Introduction
Gliomas are the most common primary brain tumors
that arise from the neuroectoderm (1). Despite significant advances in
complete surgery, radiotherapy and chemotherapy, the survival time
of patients remains ~12 months following diagnosis (2). Therefore, the identification of a
potential biological target that increases the likelihood of
patients with glioma achieving remission remains a priority.
Furthermore, the majority of prognostic factors provide no insight
into the molecular events that are responsible for tumor
proliferation, apoptosis or additional biological properties of
malignancy (3,4). Currently, emerging novel targeted
therapies, such as genetic treatment and immunological therapy, may
provide alternative strategies for the treatment of glioma
(5).
The cluster of differentiation 164 (CD164)
glycoprotein is a member of the sialomucin family, which is a mucin
that contains sialic acid (6).
CD164 was first identified as a carrier of a peanut
agglutinin-binding site, which is a tumor-associated carbohydrate
marker expressed in human gastric carcinoma cells and bone marrow
stromal reticular cells (7–9).
Previous studies have demonstrated that CD164 modulates the
proliferation, adhesion and migration of hematopoietic stem and
progenitor cells (10,11). It has been reported that CD164
regulates hematopoiesis by facilitating the adhesion of human
CD34+ cells to bone marrow stroma (12). In addition, CD164 has been
demonstrated to regulate the growth and differentiation of normal
cells, and is involved in malignant cell proliferation as well as
invasion (13). Furthermore, CD164
has been implicated in the maintenance and progression of multiple
human solid cancers, including medulloblastoma, ovarian (14) and colon (15) cancers. A previous study
demonstrated that CD164 may participate in the mediation of
prostate cancer bone metastasis (16). In addition, CD164 has been
recognized as a biomarker for the detection of acute lymphoblastic
leukemia and allergy (17,18). These studies indicated that CD164
may function as a key molecule in the modulation of tumor
progression. However, the role of CD164 in human glioma has yet to
be elucidated. The present study investigated the expression
profile of CD164 in glioma cells, and examined the correlation
between CD164 and tumorigenesis of glioma cells in vitro and
in vivo, including the proliferation and apoptosis
levels.
Materials and methods
Patients and tissue specimens
The ethics committee of The First Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) approved
the protocols employed in the present study (October, 2012).
Samples consisted of 50 paired glioma and adjacent normal brain
tissue samples (24 males and 26 females) admitted to the Department
of Neurosurgery of The First Affiliated Hospital of Wenzhou Medical
University (Wenzhou, China) between December 2013 and December
2015. The age of the patients ranged from 46 to 73 with a mean of
63±5 years. All cases were histologically confirmed by trained
pathologists. No patients had received chemotherapy or radiotherapy
prior to surgery, and informed consent was obtained from all
patients.
Cell culture
HEK-293T cells, three human glioma cell lines (U251,
SHG-44 and U87) and normal human astrocytes (NHA) were purchased
from the American Type Culture Collection (Manassas, VA, USA). All
cell lines were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and maintained at 37°C and 5% CO2.
Lentiviral infection
Short hairpin RNA (shRNA) specifically targeting
human CD164 and negative scrambled control shRNA were purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). There were
19 nucleotides of the target sequence in the shRNA expression
cassette prior to the loop sequence (TTCAAGAGA). The shRNA
sequences were as follows: CD164, sense,
5′-TGAGAAAGCTCTCCACTCTGTTCAAGAGACAGAGTGGAGAGCTTTCTCTTTTTTC-3′, and
antisense,
5′-ACTCTTTCGAGAGGTGAGACAAGTTCTCTGTCTCACCTCTCGAAAGAGAAAAAAGAGCT-3′;
negative control, sense,
5′-TAACTAGTAACGGCTGCTCCTTCAAGAGAGGAGCAGCCGTTACTAGTTTTTTTTC-3′, and
antisense,
5′-ATTGATCATTGCCGACGAGGAAGTTCTCTCCTCGTCGGCAATGATCAAAAAAAAGAGCT-3′.
Lentiviruses were produced by cotransfection of the lentiviral
packaging plasmids pMD.G and pMDLgpRRE (Ambion; Thermo Fisher
Scientific, Inc.) into HEK-293T cells using calcium phosphate. A
total of 5×105 U87 cells were transfected with 20 µg recombinant
lentivirus-transducing units plus 6 mg/ml polybrene (BD
Biosciences, San Jose, CA, USA). A total of 5×105 U87 cells
overexpressing CD164 were established by transfection with the
lentivirus-expressing pRSVRev-vector with the human CD164 coding
sequence (Ambion; Thermo Fisher Scientific, Inc.) at a
concentration of 5×109 Tu/ml. The blank control cells were treated
with PBS.
Cell proliferation
Cells were diluted to a density of 2×104 cells/ml,
and 100 µl cell solution was transferred to each well of 96-well
culture plates and incubated for 24, 48 or 72 h. Cell proliferation
was then assessed using the Cell Counting kit-8 assay (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer's protocol. Following incubation with 10 µl
CCK-8 solution at 37°C for 60 min in a CO2 incubator,
the absorbance at 490 nm was measured using a microplate
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
This experiment was repeated twice.
Annexin V/propidium iodide (PI)
staining assay
To determine the extent of early apoptosis and late
apoptosis/necrosis in cells, an Annexin V-FITC/PI apoptosis
detection kit (BD Biosciences) was used according to the
manufacturer's protocol. A total of ≥10,000 cells were analyzed for
each sample. The proportion of U87 cells in early apoptosis and
late apoptosis/necrosis were calculated by recording the percentage
of Annexin V+/PI− and Annexin V+/PI+-labeled cells, respectively.
The stained cells were analyzed directly by flow cytometry using
the FACS Calibur machine (BD Biosciences) using the Cell Quest
program (BD Biosciences) for data analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the tissue samples and
5×106 U87 cells using TRIzol reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA concentrations
were determined by spectrophotometry (DU-800; Beckman Coulter). A
260/280 absorbance ratio of 1.96 implied clean RNA at a
concentration of 0.23 mg/ml. Total 1 µg RNA was reverse transcribed
to cDNA using the PrimeScript RT Reagent kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. qPCR was performed using the ABI 7300 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
the following amplification conditions: 30 sec at 98°C, 30 cycles
of 10 sec at 98°C, 15 sec at 60°C, 15 sec at 72°C and 2 min at
72°C. The primer sequences were as follows: CD164, forward,
5′-TGAGCCCTGAACACCAGAGAG-3′, and reverse,
5′-AAAGCCAGATGAGCGCTTCTA-3′; phosphatase and tensin homolog (PTEN),
forward, 5′-TCGTGGGTGCCTCGCT-3′, and reverse,
5′-CACCACTACAGCCAGCATTTTC-3′; GAPDH, forward,
5′-AACGGATTTGGTCGTATTGGG−3′, and reverse,
5′-TCGCTCCTGGAAGATGGTGAT-3′. The expression target genes in all
samples was normalized to GAPDH. Following data collection, target
gene expression was quantified by relative quantitative analysis
using the 2-ΔΔCq method as described previously
(19).
