Introduction
Cervical cancer is the third most common malignancy
in women worldwide, with a global incidence of 500,000 and a
mortality of 250,000 in 2014 (1).
For the earliest stage of cervical cancer, >90% of women survive
at least 5 years after diagnosis. However, advanced cervical cancer
with invasion or metastasis is associated with a poor prognosis,
<20% of stage IV patients survive for ≥5 years (2). Furthermore, despite advances of
conventional therapies such as surgical treatment, radiotherapy and
chemotherapy, malignant cervical cancers still have high mortality
rate, and the mechanism underlying its aggressiveness remains
poorly understood. Thus, the identification of novel molecular
markers, which is helpful for the development of novel diagnostic
and therapeutic strategies, remains an important focus in the
current management of this malignancy.
Mammalian Large tumor suppressor kinase 1 (LATS1)
and LATS2, the major kinase components of the Hippo pathway, are
important in the control of tumor development (3,4) and
the cell cycle, via various mechanisms and signaling pathways
(5,6). It was previously reported that LATS1
protein was downregulated in various types of cancer, including
breast carcinoma (7), colorectal
carcinoma (8), gastric cancer
(9), non-small cell lung cancer
(10) and ovarian serous carcinoma
and clear cell carcinoma (11).
These results indicated that LATS1 may be an important tumor
suppressor in types of human cancer. However, whether LATS1 is a
tumor suppressor in cervical cancer remains controversial.
The present study examined LATS1 protein expression
in 80 cases of cervical carcinoma and analyzed the association
between LATS1 expression and clinicopathological factors.
Additionally, gain of function and loss of function experiments
were performed to investigate the biological roles of LATS1 in
cervical cancer. LATS1 expression was upregulated in SiHa cells and
depleted in Caski cells, and the effects on cell proliferation and
invasion were then examined. In addition, the molecular signaling
pathways underlying these biological effects of LATS1 were
investigated.
Materials and methods
Patients and specimens
The protocol of the current study was approved by
the Institutional Review Board of Kunming Maternity and Child Care
Hospital (Kunming, China). Primary tumor specimens were obtained
from 80 patients (mean age, 45.5; range, 28–72) diagnosed with
cervical carcinoma who underwent resection in Kunming Maternity and
Child Care Hospital between January 2012 and November 2014.
Informed consent was obtained. Histological diagnosis was performed
on sections stained with hematoxylin and eosin, according to the
World Health Organization classification guidelines (12). Clinical and histopathological data
were obtained from medical records.
Immunohistochemistry
Cervical cancer tissue specimens were fixed in 10%
formalin at room temperature for 24 h and embedded in paraffin.
Immunohistochemistry was carried out using Elivision™ plus Polyer
HRP IHC kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China)
according to the manufacturer-s protocol. Briefly, 4 µm thick
tissue sections were deparaffinized and rehydrated using ethanol.
Subsequently, antigen retrieval was performed using 0.01 M citrate
buffer (pH 6.0) for 2 min. H2O2 was employed
to inhibit endogenous peroxide and non-immune goat serum (Fuzhou
Maixin Biotech Co., Ltd.) was used to reduce non-specific antibody
binding at room temperature for 15 min. Sections were then
incubated with LATS1 antibody (1:200; cat. no. 9153; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight. Biotinylated
anti-rabbit horseradish peroxidase polymer (ready to use; cat. no.
9922; Fuzhou Maixin Biotech Co., Ltd.) was used as a secondary
antibody at 37°C for 2 h. Following washing, the peroxidase
reaction was developed with 3, 3-diaminobenzidine. Counterstaining
with hematoxylin was performed and the sections were dehydrated in
ethanol prior to mounting.
Two independent blinded investigators examined all
tumor slides randomly. As in previous studies (13,14),
immunostaining of LATS1 was scored on a semi-quantitative scale by
evaluating the intensity and percentage of tumor cells stained.
Cytoplasmic immunostaining was regarded as positive. The intensity
of LATS1 staining was scored as follows: 0, weak/negative; 1,
moderate; or 2 (strong). Staining percentage, the percentage of
tumor cells stained, was scored as follows: 1, 1–25%; 2, 26–50%; 3,
51–75%; or 4, 76–100%. Total score was obtained by multiplying the
staining and percentage scores. Specimens with a total score of 5–8
were considered to be positive for LATS1 expression. Specimens with
a total score of 0–4 were considered to have low LATS1
expression.
Cell culture and transfection
Caski, HeLa and SiHa cell lines were obtained from
the American Type Culture Collection (Manassas, VA, USA). SiHa and
HeLa cells were cultured in minimum essential medium (MEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
Caski cells were cultured in RPMI-1640 medium containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C for 2
days. Cells were cultured on sterilized culture plates and were
passaged every two days with trypsin.
The plasmid of pCMV6-LATS1 was purchased from
(OriGene Technologies, Inc. (Rockville, MD, USA) Plasmid was
transfected into cells using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h.
pCMV6 empty vector was used as a negative control. For transient
knockdown experiments, oligonucleotide pools of small interfering
RNA (siRNA) targeting LATS1 and non-targeting siRNA (cat. no.
