Introduction
A high blood glucose level is characteristic of
diabetes mellitus (DM), a disease resulting from abnormal insulin
levels or an abnormal response to insulin. Patients with DM are
prone to several complications, including periodontitis. A previous
longitudinal study showed that the prevalence of periodontitis
increased with an odds ratio of 4.23 in diabetic patients (1). Increased alveolar bone loss is also
found in diabetic patients with poor glycemic control (2).
The periodontal ligament, a component of periodontal
tissue, consists of heterogenous cells and is involved in tissue
regeneration. Several studies (3–5) have
shown that periodontal ligament fibroblasts (PDLFs) express
mesenchymal stem cell markers and can differentiate to become
osteoblasts, adipocytes and chondrocytes. Under hyperglycemic
conditions, PDLFs can induce apoptosis through the caspase-3
signaling pathway (6,7). In addition, high glucose levels
induce the expression of fibronectin receptor (8), which may result in delayed wound
healing in diabetic patients with severe periodontitis. However,
the stem cell properties of PDLFs in high glucose remain to be
fully elucidated. Therefore, the present study aimed to investigate
the effect of different glucose conditions on the stemness
properties of human PDLFs in order to improve understanding of the
biology of these cells in diabetic patients, which may lead to
improved dental treatment.
Materials and methods
Cell culture
The HPDLFs were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA). The HPDLFs were routinely
cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) and antibiotic/antimycotic solution containing 100 U/ml
penicillin G, 100 µg/ml streptomycin and 0.025 µg/ml amphotericin B
(Thermo Fisher Scientific, Inc.). The HPDLFs were maintained in a
humidified atmosphere at 5% CO2 at 37°C and were
subcultured at confluence. The culture medium was replaced every
2–3 days. The HPDLFs used in the present study were those in
passages 5–9.
To examine the effect of a high glucose
concentration on HPDLFs, the cells were cultured in three types of
medium: Normal glucose (NG), high mannitol (HM) and high glucose
(HG). The NG medium contained 5.5 mM of D-glucose, simulating the
normal blood glucose level in humans. The HG medium contained 25 mM
of D-glucose, whereas the HM medium was used as an osmotic pressure
control, which contained 5.5 mM of D-glucose and 19.5 mM
D-mannitol.
Proliferation assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, a quantitative colorimetric assay, was used to estimate cell
viability and cell proliferation. The principle of the assay is
that MTT, a yellow tetrazole, is metabolized by mitochondrial
succinate dehydrogenase in living cells to a purple formazan
product.
The HPDLFs were seeded in 24-well plates
(Costar®; Corning Incorporated, Corning, NY, USA) at a
density of 2×104 cells/well in a humidified atmosphere
at 5%CO2 at 37°C. Following incubation for 24 h, the
time was set as day 0 and the medium was replaced with the three
experimental media (NG DMEM, HG DMEM and HM DMEM).
On days 1, 3, 5 and 7, the cells were washed with
PBS and incubated with 500 µl of 0.5 mg/ml MTT (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) for 2 h at 37°C. The cells
were rinsed again with PBS. The formazan products were dissolved in
500 µl of dimethyl sulfoxide (Sigma-Aldrich; Merck Millipore) for
30 min at room temperature with agitation. The absorbance at 540 nm
was determined using an Epoch™ microplate spectrophotometer (Biotek
Instruments, Winooski, VT, USA). All assays were performed in
triplicate.
Osteogenic differentiation
To induce osteogenic differentiation, the HPDLFs
were seeded in 24-well plates (Costar®; Corning
Incorporated) at a density of 5×104 cells/well in a
humidified atmosphere at 5%CO2 at 37°C. When the cells
reached 90% confluence (day 0), they were induced with 50 µg/ml
ascorbic acid (Sigma-Aldrich; Merck Millipore), 10 mM
β-glycerophosphate (Sigma-Aldrich; Merck Millipore) and 100 nM
dexamethasone (Sigma-Aldrich; Merck Millipore) in each type of
DMEM. The medium was replaced every 2–3 days.
Measurement of alkaline phosphatase
activity
Following induction of the HPDLFs with osteogenic
medium for 7, 14 and 21 days, the cells were analyzed for alkaline
phosphatase activity. All groups of HPDLFs were scraped from
culture and transferred into microtubes, washed twice with 1X PBS,
and lysed with 200 µl lysis buffer containing 1 mM
phenylmethanesulfonyl fluoride (Sigma-Aldrich; Merck Millipore) in
CelLytic M (Sigma-Aldrich; Merck Millipore) for 15 min at room
temperature. The lysed cells were centrifuged at 20,598 × g for 15
min at 4°C. The supernatant was stored at −80°C until use. To
measure alkaline phosphatase activity, 50 µl of the sample
supernatant was diluted with 50 µl 0.1 M Tris-HCl, followed by the
addition of 50 µl 2 mM p-nitrophenol phosphate substrate
(Sigma-Aldrich; Merck Millipore) and incubation at 37°C for 30 min.