Western blot analysis
A total of 5×106 U87 or NHA cells were washed and
lysed with lysis buffer (20 mmol/l Tris-HCl (pH 7.4), 100 mmol/l
NaCl, 1% NP40, 0.5% sodium deoxycholate, 5 mmol/l MgCl2,
0.1 mmol/l phenylmethylsulfonyl fluoride, and 10 mg/ml protease
inhibitor mixture) from Nanjing KeyGen Biotech. Co., Ltd. (Nanjing,
China). The suspension was centrifuged at 5,000 × g at 4°C for 10
min, followed by centrifugation at 16,000 × g at 4°C for 30 min,
and then the supernatant was collected and kept at −70°C until use.
Whole cell proteins were extracted using Mammalian Protein
Extraction Reagent (Pierce; Thermo Fisher Scientific, Inc.), while
protein concentrations were measured using a bicinchoninic acid
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts
of total protein (20–40 µg) were electrophoresed in an 8% SDS-PAGE
gel with Tris-glycine, before they were transferred to a
nitrocellulose membrane. The membranes were then blocked with
Tris-buffered saline containing 5% non-fat milk powder at room
temperature for 1 h, and were incubated with specific antibodies
for CD164 (catalog no. C9618; 1:500), Bax (catalog no. B8429;
1:1,000), Bcl2 (catalog no SAB4300339; 1:1,000), caspase3 (catalog
no. SAB4503292; 1:1,000), PTEN (catalog no. SAB4300337; 1:1,000),
p53 (catalog no. P9249; 1:1,000), total AKT (catalog no.
SAB4500799; 1:1,000) and phospho-AKT (catalog no. SAB4503853;
1:1,000), all from Sigma-Aldrich, Merck KGaA, Darmstadt, Germany.
The membranes were subsequently probed with anti-rabbit lgG
(catalog no. A0545; 1:5,000) or anti-mouse lgG (catalog no.
SAB3701044; 1:5,000) secondary antibody conjugated with horseradish
peroxidase (Sigma-Aldrich, Merck KGaA) at room temperature for 1 h.
Band signals were detected using an enhanced chemiluminescence kit
(Pierce; Thermo Fisher Scientific, Inc.) and immunoreactive bands
were quantified using Alphaimager 2200 (Alpha Innotech, San
Leandro, CA, USA). β-actin was used as the internal control.
In vivo tumorigenesis
In vivo experiments were conducted as described
previously (20). A total of 50
male athymic BALB/c nu/nu mice (age, 4–6 weeks) were obtained from
the Shanghai Experimental Center, Chinese Science Academy
(Shanghai, China) and maintained under pathogen free conditions in
a temperature and humidity controlled animal care facility with a
12 h light dark cycle. Mice were allowed access to sterile food and
water ad libitum. NHA infected with vector control or CD164
lentivirus were injected subcutaneously into the flank of nude mice
at a dose of 1×107 cells/mouse. A 100 µl aliquot of the U87 cell
suspension (equivalent to 1×107 U87 cells) was injected into the
flank of nude mice in the corresponding group. Following 56 days of
tumor growth, the experiment was terminated and mice with
subcutaneous tumors were sacrificed by cervical dislocation.
Immunolocalization of the Ki-67 marker
of proliferation in tumor samples
Paraffin-embedded subcutaneous xenograft tissue
sections were fixed with 4% paraformaldehyde at room temperature
for 24 h. Following washing in phosphate-buffered saline, the
endogenous peroxidase activity of slides was blocked with protein
blocking solution (Dako, Glostrup, Denmark) at room temperature for
30 min. For Ki-67 immunohistochemistry, the samples were first
incubated with a primary antibody against Ki-67 (catalog no.
SAB5500134; 1:100) overnight at 4°C (Sigma-Aldrich, Merck KGaA),
followed by incubation with an appropriate anti-rabbit lgG (catalog
no. A0545; 1:5,000; Sigma-Aldrich, Merck KGaA) at room temperature
for 2 h. The immunogenicity of slides was detected using the
Vecstain™ ABC kit (Vector Laboratories, Burlingame, California,
USA) according to the manufacturer's protocol. The stained slides
were analyzed under a light microscope (Olympus Corporation, Tokyo,
Japan), and were analyzed using the Image-Pro Plus software system
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). A total
of 20 fields of view were assessed by an investigator who was
blinded to the experimental data.
Terminal deoxyribonucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
The number of apoptotic cells in the subcutaneous
xenograft tumors was studied using an in situ cell death
detection kit purchased from Roche Diagnostics (Basel,
Switzerland), which was performed according to the manufacturer's
protocol. Counterstaining was performed with hematoxylin (Nanjing
Keygen Biotech Co., Ltd.) at room temperature for 1 min. The tissue
sections were mounted under a glass coverslip and viewed under a
light microscope by two different pathologists unaware of the
xenograft tumor groups. The apoptotic cells were counted in 20
randomly selected fields of the most affected tumor areas under
×400 magnification.
Statistical analysis
All data were expressed as the mean ± standard
deviation for the absolute values or percentages of controls. Data
were evaluated by one-way analysis of variance followed by
Student-Newman-Keuls-q multiple comparisons tests using SPSS
software (version 17.0; SPSS, Inc. Chicago, IL, USA,). P<0.05
was considered to indicate a statistically significant
difference.
