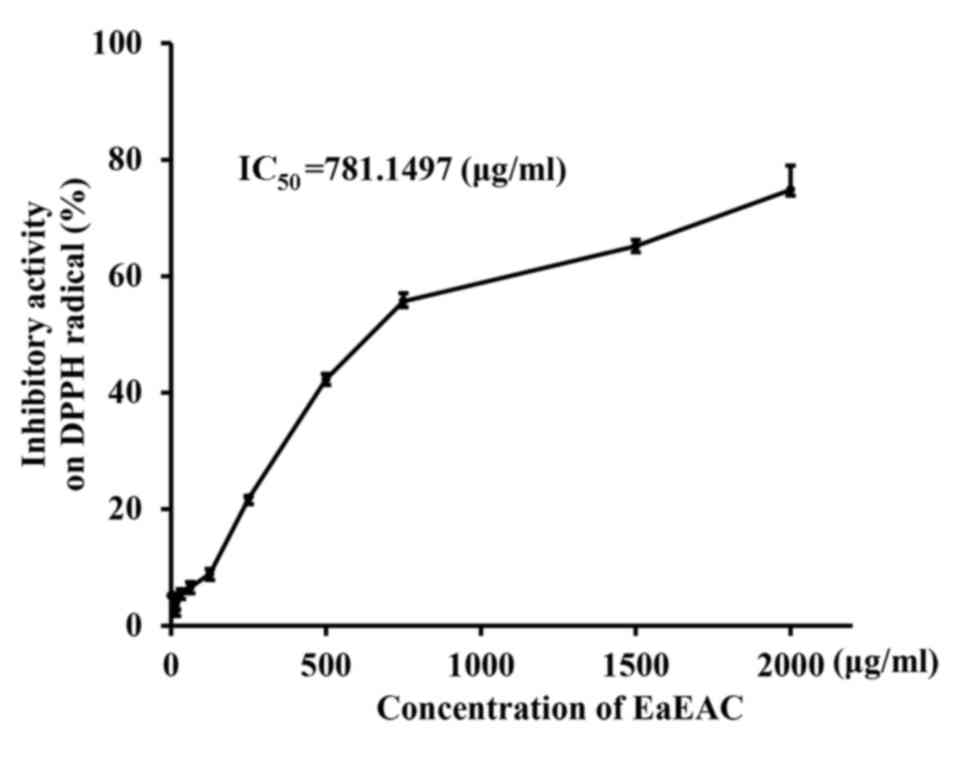
Anti-oxidant activity of EaEAC
The anti-oxidant activity of EaEAC was measured by
DPPH scavenging analysis. Scavenging activity of EaEAC against DPPH
radical was increased in a dose-dependent manner, with an
IC50 of 781.1497 µg/ml (Fig. 1). This indicated that EaEAC
exhibited DPPH radical scavenging activity.
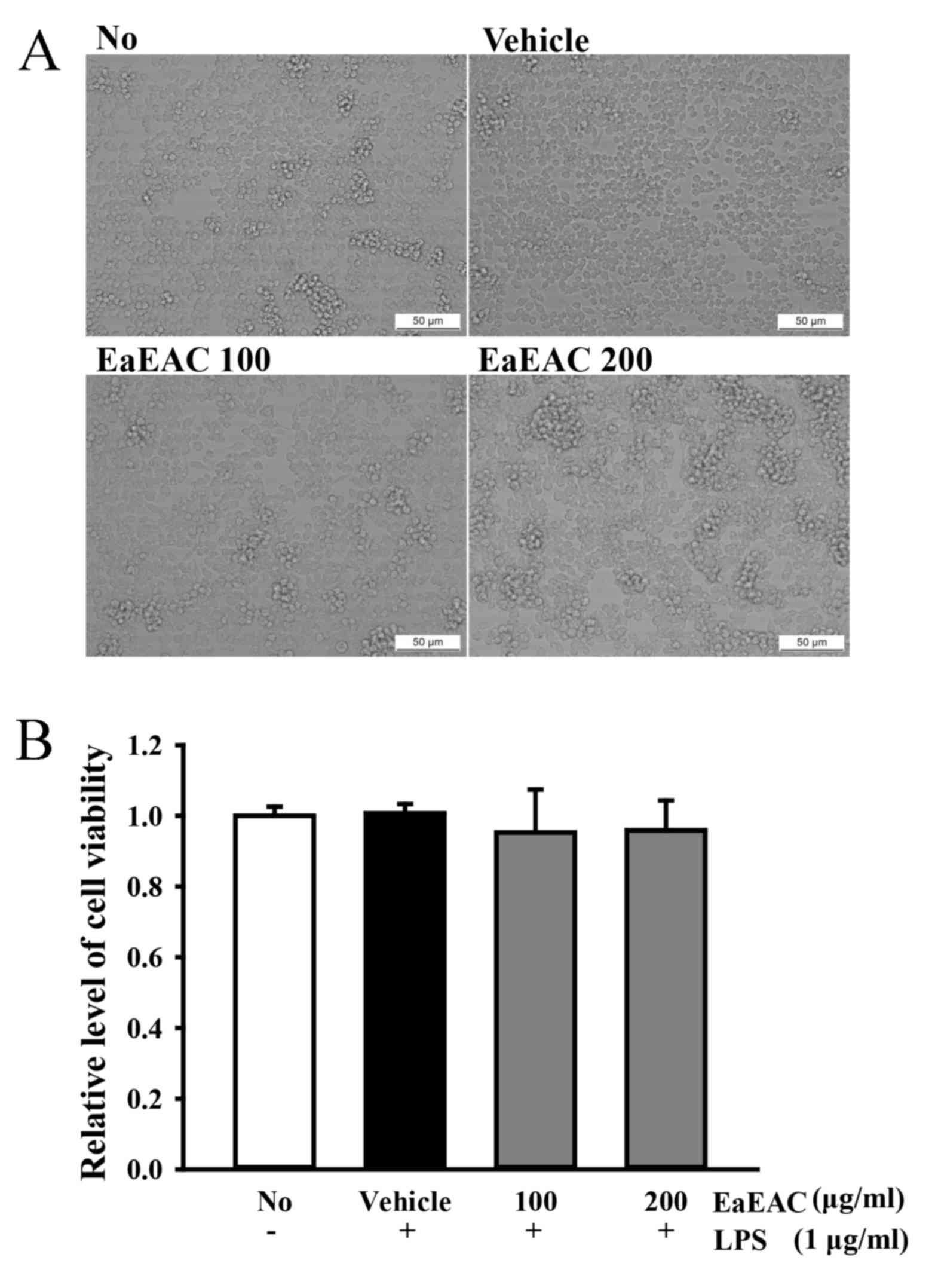
Toxicity of EaEAC
To determine the toxicity of EaEAC, cell viability
was measured by MTT assay. No marked differences in morphological
features (Fig. 2A) or significant
changes to cell viability (Fig.
