Introduction
Cardiovascular disorders claim approximately 3.6
million lives each year worldwide, and among cardiovascular
disorders, myocardial infarction is the main cause of mortality
(1). The rapidly increasing
incidence of metabolic disorders including diabetes, obesity and
metabolic syndrome, combined with more aggressive treatment of
hypertension, is a causative factor for cardiovascular diseases.
The classical approach to the treatment of acute myocardial
infarction includes the use of primary percutaneous coronary
intervention or thrombolytic therapy, which aim at limiting the
size of the infarct. However, the process of reinstating blood flow
to the ischemic myocardium can cause further injury to heart tissue
and is referred to as ischemia-reperfusion damage. Even with the
most advanced medical treatment, ~10% of the patients die following
ischemia-reperfusion procedure and ~25% die of heart failure as a
consequence of ischemia-reperfusion (1). Experimental studies have validated
that oxidative stress generated from ischemia-reperfusion can also
lead to myocardial injury (1,2).
Lycium barbarum (LB; also known as
goji berry or wolfberry) is a member of the family Solanaceae, and
its fruit is well-known in traditional Chinese herbal medicine. At
present, it is widely consumed as food, with a number of beneficial
effects, including free radical scavenging, lipid lowering,
antidiabetic, immuno-modulating and anticancer activities (3–7).
Although LB has been traditionally been used in Chinese
medicine, its efficacy has been validated recently by numerous
studies from western countries (1,2). The
beneficial effects of LB fruit have been attributed to its
bioactive polysaccharide-protein complex (LBP) (8–12).
The LBP fractions were demonstrated to contain six monosaccharides,
galactose, rhamnose, glucose, mannose, arabinose and xylose, and 18
different amino acids (8–10). The efficacy of LBP has also been
validated in various preclinical models of liver damage, immune
dysfunction, diabetes and cancer (9,10,13–15).
Taurine, one of the 18 amino acids identified in LB, is
recommended as a complementary therapeutic agent for the management
of type II diabetes complications and is also known to inhibit
caspase-3 in animal models of ischemia-reperfusion (16,17).
To the best of our knowledge, there has been no
organized research study on the prevention of cardiovascular
disorders using LB at present. Thus, the current study aimed
to characterize the extracted polysaccharides of LB and then
evaluate the level of biomarkers such as lactate dehydrogenase (LD)
and nitric oxide (NO) and the activities of sodium-potassium ATPase
and calcium ATPase in the heart tissue in a rat model of
ischemia-reperfusion injury.
Materials and methods
Materials
Commercially available heat-dried wolfberries were
purchased from Rich Nature Nutraceutical Labs (Mukilteo, WA,
USA).
Polysaccharide extraction
Dry LB fruits (100 g) were treated with 300
ml CHCl3-MeOH (3:1 v/v) at 70°C under reflux for 2 h,
and the extract was passed through an acetone extraction while
maintaining the same environment. The lipid-free substance was
removed with the aid of 400 ml of ethanol at 70°C and then
introduction to 500 ml of water at 100°C for 3 h both under
reflux.
Monosaccharide composition
analyses
The monosaccharide composition was determined using
the acid digestion method. High-performance liquid chromatography
(HPLC) was used to examine the produced hydrolyzates. The
procedures were performed following the method of Fu and Oneill
(18).
Infrared spectrum analysis of LB
polysaccharides
With the aid of a Fourier transform infrared
spectrophotometer (Shimadzu Corporation, Kyoto, Japan) running OPUS
software, version 3.1 (Bruker Corporation, Billerica, MA, USA), the
infrared spectrum of LB polysaccharides was ascertained. For
transformation infrared spectrum analysis, 5 mg LB
polysaccharides was mixed with powdered KBr and pressed into
pellets. Measurements were made in a frequency range of 400–4,000
cm−1 (19).
Animals and operative procedures
For the present study, 36 adult Wistar male rats
were obtained from the Experimental Research Section of Linyi
Peoples Hospital (Shandong, China). Each rat weighed 250±10 g. They
were kept in cages and were maintained under a constant room
temperature and 12/12 h light/dark cycle. The rats were fed with
the commercially available rat chow and tap water ad
libitum. All the experimental procedures performed were
approved by the Shandong University Ethical Committee of School of
Medicine.
The rats were randomly divided into a control group
and three surgical groups. The hearts of the rats were perfused as
follows: The rats were anesthetized with 5% napental [30 mg/kg body
weight (BW); Shanghai Kefeng Chemical Reagent Co., Ltd., Shanghai,
China] and their hearts were immediately excised and placed in cold
(4°C) Krebs-Henseleit solution containing 118 mM NaCl, 4.8 mM KCl,
1.2 mM KH2PO4, 1.6 mM CaCl2, 1.2
mM MgSO4, 25 mM NaHCO3 and 11.5 mM glucose.
Within 2 min of thoracotomy, the hearts were mounted on an
experimental setup and retrogradely perfused with Krebs-Henseleit
solution (37°C) through the aorta at 15 ml/min (Minipuls-3
peristaltic pump; Gilson SAS, Villiers-Le Bel, France) and aerated
with 95% O2 + 5% CO2 to maintain normal pH,
pO2, and pCO2 levels.
