Introduction
The number of patients undergoing surgery during
pregnancy is increasing, and an increasing number of studies are
investigating the effects of anesthesia during pregnancy (1,2).
Anesthetic exposure, particularly during the critical stages of
neurodevelopment, is considered to be safe and without any adverse
long-term consequences (3).
However, previous animal studies suggest that exposure to certain
anesthetics during periods of rapid synaptogenesis may evoke
neuronal apoptosis and inhibit neurogenesis, which may lead to
lasting cognitive impairments in the offspring (4). Therefore, it is necessary to
investigate the effects and underlying mechanisms of anesthetic
exposure on the offspring of rats that have undergone anesthesia
during pregnancy.
Sevoflurane is a volatile anesthetic and
demonstrates numerous advantages over other intravenous or
inhalation anesthetics, including reduced discomfort and a lower
blood solubility that facilitates rapid induction and recovery
(5). Additionally, it is broadly
used in a number of clinical applications (6–8).
Previous studies have been performed to determine the effects of
sevoflurane on brain development in rodents; the majority of these
studies have determined that sevoflurane exposure led to increased
apoptosis of brain cells (9,10).
However, the precise effect of sevoflurane on the offspring remains
inconclusive, and the underlying mechanisms remain to be
elucidated.
The Wnt/β-catenin signaling pathway is a crucial
regulator of the survival, growth and differentiation of neurons
(11). A previous study reported
that activation of the Wnt/β-catenin pathway improves cell
replacement therapy for Parkinson's disease (12). In addition, Wnt/β-catenin signaling
regulates hippocampal neurogenesis, with Wnt-knockout mice
exhibiting midbrain neuronal loss (13). Furthermore, the Wnt/β-catenin
pathway regulates oligodendrocyte development in a stage-dependent
manner (14). Glycogen synthase
kinase-3β (GSK-3β) is a central downstream factor of the
Wnt/β-catenin pathway. A previous study demonstrated that
GSK-3β-mediated inhibition of β-catenin potentially leads to
astrogliosis and inflammation resulting in neural stem cell
survival (15).
The molecular mechanisms involved in neuronal growth
and differentiation remain to be fully elucidated. The present
study investigated the effects of sevoflurane on the neurological
functions of the offspring of rats exposed to sevoflurane during
pregnancy, as well as the molecular mechanisms underlying these
effects.
Materials and methods
Animal model
The study protocol was approved by the Medical
Ethics and Human Clinical Trial Committee of Henan University of
Science and Technology (Luoyang, China). The procedures were
approved by the institutional Animal Research Ethics Committee of
Henan University of Science and Technology with reference to the
Chinese Community guidelines for the use of experimental animals
(16). On day 14 of pregnancy, a
total of 27 female Sprague-Dawley rats (6–8 weeks old; average
weight 200 g) that were obtained from the Laboratory Animal Centre
of Henan University of Science and Technology, and raised at room
temperature with freely available food and water and 12 h day/night
cycle, were divided into 3 groups (n=9): Control group (group C),
3% sevoflurane group and 4% sevoflurane group using the random
digit table method. The 3 groups were exposed to air, 3%
sevoflurane and 4% sevoflurane (Sangon Biotech Co., Ltd., Shanghai,
China) for 4 h, respectively. At 35 days of age, the juvenile rats
were examined using an open-field test and Morris water maze
experiments as previously reported (17). The time taken to cross the original
platform area within 90 sec was automatically recorded by image
acquisition and analysis systems (XR-XM101; Shanghai Xinruan
Information Technology Co., Ltd., Shanghai, China) as previously
reported (17). At 42 days of age,
juvenile rats were examined using the continuous passive avoidance
test to determine learning and memory abilities (18). Black boxes were used to measure the
fear memory of rats, including training phase as the rats were
stimulated by electricity and sound, and the detection phase of
memory latency, including context, altered context,
pre-conditioning-stimulus and conditioning stimulus tests in
Fig. 1. Fear memory was evaluated
based on the duration of time that rats spent frozen following
stimuli.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the hippocampus and
cerebral cortex of 6-week-old rats using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
following the manufacturer's protocol. cDNA was synthesized by
reverse transcribing a total of 20 µg RNA using the PrimeScript™
One Step RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian,
China). qPCR was performed using an Mx3000P Real-Time PCR system
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol, and SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.)