2B) were observed in RAW264.7 cells treated with 100 and 200
µg/ml EaEAC for 24 h compared with NC or vehicle group cells,
indicating EaEAC is not toxicity at concentrations up to 200
µg/ml.
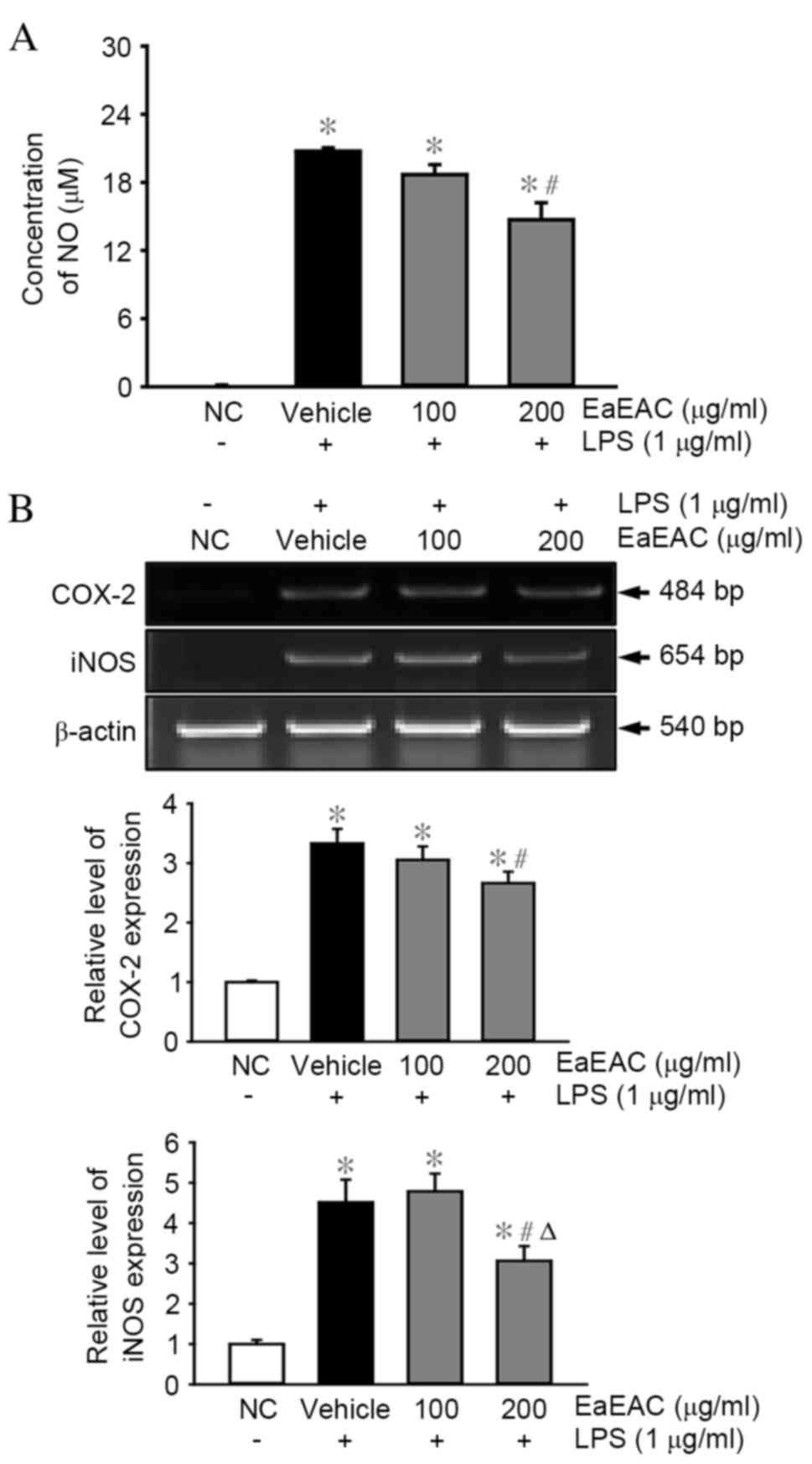
Effects of EaEAC on NO production,
iNOS and COX-2 expression
To examine the anti-inflammatory properties of
EaEAC, alterations in NO concentration, and iNOS and COX-2
transcription were measured in LPS-stimulated RAW264.7 cells
following EaEAC pretreatment. The concentration of NO was
significantly increased in the vehicle + LPS-treated group compared
with the NC group (P=0.01; Fig.
3A). However, the concentration of NO decreased by 18 and 33%
in cells pretreated with 100 and 200 µg/ml EaEAC, respectively,
with the concentration in the cells that were pretreated with 200
µg/ml EaEAC significantly reduced compared with the vehicle + LPS
group (P=0.002; Fig. 3A).
Similarly, increased levels of iNOS and COX-2 mRNA
expression were detected in the vehicle + LPS-treated group
compared with the NC group (P=0.011 and P=0.001, respectively;
Fig. 3B). However, these levels
were reduced by 21% (COX-2) and 33% (iNOS) in LPS-stimulated
RAW264.7 cells that were pretreated with 200 µg/ml of EaEAC
compared with the vehicle + LPS group (P=0.012 and P=0.003,
respectively; Fig. 3B). EaEAC
pretreatment was, therefore, demonstrated to inhibit the increase
of NO concentration, and COX-2 and iNOS mRNA expression in
LPS-activated RAW264.7 cells.
Effects of EaEAC on the expression of
inflammatory cytokines
EaEAC-induced alterations to the mRNA expression
levels of pro-inflammatory (TNF-α and IL-1β) and anti-inflammatory
(IL-10 and IL-6) cytokines in LPS-activated RAW264.7 cells were
then investigated by semi-quantitative RT-PCR. The mRNA levels of
the cytokines were significantly increased in the vehicle +
LPS-treated group compared with the NC group (P=0.002; Fig. 4A). Pretreatment with EaEAC had no
significant effect on the mRNA expression levels of TNF-α at either
100 or 200 µg/ml (Fig. 4A).