M-004632-00-0005) were purchased from Dharmacon (Lafayette, CO,
USA) and transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h,
according to the manufacturers- protocols.
Western blot analysis
Proteins were extracted and quantified using the
Bradford method, and 20 µg protein was separated by SDS-PAGE (5%
stacking gel and 10% separating gel). Proteins were transferred to
polyvinylidene fluoride membranes and 5% BSA solution (w/v) was
used to reduce non-specific antibody binding at room temperature
for 1 h. Proteins were incubated overnight at 4°C with antibodies
against LATS1 (1:1,000; cat. no. 9153; Cell Signaling Technology,
Inc.), phosphorylated-yes associated protein 1 (p-YAP; 1:1,000;
cat. no. 4911; Cell Signaling Technology, Inc.), YAP (1:1,000; cat.
no. 4912; Cell Signaling Technology, Inc.), p27 (1:1,000; cat. no.
2552; Cell Signaling Technology, Inc.), cyclin E (1:1,000; cat. no.
20808; Cell Signaling Technology, Inc.), connective tissue growth
factor (CTGF; 1:1,000; 23936-1-AP; Proteintech, Chicago, Illinois,
USA) and matrix metalloproteinase 9 (MMP9; 1:1,000; cat. no. 3852;
Cell Signaling Technology, Danvers, MA, USA) and GAPDH (1:1,000;
cat. no. sc-25778; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). PVDF membranes were washed using TBST solution
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) three times
for 5 min each. Following incubation with peroxidase-coupled
anti-mouse/rabbit IgG (1:2,000; cat. no. 5127/58802; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 37°C for 2 h, proteins were
visualized using SuperSignal West Dura Extended Duration Substrate
(Thermo Fisher Scientific, Inc.) and detected using a DNR
Bio-Imaging System (DNR Bio-Imaging Systems, Ltd., Jerusalem,
Israel).
MTT assay
Cells were plated in 96-well plates in MEM
containing 10% fetal bovine serum at approximately 1,000–1500 cells
per well. For the measurement of cell viability, 20 µl MTT solution
was added to each well and incubated at 37°C for 4 h. Subsequently,
the remaining MTT formazan was dissolved in 150 µl dimethyl