was used as a DNA-specific fluorescent dye. The primers used for
the detection of target mRNA expression were synthesized in our
lab, and the sequences are presented in Table I. Reactions were repeated >3
times. Gene expression levels were calculated by 2−∆∆Cq
method (19) relative to β-actin
using Stratagene Mx3000P software (Agilent Technologies, Inc.,
Santa Clara, CA, USA).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| Nestin |
GTAGCTCCCAGAGAGGGG |
CTCTAGAGGGCCAGGGAC |
| Ki67 |
GCCAAGGGTAACTCGTGG |
CCTCCATTGGCGTCTGAAG |
| GSK-3β |
GGTGAATCGAGAAGAGCC |
CTGTGGTTACCTTGCTGCC |
| β-catenin |
CCATCGAGAGGGCTTGTTG |
CAGTACGGAATCCACTGG |
| caspase-3 |
GTGAGGCGGTTGTAGAAG |
GCTGCATCGACATCTGTACC |
| Bcl-2 |
GGTGAACTGGGGGAGGATTG |
GGCAGGCATGTTGACTTCAC |
| Bax |
GCTGAGCGAGTGTCTCAAG |
GTCCAATGTCCAGCCCATG |
| β-actin |
CGTGCGTGACATTAAGGAG |
GCCAGGGTACATGGTGGT |
Western blotting
In order to determine protein expression levels,
whole cell extracts (lysate) were prepared from rat brain tissues
(6-weeks-old) in RIPA lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM
NaCl, 1% NP-40 and 0.1% SDS]. The concentration of protein was
determined by the BCA method. Total protein (30 µg) from each
sample was separated on 12% SDS-PAGE gels and transferred onto a
nitrocellulose membrane. Target proteins were probed with specific
primary antibodies for nestin (1:1,000; cat. no. ab18102; Abcam,
Cambridge, UK), Ki67 (1:1,000; cat. no. ab92353; Abcam), GSK-3β
(1:1,000; cat. no. 12456; Cell Signaling Technology, Inc., Danvers,
MA, USA) β-catenin (1:1,000; cat. no. 9582; Cell Signaling
Technology, Inc.), caspase 3 (1:1,000; cat. no. SC-271759, Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), BCL2 associated X (Bax;
1:200; cat. no. sc-4239; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), B cell leukemia/lymphoma 2 (Bcl-2; 1:200; cat. no.
sc-7382; Santa Cruz Biotechnology, Inc.) and β-actin (1:500; cat.
no. sc-1615; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
Subsequently, the membrane was incubated with Goat Anti-Mouse IgG
(H&L) secondary antibody (1:5,000; cat. no. PAB0096; Abnova,
Taipei, Taiwan) or Anti-Rabbit IgG (H+L) secondary antibody
(1:5,000; cat. no. 14708; Cell Signaling Technology, Inc.) at room
temperature for 1 h. Non-specific binding sites were blocked by
immersing the membrane into 5% non-fat milk in 0.1% Tween 20-TBS
(TTBS) solution for 1 h at room temperature with shaking. The
membrane was visualized by an ECL kit (Beyotime Institute of
Biotechnology) on the GelDoc™ XR+ system (Bio-rad Laboratories,
Inc., Hercules, CA, USA).
Adenosine triphosphate (ATP)
production assay
ATP production in 3-week-old rat brain tissues was
analyzed using an ATP assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Immunofluorescence staining
Rats were sacrificed and the brain cortex was
extracted. After fixing in 10% formaldehyde for 1 h at room
temperature, pathological slides (5 µm-thick sections) were
deparaffinized in xylene and rehydrated in decreasing
concentrations of ethanol to water. Then all the reactions were
performed as described previously (20). Sections were immunostained with
primary antibody against Ki67 (1:100; cat. no. ab92353; Abcam) and
horseradish peroxidase-conjugated Goat Anti-Mouse IgG secondary
antibody (1:50; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.).