However, pretreatment with 100 and 200 µg/ml EaEAC significantly
reduced mRNA expression of IL-1β (P=0.003 and P=0.003,
respectively; Fig. 4A), while 200
µg/ml EaEAC significantly reduced mRNA levels of IL-10 (P=0.008)
and IL-6 (P=0.002) compared with vehicle + LPS (Fig. 4A).
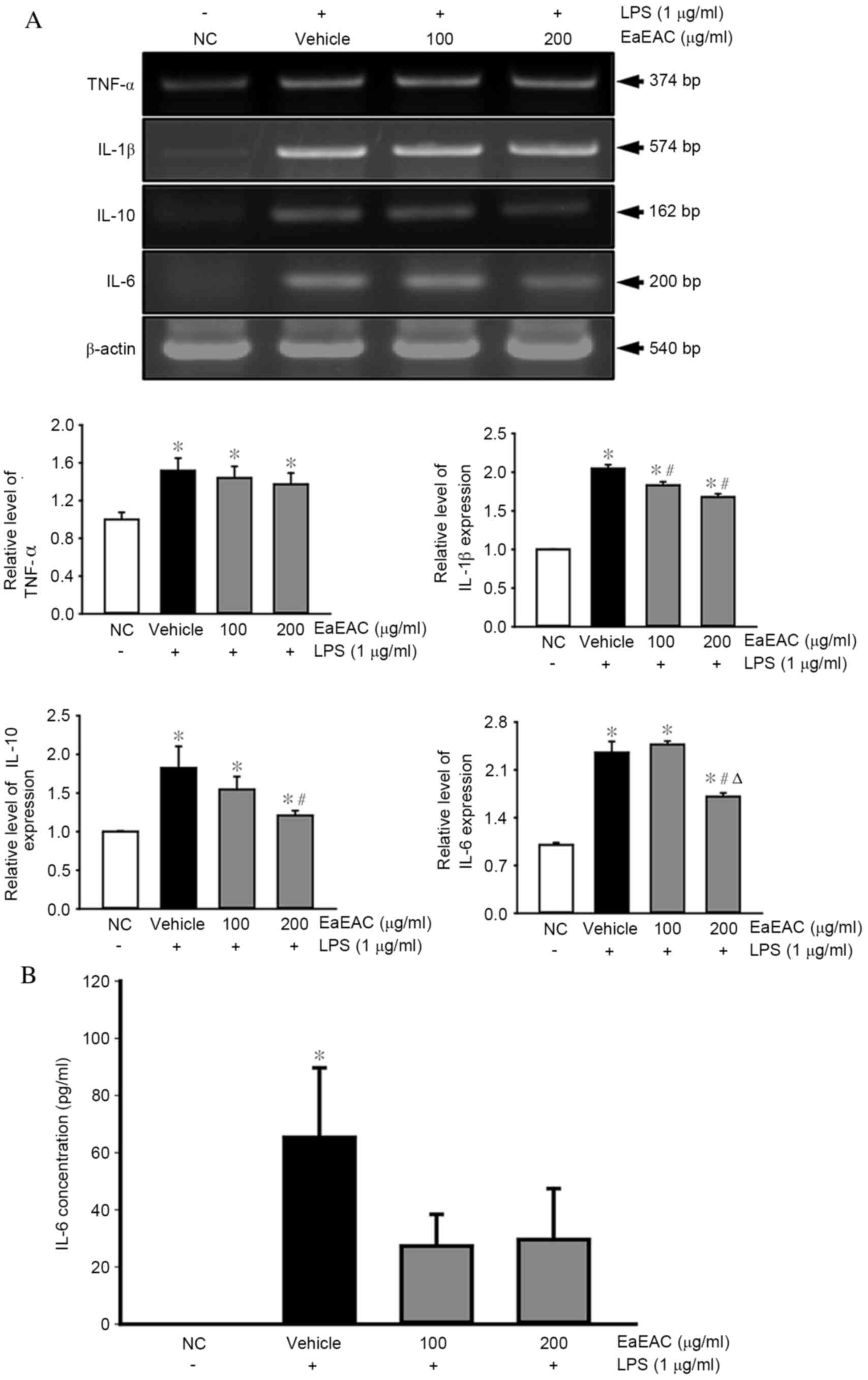 | Figure 4.Analysis of pro- and
anti-inflammatory cytokine expression. Following treatment of
RAW264.7 cells with LPS and 0 (vehicle), 100 or 200 µg/ml of EaEAC,
(A) IL-1β, TNF-α, IL-6 and IL-10 mRNA levels were assessed by
semi-quantitative reverse transcription polymerase chain reaction
using transcript-specific primers, and (B) IL-6 concentration was
detected using an enzyme-linked immunosorbent assay kit with a
minimum detection threshold of 9.3 pg/ml. Values are presented as
the mean ± standard deviation of three replicates. *P<0.05 vs.
NC; #P<0.05 vs. vehicle + LPS-treated group;
ΔP<0.05 vs. 100 µg/ml EaEAC + LPS-treated group. LPS,
lipopolysaccharide; NC, untreated control; EaEAC, ethyl acetate
extract from A. cochinchinesis root; TNF-α, tumor necrosis
factor-α; IL, interleukin. |
The concentration of IL-6 in the culture supernatant
of LPS-activated RAW264.7 cells was also measured by ELISA.
Similarly to the mRNA expression levels, the concentration of IL-6
was significantly increased in the Vehicle + LPS treated group
compared with the NC group (P=0.005; Fig. 4B). However, no significant
differences were observed between the Vehicle + LPS group and the
EaEAC + LPS groups, despite a trend towards lower concentrations
following EaEAC treatment (Fig.
4B). EaEAC pretreatment is, therefore, suggested to suppress
enhanced anti- and pro-inflammatory cytokine expression in RAW264.7
cells stimulated by LPS treatment.