sulfoxide. The plate was measured at a wavelength of 490 nm using a
plate reader.
Cell invasion analysis
Cell invasion was examined using Transwell assay
with 24-well Transwell chambers. Briefly, Transwell chamber inserts
were coated using 20–25 µl Matrigel from BD Biosciences (Franklin
Lakes, NJ, USA) with a dilution of 1:5. ~48 h after cell
transfection, ~1×105 cells were re-suspended in 100 µl
of serum-free MEM and were added to the upper chamber. MEM with
10–15% fetal bovine serum was added to the lower chamber. After
incubation at 37°C for 16–20 h, cells on the upper side of
membranes were removed using a cotton swab and the cells that had
invaded through the filter were washed with phosphate-buffered
saline and visualized with hematoxylin at room temperature for 5
min. The number of invaded cells was counted in 5 randomly selected
high power fields using a light microscope. This experiment was
performed in triplicate.
Statistical analysis
SPSS version 11.5 for Windows (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. Data was presented
as mean ± standard deviation. χ2 test was used to
examine potential associations between LATS1 expression and
clinicopathological factors and a paired Student-s t-test was used
to compare other data generated from LATS1-transfected or knockdown
cells. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical significance of LATS1 in
human cervical cancer
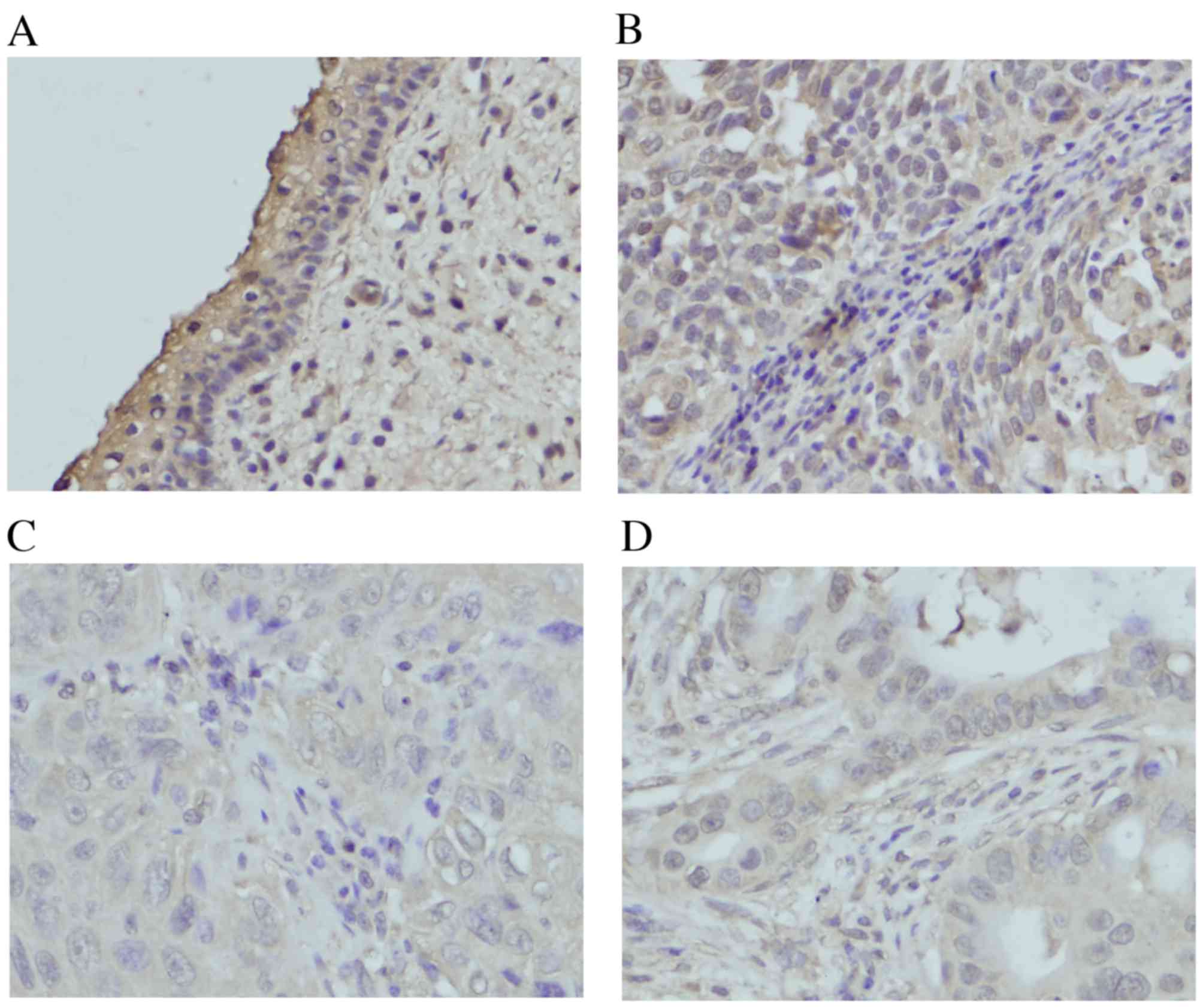
LATS1 expression was located in the cytoplasm in
normal cervical tissues (Fig. 1A).
Of the 80 cervical cancer tissues, 46 of them (45%) exhibited
decreased LATS1 staining, although others were positive for LATS1
expression (Fig. 1B-D). The
potential association of low LATS1 expression with
clinicopathological characteristics was analyzed. The results
demonstrated that low LATS1 immunostaining in cervical cancer was
significantly associated with primary tumor, node, metastasis (TNM)
stage (stages II+III vs. stage I, P=0.0102) and primary tumor (T)