Statistical analysis
Data were analyzed using SPSS software (version,
17.0; SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
was used to compare differences among treatment groups. Experiments
were performed independently at least three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
Sevoflurane reduces the learning
ability and memory in rat offspring
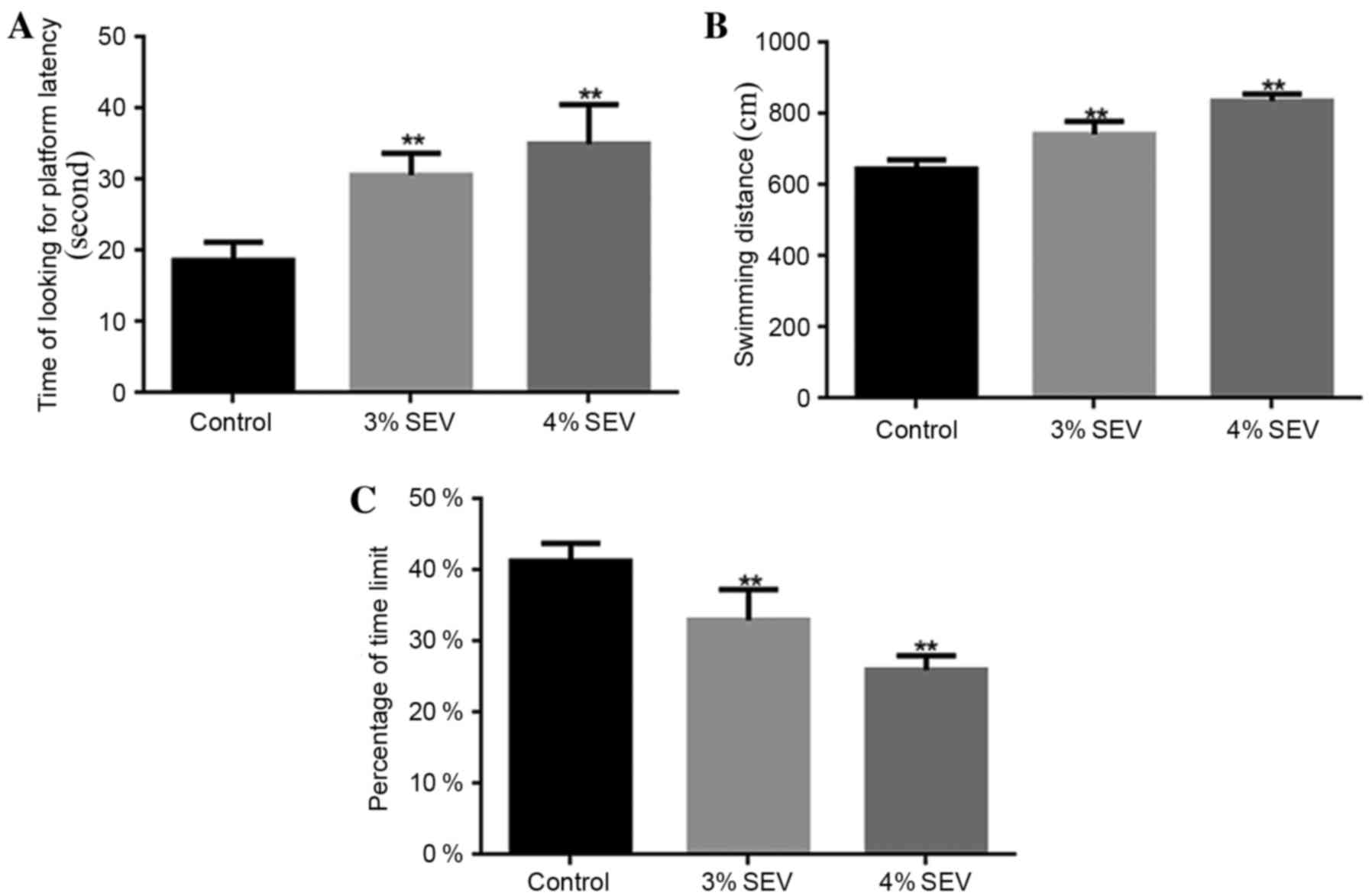
The rat offspring rats that were exposed to 3 and 4%
sevoflurane required a significantly greater amount of time to find
the platform when compared with the control group (P<0.05;
Fig. 1A). Additionally, the
swimming distance during the training period for the offspring of
rats exposed to 3 and 4% sevoflurane was significantly increased
when compared with the control group (P<0.05; Fig. 1B). Furthermore, the percentage of
platform quadrant retention time was significantly reduced in the
offspring of rats exposed to 3 and 4% sevoflurane when compared
with the control group (P<0.05; Fig. 1C). The results demonstrated that
sevoflurane reduced the learning and memory abilities of the
offspring of rats exposed to sevoflurane during pregnancy.