The reaction was terminated by adding 50 µl ice-cold 2 N NaOH. The
absorbance was measured at 450 nm. The alkaline phosphatase
concentration was calculated from the standard curve of
p-nitrophenol (Sigma-Aldrich; Merck Millipore). The alkaline
phosphatase activity was calculated in U/nmol p-nitrophenol/mg
protein/min. The alkaline phosphatase activity of the induced
HPDLFs was then divided by the basal alkaline phosphatase activity
to normalize the difference between the passages of cells. All
assays were performed in triplicate.
Nodule formation
The induced HPDLFs were evaluated for calcified
nodule formation following induction with osteogenic medium for 28
days. All groups of HPDLFs were washed twice with 1X PBS. The cells
were fixed with cold absolute methanol at room temperature for 10
min and rinsed twice with distilled deionized water. The calcified
nodules were stained with 1% Alizarin Red (pH 4.1–4.3;
Sigma-Aldrich; Merck Millipore) at room temperature for 30 min. The
excess dye was removed and nodules were rinsed with distilled
deionized water until the background became clear. The presence of
nodules was observed under an inverted microscope (Nikon
Corporation, Tokyo, Japan). All experiments were performed at least
three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
To analyze gene expression following the induction
of HPDLFs with osteogenic medium for 3 days, RNA was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
concentration and purity of RNA was determined from the ratio of
optical density (OD)260/OD280 using an Epoch™
microplate spectrophotometer (Biotek Instruments).
Contaminating DNA was removed by DNase I (Fermentas;
Thermo Fisher Scientific, Inc.). The prepared RNA was then used as
a template for RT to cDNA using iScript selected cDNA synthesis
kits (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to
the manufacturer's protocol. Briefly, cDNA was reverse transcribed
in iScript reaction mix containing iScript reverse transcriptase
together with oligo dT primer, followed by incubation at 42°C for
90 min and 85°C for 5 min.
The subsequent qPCR was performed in a StepOnePlus™
RealTime PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) to compare the expression of stem cell marker CD166,
periodontal ligament cell marker PERIOSTIN and signaling
molecule β-CATENIN between cells treated with different
glucose concentrations using the primers shown in Table I (9–15).
The 20-µl cocktail contained 10 µl 2X Maxima® SYBR green
master mix with ROX (Thermo Fisher Scientific, Inc.) 0.25 µM
forward and reverse primers, and 50 ng cDNA. The reactions were
initially preheated at 95°C for 10 min followed by 60 cycles of
denaturation at 95°C for 30 sec, annealing at the annealing
temperature of each primer (Table
I) for 30 sec and extension at 72°C for 30 sec. The comparative
quantification cycle (16) was
further analyzed for gene expression by using β-ACTIN as an
internal control.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Author, year | Target gene | Primer sequence | Length (bp) | Annealing temp.
(°C) | Refs. |
|---|
|
| Stem cell marker |
|
|
|
|
| Cao et al | OCT4
(pou5f1) | F
5′-TATACACAGGCCGATGTGG-3′ | 397 | 60 | (9) |
|
|
| R
5′-GTGCATAGTCGCTGCTTGA-3′ |
|
|
|
|
| NANOG | F
5′-ATGCCTCACACGGAGACTG-3′ | 369 | 60 |
|
|
|
| R
5′-CTGCGTCACACCATTGCTA-3′ |
|
|
|
| Saigusa et
al | SOX2 | F
5′-CAAGATGCACAACTCGGAGA-3′ | 95 | 60 | (10) |
|
|
| R
5′-GCTTAGCCTCGTCGATGAAC-3′ |
|
|
|
| Rada et
al | STRO1 | F
5′-GAAGCTAAAGTGGATTCAGGAGTA-3′ | 216 | 58 | (11) |
|
|
| R
5′-TAAGCAGGGGACCATTACA-3′ |
|
| Wang et
al | CD166 | F
5′-GAATGTCTCTGCTATAAGTATTCCAG-3′ | 157 | 62 | (12) |
|
|
| R
5′-GTACAGCCAGTAGACGACACCAGCAAC-3′ |
|
|
|
|
| Periodontal
ligament cell marker |
|
|
|
|
| Dobreva et
al |
PERIOSTIN | F
5′-GAAAGGGAGTAAGCAAGGGAG-3′ | 179 | 58 | (13) |
|
|
| R
5′-ATAATGTCCAGTCTCCAGGTTG-3′ |
|
|
|
|
| Signaling
molecule |
|
|
|
|
| Chen et
al |
β-CATENIN | F
5′-AATCTTGCCCTTTGTCCCG-3′ | 214 | 60 | (14) |
|
|
| R
5′-GGTTGTGAACATCCCGAGC-3′ |
|
|
|
|
| Internal
control |
|
|
|
|
| Herath et
al | β-ACTIN | F
5′-TTGGCAATGAGCGGTT-3′ | 93 | 60 | (15) |
|
|
| R
5′-AGTTGAAGGTAGTTTCGTGGAT-3′ |
|
|
|
Statistical analysis
Cell proliferation, gene expression and alkaline
phosphatase activity were first analyzed for data distribution
using the Shapiro-Wilk Test and the homogeneity of variance.
