Introduction
Esophageal cancer is a common malignant tumor of the
digestive tract, with high morbidity and mortality rates of all
cancers in China (1,2). The main pathological types of
esophageal cancer are esophageal adenocarcinoma and esophageal
squamous cell cancer (ESCC), which accounts for 90% of esophageal
cancer cases in China (3). The
development of radical and synchronized radiotherapy and
chemotherapy for the treatment of local advanced esophageal cancer
has improved the survival rate; however, the rate of local
recurrence and distant metastasis remains high (4). Chemo-radiotherapy resistance is
considered the most important reason for local tumor recurrence and
metastasis (5). Therefore, the
identification of clinically applicable biomarkers for early
evaluation of ESCC prognosis is important.
Long non-coding RNA (lncRNAs) are non-protein coding
RNAs >200 nucleotides long, which regulate gene expression
epigenetically, transcriptionally and post-transcriptionally.
Previous studies have suggested that lncRNAs associated with
chromatin-modifying complexes may affect epigenetic information and
confer numerous properties required for tumor progression and
metastasis (6–8).
HOX transcript antisense RNA (HOTAIR) is one of the
few well-documented lncRNAs. It consists of 2,158 bp and is located
on chromosome 12 within the homeobox C gene cluster (9). HOTAIR has been demonstrated to be
highly expressed in primary and metastatic breast cancer, thus
suggesting its involvement in the occurrence, invasion and distant
metastasis of the tumor. In vitro studies have demonstrated
that HOTAIR interacts with numerous chromatin-modifying enzymes to
regulate target gene expression (10–12).
HOTAIR acts as a bridge to coordinate the targeting of polycomb
repressive complex 2 (PRC2), a histone H3 lysine 27
(H3K27)-specific methyltransferase complex, and lysine specific
histone demethylase (LSD1)/(CoREST/REST complex) to chromatin for
coupled histone H3K27 methylation and H3 lysine 4 demethylation,
which is epigenetically involved in the silencing of genes in the
HOXD cluster and several other target genes to promote tumor
development and metastasis (13).
It has previously been confirmed that HOTAIR
regulates metastasis of hepatocellular carcinoma, suggesting that
it may be considered a useful target to reduce tumor recurrence
(14). HOTAIR has the potential to
predict tumor recurrence following liver transplantation,
demonstrating its value as a prognostic indicator (14). The upregulation of HOTAIR is also
associated with the malignant degree of colorectalcancer (15,16),
gastrointestinal stromal tumors (17), lung cancer (18), pancreatic cancer (19), nasopharyngeal carcinoma (20), laryngeal carcinoma (21) and ovarian cancer (22). In these cancers, HOTAIR is
expressed at higher levels in the presence of lymph node
involvement or organ metastasis, and higher levels correlate with a
poorer prognosis and higher mortality. However, the mechanisms
underlying the participation of HOTAIR in tumor occurrence,
invasion and metastasis remain unclear.
The epithelial-mesenchymal transition (EMT) has been
identified as one of the key mechanisms underlying ESCC tumor
invasion and metastasis, and has been clinically associated with
poor prognosis (23). EMT is
characterized by a loss of epithelial characteristics and an
acquisition of a mesenchymal state that reduces cell adhesion and
enables ESCC tumor cells to dissociate from the epithelial tissue
and migrate more effectively (24).
Notably, previous studies have reported that HOTAIR
induces zinc finger protein SNAI1 (Snail), E-cadherin, matrix
metalloproteinases (MMPs) and other EMT-related factors in breast
(25), colorectal (16) and lung cancers (18). EMT-related factors are important in
the development and metastasis of various types of cancer. In the
present study, HOTAIR mRNA expression, and EMT-related mRNA and
protein (Snail, β-catenin, E-cadherin) expression, was detected in
ESCC and para-carcinoma tissues using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. The correlation between these factors and
the clinicopathological characteristics of patients with ESCC was
also explored.
Materials and methods
Ethics
The present study was approved by the Ethics
Committee of the Tumor Hospital of Xinjiang Medical University
(Urumqi, China) and was performed in accordance with the
Declaration of Helsinki of the World Medical Association. All
patients provided written informed consent.
Clinicopathological data
A total of 96 ESCC specimens with matched
para-carcinoma tissues, which were obtained during surgery from
2009 to 2014, were collected from the Tumor Hospital of Xinjiang
Medical University (Urumqi, China). The samples were collected from
56 males and 40 females, with an average age of 60.7 years.
According to the 2010 American Joint Committee on Cancer TNM stages
(26), the 96 cases were
classified as follows: 8 cases of well-differentiated ESCC (G1); 48
cases of moderately differentiated ESCC (G2); and 40 cases of
poorly differentiated ESCC (G3). The TNM stages (26) of the 96 ESCC cases were as follows:
45 cases of stage I and II, 29 cases of stage III and 22 cases of
stage IV. The tumors were located in the upper-middle section of
the esophagus in 90 cases, and located in the lower section in 6
cases. There were 4 cases of T1 and 2, and 92 cases of T3 and 4,
indicating the size and extent of the primary tumor. There were 52
cases with lymph node metastasis and 44 cases without lymph node
metastasis, 22 cases with organ metastasis and 74 cases without
organ metastasis. All patients had complete clinical data and had
not received any pre-operative treatment.
RT-qPCR
Total RNA from homogenized cancerous and
para-carcinoma specimens was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. cDNA was obtained by
reverse transcribing total RNA using a TaqMan Reverse Transcription
kit (Takara Bio, Inc., Dalian, China). The reaction system included
2 µl 5X PrimeScript Buffer, 0.5 µl PrimeScript RT Enzyme Mix I, 0.5
µl oligo (dT) Primer (50 µM), 0.5 µl, Random 6 mers (100 µM), 500
ng Total RNA and RNase free dH2O to reach a final volume
of 10 µl. It was then reacted at 37°C for 15 min, 85°C for 5 sec,
and 4°C for preservation. RT-qPCR analyses were conducted using the
Power SYBR-Green kit (Takara Bio, Inc.), according to the
manufacturer's protocol. All RT-qPCR assays were performed on an
ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Thermo
Fisher Scientific, Inc.). The total volume of the reaction mixture
was 20 µl (2 µl reverse transcriptase, 0.8 µl each forward and
reverse template RNA primers, 6.4 µl sterilized
diethylpyrocarbonate water, and 10 µl SYBR Select Master mix). The
initialization step (uracil-DNA glycosylation activation step) was
set at 50°C for 2 min, followed by the AmpliTaq DNA polymerase
ultrapure activation step at 95°C for 2 min. The denaturation
temperature was 95°C, held for 15 sec, and annealing of primers for
E-cadherin, Snai1, and β-catenin was carried out at 55°C for 15
sec. Extension was carried out at 72°C for 1 min. The
annealing/extension steps for HOTAIR were carried out at 60°C for 1
min. The final three steps were run for 40 cycles. Expression
levels of HOTAIR, Snai1, E-cadherin, and β-catenin were normalized
to those of β-actin.