A polyethylene catheter with a small latex balloon
at the tip (size 3; Enove Precision Plastics Catheter Co., Ltd.,
Jiangsu, China) was inserted in the left ventricular cavity through
the mitral valve opening to record the pressure in the left
ventricle. Hearts that did not reach the peak left ventricular
systolic pressure of 85–90 mmHg with left ventricular end-diastolic
pressure of 5–6 mmHg (contractile performance 8–10%) were removed
(n=3). Left ventricular developed pressure was calculated as the
difference between peak left ventricular systolic pressure and
ventricular end-diastolic pressure.
The three treatment groups had nine rats each and
were as follows: Model control, low-dose and high-dose groups. The
low-dose group received 150 mg/kg BW polysaccharides and the
high-dose group received 300 mg/kg BW polysaccharides. The rats
were anesthetized with 5% napental (30 mg/kg BW; Shanghai Kefeng
Chemical Reagent, Co., Ltd.). The hearts were rapidly removed and
stored at −70°C for biochemical measurements.
Biochemical analysis
The function of sodium-potassium ATPase was assayed
as described previously (20) with
certain changes. Briefly, the assay mixture was prepared by mixing
microsome (50 µg) and a buffer containing 50 mM Tris-HCl pH 7.4,
100 mM NaCl, 20 mM KCl, 1 mM EGTA, 5 mM ATP and 5 mM
MgCl2 to a final volume of 1 ml. Controls contained the
similar reaction mixture together with 1 mM ouabain (Nanjing
Chemlin Chemical Co., Ltd, Nanjing, China). ATP was added to the
tubes to initiate the reaction and they were incubated at 37°C for
10 min. To stop the reaction, 500 µl 20% trichloroacetic acid (TCA)
was added to the tubes, and the quantity of inorganic phosphate was
determined. The sodium-potassium ATPase function (percent
inhibition against the 100% value) was determined by measuring the
difference between the activity in the presence and absence of 1 mM
ouabain.
Calcium ATPase activity was determined as indicated
in a previous study (21). The
incubation mixture was prepared by mixing 40 mM Tris-HCl buffer (pH
7.0), 50 mM KCl, 5 mM MgCl2, 2 mM ATP (neutralized with
potassium hydroxide), 0.1 mM ouabain, microsomal protein (0.5–0.8
mg/ml) with either 2 mM EGTA or 20 mM CaCl2. The enzyme
reaction was initiated by adding microsomes, and the tubes were
incubated at 37°C for 10 min. Ice cold TCA (10%) was then added to
stop the reaction, and the amount of inorganic phosphate released
in terms of nmol/min/mg protein was recorded. Calcium ATPase
activity was measured by subtracting the values obtained with EGTA
from those obtained with CaCl2.
Immunohistochemistry was performed by the
avidin-biotin-peroxidase method (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) (22) on the
300-µm thick sections of heart tissue adjoining those used for
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) detection. The sections were first dewaxed (using xylene)
and rehydrated (using distilled water), and then placed into 0.3%
H2O2 solution. The sections were then
incubated with 1.5% normal goat serum to prevent nonspecific
binding and fulfill the endogenous peroxidase function. Rat
anti-Bcl-2 (cat no. #15071, Cell Signalling Technologies, Danvers,
MA, USA) and anti-Bax (cat no. #AF820, R&D Systems, Inc.,
Minneapolis, MN, USA) antibodies were separately diluted 1:200 and
1:300, respectively, and the sections were then incubated with
these primary antibodies in a humidified chamber for 24 h at 4°C.
Negative control sections were incubated without the primary
antibody. After washing with phosphate-buffered saline (PBS), the
sections were individually incubated with biotinylated secondary
antibody, goat-anti-rat immunoglobulin G (IgG; 1:200 dilution) (cat
no. #ab97057, Abcam, Cambridge, UK), along with an
avidin-biotin-peroxidase complex (Thermo Fisher Scientific, Inc.)
for 30 min. The sections were then rinsed in PBS and exposed to
diaminobenzidine and H2O2 in 50 mM Tris-HCl
(pH 7.6) for 3 min. Following a final bath, the immunostained
sections were dehydrated in a graded ethanol series. Bcl-2- and
Bax-positive cells were quantitatively analyzed using an image
analyzer system (Aperio ePathology eIHC IVD System, Leica
Biosystems, Beijing, China). The number of cells/mm of the CA1
pyramidal cell layer was calculated for each rat, and the average
value from the adjoining two sections was used.
Nitric acid (NO−) and lactic acid
concentrations were calculated using a fluorometric nitric oxide
assay kit (cat. no. #K252-200, BioVision, Inc., Milpitas, CA, USA).
The apoptotic rate was determined using flow-cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA).
Histopathological analysis
The triphenyl tetrazolium chloride method was used
to assess the injuries to the myocardium (23). Myocardial tissue was fixed in 10%
paraformaldehyde for 24 h and embedded in paraffin wax. Sections
were cut at 3 µm thick and were stained with hematoxylin and eosin
following standard procedure and examined under a light microscope
(highest magnification, ×400). The myocardial damage was graded as
follows: Grade III, severe injury to the myocardium characterized
by severe edema and predominant contraction bands; grade II,
moderate injury to the myocardium characterized by regular presence
of contraction bands and significant interstitial and cellular
edema; grade I, minor injury to the myocardium characterized by
hydropic cardiomyocytes, few contractions, and low grade
interstitial edema; and grade 0, myocytes appear normal without
hydrops, cell disruption or interstitial edema. Myocardial damage
was measured using the semi-quantitative scale reported by Miller
et al (24).