Sevoflurane inhibits the proliferation
of nerve cells in the offspring rats
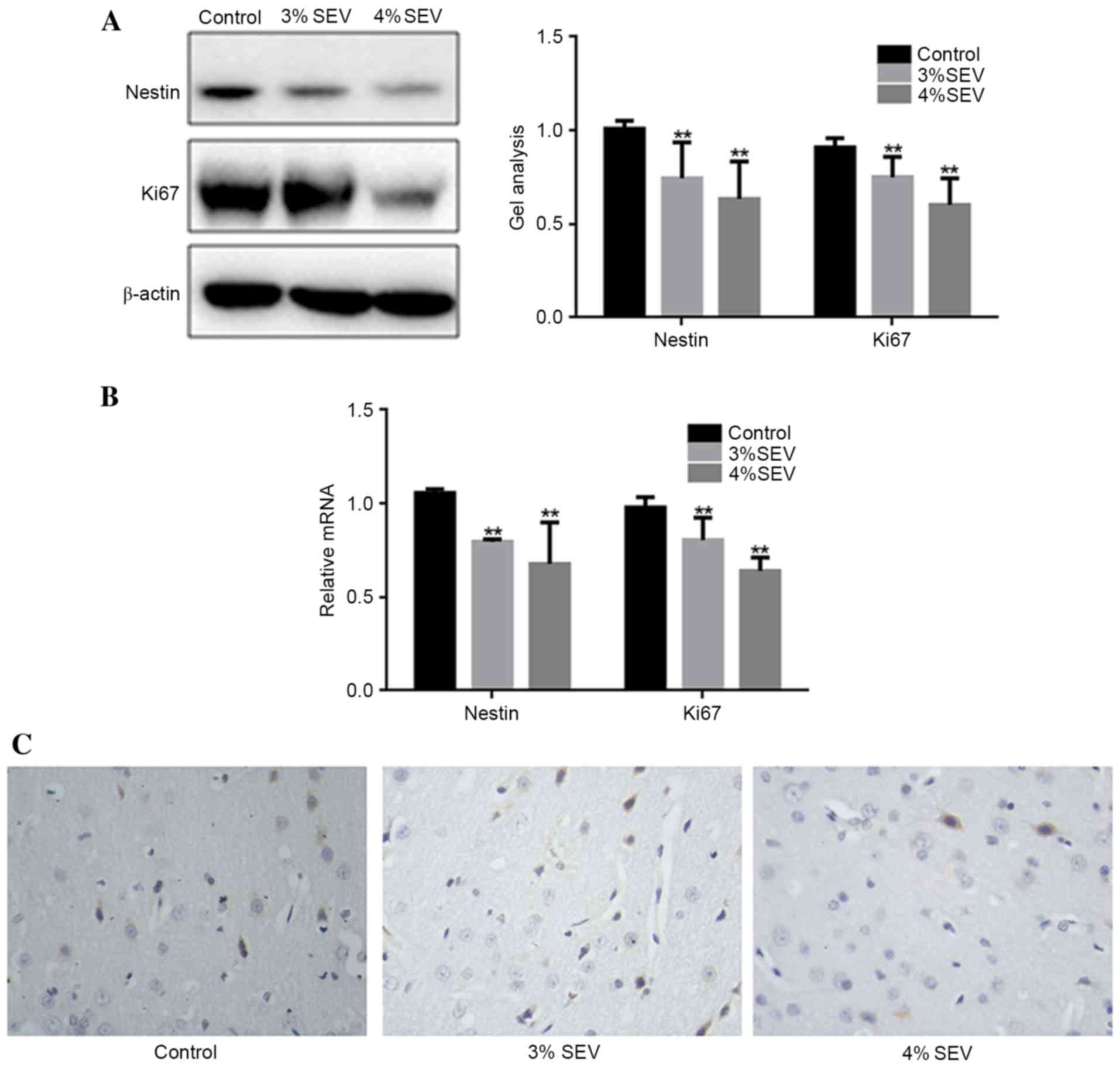
The protein and mRNA expression levels of nestin and
Ki67 were significantly reduced in groups S1 and S2 compared with
group C (Fig. 2A and B;
P<0.05). Additionally, the expression level of Ki-67 was reduced
in the cerebral cortex of the offspring rats in the 3 and 4%
sevoflurane groups in contrast with the group C (Fig. 2C). These findings demonstrated that
sevoflurane inhibited the proliferation of nerve cells in the
offspring of rats exposed during pregnancy.
Sevoflurane promotes nerve cell
apoptosis in rat offspring
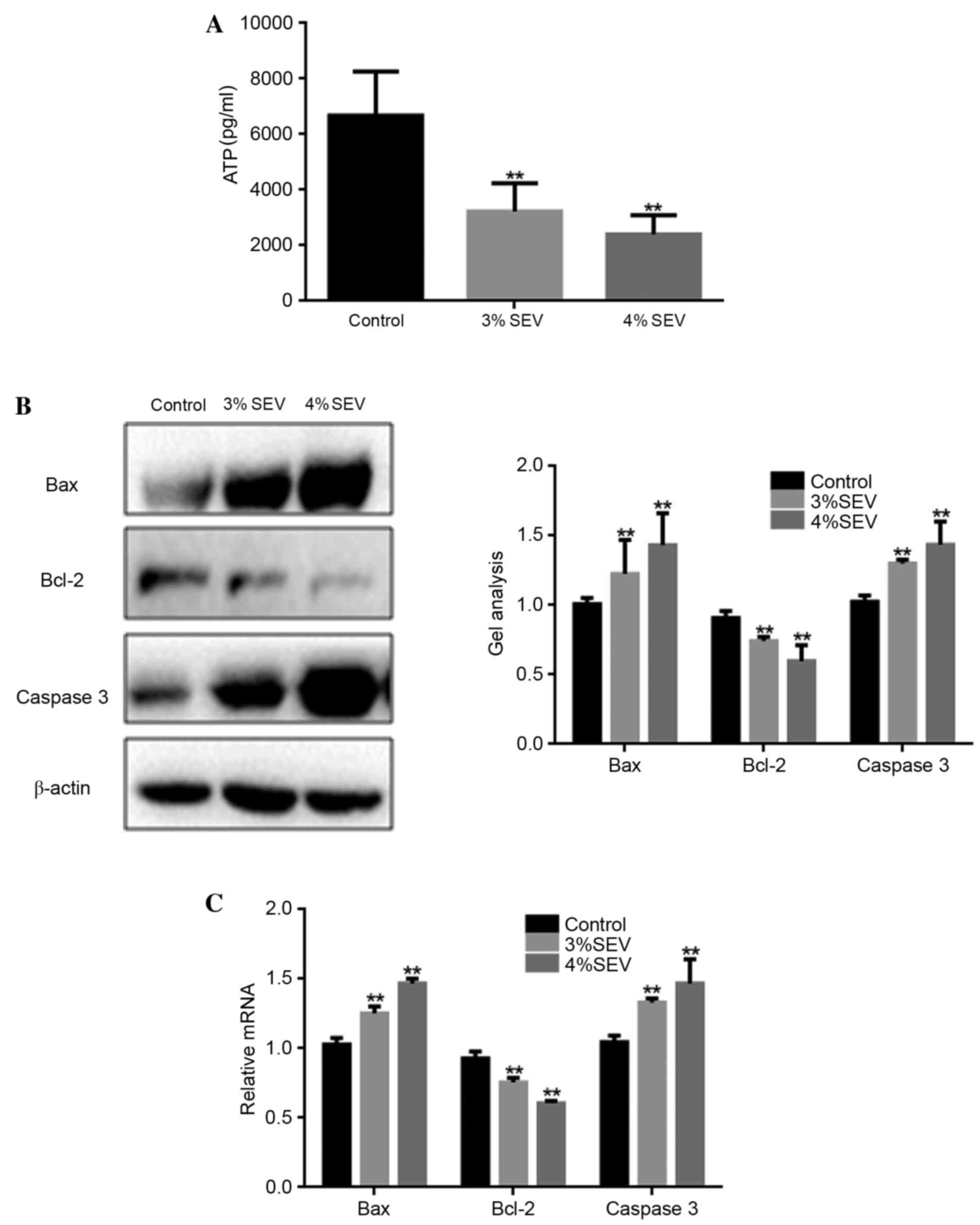
ATP production was significantly reduced in the
offspring of rats exposed to 3 and 4% sevoflurane when compared
with the control group (P<0.05; Fig. 3A). Additionally, the mRNA and
protein expression levels of Bax and caspase-3 were significantly
increased in the neuronal cells of the offspring of rats exposed to
3 and 4% sevoflurane when compared with the control group
(P<0.05; Fig. 3B and C). By
contrast, the expression level of Bcl-2 was significantly reduced
in sevoflurane-treated groups when compared with the control group
(P<0.05; Fig. 3B). These
findings suggest that sevoflurane may promote nerve cell apoptosis
in the offspring of rats exposed during pregnancy.
Sevoflurane inhibits the growth of
nerve cells in rat offspring potentially via the Wnt/β-catenin