Effects of EaEAC on MAPK
signaling
The MAPK pathway is critical in the regulation of
cell growth and differentiation, and in the control of cellular
responses to various cytokines and stresses (33). To investigate whether EaEAC
pretreatment influences activation of the MAPK pathway, the
phosphorylation levels of ERK, JNK and p38 were assessed in
LPS-stimulated RAW264.7 cells by western blotting (Fig. 5A and B). Levels of phosphorylated
ERK and p38 were significantly higher in cells stimulated with LPS
(vehicle + LPS group) compared with untreated control cells
(P=0.001 and P=0.002, respectively; Fig. 5B). EaEAC pretreatment significantly
suppressed the LPS-induced phosphorylation of ERK at concentrations
of 100 (P=0.002) and 200 µg/ml (P=0.003) compared with the vehicle
+ LPS group (Fig. 5B). JNK
phosphorylation was also suppressed by 100 (P=0.015) and 200 µg/ml
EaEAC (P=0.033) compared with the vehicle + LPS group (Fig. 5B), however, the JNK phosphorylation
level was not induced by LPS stimulation, as demonstrated by the
non-significant difference between the NC and vehicle + LPS group
(Fig. 5B). EaEAC pretreatment
resulted in no significant difference in LPS-induced
phosphorylation of p38 at concentrations of 100 or 200 µg/ml
compared with the vehicle + LPS group (Fig. 5B). Therefore, EaEAC attenuated the
enhanced phosphorylation of some MAPK proteins in LPS-stimulated
RAW264.7 macrophage cells.
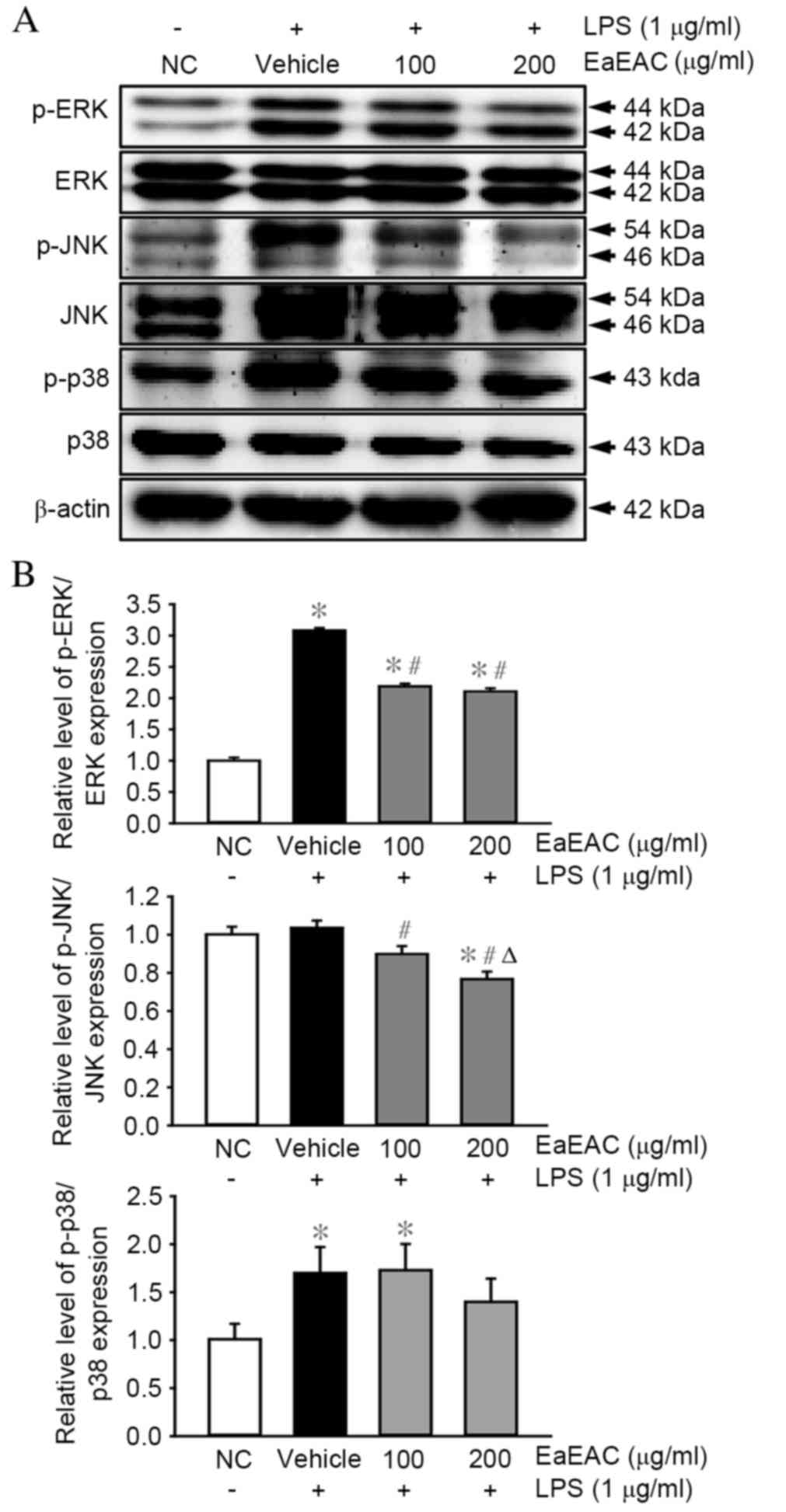 | Figure 5.Activation of the mitogen-activated
protein kinase signaling pathway proteins ERK, JNK, and p38. (A)
p-ERK, ERK, p-JNK, JNK, p38, p-p38 and β-actin protein expression
was assessed by western blot following stimulation of RAW264.7
cells with LPS and 0 (vehicle), 100 or 200 µg/ml of EaEAC. (B) Band
intensity was determined using an imaging densitometer and
expression levels calculated relative to the intensity of β-actin.