Data analysis
Statistical analysis was performed using one-way
analysis of variance, Mann-Whitney U-test and Duncan's multiple
range test, using SPSS for Windows, version 11.5 (SPSS, Inc.,
Chicago, IL, USA). Results were expressed as the mean ± standard
error, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Chemical analysis of LB
polysaccharides
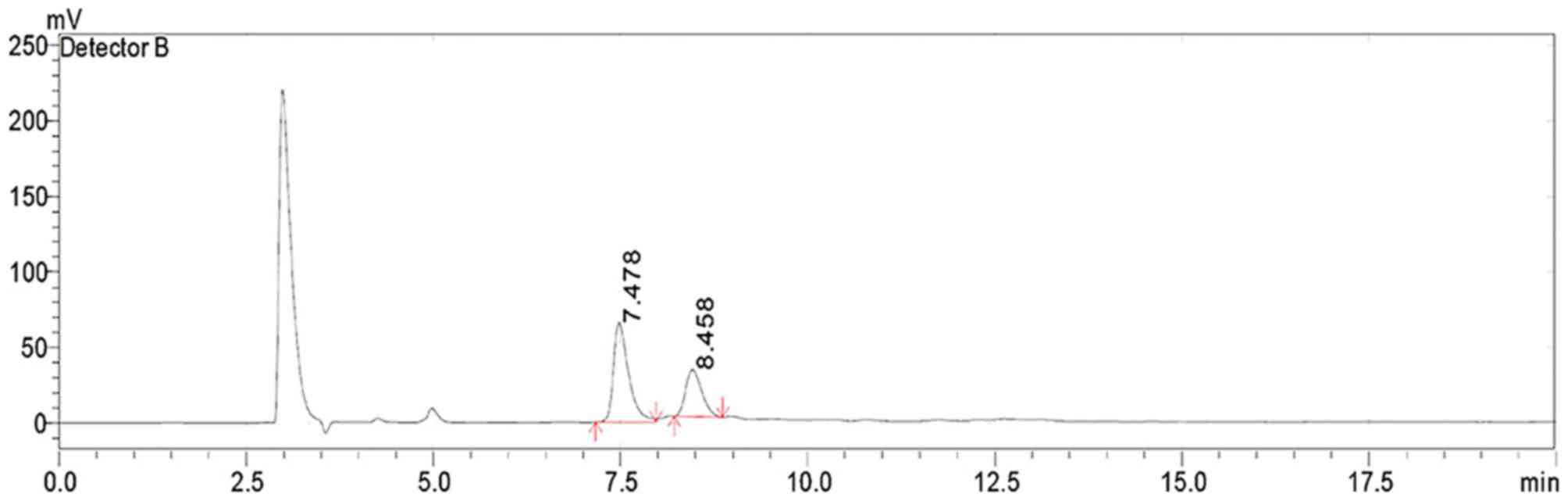
HPLC analysis identified that the LB
polysaccharides comprised two types of monosaccharides, glucose and
fructose in the molar ratio 1:2 (Fig.
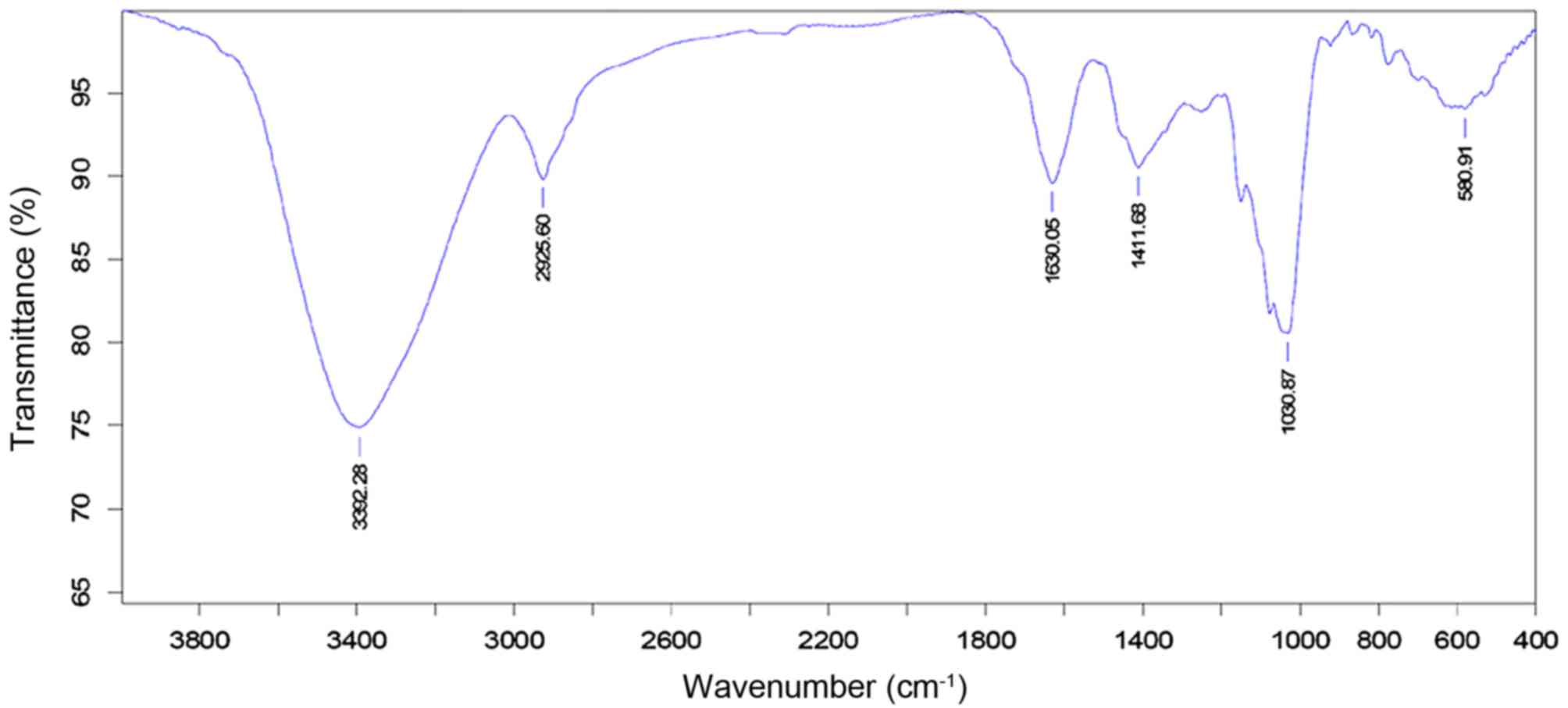
1). The infrared spectrum of LB polysaccharides depicted
a dominant, broad and stretching peak at 3,392 cm−1 for
the hydroxyl group and a weak band at 2,925 cm−1 showing
C-H stretching vibration. A band at 1,000 cm−1 referred
to the sugar units in the polysaccharide, and a band at 1,630
cm−1 to the bound water (Fig. 2).