signaling pathway
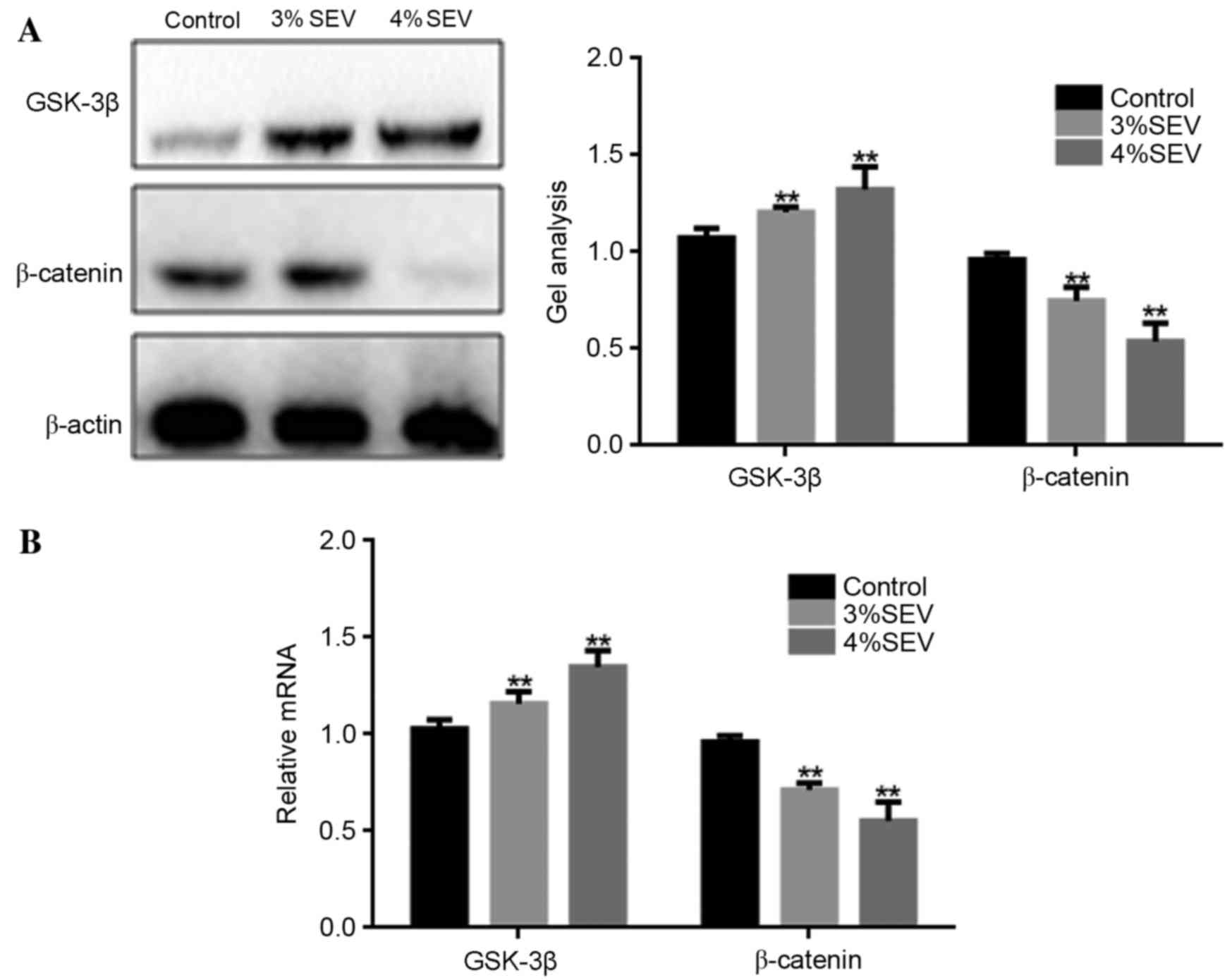
The protein and mRNA expression levels of GSK-3β and
β-catenin were significantly increased in the offspring of rats
exposed to 3 and 4% sevoflurane when compared with the control
group (P<0.05; Fig. 4). These
results suggest that sevoflurane may inhibit the growth of nerve
cells in the offspring of exposed rats via the Wnt/β-catenin
signaling pathway.
Discussion
Sevoflurane is one of the most widely used
inhalation anesthetics for pediatric surgery and cesarean delivery
due to rapid inhalational induction, tolerable odor, quick
emergence and relative cardiovascular stability for infants and
children (21). Although
sevoflurane exhibits fewer severe effects on the developing brains
of animals compared with isoflurane, which is a structurally
similar inhaled anesthetic (22),
previous studies have demonstrated that sevoflurane may lead to
neuronal apoptosis and behavioral dysfunction (23,24).
The present study assessed the neurobehavioral
effects of sevoflurane on the offspring of exposed pregnant rats
using the open-field test and the Morris water maze. The results
demonstrated the learning and memory abilities of the offspring
rats were significantly reduced in sevoflurane-exposed groups. In
addition, the western blotting and RT-qPCR analysis results
revealed that the protein and mRNA expression levels of Bax and
caspase-3 were significantly increased in sevoflurane-exposed