The mRNA expression levels of HOTAIR and EMT-related
factors were determined by RT-qPCR using the following primer
sequences: HOTAIR forward, 5′-GCCTTTCCCTGCTACTTGTG-3′, reverse,
5′GGCTGGACCTTTGCTTCTATG-3′; Snail forward,
5′-TGACCTGTCTGCAAATGCTC-3′, reverse, 5′-CAGACCCTGGTTGCTTCAA-3′;
E-cadherin forward, 5′-AGCGTGTGTGACTGTGAAGG-3′, reverse,
5′-GCTGGCTCAAGTCAAAGTCC-3′; and β-catenin forward,
5′-CCCACTAATGTCCAGCGTTT-3′ and reverse, 5′-TGTCAGTTCAGGGATTGCAC-3′.
β-actin was used as an internal control, and the primer sequences
were as follows: Forward, 5′-CATCATGAAGTGTGACGTGGA-3′ and reverse,
5′-ACATCTGCTGGAAGGTGGAC-3′. All RT-qPCR assays were performed on an
ABI 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). HOTAIR, Snail, E-cadherin, and β-catenin
values were normalized to those of β-actin, and their relative
fold-changes in mRNA expression were calculated using the
2−ΔΔCq method (27).
Western blot analysis
All homogenized tissues were lysed using the
mammalian protein extraction reagent radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with phenylmethylsulfonyl fluoride (Roche Molecular
Diagnostics, Pleasanton, CA, USA). Extracted proteins (~30 µg) were
separated by 8% SDS-PAGE, transferred to 0.45 µm polyvinylidene
fluoride (PVDF) membranes (Merck Millipore, Darmstadt, Germany),
and incubated with the indicated primary and secondary antibodies.
The PVDF membrane was blocked with 1X TBST (Beijing Solarbio
Science & Technology Co., Ltd.) with 5% milk for 2 h, before
being washed twice with TBST buffer. Primary antibodies (1:1,500)
were incubated with the membrane at 4°C overnight. The PVDF
membrane was washed 3 times for 10 min each, and incubated with
secondary antibody (1:5,000) at room temperature for 1 h. Following
this, the membrane was washed for 3 times again with TBST.
Signals were detectedusing an enhanced
chemiluminescence detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). β-actin (cat. no. sc-47778) was used as an
internal control. Rabbit-derived Snail (cat. no. sc-28199),
E-cadherin (cat. no. sc-7870) and β-catenin primary antibodies
(cat. no. sc-7199) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The secondary antibody, horseradish
peroxidase-labeled goat anti-rabbit immunoglobulin G, was purchased
from Beijing Zhongshan Golden Bridge Biotechnology; OriGene
Technologies, Inc. (cat. no. ZB-2301; Rockville, MD, USA).
Statistical analysis
All data were presented as M(P25, P75) and were
analyzed using SPSS17.0 software (SPSS, Inc., Chicago, IL, USA).
Rank-sum tests and Kruskal-Wallis tests were used to analyze
clinicopathological parameter differences between groups.
Spearman's rank correlation analysis was used to analyze the
relationship between HOTAIR and EMT-related factors. P<0.05 was
considered to indicate a statistically significant difference.
Results
HOTAIR mRNA expression levels in ESCC
and para-carcinoma tissues
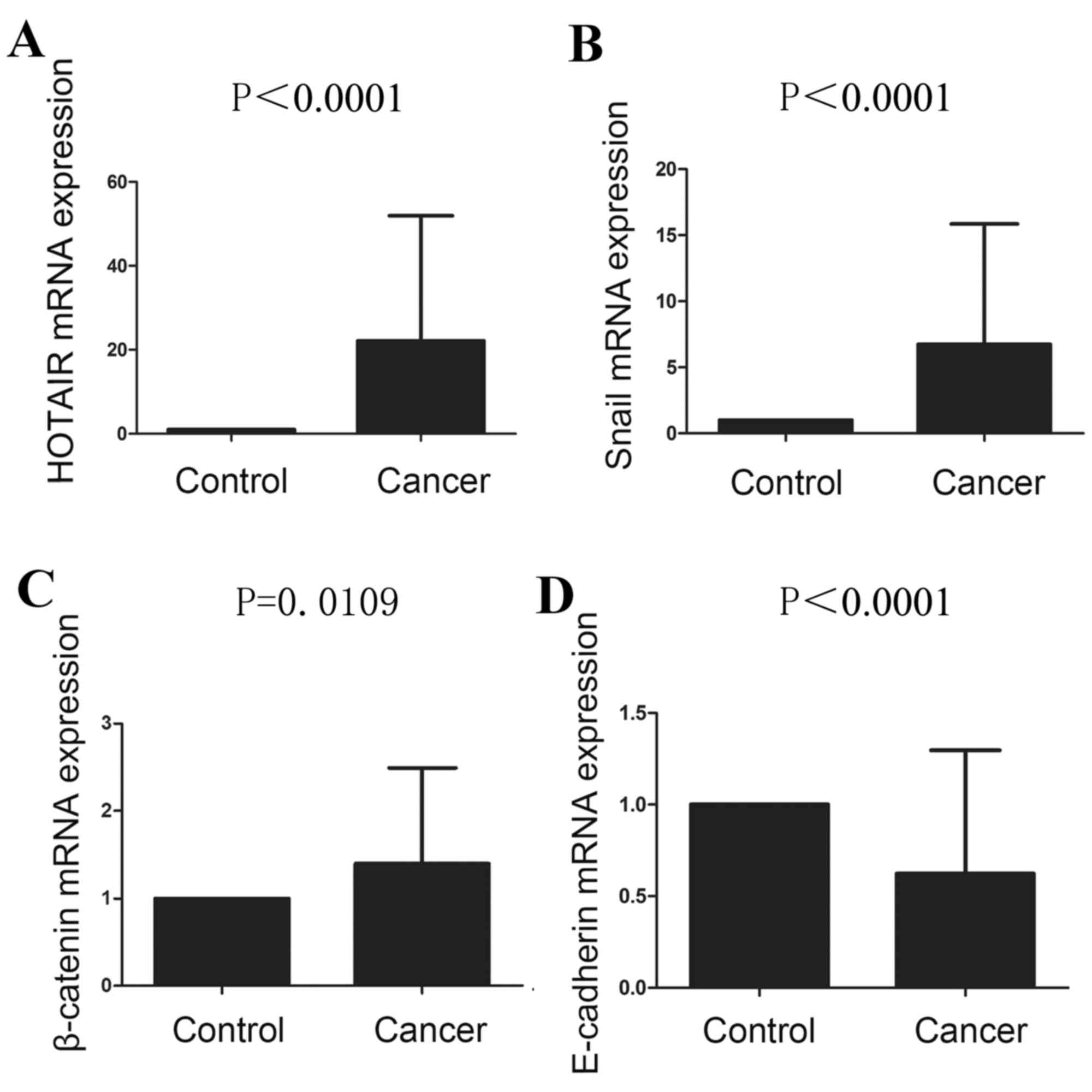
The mRNA expression levels of HOTAIR were
significantly higher in ESCC tissues compared within para-carcinoma
tissues (P<0.0001; Fig. 1A;
Table I). HOTAIR mRNA expression
levels in the groups with lymph node involvement or organ
metastasis were significantly higher compared within the group
without lymph node involvement or organ metastasis (P<0.0001 and
P=0.0003, respectively; Table
II). HOTAIR mRNA expression levels demonstrated a significantly
increasing trend from well-differentiated cancer through
moderately-differentiated cancer to poorly differentiated cancer,
and a more advanced TNM stage was significantly rank correlated
with increased HOTAIR mRNA expression levels (P<0.0001; Table II). There was no significant
correlation between HOTAIR mRNA expression levels and the tumor
location, tumor invasion depth, or the age, ethnicity or gender of
the patients (Table II).