Results
Analysis of CD164 in glioma cell lines
and clinical specimens
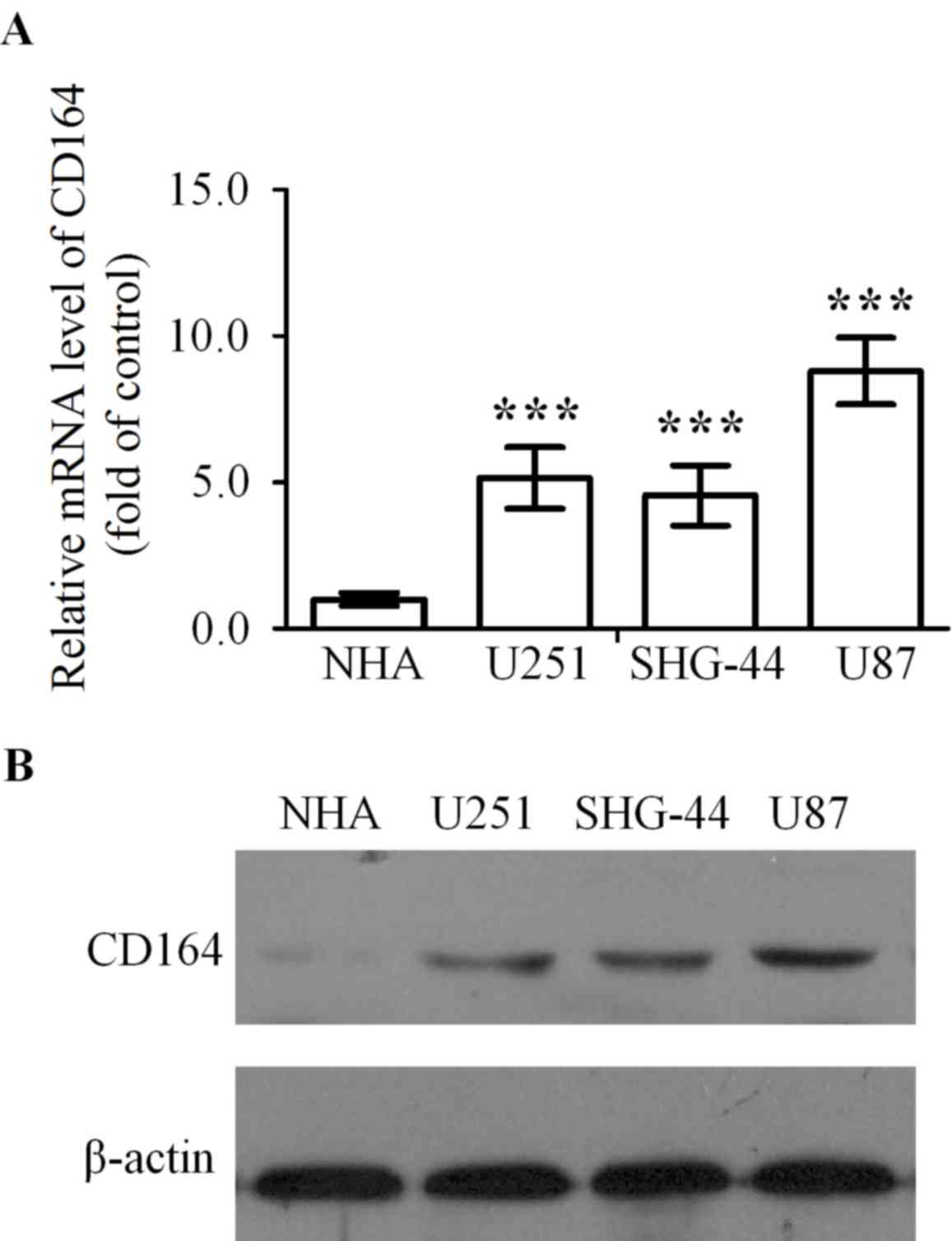
CD164 mRNA expression levels in U251, SHG-44 and U87
glioma cell lines was significantly higher when compared with NHA
cells (P<0.001, P<0.001 and P<0.001, respectively;
Fig. 1A). In addition, CD164
protein expression levels were determined in U251, SHG-44 and U87
glioma cell lines, and the NHA cell line by western blot analysis.
CD164 expression was almost undetectable in NHA cells and was
visibly elevated in U251, SHG-44 and U87 cells by comparison
(Fig. 1B). As the level of CD164
expression in U87 cells was significantly upregulated in contrast
to U251 and SHG-44 cells, U87 cells were selected for subsequent
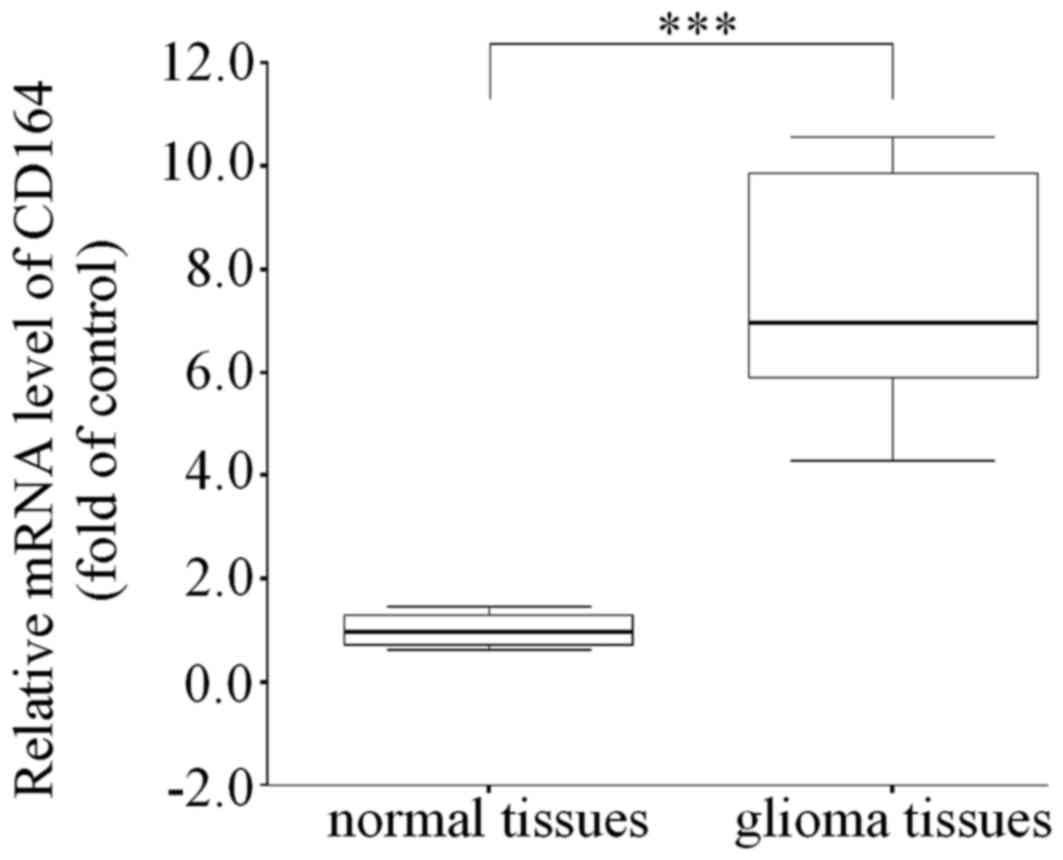
experiments. To further validate the expression of CD164 in glioma
in vivo, the expression of CD164 in 50 gliomas and normal
adjacent tissue samples was analyzed by RT-qPCR analysis. Compared
with normal brain tissues, the glioma tissues demonstrated a
significant increase in CD164 mRNA expression levels (P<0.001;
Fig. 2), indicating that CD164 may
be associated with the development and progression of glioma.
Overexpression of CD164 promotes cell
growth of NHA cells in vitro and in vivo
In order to determine the transforming effect of
CD164 in normal cells, CD164 was overexpressed in NHA cells
(Fig. 3A). Ectopic expression of
CD164 significantly promoted cell growth in NHA cells when compared
with the vector control cells, as determined using the CCK-8 assay
(Fig. 3B). A previous study
demonstrated that the Bcl-2-associated X, apoptosis regulator
(Bax)/B cell lymphoma 2 (Bcl2) ratio represents the critical
balance of regulatory pro-apoptotic and anti-apoptotic proteins in
the process of apoptosis (21). As
expected, visible increases in Bcl2 protein levels and decreases in
Bax and PTEN protein levels were detected in NHA cells
overexpressing CD164 (Fig. 3A).
