Introduction
Vascular remodeling is the primary reason for the
failure of angioplasty surgeries, including vascular stenting
(1), transplant vasculopathy
(2) and vein grafts (3), and represents a major problem for
vascular surgical procedures (4).
Coronary artery bypass grafting is widely performed using
autologous saphenous vein and internal mammary artery grafts,
however, ~50% of patients who undergo the procedure require
reoperation 10–15 years later to treat vascular restenosis
(5).
Cell proliferation, migration and apoptosis are the
main active processes in vascular remodeling (6), particularly in vascular smooth muscle
cells (VSMCs) (7,8). Several animal models of vascular
remodeling have been developed to explore the molecular mechanisms
responsible for intimal hyperplasia, including wire-insertion, vein
bypass grafting and common carotid artery (CCA) ligation models
(9). In a murine CCA ligation
model, leukocyte infiltration occurred 1 week after ligation
(10), and smooth muscle cell
(SMC)-rich neointima formation and an 80% luminal area reduction
was observed 4 weeks after ligation (11).
Numerous restenosis-associated proteins and genes
have been identified, including osteopontin (12), periostin (13), connexin 34 (14) and microRNA-21 (15), however, the association between
ubiquitin-specific peptidase 39 (USP39) and restenosis remains to
be investigated. USP39 is a 65-kDa SR protein-related member of the
ubiquitin-specific protease family, which harbors a
deubiquitinating enzyme (DUB) domain (16). Significantly increased USP39
expression has been observed in breast cancer tissues compared with
paracancer tissues and normal tissues (17) and the embryonic zebrafish brain
(18). In vitro removal of
small ubiquitin-like modifiers (SUMOs), termed deSUMOlyation,
strengthened the proliferation-enhancing effect of USP39 in
prostate cancer cells (19).
However, USP39 lacks three residues critical for protease activity
and has been revealed to be inactive as a DUB (20). However, to the best of our
knowledge, no previous study to date has assessed the involvement
of USP39 in the context of intimal hyperplasia and vascular
remodeling. In the present study, the expression and potential
novel functions of USP39 in relation to vascular remodeling were
investigated. USP39 protein expression levels were determined in
ligated arteries in mice and in a pig vein graft model, and the
involvement of USP39 in VSMC proliferation and migration was
examined.
Materials and methods
Animals and cell culture
All animal procedures were approved by the Animal
Care and Use Committee of Xiamen University [Xiamen, China; license
no: SYXK (Min) 2008-0003, issued May 6, 2008]. C57BL/6J mice (male;
8 weeks old; 27–30 g; n=18) were obtained from the Xiamen
University Class SPF Animal Laboratory Center (Xiamen, China). The
mice were assigned randomly into two groups (control and surgery)
and kept in a 12/12 h light/dark cycle, 25°C, with ad libitum
access to food and water. Large White pigs weighing 35–45 kg (male;
n=16) were obtained from the Prince of Wales Hospital Institute,
Chinese University of Hong Kong (Hong Kong, China), and were kept
in a 12/12 h light/dark cycle, 26°C, with ad libitum access to food
and water prior to surgery. Pigs were assigned randomly into four
groups, according to the time point at which vein grafts were to be
harvested: Postoperative, and at 2, 4 and 12 weeks (12). Other steps were performed as
described previously (21).
C57BL/6 mouse VSMCs (Nanjing Mucyte Bio Tech Co., Ltd.; http://www.mucyte.com/index.php) were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin in a 95% humidified, 5% CO2
incubator at 37°C. Cells from passages 3–8 were used in all
experiments. For VSMC stimulation, cells were cultured in 6-well
plates in DMEM, grown to 70% confluence and washed with
phosphate-buffered saline 12 h later. The medium was replaced with
serum-free medium and the cells were stimulated with 0, 50, 100,
200, 300 or 400 ng/ml lipopolysaccharide (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) for a further 12 h at 37°C.
Mouse CCA ligation
Mice underwent ligation of the left carotid artery
near the distal bifurcation, as described previously (11). Mice were anesthetized with 10%
chloral hydrate (400 mg/kg) by intraperitoneal injection. The left
CCA was dissected free of connective tissue and completely ligated
with 6–0 silk sutures just proximal to the common carotid
bifurcation. In control mice, the suture was passed under the
exposed carotid artery without ligation. At each time point (2 and
4 weeks after the procedure), mice were euthanized by
CO2 inhalation and the left CCAs were carefully excised
and stored in liquid nitrogen.
Pig vein-carotid artery interposition
grafting
The animals received humane care according to the
Guide for the Care and Use of Laboratory Animals. Pigs underwent
vein-carotid artery interposition grafting as follows. Anaesthesia
was induced with ketamine (30 mg/kg) and atropine (0.6 mg/kg)
administered intramuscularly. A section of the saphenous vein (~12
cm) from the right leg of each pig was dissected free from
surrounding tissue. Two para-sternocleidomastoid muscle
longitudinal neck incisions were made and the CCAs were carefully
dissected from the internal jugular vein and vagus nerve within the
carotid sheath. End-to-end anastomoses of the saphenous vein to CCA
were then performed using continuous 7–0 polypropylene sutures. The
pigs were sacrificed under general anaesthesia when the grafts were
removed using an IV injection of ketamine (>150 mg/kg). Other
steps were performed as described previously (12).