Effect of LB polysaccharides on LD and
NO levels and sodium-potassium ATPase and calcium ATPase
activities
The pharmacological actions of the natural medicines
have been demonstrated previously (25–28).
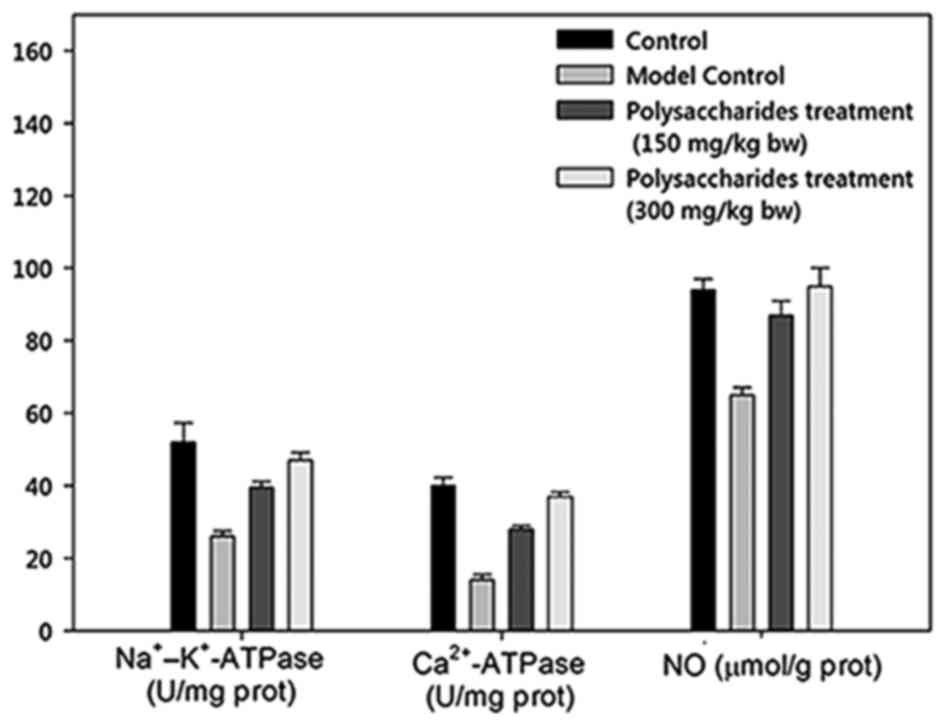
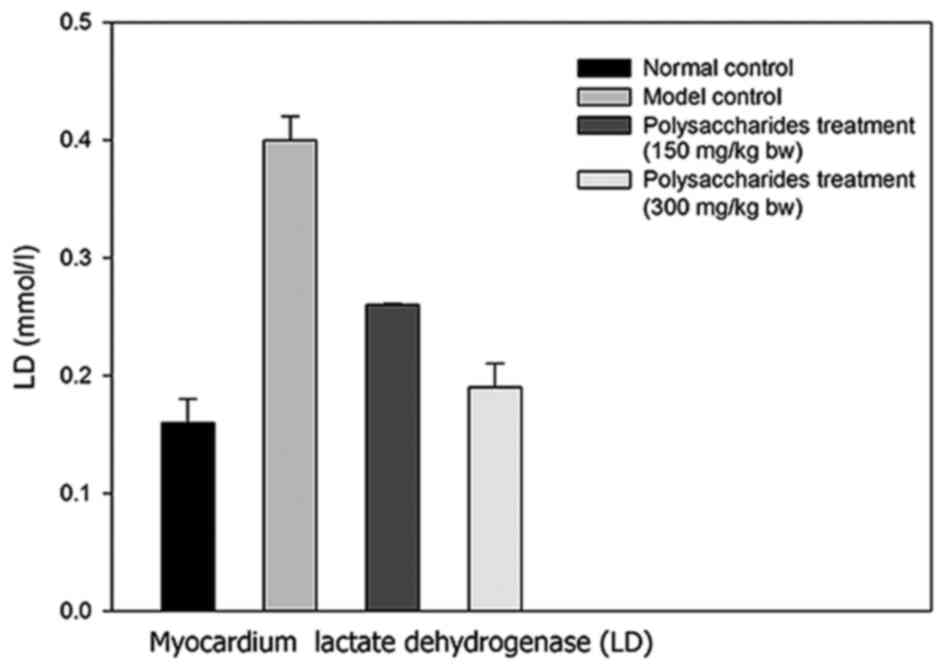
There was a marked increase in the myocardial LD concentration in
the model group rats in contrast to the healthy control rats
(Figs. 3 and 4). Notably, there was a significant
increase in myocardial LD levels in rats receiving 150 and 300
mg/kg BW LB polysaccharides. Sodium-potassium ATPase and
calcium ATPase activities were significantly decreased in the model
controlled group than in healthy controlled rats. Comparatively,
there was a significant increase in the myocardial sodium-potassium
and calcium ATPase activities in rats receiving 150 and 300 mg/kg
BW LB polysaccharides.