Differences between groups were analyzed using an
independent-samples median test and pairwise comparisons of groups
using SPSS software version 18 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
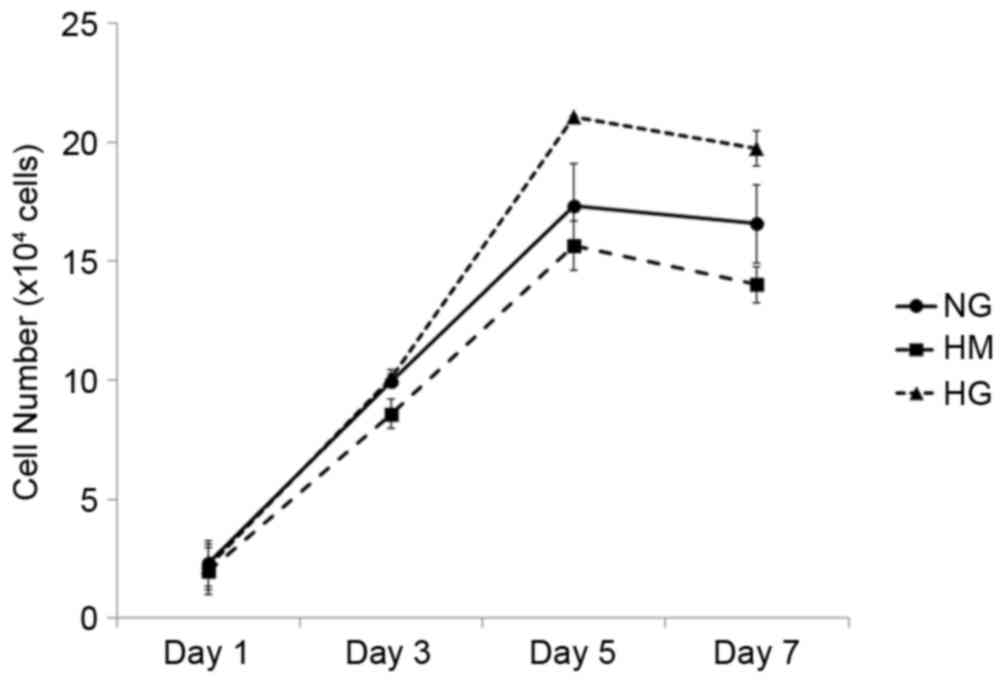
Growth of HPDLFs in different glucose
conditions
The HPDLFs were cultured in DMEM containing three
concentrations of glucose (NG, HM and HG), as described above. The
HPDLFs showed higher proliferation rates when grown in the HG
medium, compared with cells grown in the NG or HM medium, however
the differences were not statistically significant (Fig. 1).
Stemness gene expression in different
glucose conditions
The embryonic stem cell markers (OCT4, NANOG and
SOX2) and CD166 mesenchymal stem cell marker were significantly
upregulated following 3 days of induction under HG conditions,
compared with NG and HM conditions (Fig. 2A-D).