Morphometric analysis and
immunohistochemistry
The arteries (mice) and veins (pigs) were dissected,
embedded in paraffin, and serial 4 µm-sections were taken for
morphometric analysis. Sections of carotid artery and vein grafts
were stained with hematoxylin and eosin. Masson's trichrome
staining (cat. no. PT003; Shanghai Bogoo Biotechnology. Co., Ltd.,
Shanghai, China) was performed in mice carotid artery sections. The
thickness of the neointima samples was examined by light microscopy
(Olympus IX51; Olympus Corporation, Tokyo, Japan), and analyzed
using dedicated image-analysis software (Image-Pro-Plus 6.0; Media
Cybernetics, Rockville, MD, USA). Three serial sections of each
vessel were analyzed to measure neointima thickness and USP39
protein expression, and the average was calculated as a standard
for statistical analysis. Immunohistochemistry was performed using
an immunohistochemistry kit (Elivision plus kit 9901; Fuzhou Maixin
Biotechnology Co., Ltd., Fuzhou, China) and a DAB Color Developing
Reagent kit (Fuzhou Maixin Biotechnology Co., Ltd.). USP39 antibody
(1:200; cat. no. ab131244; Abcam, Cambridge, UK) was used as the
primary antibody, with phosphate-buffered saline as the negative
control. Goat anti-mouse immunoglobulin G (IgG) from the
immunohistochemistry kit (cat. no. 9901; Elivision Plus kit; Fuzhou
Maixin Biotechnology Co., Ltd., Fuzhou, China) was used as the
secondary antibody. The primary antibody was incubated 4°C
overnight and secondary antibody was incubated for 10 min at room
temperature. Slides were observed under a light microscope (x400
magnification). Immunohistochemical positivity was determined by
the ratio of USP39-positive cells to the total number of
hematoxylin-positive nuclei in a defined field (four slides
observed per condition, and three fields of view assessed per
slide).
Western blotting analysis
Mouse VSMCs were pulverized in RIPA buffer (Sangon
Biotech Co., Ltd., Shanghai, China) and sonicated to disrupt the
integrity of cell membranes and extract total proteins. Whole cell
lysates were then centrifuged at 4000 × g for 15 min at 4°C. Total
protein was quantified using the Bradford method. Total protein (20
µg per lane) was separated by 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene difluoride membrane. Other steps were performed as
described previously (14). USP39
(cat. no. ab131244; 1:1,000; Abcam), cyclin D1 (cat. no.
60186-1-Ig; 1:1,000; ProteinTech Group, Inc., Chicago, IL, USA),
and cyclin-dependent kinase 4 (CDK4; cat. no. 11026-1-AP; 1:1,000;
ProteinTech Group, Inc.) antibodies were used as primary
antibodies. β-tubulin antibody (cat. no. 10094-1-AP; ProteinTech
Group, Inc.) was used to ensure equal loading quantities of protein
samples.
Protein overexpression, virus packing
and transfection of VSMCs
In order to overexpress USP39, plasmids were
constructed in our laboratory using the pDEST_LTR_N_IRES_puro
vector, that added myc, hemagglutinin (HA) and FLAG tags to USP39.
For USP39 knockdowns, packing plasmids (pHR and pVSVG; Shanghai
Jikai Industry Co., Shanghai, China) were mixed with lentivirus
vectors containing the following small interfering RNA (siRNA)
sequences: siRNA usp39-70, 5′-GAATAACATAAAGGCCAAT-3′; siRNA
usp39-71, 5′-CGGGTATTGTGGGACTGAA-3′; siRNA usp39-72,
5′-TTCCAGACAACTATGAGAT-3′; siRNA usp39-73,
5′-TTTGGAAGAGGCGAGATAA-3′ (Shanghai Jikai Industry Co.). The
mixtures were then added to Opti-MEM medium (Gibco; Thermo Fisher
Scientific, Inc.). Lipofectamine 2000 (ProteinTech Group, Inc.) was
then mixed Opti-MEM for 5 min. The total transfection mixture was
allowed to stand for ~20 min and was then added to 6-well plates
containing 293T cells (Medical College of Xiamen University,
Xiamen, China) at 70% confluence. The virus was harvested ~48 h
later. Other steps were performed as described previously (14). For transfection, VSMCs at 70%
confluence were cultured in 12-well plates and incubated with
lentivirus for 24 h. The medium was replaced with DMEM 24 h
later.
VSMC proliferation
VSMC proliferation was quantitated by cell counting
using a hemocytometer (Bio-Rad TC20; Bio-Rad Laboratories, Inc.,
Hercules, MA, USA) and MTT assay. Transfected cells [siControl
group (control vector), siRNA usp39-73 group, control group (empty
vector) and myc-USP39 group] were cultured in 100 µl DMEM, seeded
in a 96-well plate and incubated for 3 days. Cells were
subsequently detached with trypsin and counted by a hemocytometer.
For the MTT assay, cells of each group were incubated for 1, 3 and
5 days, then 5 h before the end of the incubation 20 µl MTT (5
mg/ml) was added to each well. Culture supernatants were then
removed and resuspended in 400 µl isopropanol to dissolve the MTT
formazan, and the absorbance was measured at 490 nm using an ELISA
microplate reader (Bio-Rad Model 680; Bio-Rad Laboratories, Inc.).
Each assay was performed in triplicate.
VSMC migration
Cell migration was assessed by Transwell assay,
using the same groups as for the VSMC proliferation assays. Serum
free DMEM medium (200 µl) containing 5,000 VSMCs was seeded into
the upper chambers of 8 µm-pore size Transwell filters (cat. no.