groups when compared with the controls. These results suggest that
sevoflurane promoted nerve cell apoptosis in rat offspring, which
is consistent with the results of previous studies (23,24).
The current study determined that the protein and
mRNA expression levels of nestin and Ki-67 were significantly
reduced in the sevoflurane-treated groups when compared with the
controls. Nestin and Ki-67 are important molecules for cell growth,
which serves a role in cell division and proliferation (25). It is therefore possible that
sevoflurane inhibited the proliferation of nerve cells in the
offspring of exposed rats in the present study.
The Wnt/β-catenin signaling pathway is a crucial
regulator of the growth and proliferation of nerve cells and is
widely involved in neuronal development, differentiation,
migration, adhesion, polarization and tumorigenesis (26–28).
In addition, Wnt signaling is critical for neuronal maturation,
apoptosis, dopaminergic and hippocampal neurogenesis (29). Wnt signaling increases β-catenin
levels by inhibiting the activity of GSK-3β. GSK-3β is a
serine/threonine kinase that controls various neuronal functions,
including neurite outgrowth, synapse formation, neurotransmission
and neurogenesis (30,31). GSK-3β mediates these functions by
phosphorylating a wide range of substrates involved in gene
transcription, metabolism, apoptosis, cytoskeletal dynamics, signal
transduction, lipid membrane dynamics and trafficking (32,33).