Values are presented as the mean ± standard deviation of three
replicates. *P<0.05 vs. NC; #P<0.05 vs. vehicle +
LPS-treated group; ΔP<0.05 vs. 100 µg/ml EaEAC +
LPS-treated group. LPS, lipopolysaccharide; NC, untreated control;
EaEAC, ethyl acetate extract from Asparagus cochinchinesis
root; p-, phosphorylated; ERK, extracellular signal-regulated
kinase; JNK, c-Jun N-terminal kinase. |
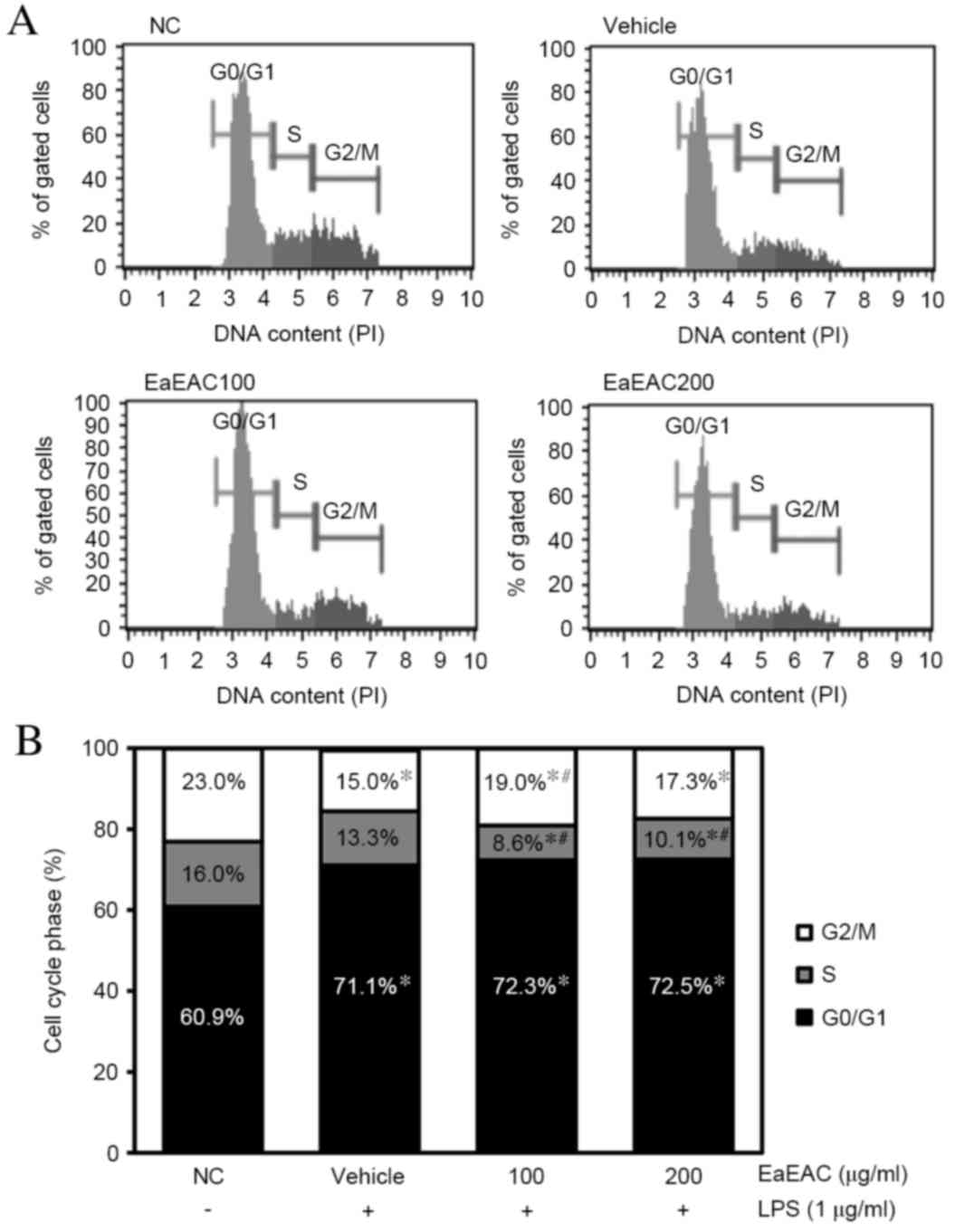
Effect of EaEAC on regulation of the
cell cycle
To examine the effect of EaEAC treatment on the cell
cycle, the number of cells in each stage of the cell cycle was
counted (Fig. 6A and B). The
number of cells in the G0/G1 stage was increased in the vehicle +
LPS group compared with untreated control cells (P=0.003; Fig. 6B), whereas those in the S and G2/M
stage were decreased (P=0.001; Fig.
6B). However, a significant increase in the number of cells in
the G2/M stage was induced by treatment with 100 µg/ml EaEAC
compared with the vehicle + LPS group (P=0.023; Fig. 6B), and a similar but
non-significant trend towards increased G2/M stage cells was
induced by treatment with 200 µg/ml EaEAC (Fig. 6B). A significant decrease in the
number of S stage cells was also observed following treatment with
100 (P=0.05) and 200 µg/ml EaEAC (P=0.008) compared with the
vehicle + LPS (Fig. 6B). EaEAC
treatment was, therefore, demonstrated to induce arrest of the cell
cycle in the G2/M stage and stimulate progression from the S stage
to the G2/M stage.
Inhibitory effects of EaEAC treatment
on ROS production
Finally, the inhibitory effects of EaEAC against
LPS-induced ROS production in RAW264.7 macrophage cells were
determined using a fluorescent oxidation-sensitive dye, DCFH-DA, to
measure ROS levels. ROS production increased rapidly in the vehicle
+ LPS-treated group compared with the NC group. However, the level
of ROS production decreased in an apparently
concentration-dependent manner, despite cell morphologies remaining
consistent. Therefore, increased ROS levels induced by LPS
stimulation were effectively suppressed by EaEAC pretreatment.
Discussion
Extracts of A. cochinchinensis roots have
been used in Korea to treat neuro-inflammatory and
skin-inflammatory diseases for many years (1,25,27).
Several studies have, therefore, focused on A.
cochinchinensis to develop therapies against lung-inflammatory
disease (28,34). In an effort to identify candidate
natural products for the treatment of chronic lung disease and
examine the mechanism of action, the present study investigated the
therapeutic effects of EaEAC in LPS-activated RAW264.7 cells. EaEAC
was demonstrated to significantly suppress inflammatory responses
through decreased NO, COX-2, iNOS, IL-6, IL-10 and IL-1β expression
levels, and attenuation of ROS production in LPS-activated RAW264.7
macrophages.