 | Table I.mRNA expression levels of HOTAIR and
epithelial mesenchymal transition-related factors in ESCC and
paired para-carcinoma tissues. |
Table I.
mRNA expression levels of HOTAIR and
epithelial mesenchymal transition-related factors in ESCC and
paired para-carcinoma tissues.
| Tissue | HOTAIR | Snail | E-cadherin | β-catenin |
|---|
| ESCC | 13.78 (0.79,
25.94) | 3.50 (1.18,
6.12) | 0.27 (0.11,
1.06) | 1.69 (0.48,
2.04) |
| Para-carcinoma | 1.00 (1.00,
1.00) | 1.00 (1.00,
1.00) | 1.00 (1.00,
1.00) | 1.00 (1.00,
1.00) |
| P-value | <0.0001 | <0.0001 | <0.0001 | 0.0109 |
 | Table II.Relationship between HOX transcript
antisense RNA mRNA expression levels and clinicopathological
factors of patients with esophageal squamous cell carcinoma. |
Table II.
Relationship between HOX transcript
antisense RNA mRNA expression levels and clinicopathological
factors of patients with esophageal squamous cell carcinoma.
| Characteristic | N |
2−∆∆Cq | P-value |
|---|
| Gender |
|
| 0.8148 |
|
Male | 56 | 8.44 (0.78,
35.90) |
|
|
Female | 40 | 13.78 (7.04,
21.84) |
|
| Age (years) |
|
| 0.1028 |
|
>60 | 60 | 12.14 (0.81,
19.89) |
|
|
≤60 | 36 | 21.84 (0.78,
35.90) |
|
| Ethnicity |
|
rk=1.71 | 0.4252 |
|
Wei | 32 | 14.00 (1.34,
20.20) |
|
|
Han | 44 | 22.12 (0.78,
54.28) |
|
| Ha | 20 | 12.14 (7.04,
25.42) |
|
| T stage |
|
| 0.0599 |
|
T1+T2 | 4 | 25.28 (24.28,
54.28) |
|
|
T3+T4 | 92 | 12.86 (0.77,
21.91) |
|
| N stage |
|
| <0.0001 |
|
Yes | 52 | 21.84 (14.59,
54.28) |
|
| No | 44 | 0.81 (0.71,
12.14) |
|
| M stage |
|
| 0.0003 |
|
Yes | 22 | 22.12 (19.89,
54.28) |
|
| No | 74 | 1.95 (0.75,
14.94) |
|
| Tumor location |
|
| 0.6615 |
|
Upper-mid | 90 | 13.57 (0.81,
22.12) |
|
|
Under | 6 | 19.89 (0.78,
27.22) |
|
| G stage |
|
rk=8.612 | 0.0135 |
| G1 | 8 | 0.81 (0.78,
7.04) |
|
| G2 | 48 | 13.57 (0.74,
21.84) |
|
| G3 | 40 | 19.89 (1.95,
35.90) |
|
| TNM stage |
|
rk=38.79 | <0.0001 |
|
I+II | 45 | 0.80 (0.71,
1.95) |
|
|
III | 29 | 14.25 (7.04,
20.20) |
|
| IV | 22 | 21.98 (15.47,
47.52) |
|
mRNA and protein expression levels of
EMT-related factors (Snail, E-cadherin, β-catenin) in ESCC and
para-carcinoma tissues
The mRNA expression levels of EMT-related factors
are presented in Fig. 1B-D. The
relative mRNA expression levels of Snail and β-catenin in ESCC were
significantly higher compared with in para-carcinoma tissues
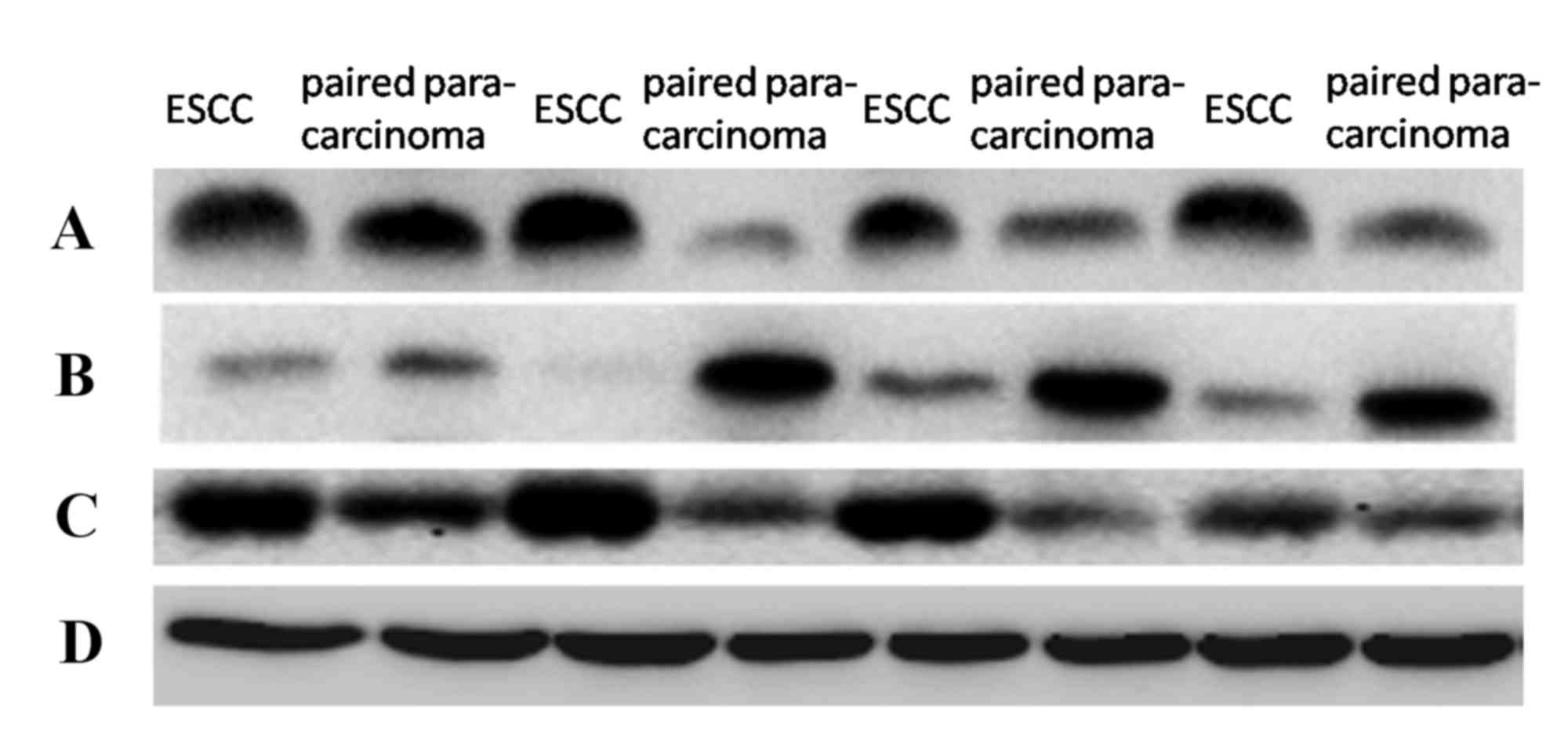
(P<0.0001 and P=0.0109, respectively; Fig. 1B and C; Table I). In addition, Snail and β-catenin
protein expression levels were significantly increased in ESCC
compared with in para-carcinoma tissues (P<0.0001 and
P<0.0001, respectively; Fig. 2;
Table III). Conversely,
E-cadherin mRNA expression level was significantly decreased in
ESCC compared with para-carcinoma tissues (P<0.0001; Fig. 1D; Table I), and the E-cadherin protein
expression level was also significantly decreased in ESCC compared
with para-carcinoma tissues (P=0.0006; Fig. 2; Table III).