These results suggest that CD164 may modulate NHA progression by
increasing cell proliferation, which is considered to be associated
with a significant decrease in the Bax/Bcl2 ratio (22). Cell viability was significantly
increased in NHA cells overexpressing CD164 when compared with the
vector control at 24, 48 and 72 h following transfection
(P<0.01, P<0.001 and P<0.001, respectively; Fig. 3B). Based on these results, it was
hypothesized that CD164 may be involved in promoting tumor growth
in vivo. Therefore, a subcutaneous tumor xenograft model in
nude mice was generated to assess the tumor formation ability of
NHA-CD164 cells, which was compared with U87 cells as a positive
control. Subcutaneous injection of NHA-CD164 cells and U87 cells
lead to tumor formation in all nude mice. NHA-CD164 and U87 tumors
were evident at 10 days following implantation, whereas NHA-vector
control tumors were too small to be measured at 56 days following
implantation. The average weight of the tumors formed by the
subcutaneous injection of NHA-CD164 cells was significantly higher
than those formed by U87 cells (1294.5±385.2 mg vs. 637.2±113.8 mg;
P<0.001; Fig. 3C).
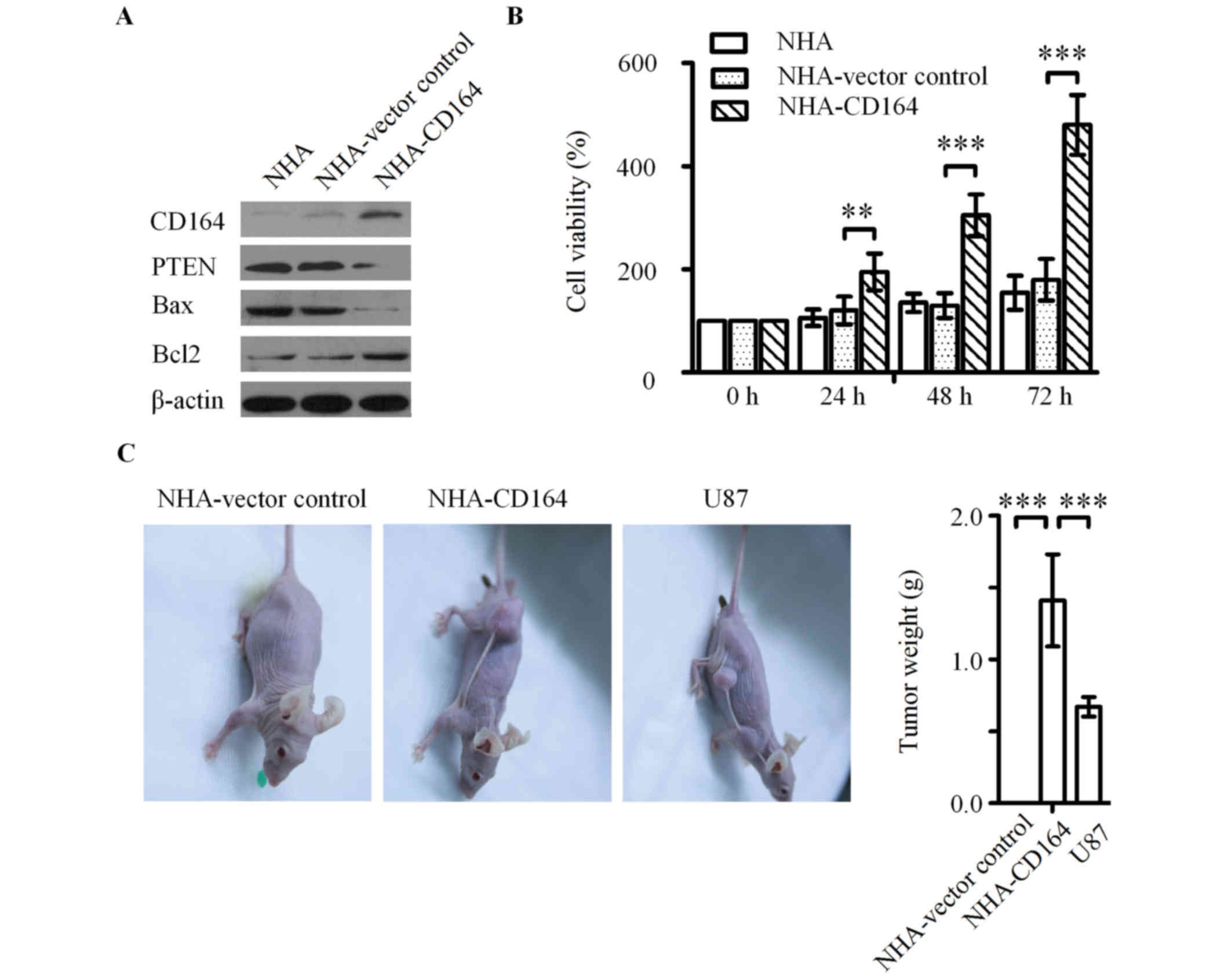 | Figure 3.Effect of CD164 on the cell growth of
NHA cells in vitro and in vivo. (A) Western blot
analysis of CD164, PTEN, apoptotic Bax and anti-apoptotic Bcl2
proteins in NHA-CD164 cell lysates. β-actin was used as a loading
control. **P<0.01 and ***P<0.001 vs. NHA-vector control. (B)
The effect of CD164 overexpression on NHA proliferation, as
determined using the Cell Counting kit-8 assay. (C) Macroscopic
appearance and quantitative analysis of xenograft tumors following
subcutaneous injection of NHA-vector control, NHA-CD164 and U87
cells in the flank of nude mice. ***P<0.001 as indicated. CD164,
cluster of differentiation 164; NHA, normal human astrocytes; PTEN,
phosphatase and tensin homolog; Bax, Bcl-2-associated X, apoptosis
regulator; Bcl2, B cell lymphoma 2. |
Downregulation of CD164 inhibits the
proliferation of glioma cells
In order to investigate the function of CD164 in
tumorigenesis in vivo, its expression was silenced in U87
cells by tumor-specific lentivirus-mediated shRNA targeting of the
CD164 gene. The U87 cells with silenced CD164 expression were then
used to determine whether this was associated with inhibition of
cell proliferation in vitro. The mRNA and protein levels of
CD164 in U87 cells were analyzed by RT-qPCR and western blot
analyses, respectively. Transfection of CD164 shRNA resulted in a
significant downregulation in CD164 mRNA expression levels when
compared with those transfected with negative control shRNA
(P<0.001; Fig. 4A). In
addition, protein expression levels were markedly decreased in
shCD164-tranfected cells when compared with negative control shRNA
or blank control cells (Fig. 4B).
CD164 mRNA and protein expression levels were not significantly
altered between cells transfected with negative control shRNA and
blank controls (Fig. 4A and
B).