stage (TII+III vs. TI, P=0.0217). No significant association was
identified between low LATS1 expression and other parameters,
including age, histological type and differentiation (Table I).
 | Table I.Distribution of LATS1 status in
cervical carcinoma according to clinicopathological
characteristics. |
Table I.
Distribution of LATS1 status in
cervical carcinoma according to clinicopathological
characteristics.
| Characteristic | Total number of
patients | Number of patients
with low LATS1 expression | Number of patients
with positive LATS1 expression | P-value |
|---|
| Age |
|
|
| 0.5445 |
|
<50 | 55 | 29 | 26 |
|
| ≥50 | 25 | 15 | 10 |
|
| Histological
type |
|
|
| 0.4552 |
| Squamous
cell carcinoma | 71 | 38 | 33 |
|
|
Adenocarcinoma | 9 | 6 | 3 |
|
| Differentiation |
|
|
| 0.2905 |
|
Well/moderate | 58 | 34 | 24 |
|
| Poor | 22 | 10 | 12 |
|
| TNM stage |
|
|
| 0.0102 |
| I | 32 | 12 | 20 |
|
|
II+III | 48 | 32 | 16 |
|
| T stage |
|
|
| 0.0217 |
| T1 | 42 | 18 | 24 |
|
| T2+3 | 38 | 26 | 12 |
|
| Lymph node
metastasis |
|
|
| 0.2458 |
|
Negative | 50 | 25 | 25 |
|
|
Positive | 30 | 19 | 11 |
|
LATS1 suppresses proliferation and
invasion of cervical cancer cells
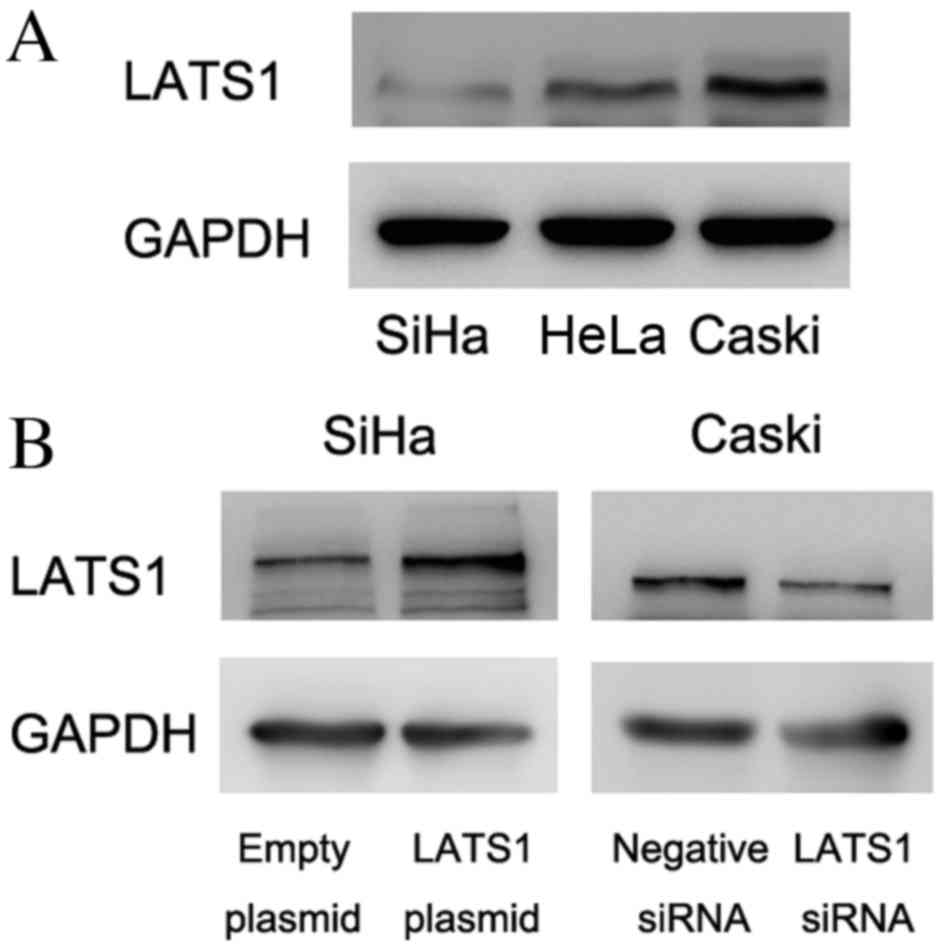
LATS1 was examined by western blot analysis in
cervical cancer cell lines (Fig.
2A). The present study demonstrated that SiHa cells had low
LATS1 protein expression and Caski cells had relatively high LATS1
protein expression. To determine its biological roles in cervical
cancer cell lines, plasmid transfection was performed in SiHa cells
and siRNA knockdown was performed in Caski cells. As presented in
Fig. 2B, LATS1 transfection
increased protein levels of LATS1 in SiHa cells and siRNA reduced
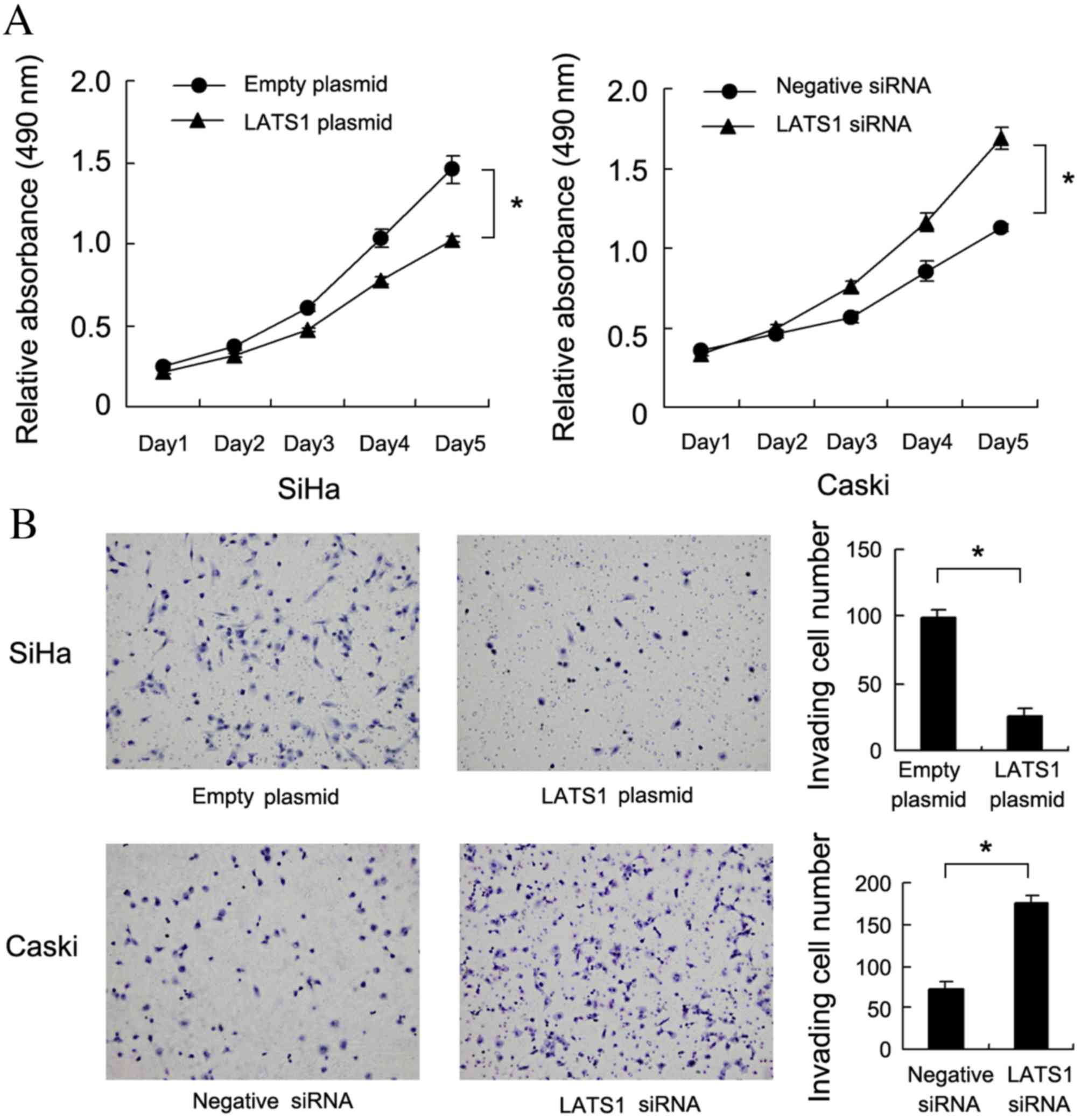
LATS1 protein levels in Caski cells. The MTT assay demonstrated
that LATS1 upregulation significantly decreased cell growth rate
compared with cells treated with empty plasmid. (SiHa:P=0.019;
Caski:P=0.006, Fig. 3A). A
Matrigel invasion assay was also performed to assess the effect of