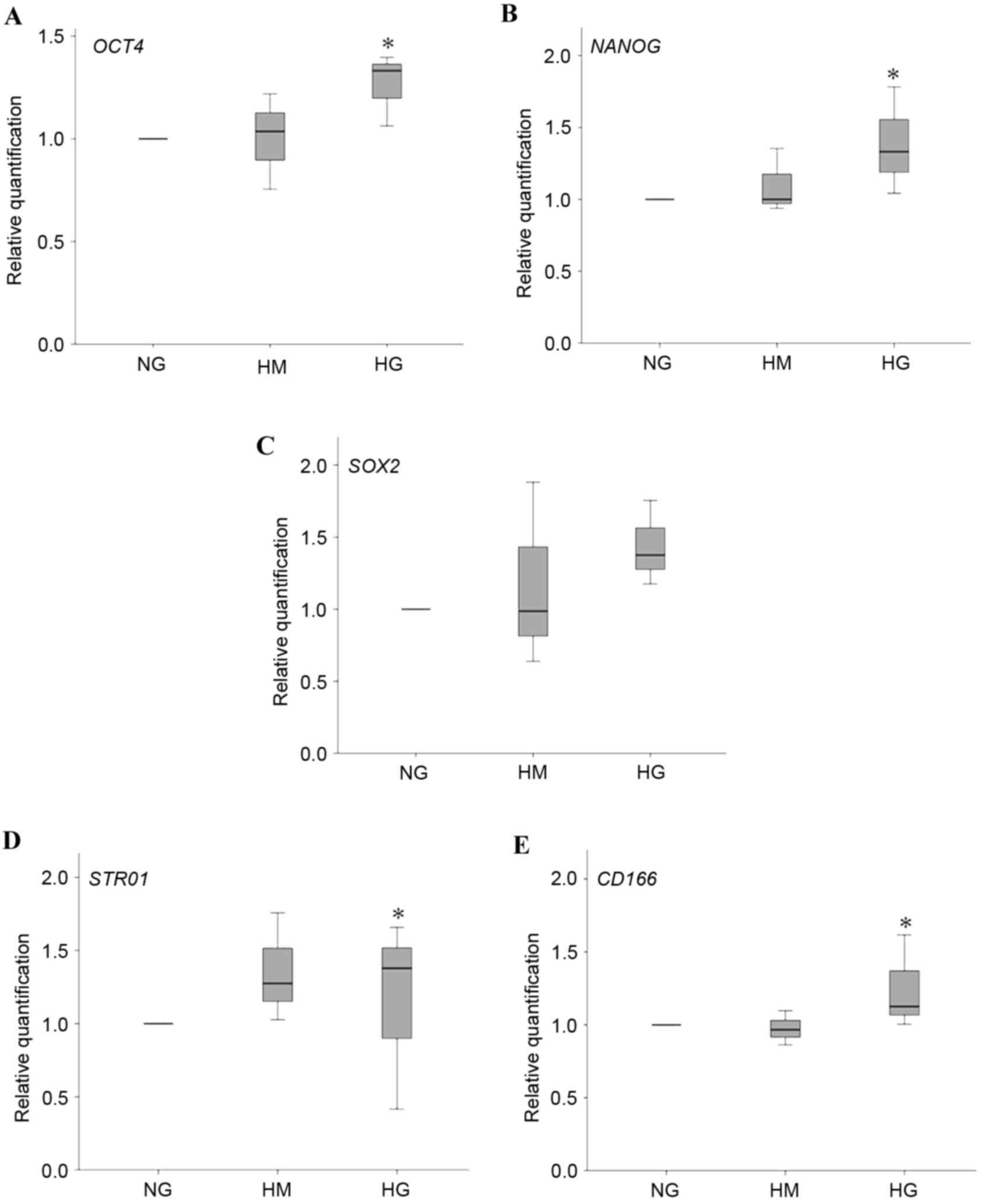 | Figure 2.Expression levels of embryonic and
mesenchymal stem cell markers. Expression of embryonic markers (A)
OCT4, (B) NANOG and (C) SOX2, and mesenchymal markers (D) STRO1 and
(E) CD166 of HPDLFs cultured in osteogenic medium containing NG, HM
and HG concentrations for 3 days. Data are presented as the median
and range. *P<0.05, compared with NG. OCT4, octamer-binding
transcription factor 4; SOX2, (sex-determining region Y)-box 2;
CD166, cluster of differentiation 166; NG, normal glucose; HM, high
mannitol; HG, high glucose. |
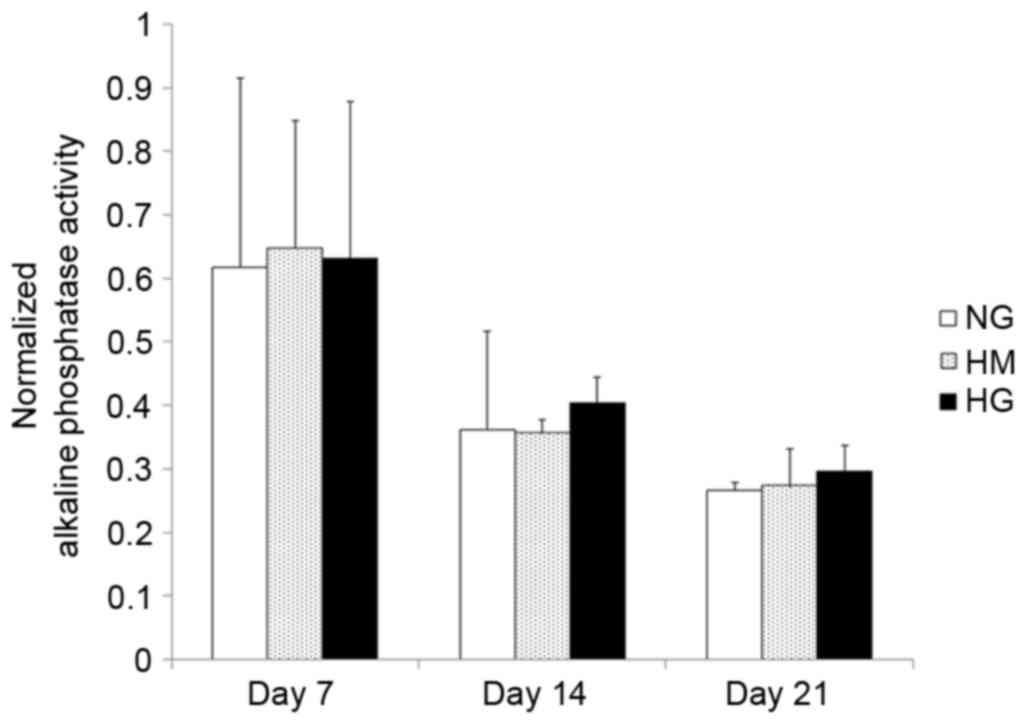
Osteogenic differentiation under
different glucose conditions
The alkaline phosphatase activity showed that HPDLFs
started to differentiate following induction with osteogenic medium
for 7 days (Fig. 3). The alkaline
phosphatase activities in all groups showed similar patterns, with
the highest activities of alkaline phosphatase found on day 7.