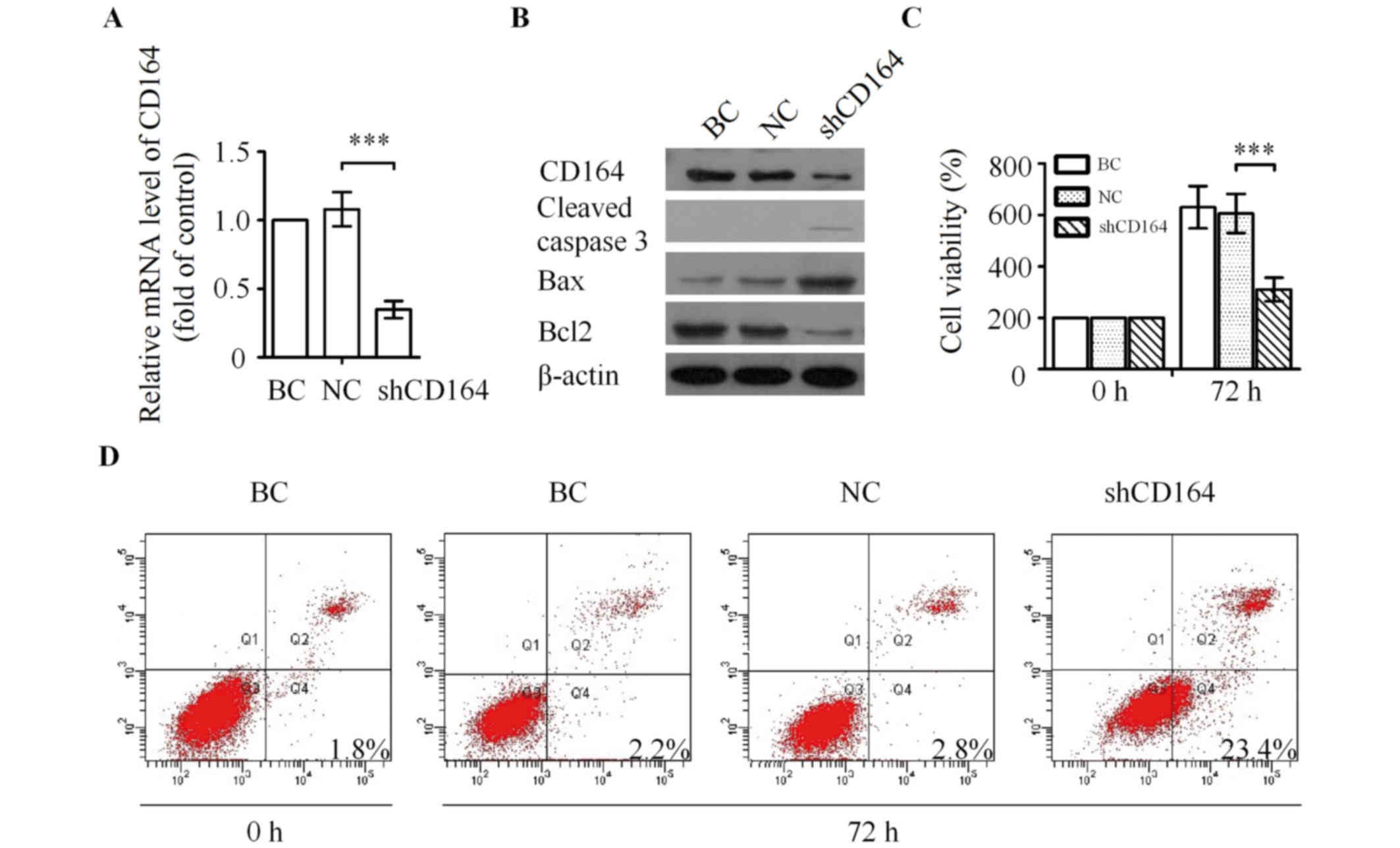 | Figure 4.Silencing of CD164 inhibits the
proliferation of U87 cells in vitro. (A) Knockdown of CD164
mRNA as determined by reverse transcription-quantitative polymerase
chain reaction analysis. ***P<0.001 vs. NC. (B) Western blot
analysis of CD164, cleaved caspase 3, Bax and Bcl2 following
transfection with CD164 shRNA. (C) Effect of CD164 shRNA on the
proliferation of U87 cells. **P<0.01 vs. NC. (D) U87 cells were
processed for Annexin V/PI staining and analyzed by flow cytometry
following transfection with CD164 shRNA for 72 h. CD164, cluster of
differentiation 164; Bax, Bcl-2-associated X, apoptosis regulator;
Bcl2, B cell lymphoma 2; shRNA, short hairpin RNA; PI, propidium
iodide; BC, blank control; NC, negative control. |
The effect of downregulated CD164
expression on the proliferation of U87 cells was then investigated
further
U87 cell viability was decreased by 45.8±7.5% in
response to CD164 knockdown when compared with cells transfected
with negative control shRNA or blank control cells (P<0.01;
Fig. 4C). In addition, the
involvement of CD164 in the apoptosis of U87 cells was assessed
using an Annexin V/PI assay. Silenced CD164 expression was
associated with a marked increase in the percentage of apoptotic
U87 cells compared with BC or NC group, demonstrating a
statistically significant difference (Fig. 4D). It has been previously reported
that caspase 3 is involved in the terminal phase of apoptosis
(23). Therefore, the activation
of apoptosis was investigated in the present study by measuring
caspase 3 cleavage in U87-shCD164 cells. Knockdown of CD164
expression was associated with a visible increase in the protein
expression levels of cleaved caspase 3 (Fig. 4B). In addition, the results
demonstrated that downregulation of CD164 visibly reduced the level
of Bcl2 and promoted the expression of Bax (Fig. 4B).