 | Table III.Protein expression levels of
epithelial mesenchymal transition-related factors in ESCC and
paired para-carcinoma tissues. |
Table III.
Protein expression levels of
epithelial mesenchymal transition-related factors in ESCC and
paired para-carcinoma tissues.
| Tissue | Snail | E-cadherin | β-catenin |
|---|
| ESCC | 1.43 (1.04,
2.09) | 0.20 (0.04,
0.47) | 1.40 (1.02,
1.89) |
| Para-carcinoma | 0.76 (0.40,
1.19) | 0.49 (0.22,
0.90) | 0.73 (0.32,
1.09) |
| P-value | <0.0001 | 0.0006 | <0.0001 |
The mRNA and protein expression levels of Snail in
the groups with lymph node involvement or organ metastasis were
significantly increased compared with the group without lymph node
involvement or organ metastasis (mRNA, P<0.001; protein,
P=0.0005; Table IV) or organ
metastasis (mRNA, P<0.001; protein, P=0.0035; Table IV). Snail expression demonstrated
an increasing trend from well-differentiated through
moderately-differentiated to poorly differentiated cancer
(PmRNA=0.0007, Pprotein<0.001; Table IV), and a more advanced TNM stage
was significantly correlated with increased Snail expression
(PmRNA<0.0001, Pprotein<0.0001;
Table IV) E-cadherin mRNA and
protein expression levels were significantly decreased in the group
with lymph node involvement (mRNA, P<0.001; protein, P=0.0016;
Table IV) and organ metastasis
(mRNA, P<0.001; protein, P=0.0140; Table IV) compared with the group without
lymph node involvement or organ metastasis. The expression of
E-cadherin demonstrated a significant decreasing trend from
well-differentiated cancer through moderately-differentiated cancer
to poorly differentiated cancer with significant rank correlation
(PmRNA=0.0007, Pprotein=0.0220; Table IV). In addition, a more advanced
TNM stage was significantly rank correlated with decreased
E-cadherin expression (PmRNA=0.0002,
Pprotein<0.0001; Table
IV).
 | Table IV.The relationship between mRNA and
protein expression levels of epithelial mesenchymal
transition-relatedfactors and clinicopathological factors of
patients with esophageal squamous cell carcinoma. |
Table IV.
The relationship between mRNA and
protein expression levels of epithelial mesenchymal
transition-relatedfactors and clinicopathological factors of
patients with esophageal squamous cell carcinoma.
|
| Snail
expression | E-cadherin
expression | β-catenin
expression |
|---|
|
|
|
|
|
|---|
| Characteristic | N |
rk/P mRNA |
rk/P Protein |
rk/P mRNA |
rk/P Protein |
rk/P mRNA |
rk/P Protein |
|---|
| Gender |
| 0.4667 | 0.4550 | 0.7017 | 0.6860 | 0.331 | 0.1820 |
|
Male | 56 | 3.33 (2.26,
4.63) | 1.16
(0.68,1.72) | 0.53
(0.14,1.58) | 0.32
(0.10,0.64) | 1.03
(0.49,2.35) | 0.94
(0.48,1.64) |
|
Female | 40 | 4.96
(0.21,21.14) | 0.94
(0.48,2.09) | 0.33
(0.11,0.90) | 0.40
(0.13,0.61) | 1.15
(0.45,1.73) | 1.14
(0.56,1.89) |
| Age (years) |
| 0.7969 | 0.6800 | 0.1047 | 0.1390 | 0.416 | 0.9730 |
|
>60 | 60 | 3.50
(2.38,4.80) | 1.16
(0.51,1.88) | 1.13
(0.11,2.29) | 0.26
(0.08,0.59) | 1.11
(0.48,2.22) | 0.98
(0.51,1.66) |
|
≤60 | 36 | 3.69
(0.38,10.09) | 1.04
(0.55,1.72) | 0.33
(0.13,1.02) | 0.46
(0.12,0.76) | 0.97
(0.41,1.80) | 1.06
(0.48,1.71) |
| Ethnicity |
|
4.287/0.1172 |
5.307/0.0700 |
4.941/0.0864 |
4.012/0.1345 |
3.011/0.2219 |
2.168/0.3390 |
|
Wei | 32 | 4.80
(0.71,19.67) | 1.43
(0.95,2.09) | 0.11
(0.06,1.13) | 0.51
(0.15,0.83) | 0.87
(0.41,1.73) | 0.79
(0.48,1.36) |
|
Han | 44 | 3.33
(2.32,4.99) | 0.82
(0.47,1.60) | 0.53
(0.14,1.57) | 0.26
(0.04,0.48) | 1.34
(0.73,2.30) | 1.14
(0.72,1.71) |
| Ha | 20 | 2.74
(0.29,3.90) | 1.32
(0.65,1.69) | 0.50
(0.39,1.82) | 0.29
(0.15,0.65) | 0.49
(0.44,2.08) | 1.0
(0.31,1.73) |
| T stage |
| 0.541 | 0.2160 | 0.122 | 0.3360 | 0.465 | 0.7620 |