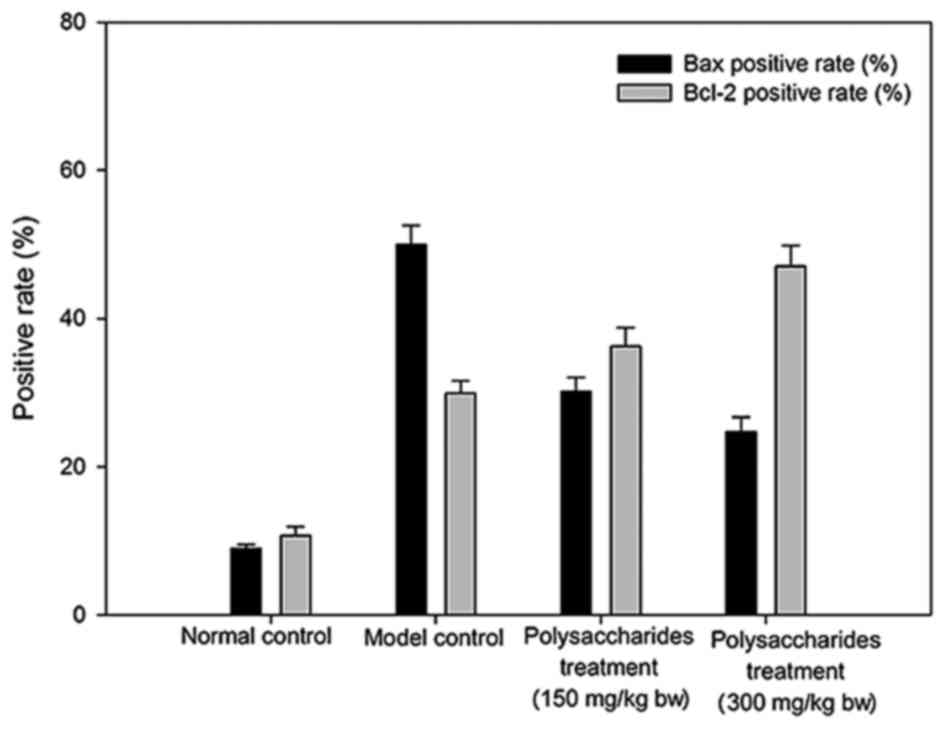
Effect of LB polysaccharides on Bax
and Bcl-2
The model group rats demonstrated markedly greater
Bax and Bcl-2 positive rates than the healthy group rats. Rats that
were administered LB polysaccharides demonstrated
comparatively higher Bcl-2 positive rates and significantly higher
Bax positive rates (P<0.01) when compared with model control
rats (Fig. 5).
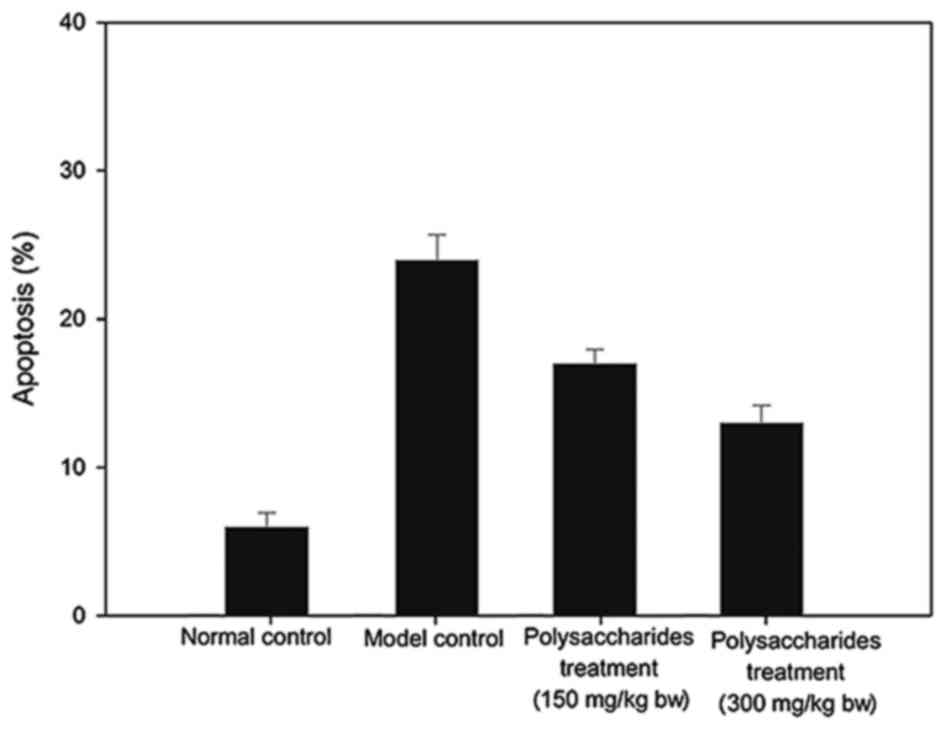
Fig. 6 presents the
myocardial apoptotic rates. Despite the comparatively significant
increase in apoptosis of the myocardium cells in the model group in
comparison with the healthy group, these variations were remedied
with an LB polysaccharides supplement.