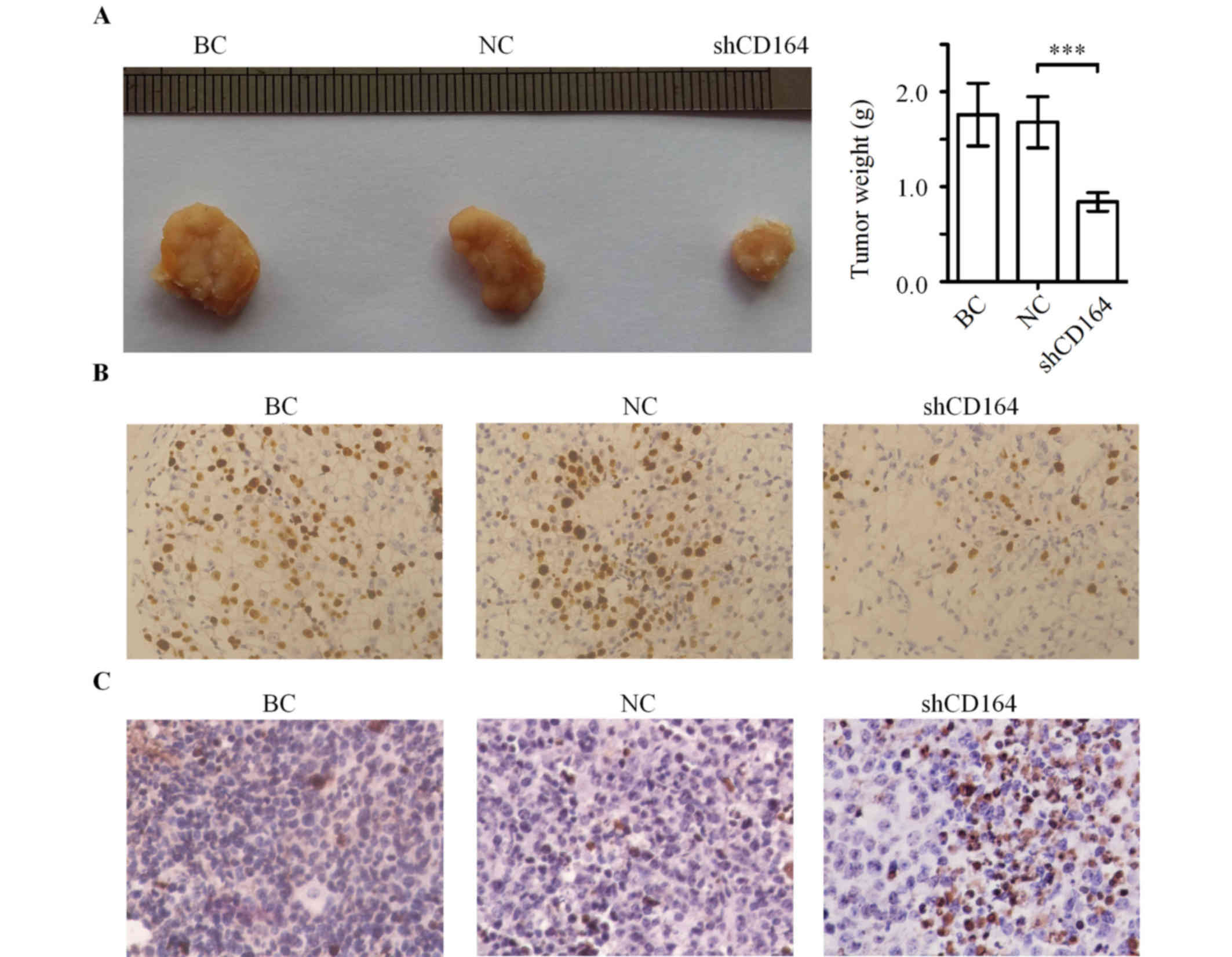
CD164 shRNA inhibits tumor growth in a
nude mouse xenograft tumor model
In order to elucidate the potential tumorigenic
function of CD164 in glioma cells in vivo, a xenograft tumor
model was established to compare the tumorigenesis of U87 cells
with or without transfection of CD164 shRNA. As shown in Fig. 5A, the final tumor weights were
significantly lower in the U87-shCD164 group when compared with the
negative control shRNA group (>50% reduction, n=8; P<0.001).
Furthermore, the average weight of tumors in the negative control
shRNA group was not statistically different from the blank control
group (Fig. 5A).
Tumor immunohistochemistry in
vivo
In order to examine the expression of Ki-67 in the
tumor tissues, immunohistochemical staining was performed. Tumors
from the U87-shCD164 group demonstrated visibly lower positive
immunohistochemical staining for Ki-67 when compared with the
negative control shRNA and blank control groups (Fig. 5B). A TUNEL staining assay for
apoptosis was subsequently performed in these tumor tissues. The
rate of apoptosis was visibly increased in the U87-shCD164 group
when compared with the negative control shRNA and blank control
group (Fig. 5C), which suggests
that downregulation of CD164 inhibited glioma growth and promoted
glioma apoptosis in vivo.
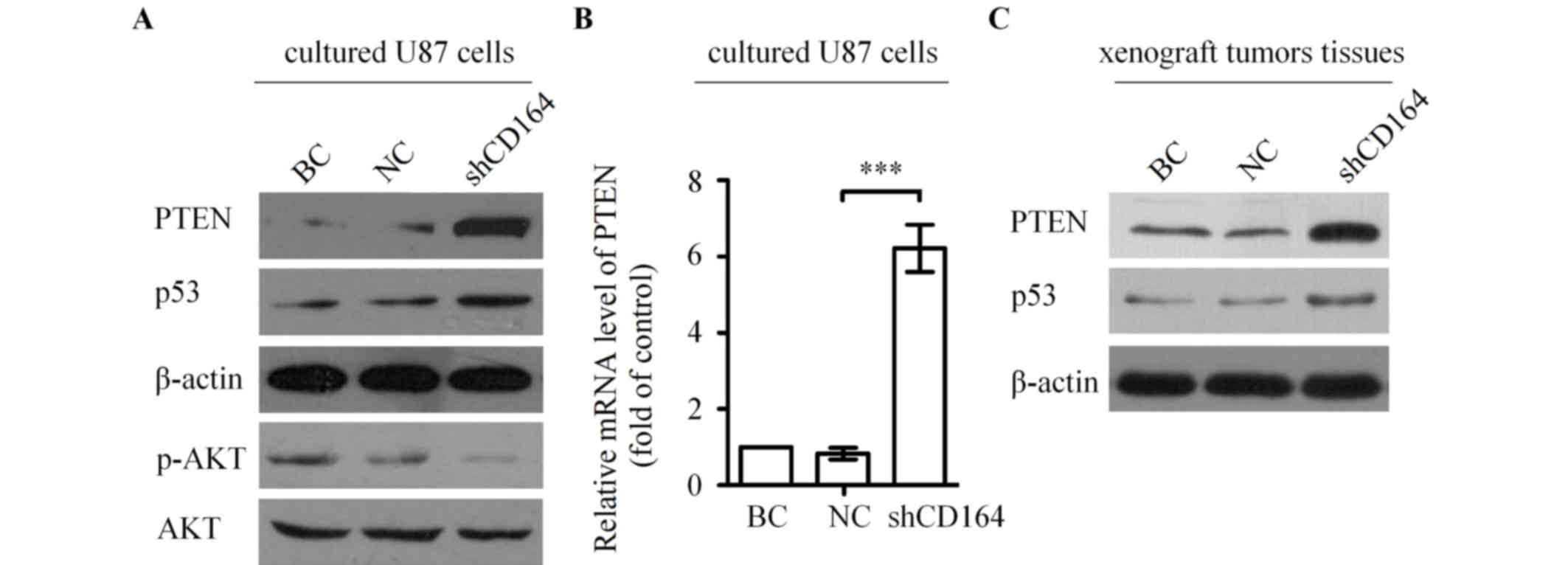
CD164 shRNA upregulated PTEN and
inhibited the phosphoinositide 3-kinase (PI3K)/AKT pathway
To further investigate the molecular mechanisms
underlying the involvement of CD164 in the regulation of glioma
growth and apoptosis, the levels of PTEN and its downstream targets
were assessed by western blot analysis in U87 cells transfected
with CD164 shRNA. PTEN and p53 protein expression levels were
markedly increased in U87 cells following CD164 shRNA transfection
compared with cells transfected with negative control shRNA or
blank control cells (Fig. 6A). By
contrast, the level of AKT phosphorylation was notably decreased in
cells following CD164 shRNA transfection when compared with cells
transfected with negative control shRNA or blank control cells
(Fig. 6A). Further investigation
was conducted to determine whether CD164 shRNA transfection
increases PTEN mRNA expression levels in U87 cells. As shown in
Fig. 6B, silencing of CD164 in U87
cells significantly increased the expression of PTEN mRNA compared
with cells transfected with negative control shRNA (P<0.001).