Subsequently, activity progressively decreased on days 14 and 21,
as shown in Fig. 3. However, the
alkaline phosphatase activity in the HG medium appeared to decrease
more slowly, compared with that in the other groups.
Following the induction of HPDLFs with osteogenic
medium for 28 days, higher numbers of calcified nodules were formed
in the HG medium, compared with the numbers formed in the NG and HM
media, as shown in Fig. 4.
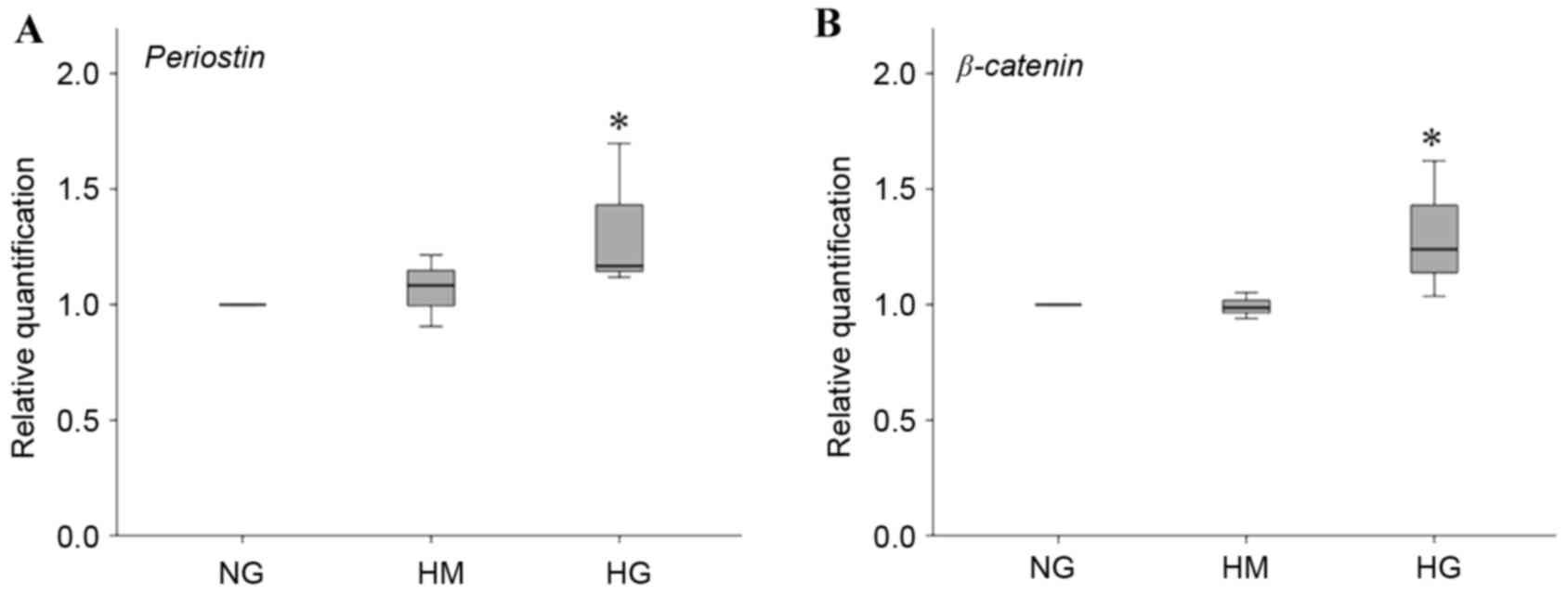
Expression of periostin and
β-CATENIN
As periostin is a marker of periodontal ligament
integrity and β-catenin is a signaling molecule associated with
osteogenesis, the expression of PERIOSTIN and β-CATENIN were also
examined in the present study. HG induced the HPDLFs to express
significantly higher levels of PERIOSTIN, compared with NG and HM
(Fig. 5A). Furthermore, the HPDLFs
showed significantly higher expression of β-CATENIN in HG (Fig. 5B).