|
T1+T2 | 4 | 3.70
(0.90,4.48) | 0.64
(0.35,1.29) | 0.39
(0.12,1.58) | 0.64
(0.19,1.76) | 1.64
(0.51,2.58) | 1.45
(0.34,1.71) |
|
T3+T4 | 92 | 3.68
(1.06,6.41) | 1.14
(0.56,1.76) | 1.22
(0.90,1.53) | 0.32
(0.10,0.61) | 1.03
(0.47,2.07) | 1.01
(0.50,1.68) |
| N stage |
|
<0.001 | 0.0005 |
<0.001 | 0.0016 | 0.076 | 0.0003 |
|
Yes | 52 | 11.36
(4.05,19.67) | 1.41
(0.96,2.12) | 0.16
(0.10,0.47) | 0.22
(0.05,0.48) | 1.13
(0.49,2.62) | 1.31
(0.94,1.76) |
| No | 44 | 1.72
(0.28,3.25) | 0.73
(0.40,1.18) | 1.13 (0.53,
3.50) | 0.48
(0.19,1.07) | 0.93
(0.41,1.73) | 0.70
(0.29,1.10) |
| M stage |
|
<0.001 | 0.0035 |
<0.001 | 0.0140 | 0.055 | 0.0099 |
|
Yes | 22 | 21.14
(4.38,24.80) | 1.68
(1.08,3.08) | 0.12
(0.10,0.27) | 0.30
(0.10,0.54) | 1.48
(0.65,2.50) | 1.56
(0.80,2.74) |
| No | 74 | 3.08
(0.53,4.51) | 1.00
(0.49,1.65) | 0.98
(0.15,1.82) | 0.63
(0.14,2.40) | 0.92
(0.41,1.80) | 0.94
(0.47,1.43) |
| Location |
| 0.537 | 0.7621 | 0.738 | 0.6280 | 0.960 | 0.8090 |
|
Upper-mid | 90 | 3.69
(0.71,6.25) | 1.14
(0.53,1.74) | 0.52
(0.14,1.58) | 0.32
(0.10,0.61) | 1.03
(0.48,2.17) | 1.06
(0.51,1.69) |
|
Under | 6 | 3.09
(2.26,4.51) | 0.85
(0.58,2.05) | 0.53
(0.11,3.50) | 0.41
(0.18,1.21) | 1.39
(0.41,1.90) | 1.02
(0.70,1.47) |
| G stage |
|
14.66/0.0007 |
18.64/<0.001 |
14.66/0.0007 |
7.61/0.022 |
12.56/0.0019 |
10.64/0.0049 |
| G1 | 8 | 2.50
(0.54,4.05) | 0.19
(0.07,0.48) | 3.50
(1.58,5.27) | 0.79
(0.31,2.67) | 0.52
(0.38,1.41) | 0.23
(0.16,1.29) |
| G2 | 48 | 3.26
(0.28,4.80) | 1.11
(0.54,1.76) | 0.90
(0.27,1.53) | 0.39
(0.17,0.61) | 1.13
(0.83,2.17) | 0.90
(0.49,1.36) |
| G3 | 40 | 14.63
(3.90,21.75) | 1.55
(0.96,2.95) | 0.11
(0.08,0.14) | 0.20
(0.08,0.51) | 2.80
(1.03,4.54) | 1.30
(0.76,2.35) |
| TNM |
|
20.61/<0.0001 |
21.40/<0.0001 | 17.23/0.0002 |
25.34/<0.0001 | 6.971/0.0306 |
15.93/0.0003 |
|
I+II | 45 | 1.72
(0.30,3.57) | 0.75
(0.35,1.16) | 1.30
(0.15,3.50) | 0.54
(0.35,0.90) | 0.59
(0.42,1.47) | 0.76
(0.30,1.02) |
|
III | 29 | 4.34
(2.99,6.25) | 1.19
(0.85,2.07) | 0.50
(0.17,1.58) | 0.25
(0.04,0.45) | 1.17
(0.83,2.17) | 1.40
(1.10,1.71) |
| IV | 22 | 21.14
(4.37,24.80) | 1.70
(1.09,3.15) | 0.12
(0.10,0.27) | 0.09
(0.03,0.22) | 1.68
(0.38,2.84) | 1.39
(1.49,3.08) |
β-catenin mRNA and protein expression levels
demonstrated a significantly increasing trend from
well-differentiated cancer through moderately-differentiated cancer
to poorly-differentiated cancer, and had a significant rank
correlation (PmRNA=0.0019,
Pprotein<0.0049; Table
IV). A more advanced TNM stage was significantly rank
correlated with increased β-catenin mRNA and protein expression
(PmRNA=0.0306, Pprotein=0.0003; Table IV). β-catenin protein expression
was significantly increased in the groups with lymph node
involvement (Pprotein=0.0003; Table IV) and organ metastasis
(Pprotein=0.0099; Table
IV), compared with the group with no lymph node or organ
involvement. However, there was no significant correlation between
β-catenin mRNA expression and lymph node or organ metastasis in the
96 esophageal cancer tissues (P>0.05; Table IV) There was no significant
correlation between Snail, E-cadherin, or β-catenin expression and
tumor location, invasion depth, or the age, ethnicity or gender of
patients (Table IV).
Correlation between the expression
levels of HOTAI RmRNA and EMT-related factors in ESCC
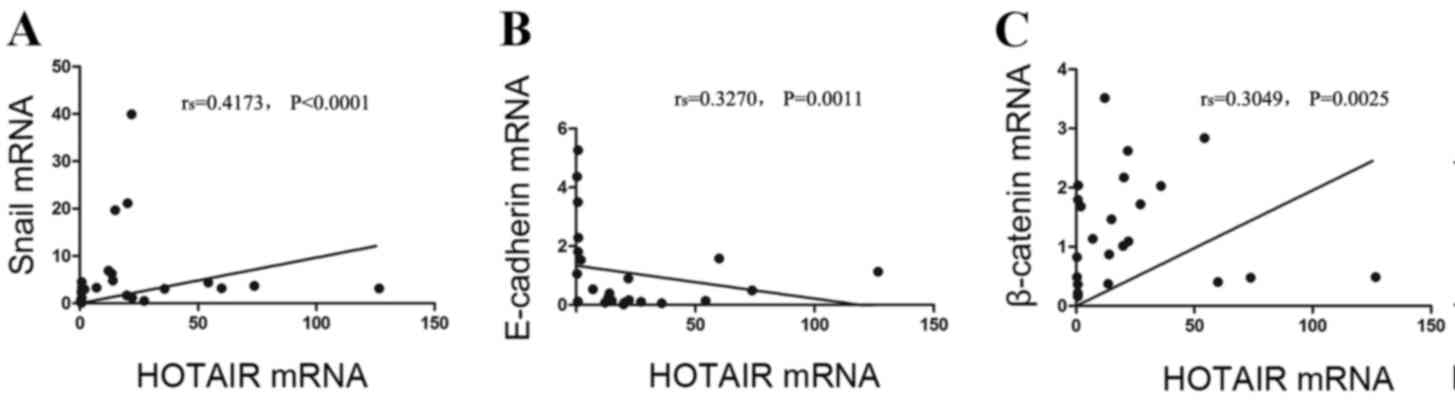
In the 96 ESCC tissues, HOTAIR mRNA expression
levels were positively correlated with Snail and β-catenin mRNA
expression levels (rs=0.4173 and 0.3049, respectively,
P<0.0001 and P=0.0025, respectively; Fig. 3). HOTAIR and E-cadherin mRNA
expression levels were negatively correlated
(rs=−0.3270, P=0.0011; Fig.
3).