14831; Corning Incorporated, Corning, NY, USA). In the lower
chamber, 500 µl DMEM containing 10% fetal bovine serum was added,
which served as a chemoattractant. Non-invading cells on the upper
chambers were removed with a cotton-tipped swab following 12 h
incubation. The lower sides of the filters were then fixed in 4%
paraformaldehyde for 30 min and stained with methylrosanilinium
chloride. The invading cells were viewed under an Olympus
microscope (Olympus IX51; Olympus Corporation) and counted at ×40
magnification (four fields of view for each group). The ability of
the cells to invade was expressed as the mean number of cells in
the entire field. The assay was performed three times.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistically significant differences were evaluated using
Student's t-tests when 2 groups were compared or for multiple
comparisons, analysis of variance followed by Student-Newman-Keuls
tests. Data were analyzed using Prism 6.0 software (GraphPad
Software, Inc., San Diego, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
USP39 is expressed in the ligated
arterial wall
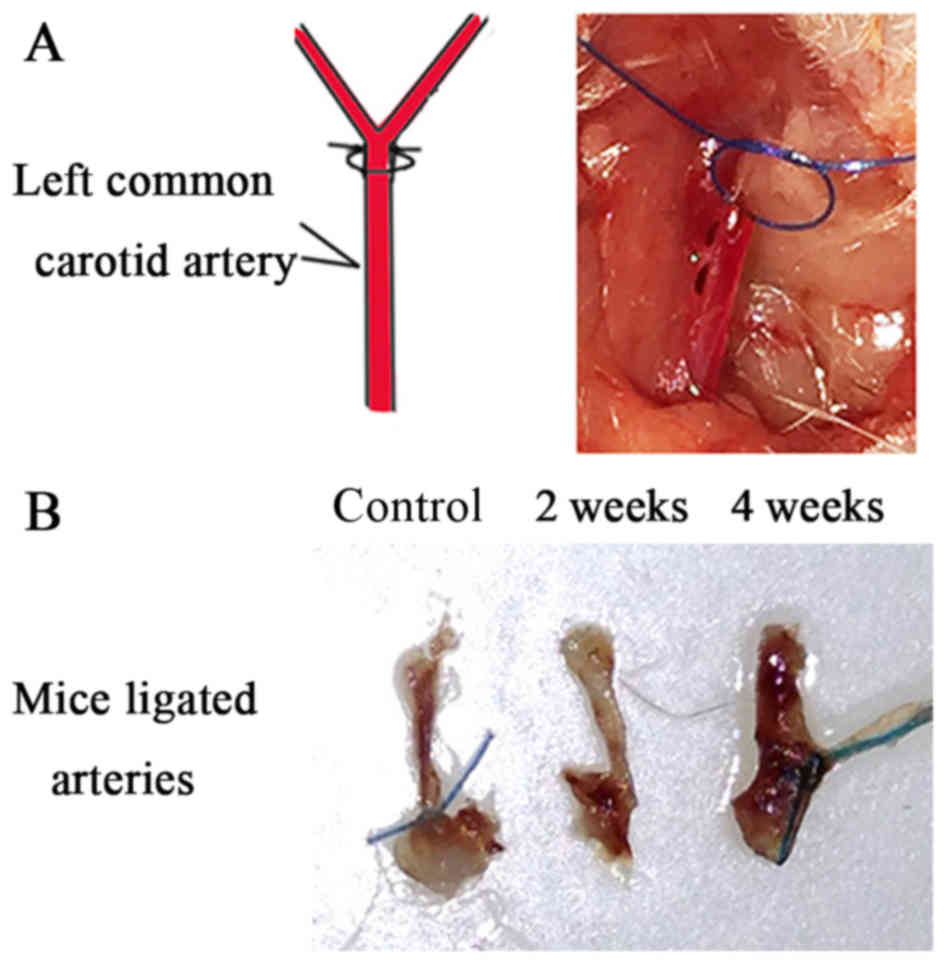
CCA ligation (Fig.
1A) resulted in vascular remodeling. The thickening of ligated
arteries in mice was assessed 2 and 4 weeks following surgery
(Fig. 1B). Morphometric analysis