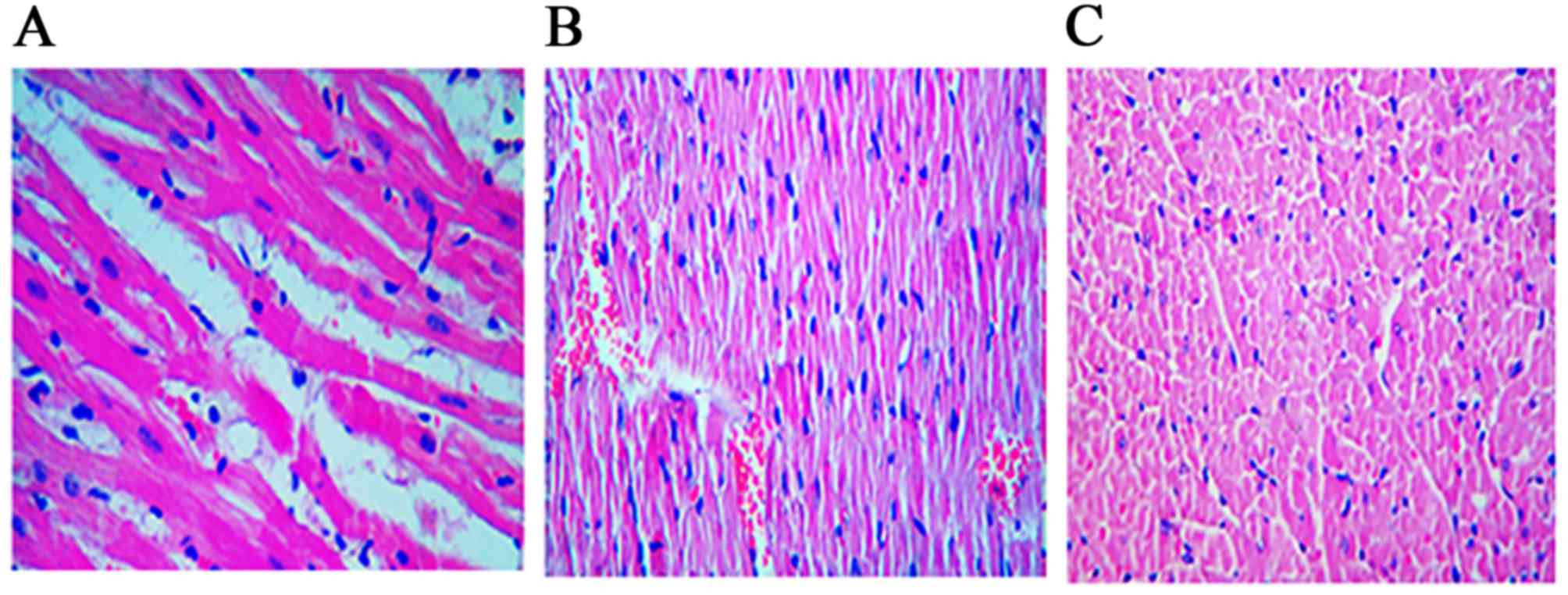
Histopathological changes
There were significant histological differences
(P=0.022) between the control and study groups. The control group
demonstrated higher grade II damage, and the study group
demonstrated decreased myocardial edema (P<0.05; Fig. 7). Student's t-test for independent
groups was performed to compare the myocardial damage in each
group.
Discussion
LB fruits have been used in Chinese
traditional medicine, however there have been limited reports on
their efficacy in ischemia-reperfusion damage. The glucose lowering
and anti-apoptotic activity of LB has been well documented
(29) and there is increasing
evidence of its efficacy in age-associated macular degeneration
(30). The present study
demonstrated that fructose and glucose were the most abundant
monosaccharides. The fruits of LB contained 18 different
amino acids, the most abundant of which was taurine (31). Although, taurine is a partially
essential amino acid in humans (32), it has demonstrated efficacy in type
II diabetes and ischemic cardiomyocytes (16). The present study also confirmed
that the cardioprotective properties of LB polysaccharide
was via increased sodium-potassium ATPase and calcium ATPase
activities.
The present study also demonstrated that the
administration of LB polysaccharides significantly decreased
myocardial LD levels. Sharikabad et al (33) demonstrated that Ca2+ and
Na+ alterations in heart failure decrease the tolerance
to ischemia by increasing Ca2+ overload. It has been
established that an increase in the sodium ion causes excessive
Ca2+ entry via reverse (Ca2+ influx mode)
Na+/Ca2+ exchange during ischemia-reperfusion
(34,35). The Na+/Ca2+
exchange is increased in myocardium disease (36,37).