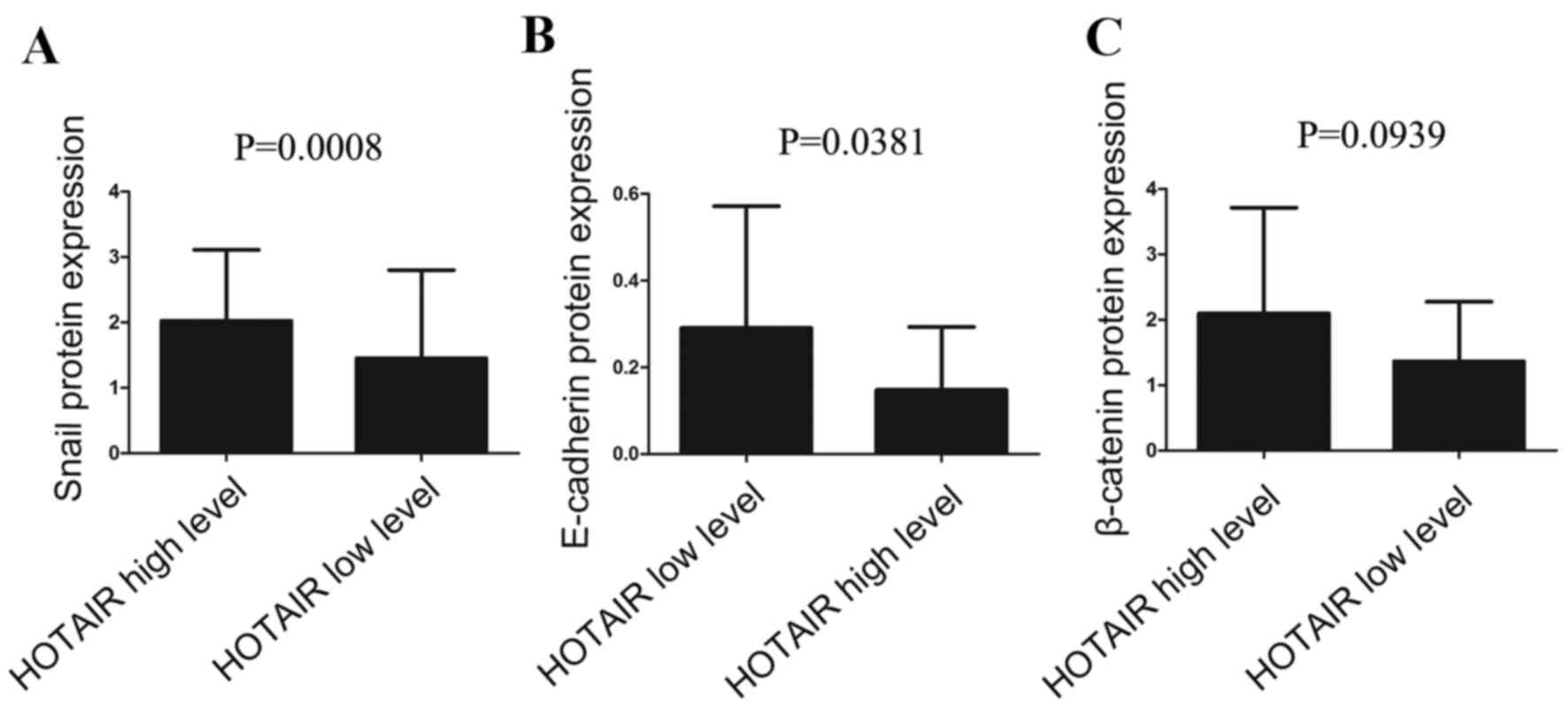
HOTAIR expression was subsequently divided into a
high level group and low level group, according to its mean
expression level (17.58). In the HOTAIR high expression group,
Snail protein expression was significantly higher compared with the
low expression group (P=0.0008; Fig.
4A; Table V), whereas
E-cadherin protein expression levels were significantly lower in
the HOTAIR high expression group compared with the HOTAIR low
expression group (P=0.0381; Fig.
4B; Table V). There was no
relationship between HOTAIR mRNA expression levels and β-catenin
protein expression levels (P>0.05; Fig. 4C; Table V).
 | Table V.Correlations between HOTAIR mRNA
expression levels and epithelial mesenchymal transition-related
protein levels in esophageal squamous cell carcinoma. |
Table V.
Correlations between HOTAIR mRNA
expression levels and epithelial mesenchymal transition-related
protein levels in esophageal squamous cell carcinoma.
| Group | Snail | E-cadherin | β-catenin |
|---|
| HOTAIR high
expression | 1.68 (1.13,
3.22) | 0.09 (0.08,
0.23) | 1.67 (1.02,
3.22) |
| HOTAIR low
expression | 1.14 (0.59,
1.68) | 0.20 (0.08,
0.45) | 1.17 (0.94,
1.69) |
| P-value | 0.0008 | 0.0381 | 0.0939 |
Discussion
Cancer has traditionally been regarded as a genetic
disease; however, but previous research has revealed that cancer
development and progression are also associated with epigenetic
abnormalities (28). Genetic
continuity requires epigenetic regulation, including DNA
methylation, histone deacetylation, chromatin remodeling, gene
imprinting and noncoding RNA (ncRNA) regulation (29,30).
MicroRNAs and lncRNAs >200 nucleotides in length serve as the
primary regulatory ncRNAs. Although microRNAs have been
comprehensively studied in several types ofcancer, they have also
been identified for potential use in esophageal cancer, and for
early clinical diagnosis, prognosis, and gene therapy (31). Emerging evidence has indicated that
lncRNAs possess more complicated and extensive regulatory functions
than microRNAs in cancer development and progression (32–36).
HOTAIR, which is one of the few well-documented
lncRNAs, is highly expressed in breast, hepatic, pancreatic, lung,
and colorectal cancers. Increased HOTAIR expression is correlated
with enhanced cancer metastasis. Conversely, HOTAIR knockdown may
inhibit cell invasion and proliferation, alter progression of the
cell cycle, induce apoptosis, and increase sensitivity to
radiochemotherapy, indicating that HOTAIR is involved in the
modulation of cancer progression (9,14,15,19,25,37,38).
In the present study, HOTAIR mRNA expression levels were
investigated in 96 ESCC and para-carcinoma tissues, and HOTAIR
expression levels were significantly higher in ESCC compared within
para-carcinoma tissues. HOTAIR expression levels in the groups with
lymph node or organ metastasis were significantly higher than in
the group without metastasis. HOTAIR also demonstrated a
significant increasing trend from well-differentiated cancer
through moderately-differentiated cancer to poorly differentiated
cancer, and the more advanced TNM stage was also significantly rank
correlated with increased HOTAIR expression. Based on these
results, upregulated HOTAIR mRNA expression appears to be
heterogeneous among patients and is associated with various
clinicopathological factors, including lymph node/organ metastasis,
cancer differentiation and TNM stage. HOTAIR may participate in
ESCC occurrence, differentiation and metastasis, and it may be a
useful early diagnostic and prognostic marker.