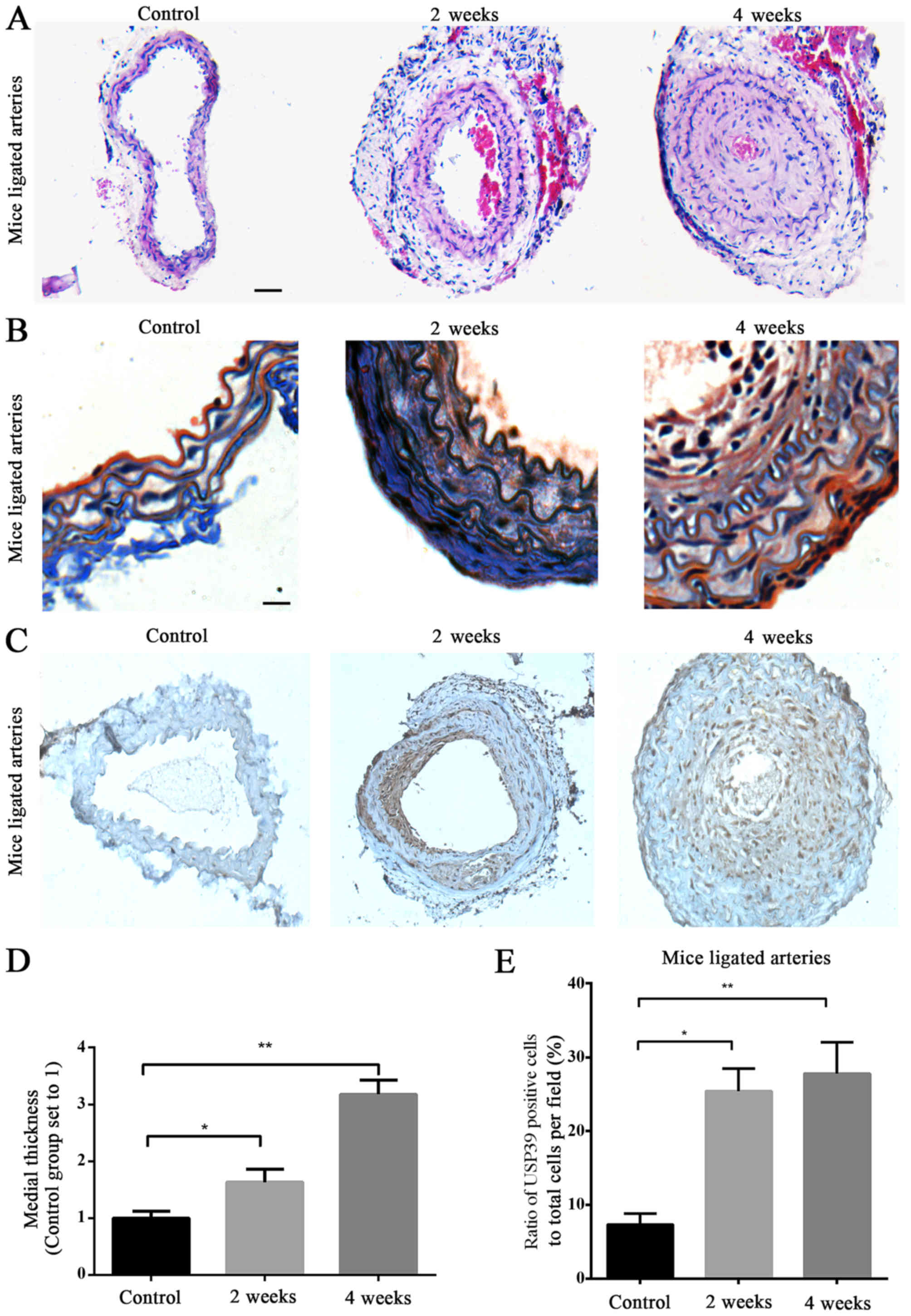
of hematoxylin and eosin (Fig. 2A)
and Masson's trichrome-stained sections (Fig. 2B) was performed.
Immunohistochemical analysis revealed positive staining for USP39,
which was predominantly localized in the neointimal layer, and was
also present in the nucleus (Fig.
2C). Analysis of mice carotid artery sections confirmed that
the neointima thickness was increased in the ligated arteries 2 and
4 weeks after surgery, compared with the non-ligated control
arteries (P=0.0125 and P=0.0046, respectively; Fig. 2D). Significantly increased USP39
protein expression was observed at 2 and 4 weeks in ligated carotid
arteries compared with the non-ligated control arteries (P=0.0136
and P=0.0014, respectively; Fig.
2E) and USP39 was more abundant at 4 weeks in ligated carotid
arteries compared with at 2 weeks (Fig. 2E).
USP39 expression is elevated during
restenosis in pig vein grafts
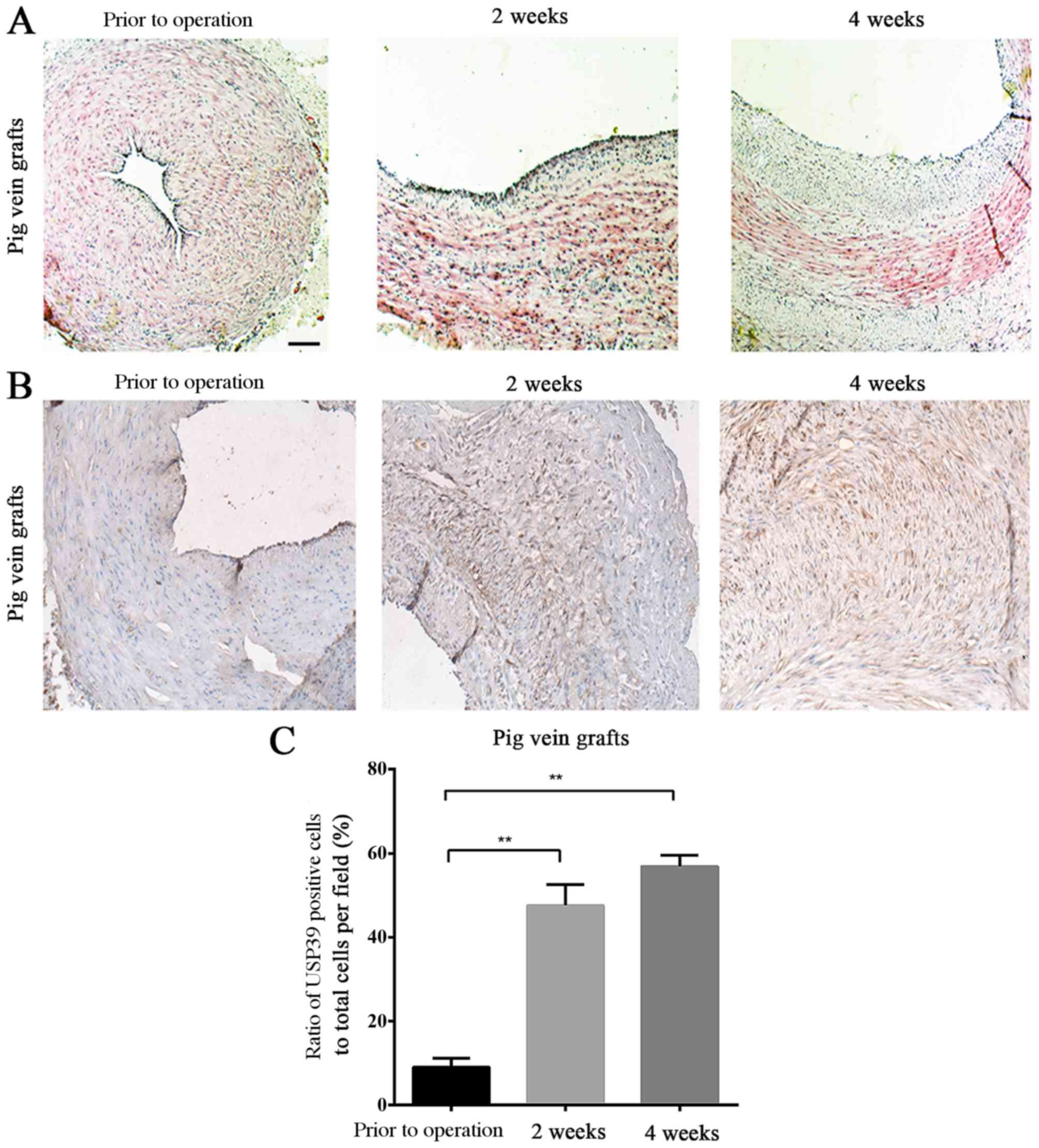
The thickening of vein-grafts in the pig was
assessed at 2 and 4 weeks after surgery (Fig. 3A). USP39 protein expression levels
in the pig vein grafts at different time points following
transplantation confirmed the results observed in the ligated mouse
carotid arteries (Fig. 3B), and
there were significantly more USP39-positive cells in the grafts at