Sjaastad et al (37)
reported that cardiomyocytes from rats with chronic heart failure
demonstrated higher Ca2+ influx in an experimental model
of Na+-loaded cells. It has also been reported that
intracellular Na+ concentration is increased in chronic
heart disease (38), which causes
an increase in Ca2+ influx through the
Na+/Ca2+ exchanger (39).
Calcium overload is the determinant to
ischemia-reperfusion damage of the myocardium. Increased
concentration of intracellular Ca2+ also increases the
activity of degrading enzymes (40,41),
which damages mitochondria and causes arrhythmias (35,42).
Therefore, alterations in Na+ and Ca2+ may
render the myocardium prone to ischemia-reperfusion damage.
In conclusion, the data collected from the
ischemia-reperfusion model confirmed that LB polysaccharides
prevent damage to heart tissue by decreasing the myocardium LD and
NO levels and increasing sodium-potassium ATPase and calcium ATPase
activities. The present study requires further validation in a
clinical setting and its efficacy in patients with myocardial
infarction remains to be established.
References
|
1
|
Keeley EC, Boura JA and Grines CL: Primary
angioplasty versus intravenous thrombolytic therapy for acute
myocardial infarction: A quantitative review of 23 randomised
trials. Lancet. 361:13–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zweier JL: Measurement of
superoxide-derived free radicals in the reperfused heart. Evidence
for a free radical mechanism of reperfusion injury. J Biol Chem.
263:1353–1357. 1988.PubMed/NCBI
|
|
3
|
Mocan A, Vlase L, Vodnar DC, Gheldiu AM,
Oprean R and Crişan G: Antioxidant, antimicrobial effects and
phenolic profile of Lycium barbarum L. Flowers. Molecules.
20:15060–15071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gündüz E, Dursun R, Zengin Y, İçer M,
Durgun HM, Kanıcı A, Kaplan İ, Alabalık U, Gürbüz H and Güloğlu C:
Lycium barbarum extract provides effective protection against
paracetamol-induced acute hepatotoxicity in rats. Int J Clin Exp
Med. 8:7898–7905. 2015.PubMed/NCBI
|
|
5
|
Tang HL, Chen C, Wang SK and Sun GJ:
Biochemical analysis and hypoglycemic activity of a polysaccharide
isolated from the fruit of Lycium barbarum L. Int J Biol Macromol.
77:235–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao K, Liu M, Cao J, Yao M, Lu Y, Li J,
Zhu X, Yang Z and Wen A: Protective effects of Lycium barbarum
polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through
the ROS-NO pathway. Molecules. 20:293–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW,
He ZX, Sun T, Zhang X, Zhao RJ, Gu L, et al: An evidence-based
update on the pharmacological activities and possible molecular
targets of Lycium barbarum polysaccharides. Drug Des Devel Ther.
9:33–78. 2014.PubMed/NCBI
|
|
8
|
Huang L, Lin Y, Tian G and Ji G:
Isolation, purification and physico-chemical properties of
immunoactive constituents from the fruit of Lycium barbarum L. Yao
Xue Xue Bao. 33:512–516. 1998.(In Chinese). PubMed/NCBI
|
|
9
|
Gan L, Zhang SH, Liu Q and Xu HB: A
polysaccharide-protein complex from Lycium barbarum upregulates
cytokine expression in human peripheral blood mononuclear cells.
Eur J Pharmacol. 471:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gan L, Hua ZS, Liang YX and Xu Bi H:
Immunomodulation and antitumor activity by a polysaccharide-protein
complex from Lycium barbarum. Int Immunopharmacol. 4:563–569. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu AJ, Wu JM, Yu DH and Wang R:
Protective effect of Lycium barbarum polysaccharides on oxidative
damage in skeletal muscle of exhaustive exercise rats. Int J Biol
Macromol. 42:447–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Xu J, Zhu R, Zhu Y, Zhao Y, Chen P,
Pan C, Yao W and Gao X: Fingerprinting profile of polysaccharides
from Lycium barbarum using multiplex approaches and chemometrics.
Int J Biol Macromol. 78:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK
and Kim CH: Protective effect of Lycium chinense fruit on carbon
tetrachloride-induced hepatotoxicity. J Ethnopharmacol. 96:529–535.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo Q, Cai Y, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong H, Shen P, Jin L, Xing C and Tang F:
Therapeutic effects of Lycium barbarum polysaccharide (LBP) on
irradiation or chemotherapy-induced myelosuppressive mice. Cancer
Biother Radiopharm. 20:155–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu X, Xu Z, Mi M, Xu H, Zhu J, Wei N, Chen
K, Zhang Q, Zeng K, Wang J, et al: Dietary taurine supplementation
ameliorates diabetic retinopathy via anti-excitotoxicity of
glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem
Res. 33:500–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takatani T, Takahashi K, Uozumi Y, Matsuda
T, Ito T, Schaffer SW, Fujio Y and Azuma J: Taurine prevents the
ischemia-induced apoptosis in cultured neonatal rat cardiomyocytes
through Akt/caspase-9 pathway. Biochem Biophys Res Commun.