EMT is involved in cancer development and
metastasis, and this process is characterized by the loss of
epithelial markers, including E-cadherin, and the gain of
mesenchymal markers, including Snail and β-catenin. β-catenin is
also a vital component of the Wnt signaling pathway.
Snail, which is a critical mesenchymal transcription
factor, is highly expressed and closely correlated with poor tumor
differentiation, metastasis, radiochemotherapy resistance, and
short survival in lung (39),
breast (40) and colon tumors
(41). The injection of Snail into
a pancreatic tumor mouse model significantly promoted metastasis
in vivo (42). Inhibition
of Snail expression also reduces the metastatic ability of ovarian
carcinoma cells in vitro (43). Snail expression demonstrated a
gradual increasing trend from well-differentiated cancer through
moderated-differentiated to poorly-differentiated cancer, and a
more advanced TNM stage was associated with increased Snail
expression with a significant rank correlation, indicating that
Snail may be involved in ESCC incidence, development and lymph
node/organ metastasis. Therefore, Snail may also be considered a
useful prognostic and predictive indicator for ESCC.
E-cadherin, which is expressed in epithelial cells,
is thought to be a metastatic suppressor during tumor progression.
E-cadherin mediates homotypic cell adhesion and maintains normal
morphology, epithelial cell polarity, and tissue structural
integrity by binding to cytosolic β-catenin, which is required for
EMT, to form the E-cadherin/β-catenin complex. The destruction of
the E-cadherin/β-catenin complex is closely correlated with the
recurrence and metastasis of colon (44,45),
lung (46) and bladder cancers
(47).
If the structure or function of β-catenin is
abnormal, E-cadherin expression is lost, or the
E-cadherin/β-catenin complex is broken, tumor cells may become
metastatic through reduced intracellular adherence and stimulated
cell proliferation. This is often observed in breast, lung,
gastrointestinal and other cancers (48–51).
Zhao et al (52)
demonstrated that the loss of E-cadherin and nuclear accumulation
of β-catenin were correlated with poor prognosis for patients with
head and neck squamous cell carcinoma.
The present study demonstrated that the E-cadherin
mRNA and protein expression levels were significantly lower in ESCC
compared with para-carcinoma tissues, whereas β-catenin mRNA and
protein expression levels were significantly higher in ESCC
compared with para-carcinoma tissues. Combined with previous
reports, this indicates that E-cadherin and β-catenin are important
to tumor development and metastasis. The present investigation
indicated that E-cadherin and β-catenin may participate in the
incidence, differentiation, invasion and metastasis of ESCC. These
factors may therefore be useful for the early diagnosis and
prognosis of ESCC.
Ge et al (53) revealed that high expression of
HOTAIR activates the Wnt pathway by inhibiting Wnt inhibitory
factor 1 expression, which leads to the accumulation of cytosolic
β-catenin. This translocates to the nucleus to promote the
expression of Snail, MMP13A, and other EMT-related factors.
Although direct evidence is lacking, the involvement of HOTAIR in
the regulation of EMT has been hypothesized. In addition to
previous data demonstrating that HOTAIR affects β-catenin (53), other investigations have
demonstrated that HOTAIR induces the expression of Snail (19,25).
Kogo et al (15) used gene
set enrichment analysis and demonstrated that HOTAIR overexpression
in colorectal cancer maybe associated with the multipotent
differentiation of colorectal cancer cells. Gene pathway analysis
also indicated that HOTAIR-regulated gene sets included E-cadherin,
which was lost in tissues with elevated HOTAIR expression. Xu et
al (54) demonstrated that
HOTAIR knockdown reversed EMT progression, leading to the
upregulation of E-cadherin and the downregulation of N-cadherin,
the marker of the mesenchymal phenotype. Gastric cancer
invasiveness, suppressed by HOTAIR knockdown, was also restored by
exogenous Snail.
However, the relationship between HOTAIR and
EMT-related factors in ESCC tissues remains unclear. The present
study demonstrated that HOTAIR expression levels were positively
correlated with Snail and β-catenin protein expression levels,
whereas it was negatively correlated with E-cadherin protein
expression levels.
Combined with these other results, HOTAIR appears to
participate in tumor invasion and metastasis by directly or
indirectly affecting the expression of EMT-related factors.
Therefore, HOTAIR expression may be used as a specific indicator of
tumor metastasis and prognosis and assist in therapeutic
planning.
In conclusion, HOTAIR, together with EMT-related
factors, may be a specific indicator for the occurrence, metastasis
and prognosis of ESCC. The results of the present study supported
the use of HOTAIR as a potential novel tumor molecular marker for
use in future therapies. However, there are some limitations to the
present study, including a small sample size. The specific
mechanisms underlying how HOTAIR regulates EMT-related factors in
ESCC require further investigation in future studies.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Xinjiang Uygur Autonomous Region (grant no.
2015211C115).
References
|
1
|
Pakzad R, Mohammadian-Hafshejani A,
Khosravi B, Soltani S, Pakzad I, Mohammadian M, Salehiniya H and
Momenimovahed Z: The incidence and mortality of esophageal cancer
and their relationship to development in Asia. Ann Transl Med.
4:292016.PubMed/NCBI
|
|
2
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tepper JE: Is radiation therapy needed in
the treatment of gastroesophageal junction adenocarcinoma?
Gastrointest Cancer Res. 2(4 Suppl): S2–S5. 2008.PubMed/NCBI
|
|
5
|
Linkous AG and Yazlovitskaya EM: Novel
radiosensitizing anticancer therapeutics. Anticancer Res.
32:2487–2499. 2012.PubMed/NCBI
|
|
6
|
Kotake Y, Nakagawa T, Kitagawa K, Suzuki
S, Liu N, Kitagawa M and Xiong Y: Long non-coding RNA ANRIL is
required for the PRC2 recruitment to and silencing of p15(INK4B)
tumor suppressor gene. Oncogene. 30:1956–1962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu HA and Bernstein E: Partners in
imprinting: Noncoding RNA and polycomb group proteins. Dev Cell.
15:637–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khalil AM, Guttman M, Huarte M, Garber M,
Raj A, Morales D Rivea, Thomas K, Presser A, Bernstein BE, van
Oudenaarden A, et al: Many human large intergenic noncoding RNAs
associate with chromatin-modifying complexes and affect gene
expression. Proc Natl Acad Sci USA. 106:11667–11672. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu C, Qu K, Zhong FL, Artandi SE and
Chang HY: Genomic maps of long noncoding RNA occupancy reveal
principles of RNA-chromatin interactions. Mol Cell. 44:667–678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|
|
13
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in hepatocellular carcinoma patients
following liver transplantation. Ann Surg Oncol. 18:1243–1250.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu ZH, Wang XL, Tang HM, Jiang T, Chen J,
Lu S, Qiu GQ, Peng ZH and Yan DW: Long non-coding RNA HOTAIR is a
powerful predictor of metastasis and poor prognosis and is
associated with epithelial-mesenchymal transition in colon cancer.
Oncol Rep. 32:395–402. 2014.PubMed/NCBI
|
|
17
|
Niinuma T, Suzuki H, Nojima M, Nosho K,
Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki
Y, et al: Upregulation of miR-196a and HOTAIR drive malignant
character in gastrointestinal stromal tumors. Cancer Res.