Western blot analysis was subsequently performed to determine
whether CD164 shRNA-mediated suppression of xenograft tumor growth
in mice was associated with upregulation of PTEN in tumors, as
observed in cultured cells. PTEN protein levels were markedly
increased in tumors from the U87-shCD164 mice when compared with
those from the control groups (Fig.
6C). In addition, there was a visible increase in p53 protein
levels in U87-shCD164 xenograft tumors tissues compared with the
controls (Fig. 6C). These
alterations in PTEN and p53 protein levels in U87-shCD164 mice were
consistent with the observed expression alterations of these
proteins in the cultured cells following transfection with CD164
shRNA (Fig. 6A). These results
indicate that the tumorigenicity of CD164 in glioma cells may be
mediated by the PTEN/PI3K/AKT pathway.
Discussion
RNA interference (RNAi) techniques have been proven
to be a powerful tool for identifying novel and unexpected tumor
promotors and suppressors. Multiple previous studies have used the
RNAi approach for the treatment of cancer, in particular those
caused by overexpression of oncogenes (14). The present study utilized the RNAi
approach to investigate the tumorigenic function of CD164 in
glioma. The results of the present study demonstrated that the
level of CD164 was increased in malignant glioma cells when
compared with normal cells. In addition, RT-qPCR results revealed
that CD164 expression was higher in glioma samples when compared
with paired normal adjacent brain tissues. This differential
expression of CD164 suggests that CD164 may be involved in glioma
malignancy. Previous studies have demonstrated that CD164
expression is upregulated in colon cancer, which suggests that
CD164 may function as a tumor promoter, and may therefore present a
potential target for the treatment of cancer (16). These results are consistent with
those presented in the current study.
Previous studies have revealed that CD164 is
involved in cancer development via the regulation of cell apoptosis
and survival in several cancers (14–18).
In addition, CD164 has been implicated in the regulation of human
ovarian cancer invasion, and silencing of CD164 expression
significantly decreased the metastasis of ovarian cancer cells
(20). In the present study, CD164
expression levels were upregulated in NHA cells to investigate the
tumorigenic effects of CD164 on NHA cells. The results demonstrated
that the overexpression of CD164 promotes the growth of NHA cells
in vitro and in vivo, which supports the hypothesis
that CD164 may function as a tumor promoter. Previous studies have
reported that CD164 is a potential oncogene, based on its ability
to target the C-X-C motif chemokine receptor 4 (15,20).
In the present study, CD164 was implicated in the regulation of NHA
proliferation and apoptosis by suppressing Bax expression and
promoting Bcl-2 expression.
To further elucidate the tumorigenicity of CD164 in
glioma, a CD164-silenced cell line was successfully generated by
infecting lentivirus CD164 shRNA into U87 cells. The results
demonstrated that CD164 was associated with glioma cell growth.
Downregulation of CD164 inhibited cell growth and induced cell
apoptosis in U87 cells. Previous studies have indicated that the
Bax/Bcl2 ratio is involved in the release of cytochrome c to the
cytosol, resulting in caspase activation (21). In addition, overexpression of the
anti-apoptotic Bcl2 protein protects cells from apoptosis induced
by stimulants (22). The present
study demonstrated that silencing of CD164 in U87 cells results in
upregulation of cleaved caspase 3 and alters the Bax/Bcl2 ratio by
increasing Bax and decreasing Bcl2 expression. These in
vitro results indicated that a decrease in CD164 expression may
have participated in the apoptosis of U87 cells.
To further investigate whether the
anti-proliferative effects of CD164 silencing on glioma cells is
sustained in vivo, CD164-silenced and non-silenced U87 cells
were subcutaneously injected into mice. The in vivo results
of the present study were similar to the in vitro results.
CD164 knockdown significantly inhibited tumor growth in
vivo. These results are consistent with the observed decrease
in Ki-67 immunoreactivity and increased TUNEL staining observed in
the xenograft tumors. Therefore, the in vitro and in
vivo results of the present study indicated that CD164
dysfunction may lead to decreased glioma tumor growth.
In order to investigate the molecular mechanisms
underlying the CD164-associated promotion of tumorigenesis in
glioma, the expression PTEN in glioma was detected. During normal
tissue development, PTEN functions as an essential regulator of
cell proliferation, apoptosis, migration and differentiation
(17). Furthermore, PTEN is an
established tumor suppressor gene that possesses dual-specificity
phosphatase activities. Dysregulation of PTEN in mice results in
the development of multiple solid tumors, and depletion of PTEN
promotes the development of multiple cancers (23,24).
The PI3K/AKT signaling pathway is an intracellular signaling
pathway, and activation of this pathway has been observed in a
variety of tumors. Phosphorylation of AKT exerts anti-apoptotic
effects by regulating downstream substrates, including Bax and
Bcl2. Loss of PTEN results in hyperactivation of PI3K/AKT pathway
(25). In addition, previous
studies have revealed that PTEN is implicated in glioma; however,
the regulation of PTEN during glioma progression remains unclear
(26,27). Consistent with these observations,
the results of the present study have provided novel evidence
demonstrating that the expression of PTEN at the mRNA and protein
level was increased in response to CD164 depletion. The observed
upregulation in PTEN expression was associated with decreased AKT
phosphorylation, and an increase in p53 expression, as well as the
growth inhibition of glioma cells in vitro. Notably, the
in vitro results were confirmed in vivo. These
results implied that the tumorigenic effects of CD164 in the
progression of glioma may be dependent on the PTEN/PI3K/AKT
signaling pathway.
In conclusion, the present study revealed for the
first time, that the expression of CD164 may be involved in the
tumorigenesis of glioma via the PTEN/PI3K/AKT signaling pathway.
These results provide an improved understanding of the mechanisms
of tumorigenesis in glioma, and CD164 may therefore present a novel
candidate therapeutic target for the treatment of patients with
glioma.
Glossary
Abbreviations
Abbreviations:
|
NHA
|
normal human astrocytes
|
|
shRNA
|
short hairpin RNA
|
|
CCK-8
|
cell counting kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
BC
|
blank control
|
|
NC
|
negative control
|
|
TUNEL
|
terminal deoxyribonucleotidyl
transferase-mediated dUTP nick end labeling
|
References
|
1
|
Rodon J, Carducci MA, Sepulveda-Sánchez
JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I,
Cleverly AL, et al: First-in-human dose study of the novel
transforming growth factor-β receptor I kinase inhibitor LY2157299
monohydrate in patients with advanced cancer and glioma. Clin
Cancer Res. 21:553–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peters KB, West MJ, Hornsby WE, Waner E,
Coan AD, McSherry F, JE II Herndon, Friedman HS, Desjardins A and
Jones LW: Impact of health-related quality of life and fatigue on
survival of recurrent high-grade glioma patients. J Neurooncol.