Discussion
In the oral cavity, the periodontal ligament anchors
the tooth in the alveolar bone. It is an important source of stem
cells, lying between the alveolar bone and the cementum, which is
involved in regeneration by differentiating to replace the
destroyed attachment tissue. As HPDLFs are readily obtained from
extracted teeth by scraping the periodontal tissue out from the
root of extracted teeth, they offer potential as a source of adult
stem cells. HPDLFs have been reported to possess mesenchymal stem
cell properties, as they can differentiate into osteoblasts,
adipocytes, chondrocytes and neurons (3–5).
However, their differentiation activities under different glucose
conditions remain to be fully elucidated. As the progression of
periodontitis destroys periodontal tissue, increased alveolar bone
loss has been found in diabetic patients with poor glycemic control
(2). The effect of high glucose
conditions has been investigated in several cell types, including
fibroblasts (6–8). Therefore, the regeneration processes
of periodontal ligament tissue may be affected in periodontitis,
particularly in patients with poor glycemic control.
In the present study, HPDLFs in the HG medium tended
to proliferate more, compared with those in the NG and HM medium,
although differences were not statistically significant. The
glucose concentrations used in the present study simulated the
normal blood glucose levels found in healthy individuals (100
mg/dl) and the high blood glucose level found in diabetic patients
(400 mg/dl). The higher proliferation rate in the HG group was not
unexpected, as glucose is an energy source for all cells. In
addition, the HM group, an osmotic pressure control group, showed
the same effect as the NG group. This suggested that the higher
growth rate was due to the effect of glucose and not the effect of
osmotic pressure. Previous studies on mouse bone mesenchymal stem
cells support these findings, which showed that 25 mM
glucose-containing medium promoted growth of cells on day 17
(17). These findings suggest that
high glucose conditions alone, as found in diabetic patients, may
not harm the growth of PDLFs.
When HPDLFs were induced with osteogenic medium for
3 days, the embryonic stem cell markers, OCT4, NANOG and
SOX2, were upregulated in the HG condition, similar to the
results reported by Madonna et al in 2014 (18). These results showed that HG levels
can enhance the expression of embryonic stem cell markers in HPDLFs
at early stages of osteogenesis. The mesenchymal stem cell marker,
CD166, was also upregulated on day 3. The upregulation of
stem cell marker genes may be associated with the potential of
cells to differentiate, which correlates with the increased number
of calcified nodules formed in this condition at later stages. In
addition, Saito et al in 2014 (19) suggested that the expression of
CD166 is associated with the capacity of cells to
differentiate into osteoblasts/cementoblasts.
Following osteogenic induction for 14 days, the
HPDLFs in the HG concentration medium expressed higher levels of
alkaline phosphatase activity, compared with those in the NG and HM
media. Again, this suggested that the increased alkaline
phosphatase activity was due to the metabolic activity of cells,
whereas increased osmotic pressure did not affect activity. On day
28, higher numbers of calcified nodules were also formed in the HG
condition, compared with those in the NG condition. In 2014, Li and
Li (17) also showed that
periodontal ligament cells cultured under HG conditions also had
higher alkaline phosphatase activities on days 14 and 21, but found
that fewer nodules were formed in the HG medium. These differences
may be due to the different sources of cells and their ability to
express stem cell markers. In the present study, following
induction, the cells started to differentiate and form calcified
nodules. The HG condition was found to stimulate the formation of
calcified nodules, and this may be due to higher metabolic activity
producing more ATP and increasing calcium transport into cells,
which leads to increased calcium concentration inside cells,
followed by calcium deposition.