2 and 4 weeks after surgery compared with the baseline levels prior
to the operation (P=0.0028 and P=0.0023, respectively; Fig. 3C).
Knockdown of USP39 inhibits VSMC
proliferation and the expression of cyclin D1 and CDK4
Wang et al (17) previously reported that RNA
interference-mediated downregulation of USP39 markedly reduced the
proliferation and colony-forming ability of MCF-7 cells, and
induced G0/G1-phase arrest and apoptosis. The effect of
overexpression (Fig. 4A) and
knockdown (Fig. 4B) of USP39 on
the USP39 protein was determined in the present study. USP39-73
siRNA was selected as the most effective siRNA at knocking down
USP39 protein levels (Fig. 4B) and
was, therefore, used for further experiments. Knockdown of USP39 in
VSMCs inhibited cyclinD1 and CDK4 protein expression compared with
transfection with control siRNA (Fig.
4C), which are essential proteins for G1/S phase
transformation. Furthermore, USP39 knockdown in VSMCs significantly
reduced proliferation compared with cells transfected with control
siRNA (P=0.0238; Fig. 4D). The
growth rate of VMSCs was demonstrated to be significantly
suppressed by USP39 knockdown compared with cells transfected with
control siRNA at 3 and 5 days (P=0.0478 and P=0.0002, respectively;
Fig. 4E) via an MTT assay.
However, USP39 overexpression had no significant effect on cell
growth rate compared with cells transfected with empty vector