LPS is the major component of the external membrane
of gram-negative bacteria, and an important molecule in the
pathogenesis of sepsis and septic shock. LPS has also been widely
used as a prototypical endotoxin to activate numerous cell types,
including monocytes, dendritic cells, macrophages and B cells
(35,36). Among these cells, macrophages are
crucial for regulating NO, TNF-α, and IL-6 production, which
mediate the development of various inflammatory diseases, including
sepsis-related multiple organ dysfunction/multiple organ failure,
microbial infection, acute brain/lung/hepatic/renal injuries,
neurodegenerative disorders, tumorigenesis,
osteoporosis/osteonecrosis, and cardiovascular, metabolic and
autoimmune diseases (37–39). Furthermore, various concentrations
of LPS (50 ng/ml to 10 µg/ml) have been applied to activate
RAW264.7 cells (40–42). Preliminary data from the present
study demonstrated that an inflammatory response was induced in
RAW264.7 cells stimulated with 0.1, 0.5, 1.0 and 1.5 µg/ml LPS
(data not shown). A concentration of 1 µg/ml LPS was, therefore,
selected as the optimal concentration for activation of macrophages
in subsequent experiments.
NO, which include nitroglycerine and amyl nitrite
precursor, are considered to be important cellular signaling
molecules in various mammalian physiological and pathological
processes (33). NO is produced by
NOS in the majority of organisms, including bacteria, fungi and
mammals. In humans, NO is primarily produced by iNOS in phagocytic
cells during an immune response (43). LPS-induced NO production in BV-2
cells has previously been demonstrated to be significantly
suppressed by three compounds originating from A.
cochinchinensis: Aspacochinoside O, Aspacochinoside P and
3-O-β-d-xylopyranosyl(1→4)-[β-d-glucopyranosyl
(1→2)]-β-d-glucopyranosyl-26-O-β-d-glucopyranosyl-(25S)-5β-furostane-3β,
22α, 26-triol (44). The present
study demonstrated similar inhibitory effects on NO production,
although the cell line and solvent used for extraction in the
studies differed. EaEAC pretreatment induced the restoration of
iNOS and COX-2 transcription levels in LPS-activated macrophage
cells. The results of the current study suggest that the inhibitory
effects of EaEAC on LPS-stimulated NO production are a result of
iNOS and COX-2 transcriptional suppression. However, additional
research is required to determine the key compounds within EaEAC
that are responsible for the regulation of NO production.
Secretion of cytokines, including interleukins,
interferon, colony stimulating factors and numerous growth factors
regulate the differentiation, proliferation and development of
cells (45). The pro-inflammatory
cytokines secreted by stimulated macrophages are important in
inflammatory diseases, including sepsis and arthritis (46,47).
Furthermore, extracts from A. cochinchinensis have
previously been demonstrated to effectively regulate the expression
of various cytokines; the increase of TNF-α secretion in astrocytes
induced by substance P + LPS and ethanol treatment was
significantly inhibited by ethanol extracts (70%) of A.
cochinchinensis (ACE) in a dose dependent manner (21,25).
Production of pro-inflammatory cytokines, including IL-1β and TNF-α
has also been previously demonstrated to be suppressed by ACE in
mice with phorbol ester-induced dermatitis (27). In the present study, the transcript
and protein levels of pro- and anti-inflammatory cytokines in
RAW264.7 cells were measured following EaEAC + LPS treatment,
revealing similar suppressive activities compared with previous
studies, despite certain differences in the reduction rate and the
composition of the extracts.
To the best of our knowledge, the present study
presents the first evidence of the therapeutic effect of EaEAC in
LPS-activated RAW264.7 cells accompanied by alterations in MAPK
signaling and the cell cycle. MAPKs are important in the regulation
of cell growth and differentiation, and in the control of cellular
responses to cytokines and stresses (48). In several previous studies, the
expression levels of three major members of the MAPK signaling
pathway, JNK, ERK and p38, were significantly increased by LPS
treatment in macrophage cells (49,50).
LPS also increased the expression of several inflammatory genes,
including TNF-α, IL-6, iNOS and COX-2, through regulation of MAPKs
(51,52). Furthermore, pathological
conditions, including inflammation and oxidative stress, have been
demonstrated to induce alteration of the cell cycle in numerous
diseases (53,54). Certain natural products have also
been previously demonstrated to induce alteration of MAPK
expression and the cell cycle in RAW264.7 cells. For example,
triterpene extract from G. lucidum suppressed the
LPS-induced phosphorylation of ERK1/2 and JNK, and arrested these
cells at the G0/G1-M stage (55).
Wild grape seed procyanidins inhibited the LPS-induced
phosphorylation of p38 (56), and
Rhus verniciflua Stokes arrested RAW264.7 cells at the G1
stage of the cell cycle (57). In
the present study, EaEAC inhibited the phosphorylation of three
members of the MAPK signaling pathway and arrested the cell cycle
at the G2/M stage. The results of the present study were similar to
those of previous studies, although certain differences in the
suppression of MAPK signaling were observed. These differences may
be due to factors including the innate composition of the extracts
and key molecules within the herbal medicine.
Oxidative stress, cell dysfunction and, ultimately,
apoptosis and necrosis are induced by excessive production of ROS
(58). The activity of SOD and the
content of MDA have been demonstrated to be differentially
regulated by the roots and stems of A. cochinchinensis, and
polysaccharide and aqueous extracts of A. cochinchinensis
roots increased SOD activity, but decreased MDA content (59). By contrast, A.
cochinchinensis stem extracts decreased SOD activity and
enhanced MDA accumulation in the brains and livers of mice
(1). Similar effects on
anti-oxidant state were detected in the present study: ROS
production was increased in the vehicle + LPS-treated group, but
was recovered in a dose dependent manner in the EaEAC + LPS-treated
group, therefore providing additional evidence that the roots of
A. cochinchinensis have anti-oxidant activity and may
suppress ROS production. In summary, the present study suggests
that EaEAC may suppress inflammatory responses in macrophages
through stimulation of anti-inflammatory effects, decreased
pro-inflammatory cytokine expression and inhibition of ROS
production. The inhibitory effects of EaEAC were observed to be
associated with attenuation of the enhanced phosphorylation of MAPK
proteins following LPS treatment. The MAPK signaling pathway
regulates the transcription of a number of genes associated with
inflammation, thus, its inhibition by EaEAC offers a potential
approach for the treatment of severe inflammatory disease.
Acknowledgements
This study was supported by grants to Professor Dae
Youn Hwang from the Korea Institute of Planning Evaluation for
Technology of Food, Agriculture, Forestry and Fisheries (grant no.
114034-03-1-HD030).
References
|
1
|
Xiong DS, Yu LX, Yan X, Guo C and Xiong Y:
Effects of root and stem extracts of Asparagus cochinchinensis on
biochemical indicators related to aging in the brain and liver of
mice. Am J Chinese Med. 39:719–726. 2011. View Article : Google Scholar
|
|
2
|
Xiao PG: Modern Chinese material medica.