In terms of the association between diabetes and
periodontal disease in bone metabolism (20), diabetic animals show delayed bone
healing. Patients with diabetes have been shown to have lower
radial bone density, compared with non-diabetic patients (21). By contrast, the results of the
present study showed the enhancement of osteogenesis in HPFLFs
cultured in HG medium. However, other factors may also require
consideration in examining the effect of the diabetic condition on
cells. Diabetic animals may not only exhibit hyperglycemia, but may
exhibit other conditions, including the production of advanced
glycation end products, lipotoxicity from long-chain free fatty
acids and immune responses due to the diabetic condition.
Consequently, the HG levels alone may not have been sufficient to
negatively affect the osteogenic differentiation of HPDLFs in the
present study, compared with diabetic animals. Cells under chronic
diabetic conditions may have alterations in protein modification,
which lead to a different response to hyperglycemia, compared with
the response of cells in in vitro HG conditions.
Intracellular hyperglycemia initiates intracellular and
extracellular advanced glycation end product formation. In
addition, several immune cells in the body of animals and humans
respond to hyperglycemia. Neutrophils in diabetic patients with
moderate and poor diabetic control have been found to release
higher levels of superoxide, compared with healthy subjects
(22). Thus, it is possible that
fibroblasts in vitro may respond differently to HG, compared
with in vivo. The cocktail of inflammatory cytokines
secreted may also affect in vivo results. An example of this
is the increased level of TNF-α in a diabetic mouse model, which
induces the apoptosis of mesenchymal stem cells (23).
The findings of the present study have various
implications. In terms of clinical implications, the results showed
that HG increased all cellular processes, including the
proliferation and differentiation of HPDLFs. Therefore, in diabetic
patients with periodontitis, once the periodontal pathogen has been
removed and inflammation subsides, HPDLFs can differentiate into
the osteoblast lineage, which assists in bone regeneration.
Intensive periodontal therapy can significantly reduce periodontal
indices and HbA1c in diabetic patients with moderate periodontitis
(24).
The in vitro results of the present study are
also of interest in terms of cell-based experiments as HG caused
HPDLFs to differentiate into osteoblasts and form calcified
nodules. Accordingly, the glucose level in the medium may be
important when examining the process of osteogenesis in cells.
Periostin is a tissue specific protein in
periodontal ligaments (25). It is
reported to be secreted in adipose tissue and is significantly
positively correlated with blood glucose levels in obese rats
(Psammomys obesus) with type 2 diabetes mellitus (26). In addition, a study by Horiuchi
et al in 1999 (27)
suggested that periostin is involved in the recruitment and
attachment of osteoblast precursors. The results of the present
study showed increased expression of PERIOSTIN in the HG
osteogenic medium. This suggested that the cells retained tissue
specific origin, and that this protein may be involved in
osteogenic differentiation in HG medium.
The results of the present study also showed that
β-CATENIN was upregulated in HG medium. As several reports
(28–30) have suggested that Wnt/β-catenin is
involved in osteogenesis, HG conditions may induce osteoblast
differentiation in HPDLFs through the Wnt/β-catenin pathway.
However, further studies are required to understand the mechanisms
involved.
In terms of tissue regeneration in diabetes and
periodontitis, the results of the present study showed that HG
increased all cellular processes, including the proliferation and
differentiation of HPDLFs. Therefore, in diabetic patients with
periodontitis, once the periodontal pathogen has been removed and
inflammation subsides, HPDLFs can differentiate into the osteoblast
lineage, which will assist bone regeneration. Thus, intensive
periodontal therapy can significantly reduce periodontal indices
and HbA1c in diabetic patients with moderate periodontitis
(24). The results of the present
study also suggested that calcification in cell-based
transplantation may be performed successfully in diabetic patients.
Furthermore, reagents associated with the Wnt/β-catenin pathway may
be useful in stem cell therapy to assist in inducing osteogenic
differentiation, as suggested by Liu et al in 2015 (31). This may improve the treatment of
bone regeneration in the future.
In conclusion, the present study indicated that HG
medium (25 mM) was non-toxic to HPDLFs. The HPDLFs responded to HG
conditions by increasing the expression of embryonic and
mesenchymal stem cell markers, as well as the tissue specific
protein, periostin. Calcified nodules were also formed in higher
numbers when the cells were induced in osteogenic medium containing
HG concentration. This effect may be regulated by β-catenin, which
was induced by HG levels. Further investigation of HPDLFs
transplantation in animal models is required to confirm these in
vitro results. However, these cells are likely to be useful for
tissue regeneration in diabetic patients.