LATS1 on cell invasion. As presented in Fig. 3B, LATS1 transfection significantly
decreased the invasiveness of SiHa cells compared with cells
transfected with empty plasmid (P=0.007), while LATS1 knockdown
significantly increased the invasiveness of Caski cells compared
with cells treated with negative siRNA (P=0.005).
LATS1 regulates the expression of
cyclin E, p27, MMP9 and YAP in cervical cancer cells
In order to investigate the molecular mechanism
underlying LATS1-induced cell growth and invasion, the present
study examined the expression of growth and invasion-associated
proteins. The results demonstrated that the cyclin E expression was
notably decreased following LATS1 overexpression in SiHa cells,
compared with cells treated with empty plasmid, and increased
following LATS1 knockdown in Caski cells, compared with cells
treated with negative siRNA. Protein expression of cell cycle
inhibitor p27 was increased compared to control following LATS1
transfection in SiHa cells and decreased compared to the control
following LATS1 knockdown in Caski cells. (Fig. 4A). The present study also examined
invasion-associated protein MMP9, and demonstrated that MMP9 was
downregulated in LATS1-transfected SiHa cells and upregulated in
LATS1-knockdown Caski cells, compared to respective controls
(Fig. 4A). LATS1 is the upstream
inhibitor of Hippo signaling. Activation of the Hippo signaling
pathway inhibits the expression and function of YAP oncoprotein and
suppresses cancer growth and invasion. The current study examined
YAP expression, and its downstream factor CTGF, in LATS1
overexpressing and knockdown cervical cancer cells. As presented in
Fig. 4B, in LATS1-transfected SiHa
cells, CTGF and YAP levels were decreased, compared to the control
SiHa cells. In LATS1 knockdown Caski cells, their expression (YAP
and CTGF) was upregulated compared with control Caski cells.
Changes in the levels of YAP phosphorylation were also examined.
Western blot analysis demonstrated that LATS1 increased the
phosphorylation of YAP.