Chemical Industry Press; Beijing: pp. 1502002
|
|
3
|
Liu YZ, Qu FY and Zhang PX: Effect of
chloroform extract of Tiandong on the brain antioxidation of
D-galatose-induced senile mice. Heilongjiang Med Pharm. 24:7–8.
2001.
|
|
4
|
Ni JM, Zhao R and Wang R: Comparison on
amino acid content in prepared and unprepared Asparagus
cochinchinensis. Chin Tradit Herb Drugs. 23:182–183. 1992.
|
|
5
|
Tenji K and Junzo S: Studies on the
constituents of Asparagi Radix. I. On the structures of furostanol
oligosides of Asparagus cochinchinensis (LOUREIO) MERRILL. Chem
Pharm Bull. 27:3086–3094. 1979. View Article : Google Scholar
|
|
6
|
Liang ZZ, Aquino R, De Simone F, Dini A,
Schettino O and Pizza C: Oligofurostanosides from Asparagus
cochinchinensis. Planta Med. 54:344–346. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YC, Huang SY and Shi JG: Two new
furostanol glycosides from Asparagus cochinchinensis. Chin Chem
Lett. 13:1185–1188. 2002.
|
|
8
|
Cong PZ and Keman S: Handbook of
analytical chemistry-mass volume. Chemical Industry Publishing
House; 2. Beijing: pp. 296–298. 2000
|
|
9
|
Gong YH: 13C NMR chemical shifts of
natural organic compounds. Yunnan Science and Technology Publishing
House Kunming. 2:2521986.
|
|
10
|
Yang MH: Steroidal sapogenins of
dioscorea. Chin Tradit Herb Drugs. 12:43–44. 1981.
|
|
11
|
Xu CL, Chen HS and Tan XQ: Studies on the
active constituents of Asparagi Radix. Nat Prod Res Dev.
17:128–130. 2005.
|
|
12
|
Shen Y, Chen HS and Wang Q: Studies on
chemical constituents of Asparagus cochinchinensis (II). J Second
Med Univ. 28:1241–1244. 2007.
|
|
13
|
Shen Y, Xu Cl, Xuan WD, Li HL, Liu RH, Xu
XK and Chen HS: A new furostanol saponin from Asparagus
cochinchinensis. Arch Pharm Res. 34:1587–1591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XN, Chu C, Cheng DP, Tong SQ and Yan
JZ: Norlignans from Asparagus Cochinchinensis. Nat Prod Commun.
7:1357–1358. 2012.PubMed/NCBI
|
|
15
|
Zhu GL, Hao Q, Li RT and Li HZ: Steroidal
saponins from the roots of Asparagus cochinchinensis. Chin J Nat
Med. 12:213–217. 2014.PubMed/NCBI
|
|
16
|
Li M, Fei Y and Wang JK: Studies on
pharmacologic effects of Radix Asparagi. LiShiZhen Med Mater Med
Res. 16:580–582. 2005.
|
|
17
|
Qu FY, Wei XD, Li SL, Wang YM and Bai SG:
Experimental study of Asparagus cochinchinensis delay aging. Acta
Chin Med Pharm. 2:68–70. 1999.
|
|
18
|
Zhao YJ, Meng XL, Li XL and Qu FY:
Influence of Radix Asparagi nano-pharmaceutics on NOS NO, LPF of
aging mice. Chin Wild Plant Resour. 24:49–51. 2005.
|
|
19
|
Wen JY, Li Y, Ding SS and Li QH: Nine
Pharmacological screening of medicinal plants of China Liliaceae
Asparagus. J Acta Acad Med Shanghai. 20:107–111. 1993.
|
|
20
|
Luo J, Long Q, Li C, Li L and Huang N:
Inhibitory effects of ALWB and ACM on mice bearing tumor. J GuiYang
Med Coll. 25:15–16. 2000.
|
|
21
|
Koo HN, Jeong HJ, Choi JY, Choi SD, Choi
TJ, Cheon YS, Kim KS, Kang BK, Park ST, Chang CH, et al: Inhibition
of tumor necrosis factor-alpha-induced apoptosis by Asparagus
cochlnchinensis in Hep G2 cells. J Ethnopharmacol. 73:137–143.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu FR, Lian XZ and Guo HY: Effect of lucid
asparagus extract on the regulation of blood sugar. Chin J Clin
Rehabil. 10:57–59. 2006.
|
|
23
|
Jun L, Qingde L, Chengxiu L, Ling L,
Nenghui H, Min N and Peixian T: Comparison of antitussive,
expectorant and anti-asthmatic effect between ALWB and ACM. J
GuiYang Med Coll. 23:132–134. 1998.
|
|
24
|
Lv B and Liu WZ: Aspartate treatment of
hemodialysis patients with hypertension in 22 cases. J Tradit Chin
Med. 19:43–44. 2004.
|
|
25
|
Kim HM, Lee E, Lim T, Jung J and Lyu Y:
Inhibitory effect of Asparagus cochinchinensis on tumor necrosis
factor-alpha secretion from astrocytes. Int J Immunopharmacol.
20:153–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jian R, Zeng KW, Li J, Li N, Jiang Y and
Tu P: Anti-neuroinflammatory constituents from Asparagus
cochinchinensis. Fitoterapia. 84:80–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW,
Lee YA and Kim HK: Anti-inflammatory effects of Asparagus
cochinchinensis extract in acute and chronic cutaneous
inflammation. J Ethnopharmacol. 121:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung KH, Choi HL, Park SJ, Lee GH, Kim MR,
Min JK, Min BI and Bae H: The effects of the standardized herbal
formula PM014 on pulmonary inflammation and airway responsiveness
in a murine model of cockroach allergen-induced asthma. J
Ethnopharmacol. 155:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA,
Bennett AM and Flavell RA: Dynamic regulation of pro- and
anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate
immune responses. Proc Natl Acad Sci USA. 103:2274–2279. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh H, Ko EK, Kim DH, Jang KK, Park SE, Lee
HS and Kim YC: Secoiridoid glucosides with free radical scavenging
activity from the leaves of Syringa dilatata. Phytother Res.