120:499–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu H, Lv Z and Guo E: Knockdown of long
noncoding RNA SPRY4-IT1 suppresses glioma cell proliferation,
metastasis and epithelial-mesenchymal transition. Int J Clin Exp
Pathol. 8:9140–9146. 2015.PubMed/NCBI
|
|
4
|
Chen XH, Ling XM and Shi S: microRNA-106a
induces the proliferation and apoptosis of glioma cells through
regulating JNK/MAPK pathway. Eur Rev Med Pharmacol Sci.
19:3412–3417. 2015.PubMed/NCBI
|
|
5
|
Kwiatkowska A and Symons M: Signaling
determinants of glioma cell invasion. Adv Exp Med Biol.
986:121–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Havens AM, Jung Y, Sun YX, Wang J, Shah
RB, Bühring HJ, Pienta KJ and Taichman RS: The role of sialomucin
CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC
Cancer. 6:1952006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Darash-Yahana M, Pikarsky E, Abramovitch
R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E
and Peled A: Role of high expression levels of CXCR4 in tumor
growth, vascularization and metastasis. FASEB J. 18:1240–1242.
2004.PubMed/NCBI
|
|
8
|
Tabaczar S, Domeradzka K, Czepas J,
Piasecka-Zelga J, Stetkiewicz J, Gwoździński K and Koceva-Chyła A:
Anti-tumor potential of nitroxyl derivative Pirolin in the
DMBA-induced rat mammary carcinoma model: A comparison with
quercetin. Pharmacol Rep. 67:527–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murad N, Kokkinaki M, Gunawardena N,
Gunawan MS, Hathout Y, Janczura KJ, Theos AC and Golestaneh N:
miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in
human retinal pigment epithelium and is downregulated in
age-related macular degeneration. FEBS J. 281:5251–5264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forde S, Tye BJ, Newey SE, Roubelakis M,
Smythe J, McGuckin CP, Pettengell R and Watt SM: Endolyn (CD164)
modulates the CXCL12-mediated migration of umbilical cord blood
CD133+ cells. Blood. 109:1825–1833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Doyonnas R, Yi-Hsin Chan J, Butler LH,
Rappold I, Lee-Prudhoe JE, Zannettino AC, Simmons PJ, Bühring HJ,
Levesque JP and Watt SM: CD164 monoclonal antibodies that block
hemopoietic progenitor cell adhesion and proliferation interact
with thefirst mucin domain of the CD164 receptor. J Immunol.
165:840–851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jorgensen-Tye B, Levesque JP, Royle L,
Doyonnas R, Chan JY, Dwek RA, Rudd PM, Harvey DJ, Simmons PJ and
Watt SM: Epitope recognition of antibodies that define the
sialomucin, endolyn (CD164), a negative regulator of
haematopoiesis. Tissue Antigens. 65:220–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin J, Xu K, Wei J, Heimberger AB, Roth JA
and Ji L: MicroRNA-124 suppresses tumor cell proliferation and
invasion by targeting CD164 signaling pathway in non-small cell
lung cance. J Gene Ther. 2:62016.PubMed/NCBI
|
|
14
|
Shi JA, Lu DL, Huang X and Tan W: miR-219
inhibits the proliferation, migration and invasion of
medulloblastoma cells by targeting CD164. Int J Mol Med.
34:237–243. 2014.PubMed/NCBI
|
|
15
|
Tang J, Zhang L, She X, Zhou G, Yu F,
Xiang J and Li G: Inhibiting CD164 expression in colon cancer cell
line HCT116 leads to reduced cancer cell proliferation, mobility,
and metastasis in vitro and in vivo. Cancer Invest. 30:380–389.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Havens AM, Jung Y, Sun YX, Wang J, Shah
RB, Bühring HJ, Pienta KJ and Taichman RS: He role of sialomucin
CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC
Cancer. 6:1952006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coustan-Smith E, Song G, Clark C, Key L,
Liu P, Mehrpooya M, Stow P, Su X, Shurtleff S, Pui CH, et al: New
markers for minimal residual disease detection in acute
lymphoblastic leukemia. Blood. 117:6267–6676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chirumbolo S: CD164 and other recently
discovered activation markers as promising tools for allergy
diagnosis: What's new? Clin Exp Med. 11:255–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang AF, Chen MW, Huang SM, Kao CL, Lai
HC and Chan JY: CD164 regulates the tumorigenesis of ovarian
surface epithelial cells through the SDF-1α/CXCR4 axis. Mol Cancer.
12:1152013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kordezangeneh M, Irani S, Mirfakhraie R,
Esfandyari-Manesh M, Atyabi F and Dinarvand R: Regulation of
BAX/BCL2 gene expression in breast cancer cells by docetaxel-loaded
human serum albumin nanoparticles. Med Oncol. 32:2082015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodríguez-Berriguete G, Torrealba N,
Ortega MA, Martínez-Onsurbe P, Olmedilla G, Paniagua R, Guil-Cid M,
Fraile B and Royuela M: Prognostic value of inhibitors of apoptosis
proteins (IAPs) and caspases in prostate cancer: Caspase-3 forms
and XIAP predict biochemical progression after radical
prostatectomy. BMC Cancer. 15:8092015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shang Y, Guo XX, Li WW, Rao W, Chen ML, Mu
LN and Li SJ: Cucurbitacin-B inhibits neuroblastoma cell
proliferation through up-regulation of PTEN. Eur Rev Med Pharmacol
Sci. 18:3297–3303. 2014.PubMed/NCBI
|
|
24
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
regulates cellular proliferation, invasion, migration and apoptosis
by PTEN/AKT signaling in human glioblastoma cells. Cell Mol
Neurobiol. 35:679–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Q, Zhang Y, Meng R, Xie KM, Xiong Y,
Lin S, He ZL, Tao T, Yang Y, Zhao JZ and He JQ: MAGI3 suppresses
glioma cell proliferation via upregulation of PTEN expression.
Biomed Environ Sci. 28:502–509. 2015.PubMed/NCBI
|
|
26
|
Wang LJ, He CC, Sui X, Cai MJ, Zhou CY, Ma
JL, Wu L, Wang H, Han SX and Zhu Q: MiR-21 promotes intrahepatic
cholangiocarcinoma proliferation and growth in vitro and in vivo by
targeting PTPN14 and PTEN. Oncotarget. 6:5932–5946. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang WR, Chiu HC, Liao TL, Chuang KP,
Shih WL and Liu HJ: Correction: avian reovirus protein p17
functions as a nucleoporin Tpr suppressor Leading to activation of
p53, p21 and PTEN and inactivation of PI3K/AKT/mTOR and ERK
signaling pathways. PLoS One. 10:e01386272015. View Article : Google Scholar : PubMed/NCBI
|