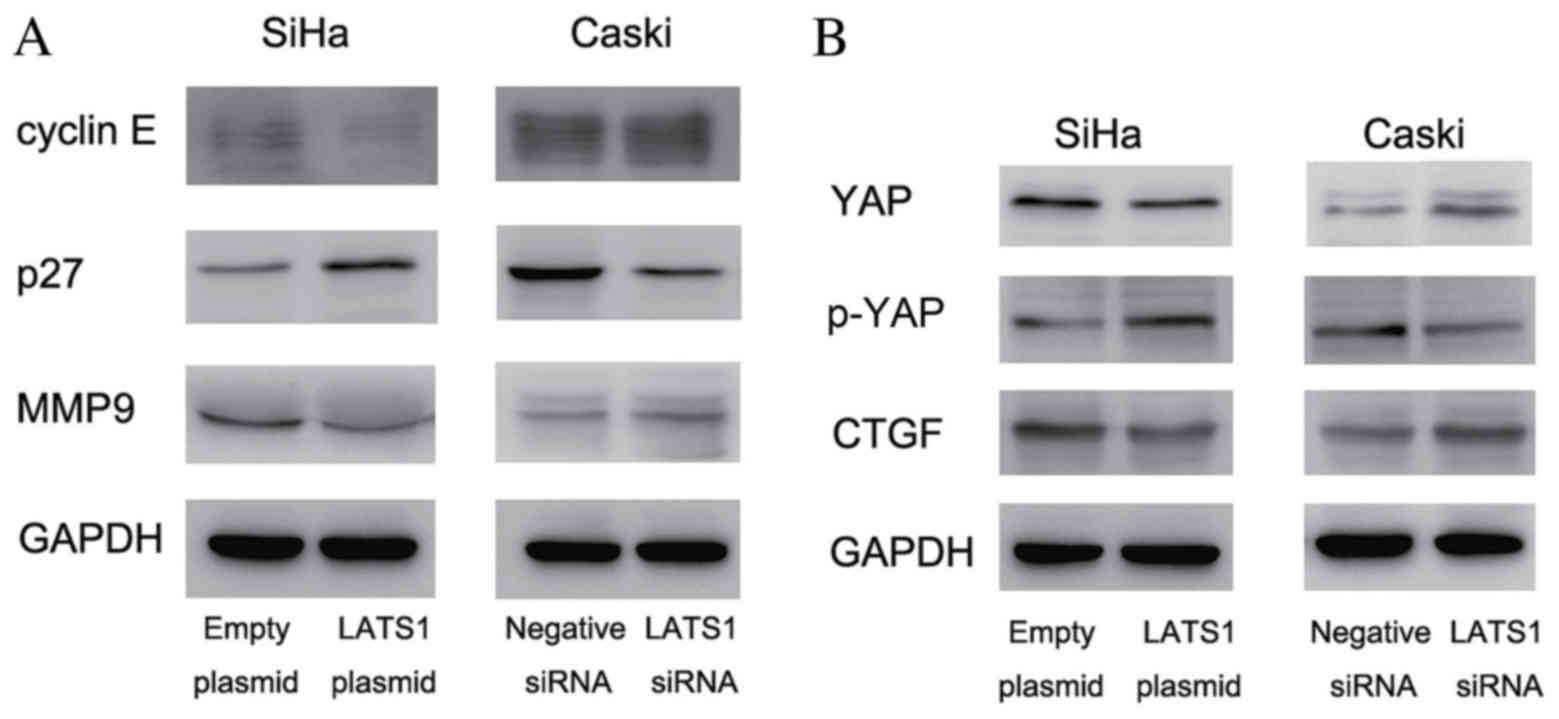 | Figure 4.LATS1 inhibits cervical cancer cell
proliferation and invasion via regulation of cyclin E, p27, MMP9,
YAP and CTGF. (A) Western blot analysis demonstrated that LATS1
transfection downregulated the levels of cyclin E and MMP9, and
upregulated p27 expression in SiHa cells compared with the control.
LATS1 knockdown in Caski cells upregulated cyclin E and MMP9, and
downregulated p27 expression. (B) LATS1 overexpression in SiHa
cells decreased YAP and CTGF protein levels, and increased YAP
phosphorylation compared with the control. LATS1 knockdown in Caski
cells resulted in the opposite effects, which upregulated YAP and
downregulated p-YAP. LATS1, large tumor suppressor kinase 1; MMP9,
matrix metalloproteinase 9; YAP, yes associated protein 1; p-YAP,
phosphorylated YAP; CTGF, connective tissue growth factor; siRNA,
small interfering RNA. |
Discussion
LATS1 is the mammalian homolog of Drosophila LATS,
originally identified as a cell proliferation inhibitor (15). It is a serine/threonine kinase that
localizes to the mitotic apparatus. LATS1 may form a complex with
cyclin-dependent kinase 1 in early mitosis, decreasing H1 histone
kinase activity, which indicates its role as a cell cycle inhibitor
(16). LATS1-knockout mice
developed sarcomas and ovarian tumors, indicating that it may also
function as a tumor suppressor (3). The expression and biological roles of
LATS1 have been implicated in a number of human malignancies,
including gastric cancer, breast cancer, glioma, renal cell
carcinoma and ovarian cancer (9,11,17–19).
However, to the best of our knowledge, the involvement of LATS1 in
human cervical carcinoma has not been reported. The present study
examined LATS1 protein expression in 80 cases of cervical cancer
and demonstrated that LATS1 was downregulated in 45% of tissue
specimens. Statistical analysis demonstrated that LATS1
downregulation was positively associated with advanced TNM stage
and tumor status, indicating that loss of LATS1 is associated with
cervical cancer progression. The results of the current study are
consistent with previous studies (20–22),
indicating that LATS1 may be a potential tumor suppressor in human
cervical cancer.
To identify its biological roles in cervical cancer
cells, the present study transfected SiHa cells with a LATS1
plasmid and Caski cells were transfected with LATS1 siRNA.
Subsequently, the effects on cell proliferation and invasion were
examined. Consistent with the immunohistochemical results of the
present study, LATS1 transfection significantly inhibited cell
growth and invasiveness of SiHa cells, while LATS1 siRNA had the
opposite effect in Caski cells. The current study further
investigated the potential underlying mechanisms by which LATS1
inhibited cell proliferation and invasion in cervical cancer.
Previous studies demonstrated that LATS1 functions as a cell cycle
regulator (20,21). Thus, the protein levels of cyclin E
and p27 in LATS1-overexpressing SiHa cells and LATS1-knockdown
Caski cells were investigated in the present study. The results
demonstrated that LATS1 downregulated cyclin E and upregulated p27.