(P>0.05; Fig. 4F).
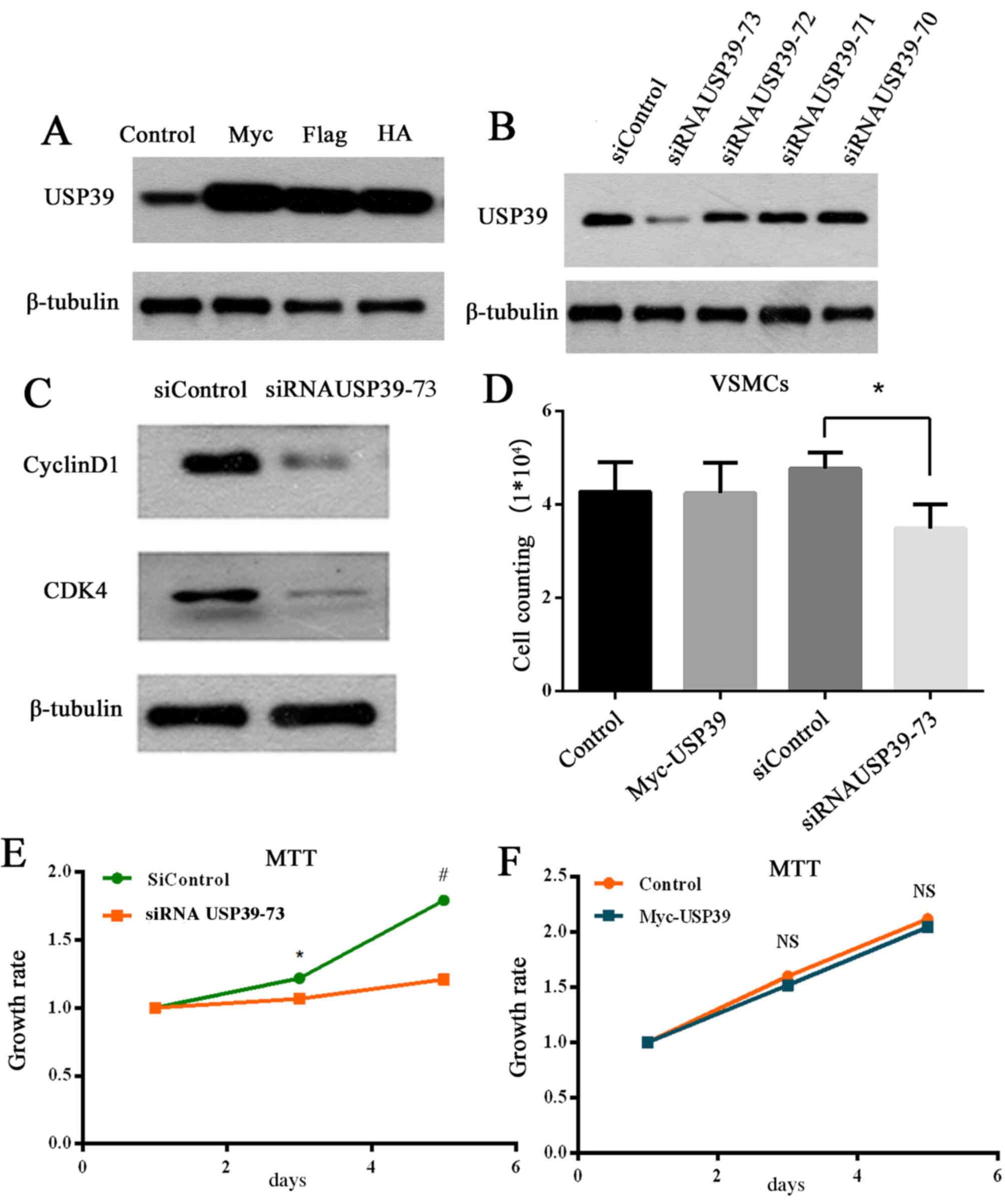 | Figure 4.(A) Overexpression of myc-, Flag- and
HA-tagged USP39 in VSMCs. (B) Knockdown of USP39 (siRNA USP39-70,
71, 72, and 73) in VSMCs. (C) Knockdown of USP39 in VSMCs decreased
expression of cyclin D1 and CDK4. (D) USP39 knockdown inhibited
proliferation in VSMCs. *P<0.05 comparison indicated by
brackets. (E) Growth rate of siRNA USP39-73 group compared with
siControl group VSMCs. *P<0.05 vs. siControl at 3 days,
#P<0.001 vs. siControl at 5 days. (F) No significant
difference in VSMC proliferation between the myc-USP39 group and
the control VSMCs. HA, hemagglutinin; VSMC, vascular smooth muscle
cell; USP39, ubiquitin-specific peptidase 39; siControl, control
small interfering RNA; siRNA, small interfering RNA; CDK4,
cyclin-dependent kinase 4; NS, no significance. |
USP39 is associated with VSMC
migration
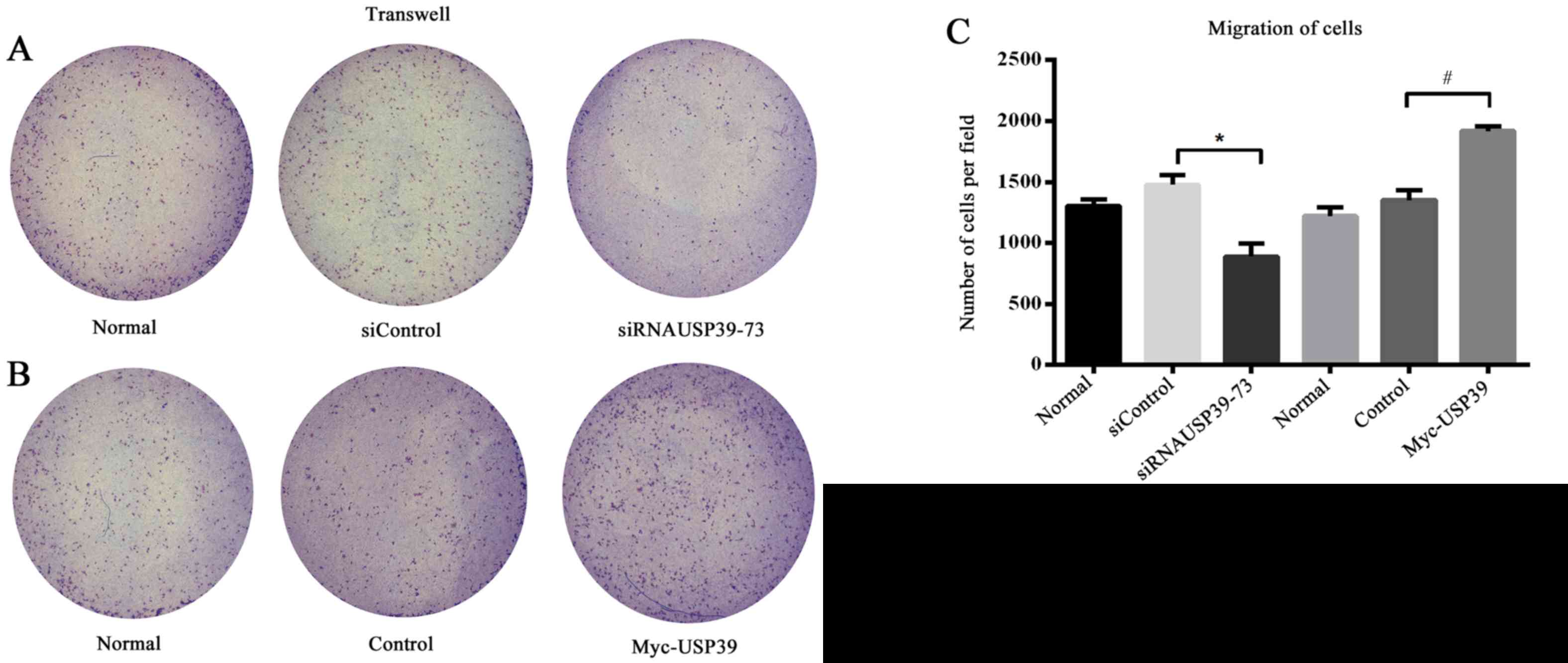
The migration of VSMC is a crucial event in the
pathogenesis of vascular diseases and is characterized by intimal
thickening (22). In the present
study, VSMC migration was suppressed by knockdown of USP39 compared
with transfection with control siRNA (P=0.0111; Fig. 5A) and migration was significantly
increased in VSMCs overexpressing USP39 compared with control cells
(P=0.0028; Fig. 5B and C).