316:484–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu DT and O'Neill RA: Monosaccharide
composition analysis of oligosaccharides and glycoproteins by
high-performance liquid chromatography. Anal Biochem. 227:377–384.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parikh A and Madamwar D: Partial
characterization of extracellular polysaccharides from
cyanobacteria. Bioresour Technol. 97:1822–1827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ver A, Csermely P, Bányász T, Kovács T and
Somogyi J: Alterations in the properties and isoform ratios of
brain Na+/K(+)-ATPase in streptozotocin diabetic rats. Biochim
Biophys Acta. 1237:143–150. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grosman N: Similar effects of ether
phospholipids, PAF and lyso-PAF on the Ca(2+)-ATPase activity of
rat brain synaptosomes and leukocyte membranes. Int
Immunopharmacol. 1:1321–1329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jordan RC, Catzavelos GC, Barrett AW and
Speight PM: Differential expression of bcl-2 and bax in squamous
cell carcinomas of the oral cavity. Eur J Cancer B Oral Oncol.
32B:394–400. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buerke M, Murohara T, Skurk C, Nuss C,
Tomaselli K and Lefer AM: Cardioprotective effect of insulin-like
growth factor I in myocardial ischemia followed by reperfusion.
Proc Natl Acad Sci USA. 92:8031–8035. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller S, Schick F, Scheule AM, Vogel U,
Hiller R, Strotmann C, Naegele T, Hahn U and Claussen CD:
Conventional high resolution versus fast T(2)-weighted MR imaging
of the heart: Assessment of reperfusion induced myocardial injury
in an animal model. Magn Reson Imaging. 18:1069–1077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mocan A, Vlase L, Vodnar DC, Bischin C,
Hanganu D, Gheldiu AM, Oprean R, Silaghi-Dumitrescu R and Crișan G:
Polyphenolic content, antioxidant and antimicrobial activities of
Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules.
19:10056–10073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Potterat O: Goji (Lycium barbarum and L.
chinense): Phytochemistry, pharmacology and safety in the
perspective of traditional uses and recent popularity. Planta Med.
76:7–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin M, Huang Q, Zhao K and Shang P:
Biological activities and potential health benefit effects of
polysaccharides isolated from Lycium barbarum L. Int J Biol
Macromol. 54:16–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li XM, Ma YL and Liu XJ: Effect of the
Lycium barbarum polysaccharides on age-related oxidative stress in
aged mice. J Ethnopharmacol. 111:504–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao H, Alexeev A, Chang E, Greenburg G
and Bojanowski K: Lycium barbarum glycoconjugates: Effect on human
skin and cultured dermal fibroblasts. Phytomedicine. 12:131–137.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng CY, Chung WY, Szeto YT and Benzie
IF: Fasting plasma zeaxanthin response to Fructus barbarum L.
(wolfberry; Kei Tze) in a food-based human supplementation trial.
Br J Nutr. 93:123–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie H and Zhang S: Determination of
taurine in Lycium barbarum L. by high performance liquid
chromatography with OPA-urea pre-column derivatization. Se Pu.
15:54–56. 1997.(In Chinese). PubMed/NCBI
|
|
32
|
Gaull GE: Taurine as a conditionally
essential nutrient in man. J Am Coll Nutr. 5:121–125. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharikabad MN, Aronsen JM, Haugen E,
Pedersen J, Møller AS, Mørk HK, Aass HC, Sejersted OM, Sjaastad I
and Brørs O: Cardiomyocytes from postinfarction failing rat hearts
have improved ischemia tolerance. Am J Physiol Heart Circ Physiol.
296:H787–H795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takayama E, Guo LL, Digerness SB and Pike
MM: Early reperfusion levels of Na(+) and Ca(2+) are strongly
associated with postischemic functional recovery but are
disassociated from K(ATP) channel-induced cardioprotection. J Mol
Cell Cardiol. 37:483–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tani M and Neely JR: Role of intracellular
Na+ in Ca2+ overload and depressed recovery of ventricular function
of reperfused ischemic rat hearts. Possible involvement of H+-Na+
and Na+-Ca2+ exchange. Circ Res. 65:1045–1056. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hasenfuss G and Pieske B: Calcium cycling
in congestive heart failure. J Mol Cell Cardiol. 34:951–969. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sjaastad I, Bentzen JG, Semb SO, Ilebekk A
and Sejersted OM: Reduced calcium tolerance in rat cardiomyocytes
after myocardial infarction. Acta Physiol Scand. 175:261–269. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pieske B, Kretschmann B, Meyer M,
Holubarsch C, Weirich J, Posival H, Minami K, Just H and Hasenfuss
G: Alterations in intracellular calcium handling associated with
the inverse force-frequency relation in human dilated
cardiomyopathy. Circulation. 92:1169–1178. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pieske B and Houser SR: [Na+]i handling in
the failing human heart. Cardiovasc Res. 57:874–886. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawasaki H and Kawashima S: Regulation of
the calpain-calpastatin system by membranes (review). Mol Membr
Biol. 13:217–224. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Van der Vusse GJ, Reneman RS and van
Bilsen M: Accumulation of arachidonic acid in ischemic/reperfused
cardiac tissue: Possible causes and consequences. Prostaglandins
Leukot Essent Fatty Acids. 57:85–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyamae M, Camacho SA, Weiner MW and
Figueredo VM: Attenuation of postischemic reperfusion injury is
related to prevention of [Ca2+]m overload in rat hearts. Am J
Physiol. 271:H2145–H2153. 1996.PubMed/NCBI
|