72:1126–1136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nie Y, Liu X, Qu S, Song E, Zou H and Gong
CL: Non-coding RNA HOTAIR is an independent prognostic marker for
nasopharyngeal carcinoma progression and survival. Cancer Sci.
104:458–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J
and Liu M: Long intergenic noncoding RNA HOTAIR is overexpressed
and regulates PTEN methylation in laryngeal squamous cell
carcinoma. Am J Pathol. 182:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui L, Xie XY, Wang H, Chen XL, Liu SL and
Hu LN: Expression of long non-coding RNA HOTAIR mRNA in ovarian
cancer. Sichuan Da Xue Xue Bao Yi Xue Ban. 44:57–59. 2013.(In
Chinese). PubMed/NCBI
|
|
23
|
Sung CO, Park CK and Kim SH:
Classification of epithelial-mesenchymal transition phenotypes in
esophageal squamous cell carcinoma is strongly associated with
patient prognosis. Mod Pathol. 24:1060–1068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelialmesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen LQ: Understanding and appraisal of
the new TNM classification for esophageal cancer in the AJCC Cancer
Staging Manual (7th edition). Zhonghua Zhong Liu Za Zhi.
32:237–240. 2010.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogemesis. 31:27–36. 2010. View Article : Google Scholar
|
|
29
|
Banerjee HN and Verma M: Epigenetic
mechanisms in cancer. Biomark Med. 3:397–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li SQ, Wang HM and Cao XF: Potential
clinical insights into microRNAs and their target genes in
esophageal carcinoma. Biomarkers. 16:629–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin R, Maeda S, Liu C, Karin M and
Edgington TS: A large noncoding RNA is a marker for murine
hepatocellular carcinomas and a spectrum of human carcinomas.
Oncogene. 26:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S,
Abu-lail R, Hochberg A and Galun E: The H19 non-coding RNA is
essential for human tumor growth. PLoS One. 2:e8452007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pasmant E, Sabbagh A, Masliah-Planchon J,
Ortonne N, Laurendeau I, Melin L, Ferkal S, Hernandez L, Leroy K,
Valeyrie-Allanore L, et al: Role of noncoding RNA ANRIL in genesis
of plexiform neurofibromas in neurofibromatosis type 1. J Natl
Cancer Inst. 103:1713–1722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Silva JM, Boczek NJ, Berres MW, Ma X and
Smith DI: LSINCT5 is overexpressed in breast and ovarian cancer and
affects cellular proliferation. RNA Biol. 8:496–505. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Srikantan V, Zou Z, Petrovics G, Xu L,
Augustus M, Davis L, Livezey JR, Connell T, Sesterhenn IA, Yoshino
K, et al: PCGEM1, a prostate-specific gene, is overexpressed in
prostate cancer. Proc Natl Acad Sci USA. 97:12216–12221. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galván JA, González MV, Crespo G,
Folgueras MV and Astudillo A: Snail nuclear expression parallels
higher malignancy potential in neuroendocrine lung tumors. Lung
Cancer. 69:289–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olmeda D, Moreno-Bueno G, Flores JM, Fabra
A, Portillo F and Cano A: SNAI1 is required for tumor growth and
lymph node metastasis of human breast carcinoma MDA-MB-231 cells.
Cancer Res. 67:11721–11731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Francí C, Gallén M, Alameda F, Baró T,
Iglesias M, Virtanen I and García de Herreros A: Snail1 protein in
the stroma as a new putative prognosis marker for colon tumours.
PLoS One. 4:e55952009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishioka R, Itoh S, Gui T, Gai Z, Oikawa
K, Kawai M, Tani M, Yamaue H and Muragaki Y: SNAIL induces
epithelial-to-mesenchymal transition in a human pancreatic cancer
cell line (BxPC3) and promotes distant metastasis and invasiveness
in vivo. Exp Mol Pathol. 89:149–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia
L, Wu Y, Zhou BP and Feng Y: Snail is critical for tumor growth and
metastasis of ovarian carcinoma. Int J Cancer. 126:2102–2111.
2010.PubMed/NCBI
|
|
44
|
Aamodt R, Bondi J, Andersen SN, Bakka A,
Bukholm G and Bukholm IR: The prognostic impact of protein
expression of E-cadherin-catenin complexes differs between rectal
and colon carcinoma. Gastroenterol Res Pract. 2010:6160232010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang H, Min BS, Lee KY, Kim NK, Kim SN,
Choi J and Kim H: Loss of E-cadherin and MUC2 expressions
correlated with poor survival in patients with stages II and III
colorectal carcinoma. Ann Surg Oncol. 18:711–719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chelidonis G, Kavantzas N, Patsouris E,
Pagaki E, Agrogiannis G and Athanasiadou P: DNA ploidy, E-cadherin,
beta-catenin expression and their clinicopathologic significance in
imprints of non-small cell lung cancer. Anal Quant Cytol Histol.
31:332–339. 2009.PubMed/NCBI
|
|
47
|
Baumgart E, Cohen MS, Neto B Silva, Jacobs
MA, Wotkowicz C, Rieger-Christ KM, Biolo A, Zeheb R, Loda M,
Libertino JA and Summerhayes IC: Identification and prognostic
significance of an epithelial-mesenchymal transition expression
profile in human bladder tumors. Clin Cancer Res. 13:1685–1694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Christofori G and Semb H: The role of the
cell-adhesion molecule E-cadherin as a tumour suppressor gene.
Trends Biochem Sci. 24:73–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Adhikary A, Chakraborty S, Mazumdar M,
Ghosh S, Mukherjee S, Manna A, Mohanty S, Nakka KK, Joshi S, De A,
et al: Inhibition of epithelial to mesenchymal transition by
E-cadherin up-regulation via repression of slug transcription and
inhibition of E-cadherin degradation: Dual role of scaffold/matrix
attachment region-binding protein 1 (SMAR1) in breast cancer cells.
J Biol Chem. 289:25431–25444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Beck TN, Chikwem AJ, Solanki NR and
Golemis EA: Bioinformatic approaches to augment study of
epithelial-to-mesenchymal transition (EMT) in lung cancer. Physiol
Genomics. 2014:699–724. 2014. View Article : Google Scholar
|
|
51
|
Zheng H, Li W, Wang Y, Xie T, Cai Y, Wang
Z and Jiang B: miR-23a inhibits E-cadherin expression and is
regulated by AP-1 and NFAT4 complex during Fas-induced EMT in
gastrointestinal cancer. Carcinogenesis. 35:173–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao Z, Ge J, Sun Y, Tian L, Lu J, Liu M
and Zhao Y: Is E-cadherin immunoexpression a prognostic factor for
head and neck squamous cell carcinoma (HNSCC)? A systematic review
and meta-analysis. Oral Oncol. 48:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, A prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ,
Huang L, Yu PF and Cheng XD: Knockdown of long non-coding RNA
HOTAIR suppresses tumor invasion and reverses epithelial-
mesenchymal transition in gastric cancer. Int J Biol Sci.
9:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|