Discussion
The ubiquitin specific protease USP39 lacks three
residues critical for protease activity, and thus lacks DUB
activity despite the presence of a DUB domain (17,20).
USP39 has previously been suggested to be involved in controlling
cell growth in the pituitary gland, thus maintaining pituitary
homeostasis in zebrafish (18).
However, no exact mechanisms, specific substrates or other
functions of USP39 have been identified.
To the best of our knowledge, the present study
provides the first evidence for an association between USP39
expression and remodeling in ligated arteries and vein grafts, and
USP39 may be involved in this pathological process in vein grafts
and ligated arteries. The mouse carotid artery ligation and pig
vein graft models closely resemble human venous bypass graft
failure in terms of their morphological characteristics and
development (23,24), and are thus useful for exploring
the potential pathogenesis and novel therapies.
It has previously been reported that USP39 silencing
affects spindle checkpoint function and cytokinesis by reducing
Aurora B kinase levels (20).
Furthermore, inhibition of USP39 induced G0/G1-phase arrest and
apoptosis in breast cancer cells (17). Similarly, cyclin D1 and CDK4
protein expression levels, which maintain the cell cycle process,
were decreased in USP39-knockout VSMCs compared with control cells,
and VSMC proliferation was also significantly reduced by
USP39-knockdown in the present study. Although it is not possible
to conclude that USP39 has a direct effect on VSMC proliferation
from these results alone, it appears to be associated with SMC
proliferation, and may provide a novel target for the treatment of
vascular restenosis. Vascular remodeling is the structural
reorganization of a vessel and involves multiple cell activities,
including SMC proliferation, migration and extracellular membrane
restriction (7). The present study
also demonstrated that VSMC migration was suppressed by
downregulation of USP39 and enhanced by upregulation of USP39. The
proliferation and migration of VSMCs is a key event in the
pathogenesis of vascular diseases and is characterized by intimal
thickening, which is most commonly seen in atherosclerosis,
vascular rejection, restenosis following vein grafting and coronary
intervention (22,25,26).
Multiple factors that affect the proliferation and migration of
VSMCs. USP39 appears to be involved in proliferation and migration,
but further studies are needed to assess potential underlying
molecular mechanisms.
Once vascular injury occurs, the exposed
subendothelial matrix rapidly attracts platelets and leukocytes,
and infiltrated leukocytes may then release inflammatory cytokines
several days following injury (12,24).
Inflammation is believed to be associated with altered gene
expression during the early stage of vascular remodeling (27–29).
In the present study, USP39 expression was significantly increased
in pig vein grafts and ligated mouse carotid arteries at 2 weeks
after surgery.
The results of the present study indicate that USP39
may be involved in the early stages and development of vascular
injury. However, inflammation and vascular remodeling are
continuous processes, and specific mechanisms of USP39 throughout
these processes require further investigation.
The present study provides the first evidence for an
association between high USP39 protein levels and the development
of vascular remodeling. USP39 regulates the cell cycle and affects
proliferation and migration of VSMCs. USP39 may thus represent a
novel therapeutic target for treating vascular injury and
preventing vein-graft failure.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270192) and the
Summit of the Six Top Talents Program of Jiangsu Province (grant
no. 2014-WSW-052).
References
|
1
|
Liuzzo JP, Ambrose JA and Coppola JT:
Sirolimus- and taxol-eluting stents differ towards intimal
hyperplasia and re-endothelialization. J Invasive Cardiol.
17:497–502. 2005.PubMed/NCBI
|
|
2
|
Weis M and von Scheidt W: Cardiac
allograft vasculopathy: A review. Circulation. 96:2069–2077. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newby AC and George SJ: Proliferation,
migration, matrix turnover, and death of smooth muscle cells in
native coronary and vein graft atherosclerosis. Curr Opin Cardiol.
11:574–582. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Task Force on Myocardial Revascularization
of the European Society of Cardiology (ESC) and the European
Association for Cardio-Thoracic Surgery (EACTS)1; European
Association for Percutaneous Cardiovascular Interventions (EAPCI).
Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg
S, Huber K, et al: Guidelines on myocardial revascularization. Eur
Heart J. 31:2501–2555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schamroth CL and Sacks AD: Aortocoronary
saphenous vein bypass aneurysm-an unusual presentation. S Afr Med
J. 88:(Suppl 2). C91–C93. 1998.PubMed/NCBI
|
|
6
|
Gibbons GH and Dzau VJ: The emerging
concept of vascular remodeling. N Engl J Med. 330:1431–1438. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehta RH, Ferguson TB, Lopes RD, Hafley
GE, Mack MJ, Kouchoukos NT, Gibson CM, Harrington RA, Califf RM,
Peterson ED, et al: Saphenous vein grafts with multiple versus
single distal targets in patients undergoing coronary artery bypass
surgery: One-year graft failure and five-year outcomes from the
Project of Ex-Vivo Vein Graft Engineering via Transfection
(PREVENT) IV trial. Circulation. 124:280–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haruguchi H and Teraoka S: Intimal
hyperplasia and hemodynamic factors in arterial bypass and
arteriovenous grafts: A review. J Artif Organs. 6:227–235. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LN, Parkinson JF, Haskell C and Wang
YX: Mechanisms of intimal hyperplasia learned from a murine carotid
artery ligation model. Curr Vasc Pharmacol. 6:37–43. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar A, Hoover JL, Simmons CA, Lindner V
and Shebuski RJ: Remodeling and neointimal formation in the carotid
artery of normal and P-selectin-deficient mice. Circulation.