17:417–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jie S, Xueji Z, Mark B and Harry F:
Measurement of nitric oxide production in biological systems by
using griess reaction assay. Sensors. 3:276–284. 2003. View Article : Google Scholar
|
|
32
|
Kim JE, Park SH, Kwak MH, Go J, Koh EK,
Song SH, Sung JE, Lee HS, Hong JT and Hwang DY: Characterization of
changes in global genes expression in the distal colon of
loperamide-induced constipation SD rats in response to the laxative
effects of Liriope platyphylla. PLoS One. 10:e01296642015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou YC, Janczuk A and Wang PG: Current
trends in the development of nitric oxide donors. Curr Pharm Des.
5:417–441. 1999.PubMed/NCBI
|
|
34
|
Lee JH, Lim HJ, Lee CW, Son KH, Son JK,
Lee SK and Kim HP: Methyl protodioscin from the roots of Asparagus
cochinchinensis attenuates airway inflammation by inhibiting
cytokine production. Evid Based Complement Alternat Med.
2015:6408462015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rietschel ET, Kirikae T, Schade FU, Mamat
U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U and Di
Padova F: Bacterial endotoxin: Molecular relationships of structure
to activity and function. FASEB J. 8:217–25. 1994.PubMed/NCBI
|
|
36
|
Stewart I, Schluter PJ and Shaw GR:
Cyanobacterial lipopolysaccharides and human health-a review.
Environ Health. 5:72006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dalmas E, Tordjman J, Guerre-Millo M and
Clément K: Macrophages and inflammationAdipose Tissue Biology.
Symonds ME: Springer; New York, NY: pp. 167–193. 2012, View Article : Google Scholar
|
|
39
|
Jou IM, Lin CF, Tsai KJ and Wei SJ:
Macrophage-mediated inflammatory disorders. Mediators Inflamm.
2013:3164822013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang GY, Liao J, Li C, Chung J, Yurkow EJ,
Ho CT and Yang CS: Effect of black and green tea polyphenols on
c-jun phosphorylation and H(2)O(2) production in transformed and
non-transformed human bronchial cell lines: Possible mechanisms of
cell growth inhibition and apoptosis induction. Carcinogenesis.
21:2035–2039. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee SJ, Kang HY, Lee SY and Hur SJ: Green
tea polyphenol Epigallocatechin-3-O-Gallate attenuates
lipopolysaccharide-induced nitric oxide production in RAW264.7
cells. J Food Nutr Res. 2:425–428. 2014. View Article : Google Scholar
|
|
42
|
Hashimoto K and Sakagami H: Induction of
apoptosis by Epigallocatechin gallate and autophagy inhibitors in a
mouse macrophage-like cell line. Anticancer Res. 28:1713–1718.
2008.PubMed/NCBI
|
|
43
|
Kamijo R, Gerecitano J, Shapiro D, Green
SJ, Aguet M, Le J and Vilcek J: Generation of nitric oxide and
clearance of interferon-gamma after BCG infection are impaired in
mice that lack the interferon-gamma receptor. J Inflamm. 46:23–31.
1995.PubMed/NCBI
|
|
44
|
Jian R, Zeng KW, Li J, Li N, Jiang Y and
Tu P: Anti-neuroinflammatory constituents from Asparagus
cochinchinensis. Fitoterapia. 84:80–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Szabó C: Role of nitric oxide in endotoxic
shock. An overview of recent advances. Ann N Y Acad Sci.
851:422–425. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Martel-Pelletier J, Pelletier JP and Fahmi
H: Cyclooxygenase-2 and prostaglandins in articular tissues. Semin
Arthritis Rheum. 33:155–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Berghe W Vanden, Plaisance S, Boone E, De
bosscher K, Schmitz ML, Fiers W and Haegeman G: p38 and
extracellular signal-regulated kinase mitogen-activated protein
kinase pathways are required for nuclear factor-kappaB p65
transactivation mediated by tumor necrosis factor. J Biol Chem.
273:3285–3290. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weinstein SL, Sanghera JS, Lemke K,
DeFranco AL and Pelech SL: Bacterial lipopolysaccharide induces
tyrosine phosphorylation and activation of mitogen-activated
protein kinases in macrophages. J Biol Chem. 267:14955–14962.
1992.PubMed/NCBI
|
|
50
|
Comalada M, Xaus J, Valledor AF,
López-López C, Pennington DJ and Celada A: PKC epsilon is involved
in JNK activation that mediates LPS-induced TNF-alpha, which
induces apoptosis in macrophages. Am J Physiol Cell Physiol.
285:C1235–C1245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ulevitch RJ and Tobias PS:
Receptor-dependent mechanisms of cell stimulation by bacterial
endotoxin. Annu Rev Immunol. 13:437–457. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weinstein SL, Sanghera JS, Lemke K,
DeFranco AL and Pelech SL: Bacterial lipopolysaccharide induces
tyrosine phosphorylation and activation of mitogen-activated
protein kinases in macrophages. J Biol Chem. 267:14955–14962.
1992.PubMed/NCBI
|
|
53
|
Kirouac L, Mathew M and Padmanabhan J:
Interplay between inflammation and cell cycle deregulation in
Alzheimer's disease. JSM Alzheimer's Dis and Related Dementia.
2:10182015.
|
|
54
|
Stockley JA, Walton GM, Lord JM and Sapey
E: Aberrant neutrophil functions in stable chronic obstructive
pulmonary disease: The neutrophil as an immunotherapeutic target.
Int Immunopharmacol. 17:1211–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dudhgaonkar S, Thyagarajan A and Sliva D:
Suppression of the inflammatory response by triterpenes isolated
from the mushromm Ganoderma lucidum. Int Immunopharmacol.
9:1272–1280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bak MJ, Truong VL, Kang HS, Jun MR and
Jeong WS: Anti-inflammatory effect of procyanidins from wild grape
(Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid Med
Cell Longev. 2013:4093212013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi HS, Seo HS, Kim SR, Choi YK, Shin YC
and Ko SG: Anti-inflammatory and anti-proliferative effect of
herbal medicines (APR) in RAW264.7 cells. Mol Med Rep. 9:1569–1574.
2014.PubMed/NCBI
|
|
58
|
Halliwell B: Reactive oxygen species and
the central nervous system. J Neurochem. 59:1609–1623. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xiong D, Yu LX, Yan X, Guo C and Xiong Y:
Effects of root and stem extracts of Asparagus cochinchinensis on
biochemical indicators related to aging in the brain and liver of
mice. Am J Chin Med. 39:719–726. 2011. View Article : Google Scholar : PubMed/NCBI
|