Cyclin E facilitates and p27 inhibits cell cycle progression
(23,24). Cycle E overexpression is important
during cervical cancer proliferation (25), and p27 downregulation has been
previously identified in cervical cancer tissues where it may cause
cell cycle arrest in cervical cancer cells (26,27).
In addition, the present study demonstrated that
invasion-associated protein MMP9 was negatively regulated by LATS1
expression. MMP9 is considered to be associated with cervical
cancer invasion (28–30). Thus LATS1 may regulate cervical
cancer growth and invasion through modulation of cell cycle
proteins and MMP9. As LATS1 is the upstream positive regulator of
Hippo pathway, the current study investigated changes in the
expression levels of downstream proteins, including YAP and CTGF.
The results demonstrated that LATS1 overexpression inhibited CTGF
and YAP expression, and increased YAP phosphorylation in SiHa cells
compared with control SiHa cells. YAP is a positive regulator of
growth and invasion in various types of human cancers, including
cervical cancer (31). CTGF is the
target of YAP and functions as a growth promoter. CTGF has been
reported to positively regulate the cell cycle and MMP proteins
(32,33). Thus, the role of LATS1 in cervical
cancer invasion and proliferation may depend on its regulation on
YAP, and its downstream target CTGF.
In conclusion, LATS1 is downregulated in human
cervical cancers and LATS1 expression is associated with TNM stage.
LATS1 inhibited cervical cancer cell proliferation and invasion,
potentially through regulation of p27 and MMP9. LATS1 may activate
the Hippo pathway through downregulation of YAP and CTGF. Loss of
LATS1 may serve as an indicator of malignant phenotype in human
cervical cancer.
Acknowledgements
The present study was supported by the Yunnan
Provincial Science and Technology Project Fund (grant no.
2014FB200).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song C, Zhu S, Wu C and Kang J: Histone
deacetylase (HDAC) 10 suppresses cervical cancer metastasis through
inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J
Biol Chem. 288:28021–28033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
St John MA, Tao W, Fei X, Fukumoto R,
Carcangiu ML, Brownstein DG, Parlow AF, McGrath J and Xu T: Mice
deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours
and pituitary dysfunction. Nat Genet. 21:182–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huntoon CJ, Nye MD, Geng L, Peterson KL,
Flatten KS, Haluska P, Kaufmann SH and Karnitz LM: Heat shock
protein 90 inhibition depletes LATS1 and LATS2, two regulators of
the mammalian hippo tumor suppressor pathway. Cancer Res.
70:8642–8650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furth N, Bossel B, en-Moshe N, Pozniak Y,
Porat Z, Geiger T, Domany E, Aylon Y and Oren M: Down-regulation of
LATS kinases alters p53 to promote cell migration. Genes Dev.
29:2325–2330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Visser S and Yang X: LATS tumor
suppressor: A new governor of cellular homeostasis. Cell Cycle.
9:3892–3903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morinaga N, Shitara Y, Yanagita Y, Koida
T, Kimura M, Asao T, Kimijima I, Takenoshita S, Hirota T, Saya H
and Kuwano H: Molecular analysis of the h-warts/LATS1 gene in human
breast cancer. Int J Oncol. 17:1125–1129. 2000.PubMed/NCBI
|
|
8
|
Wierzbicki PM, Adrych K, Kartanowicz D,
Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I,
Celinski K, Gach T, Kulig J, Korybalski B and Kmiec Z:
Underexpression of LATS1 TSG in colorectal cancer is associated
with promoter hypermethylation. World J Gastroenterol.
19:4363–4373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu ZP, Zhu JS, Zhang Q and Wang XY: A
breakdown of the Hippo pathway in gastric cancer.
Hepatogastroenterology. 58:1611–1617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin XY, Zhang XP, Wu JH, Qiu XS and Wang
EH: Expression of LATS1 contributes to good prognosis and can
negatively regulate YAP oncoprotein in non-small-cell lung cancer.