96:4333–4342. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A and Lindner V: Remodeling with
neointima formation in the mouse carotid artery after cessation of
blood flow. Arterioscler Thromb Vasc Biol. 17:2238–2244. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang N, Ng CS, Hu J, Qiu ZB, Underwood MJ,
Jeremy JY and Wan S: Role of osteopontin in the development of
neointimal hyperplasia in vein grafts. Eur J Cardiothorac Surg.
41:1384–1389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Oparil S, Sanders JM, Zhang L, Dai
M, Chen LB, Conway SJ, McNamara CA and Sarembock IJ:
Phosphatidylinositol-3-kinase signaling mediates vascular smooth
muscle cell expression of periostin in vivo and in vitro.
Atherosclerosis. 188:292–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han XJ, Chen M, Hong T, Zhu LY, He D, Feng
JG and Jiang LP: Lentivirus-mediated RNAi knockdown of the gap
junction protein, Cx43, attenuates the development of vascular
restenosis following balloon injury. Int J Mol Med. 35:885–892.
2015.PubMed/NCBI
|
|
15
|
McDonald RA, White KM, Wu J, Cooley BC,
Robertson KE, Halliday CA, McClure JD, Francis S, Lu R, Kennedy S,
et al: miRNA-21 is dysregulated in response to vein grafting in
multiple models and genetic ablation in mice attenuates neointima
formation. Eur Heart J. 34:1636–1643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makarova OV, Makarov EM and Lührmann R:
The 65 and 110 kDa SR-related proteins of the U4/U6.U5 tri-snRNP
are essential for the assembly of mature spliceosomes. EMBO J.
20:2553–2563. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Ji X, Liu X, Yao R, Chi J, Liu S,
Wang Y, Cao W and Zhou Q: Lentivirus-mediated inhibition of USP39
suppresses the growth of breast cancer cells in vitro. Oncol Rep.
30:2871–2877. 2013.PubMed/NCBI
|
|
18
|
Rios Y, Melmed S, Lin S and Liu NA:
Zebrafish usp39 mutation leads to rb1 mRNA splicing defect and
pituitary lineage expansion. PLoS Genet. 7:e10012712011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen D, Xu Z, Xia L, Liu X, Tu Y, Lei H,
Wang W, Wang T, Song L, Ma C, et al: Important role of SUMOylation
of Spliceosome factors in prostate cancer cells. J Proteome Res.
13:3571–3582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Leuken RJ, Luna-Vargas MP, Sixma TK,
Wolthuis RM and Medema RH: Usp39 is essential for mitotic spindle
checkpoint integrity and controls mRNA-levels of aurora B. Cell
Cycle. 7:2710–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wan S, George SJ, Nicklin SA, Yim AP and
Baker AH: Overexpression of p53 increases lumen size and blocks
neointima formation in porcine interposition vein grafts. Mol Ther.
9:689–698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehta D, George SJ, Jeremy JY, Izzat MB,
Southgate KM, Bryan AJ, Newby AC and Angelini GD: External stenting
reduces long-term medial and neointimal thickening and platelet
derived growth factor expression in a pig model of arteriovenous
bypass grafting. Nat Med. 4:235–239. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davies MG and Hagen PO: Pathobiology of
intimal hyperplasia. Br J Surg. 81:1254–1269. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Motwani JG and Topol EJ: Aortocoronary
saphenous vein graft disease: Pathogenesis, predisposition, and
prevention. Circulation. 97:916–931. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SY, Jeoung NH, Oh CJ, Choi YK, Lee HJ,
Kim HJ, Kim JY, Hwang JH, Tadi S, Yim YH, et al: Activation of
NAD(P)H: Quinone oxidoreductase 1 prevents arterial restenosis by
suppressing vascular smooth muscle cell proliferation. Circ Res.
104:842–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vogt F, Zernecke A, Beckner M, Krott N,
Bosserhoff AK, Hoffmann R, Zandvoort MA, Jahnke T, Kelm M, Weber C
and Blindt R: Blockade of angio-associated migratory cell protein
inhibits smooth muscle cell migration and neointima formation in
accelerated atherosclerosis. J Am Coll Cardiol. 52:302–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mayr M, Li C, Zou Y, Huemer U, Hu Y and Xu
Q: Biomechanical stress-induced apoptosis in vein grafts involves
p38 mitogen-activated protein kinases. FASEB J. 14:261–270.
2000.PubMed/NCBI
|
|
28
|
Zou Y, Hu Y, Mayr M, Dietrich H, Wick G
and Xu Q: Reduced neointima hyperplasia of vein bypass grafts in
intercellular adhesion molecule-1-deficient mice. Circ Res.
86:434–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Zou Y, Dietrich H, Wick G and Xu Q:
Inhibition of neointima hyperplasia of mouse vein grafts by locally
applied suramin. Circulation. 100:861–868. 1999. View Article : Google Scholar : PubMed/NCBI
|