β-catenin is a central component of the Wnt/β-catenin signaling
pathway, which transmits information to the cytoplasm and
translocates to the nucleus in order to activate target gene
transcription (34). Previous
studies have suggested that the Wnt/β-catenin pathway stimulates
the self-renewal of neural stem cells and their differentiation
towards neuronal phenotype (35)
by inhibiting gliogenesis (36).
Downregulation of this pathway may lead to striatal synaptic
degeneration, resulting in impaired motor behavior (37). The present study demonstrated that
the protein and mRNA expression levels of GSK-3β and β-catenin in
sevoflurane-exposed groups were significantly increased compared
with the control group. Therefore, it is possible that sevoflurane
may suppress the proliferation of nerve cells in the offspring of
rats exposed during pregnancy, potentially via inhibition of the
Wnt/β-catenin signaling pathway.
In conclusion, the results of the present study
indicate that sevoflurane may inhibit the proliferation of neural
cells and promote their apoptosis in the offspring of rats exposed
during pregnancy. In addition, sevoflurane may affect the learning
and memory abilities of the rat offspring. Furthermore, the
Wnt/β-catenin pathway may be involved in the negative effects of
sevoflurane exposure in rat offspring. Ultimately, the exposure of
pregnant mice to sevoflurane anesthesia demonstrated a negative
effect on the learning and memory abilities of their offspring; an
effect that may be meditated by the Wnt/β-catenin signaling
pathway.
References
|
1
|
Heesen M and Klimek M: Nonobstetric
anesthesia during pregnancy. Curr Opin Anaesthesiol. 29:297–303.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Owsiak JN and Bullough AS: Chronic myeloid
leukemia in pregnancy: An absolute contraindication to neuraxial
anesthesia? Int J Obstet Anesth. 25:85–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller TL, Park R and Sun LS: Report on
the Fifth PANDA Symposium on ‘Anesthesia and Neurodevelopment in
Children’. J Neurosurg Anesthesiol. 28:350–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stratmann G: Review article: Neurotoxicity
of anesthetic drugs in the developing brain. Anesth Analg.
113:1170–1179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Costi D, Cyna AM, Ahmed S, Stephens K,
Strickland P, Ellwood J, Larsson JN, Chooi C, Burgoyne LL and
Middleton P: Effects of sevoflurane versus other general
anaesthesia on emergence agitation in children. Cochrane Database
Syst Rev CD007084. 2014. View Article : Google Scholar
|
|
6
|
Lorsomradee S, Cromheecke S, Lorsomradee S
and De Hert SG: Effects of sevoflurane on biomechanical markers of
hepatic and renal dysfunction after coronary artery surgery. J
Cardiothorac Vasc Anesth. 20:684–690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Head BP and Patel P: Anesthetics and brain
protection. Curr Opin Anaesthesiol. 20:395–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Istaphanous GK, Howard J, Nan X, Hughes
EA, McCann JC, McAuliffe JJ, Danzer SC and Loepke AW: Comparison of
the neuroapoptotic properties of equipotent anesthetic
concentrations of desflurane, isoflurane, or sevoflurane in
neonatal mice. Anesthesiology. 114:578–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen X, Dong Y, Xu Z, Wang H, Miao C,
Soriano SG, Sun D, Baxter MG, Zhang Y and Xie Z: Selective
anesthesia-induced neuroinflammation in developing mouse brain and
cognitive impairment. Anesthesiology. 118:502–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joksimovic M and Awatramani R:
Wnt/β-catenin signaling in midbrain dopaminergic neuron
specification and neurogenesis. J Mol Cell Biol. 6:27–33. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parish CL and Thompson LH: Modulating Wnt
signaling to improve cell replacement therapy for Parkinson's
disease. J Mol Cell Biol. 6:54–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lange C, Mix E, Rateitschak K and Rolfs A:
Wnt signal pathways and neural stem cell differentiation.
Neurodegener Dis. 3:76–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai ZM, Sun S, Wang C, Huang H, Hu X,
Zhang Z, Lu QR and Qiu M: Stage-specific regulation of
oligodendrocyte development by Wnt/β-catenin signaling. J Neurosci.
34:8467–8473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
L'Episcopo F, Tirolo C, Testa N, Caniglia
S, Morale MC, Deleidi M, Serapide MF, Pluchino S and Marchetti B:
Plasticity of subventricular zone neuroprogenitors in MPTP
(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of
Parkinson's disease involves cross talk between inflammatory and
Wnt/β-catenin signaling pathways: Functional consequences for
neuroprotection and repair. J Neurosci. 32:2062–2085. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H and Lou W: Functioning
remobilization of the paralyzed vocal cord using the split-vagus
nerve procedure in rats the split-vagus nerve procedure in rats.
Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 24:273–275.
2010.(In Chinese). PubMed/NCBI
|
|
17
|
Schoenfeld R, Schiffelholz T, Beyer C,
Leplow B and Foreman N: Variations of the Morris water maze task to
comparatively assess human and rodent place navigation. Neurobiol
Learn Mem. 139:117–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radahmadi M, Alaei H, Sharifi MR and
Hosseini N: Effect of forced exercise and exercise withdrawal on
memory, serum and hippocampal corticosterone levels in rats. Exp
Brain Res. 233:2789–2799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel D and Chaudhary J: Increased
expression of bHLH transcription factor E2A (TCF3) in prostate
cancer promotes proliferation and confers resistance to doxorubicin
induced apoptosis. Biochem Biophys Res Commun. 422:146–151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lerman J, Sikich N, Kleinman S and Yentis
S: The pharmacology of sevoflurane in infants and children.
Anesthesiology. 80:814–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen X, Liu Y, Xu S, Zhao Q, Guo X, Shen R
and Wang F: Early life exposure to sevoflurane impairs adulthood
spatial memory in the rat. Neurotoxicology. 39:45–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takaenoki Y, Satoh Y, Araki Y, Kodama M,
Yonamine R, Yufune S and Kazama T: Neonatal exposure to sevoflurane
in mice causes deficits in maternal behavior later in adulthood.
Anesthesiology. 120:403–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kruger K, Stefansson IM, Collett K, Arnes
JB, Aas T and Akslen LA: Microvessel proliferation by co-expression
of endothelial nestin and Ki-67 is associated with a basal-like
phenotype and aggressive features in breast cancer. Breast.
22:282–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirabayashi Y, Itoh Y, Tabata H, Nakajima
K, Akiyama T, Masuyama N and Gotoh Y: The Wnt/beta-catenin pathway
directs neuronal differentiation of cortical neural precursor
cells. Development. 131:2791–2801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rudloff S and Kemler R: Differential
requirements for β-catenin during mouse development. Development.
139:3711–3721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lupo G, Novorol C, Smith JR, Vallier L,
Miranda E, Alexander M, Biagioni S, Pedersen RA and Harris WA:
Multiple roles of Activin/Nodal, bone morphogenetic protein,
fibroblast growth factor and Wnt/β-catenin signalling in the
anterior neural patterning of adherent human embryonic stem cell
cultures. Open Biol. 3:1201672013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Castelo-Branco G and Arenas E: Function of
Wnts in dopaminergic neuron development. Neurodegener Dis. 3:5–11.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cole AR: GSK3 as a Sensor Determining Cell
Fate in the Brain. Front Mol Neurosci. 5:42012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fuchs C, Trazzi S, Torricella R, Viggiano
R, De Franceschi M, Amendola E, Gross C, Calzà L, Bartesaghi R and
Ciani E: Loss of CDKL5 impairs survival and dendritic growth of
newborn neurons by altering AKT/GSK-3β signaling. Neurobiol Dis.
70:53–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim WY, Wang X, Wu Y, Doble BW, Patel S,
Woodgett JR and Snider WD: GSK-3 is a master regulator of neural
progenitor homeostasis. Nat Neurosci. 12:1390–1397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morales-Garcia JA, Luna-Medina R,
Alonso-Gil S, Sanz-Sancristobal M, Palomo V, Gil C, Santos A,
Martinez A and Perez-Castillo A: Glycogen synthase kinase 3
inhibition promotes adult hippocampal neurogenesis in vitro and in
vivo. ACS Chem Neurosci. 3:963–971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Munji RN, Choe Y, Li G, Siegenthaler JA
and Pleasure SJ: Wnt signaling regulates neuronal differentiation
of cortical intermediate progenitors. J Neurosci. 31:1676–1687.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalani MY, Cheshier SH, Cord BJ, Bababeygy
SR, Vogel H, Weissman IL, Palmer TD and Nusse R: Wnt-mediated
self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci
USA. 105:16970–16975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kunke D, Bryja V, Mygland L, Arenas E and
Krauss S: Inhibition of canonical Wnt signaling promotes
gliogenesis in P0-NSCs. Biochem Biophys Res Commun. 386:628–633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galli S, Lopes DM, Ammari R, Kopra J,
Millar SE, Gibb A and Salinas PC: Deficient Wnt signalling triggers
striatal synaptic degeneration and impaired motor behaviour in
adult mice. Nat Commun. 5:49922014. View Article : Google Scholar : PubMed/NCBI
|