Tumour Biol. 35:6435–6443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu B, Sun D, Wang Z, Weng H, Wu D, Zhang
X, Zhou Y and Hu W: Expression of LATS family proteins in ovarian
tumors and its significance. Hum Pathol. 46:858–867. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puppo P, Conti G, Francesca F, Mandressi A
and Naselli A: AURO. it guideline committee: New Italian guidelines
on bladder cancer, based on the World Health Organization 2004
classification. BJU Int. 106:168–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bocchi EA, Tanigawa RY, Brandão SM, Cruz
F, Issa V, Ayub-Ferreira S, Chizzola P, Souza G, Fiorelli AI, Bacal
F, et al: Immunohistochemical quantification of inflammatory cells
in endomyocardial biopsy fragments after heart transplantation: A
new potential method to improve the diagnosis of rejection after
heart transplantation. Transplant Proc. 46:1489–1496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roussel AJ, Knol AC, Bourdeau PJ and Bruet
V: Optimization of an immunohistochemical method to assess
distribution of tight junction proteins in canine epidermis and
adnexae. J Comp Pathol. 150:35–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Justice RW, Zilian O, Woods DF, Noll M and
Bryant PJ: The Drosophila tumor suppressor gene warts encodes a
homolog of human myotonic dystrophy kinase and is required for the
control of cell shape and proliferation. Genes Dev. 9:534–546.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao W, Zhang S, Turenchalk GS, Stewart RA,
St John MA, Chen W and Xu T: Human homologue of the Drosophila
melanogaster lats tumour suppressor modulates CDC2 activity. Nat
Genet. 21:177–181. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji T, Liu D, Shao W, Yang W, Wu H and Bian
X: Decreased expression of LATS1 is correlated with the progression
and prognosis of glioma. J Exp Clin Cancer Res. 31:672012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi Y, Miyoshi Y, Takahata C,
Irahara N, Taguchi T, Tamaki Y and Noguchi S: Down-regulation of
LATS1 and LATS2 mRNA expression by promoter hypermethylation and
its association with biologically aggressive phenotype in human
breast cancers. Clin Cancer Res. 11:1380–1385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen KH, He J, Wang DL, Cao JJ, Li MC,
Zhao XM, Sheng X, Li WB and Liu WJ: Methylation-associated
inactivation of LATS1 and its effect on demethylation or
overexpression on YAP and cell biological function in human renal
cell carcinoma. Int J Oncol. 45:2511–2521. 2014.PubMed/NCBI
|
|
20
|
Visser-Grieve S, Zhou Z, She YM, Huang H,
Cyr TD, Xu T and Yang X: LATS1 tumor suppressor is a novel
actin-binding protein and negative regulator of actin
polymerization. Cell Res. 21:1513–1516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao Y, Chun A, Cheung K, Rashidi B and
Yang X: Tumor suppressor LATS1 is a negative regulator of oncogene
YAP. J Biol Chem. 283:5496–5509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia H, Qi H, Li Y, Pei J, Barton J,
Blackstad M, Xu T and Tao W: LATS1 tumor suppressor regulates G2/M
transition and apoptosis. Oncogene. 21:1233–1241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lopez-Beltran A, MacLennan GT and
Montironi R: Cyclin E as molecular marker in the management of
breast cancer: A review. Anal Quant Cytol Histol. 28:111–114.
2006.PubMed/NCBI
|
|
24
|
Guan X, Wang Y, Xie R, Chen L, Bai J, Lu J
and Kuo MT: p27 (Kip1) as a prognostic factor in breast cancer: A
systematic review and meta-analysis. J Cell Mol Med. 14:944–953.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zubillaga-Guerrero MI, Alarcón-Romero Ldel
C, Illades-Aguiar B, Flores-Alfaro E, Bermúdez-Morales VH, Deas J
and Peralta-Zaragoza O: MicroRNA miR-16-1 regulates CCNE1 (cyclin
E1) gene expression in human cervical cancer cells. Int J Clin Exp
Med. 8:15999–16006. 2015.PubMed/NCBI
|
|
26
|
Prasad SB, Yadav SS, Das M, Modi A, Kumari
S, Pandey LK, Singh S, Pradhan S and Narayan G: PI3K/AKT
pathway-mediated regulation of p27(Kip1) is associated with cell
cycle arrest and apoptosis in cervical cancer. Cell Oncol (Dordr).
38:215–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van De Putte G, Holm R, Lie AK, Tropé CG
and Kristensen GB: Expression of p27, p21, and p16 protein in early
squamous cervical cancer and its relation to prognosis. Gynecol
Oncol. 89:140–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu D, Ye M and Zhang W: E6/E7oncoproteins
of high risk HPV-16 upregulate MT1-MMP, MMP-2 and MMP-9 and promote
the migration of cervical cancer cells. Int J Clin Exp Pathol.
8:4981–4999. 2015.PubMed/NCBI
|
|
29
|
Pahne-Zeppenfeld J, Schröer N,
Walch-Rückheim B, Oldak M, Gorter A, Hegde S and Smola S: Cervical
cancer cell-derived interleukin-6 impairs CCR7-dependent migration
of MMP-9-expressing dendritic cells. Int J Cancer. 134:2061–2073.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in
human cervical and ovarian cancer cell lines by cytokines, inducers
and inhibitors. Oncol Rep. 23:605–614. 2010.PubMed/NCBI
|
|
31
|
He C, Mao D, Hua G, Lv X, Chen X,
Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, et al: The
Hippo/YAP pathway interacts with EGFR signaling and HPV
oncoproteins to regulate cervical cancer progression. EMBO Mol Med.
7:1426–1449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhen Y, Ye Y, Yu X, Mai C, Zhou Y, Chen Y,
Yang H, Lyu X, Song Y, Wu Q, et al: Reduced CTGF expression
promotes cell growth, migration, and invasion in nasopharyngeal
carcinoma. PLoS One. 8:e649762013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ
and Tang CH: CTGF increases matrix metalloproteinases expression
and subsequently promotes tumor metastasis in human osteosarcoma
through down-regulating miR-519d. Oncotarget. 5:3800–3812. 2014.
View Article : Google Scholar : PubMed/NCBI
|