Acknowledgements
The present study was supported by the project for
Higher Education Research Promotion and National Research
University Development, the Office of the Higher Education
Commission and the Thailand and Mahidol University Medical Scholar
Program.
References
|
1
|
Taylor GW, Burt BA, Becker MP, Genco RJ
and Shlossman M: Glycemic control and alveolar bone loss
progression in type 2 diabetes. Ann Periodontol. 3:30–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor GW, Burt BA, Becker MP, Genco RJ,
Shlossman M, Knowler WC and Pettitt DJ: Non-insulin dependent
diabetes mellitus and alveolar bone loss progression over 2 years.
J Periodontol. 69:76–83. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SS, Kwon DW, Im I, Kim YD, Hwang DS,
Holliday LS, Donatelli RE, Son WS and Jun ES: Differentiation and
characteristics of undifferentiated mesenchymal stem cells
originating from adult premolar periodontal ligaments. Korean J
Orthod. 42:307–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JC, Kim JM, Jung IH, Kim JC, Choi SH,
Cho KS and Kim CS: Isolation and characterization of human
periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed
PDL tissue: In vitro and in vivo evaluations. J Clin Periodontol.
38:721–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagatomo K, Komaki M, Sekiya I, Sakaguchi
Y, Noguchi K, Oda S, Muneta T and Ishikawa I: Stem cell properties
of human periodontal ligament cells. J Periodont Res. 41:303–310.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Wu Y, Wang B, Yuan X and Fang B:
High levels of glucose induced the caspase-3/PARP signaling
pathway, leading to apoptosis in human periodontal ligament
fibroblasts. Cell Biochem Biophys. 66:229–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Jiang Y, Mao J, Gu B, Liu H and
Fang B: High levels of glucose induces a dose-dependent apoptosis
in human periodontal ligament fibroblasts by activating caspase-3
signaling pathway. Appl Biochem Biotechnol. 170:1458–1471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura F, Terranova V, Foo H, Kuriharal
M, Kuriharal H and Murayama Y: Glucose-mediated alteration of
cellular function in human periodontal ligament cells. J Dent Res.
75:1664–1671. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X and Xu X, Li J, Chen Z and Xu X: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Ioue Y, Miki C and Kusunoki M: Correlation of CD133,
OCT4 and SOX2 in rectal cancer and their association with distant
recurrence after chemoradiotherapy. Ann Surg Oncol. 16:3488–3498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rada T, Reis RL and Gomes ME: Distinct
stem cells subpopulations isolated from human adipose tissue
exhibit different chondrogenic and osteogenic differentiation
potential. Stem Cell Rev. 7:64–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Gu Z, Ni P, Qiao Y, Chen C, Liu X,
Lin J, Chen N and Fan Q: NF-kappaB P50/P65 hetero-dimer mediates
differential regulation of CD166/ALCAM expression via interaction
with micoRNA-9 after serum deprivation, providing evidence for a
novel negative auto-regulatory loop. Nucleic Acids Res.
39:6440–6455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dobreva MP, Lhoest L, Pereira PN, Umans L,
Camus A, de Chuva Sousa Lopes SM and Zwijsen A: Periostin as a
biomarker of the amniotic membrane. Stem Cells Int.
2012:9871852012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Hu C, Wang G, Li L, Kong X, Ding Y
and Jin Y: Nuclear factor-κB modulates osteogenesis of periodontal
ligament stem cells through competition with β-catenin signaling in
inflammatory microenvironments. Cell Death Dis. 4:e5102013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herath TD, Wang Y, Seneviratne CJ, Lu Q,
Darveau RP, Wang CY and Jin L: Porphyromonas gingivalis
lipopolysaccharide lipid a heterogeneity differentially modulates
the expression of IL-6 and IL-8 in human gingival fibroblasts. J
Clin Periodontol. 38:694–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M and Li CZ: High glucose improves
healing of periodontal wound by inhibiting proliferation and
osteogenetic differentiation of human PDL cells. Int Wound J.
13:39–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madonna R, Geng YJ, Shelat H, Ferdinandy P
and De Caterina R: High glucose-induced hyperosmolarity impacts
proliferation, cytoskeleton remodeling and migration of human
induced pluripotent stem cells via aquaporin-1. Biochim Biophys
Acta. 1842:2266–2275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saito MT, Salmon CR, Amorim BR, Ambrosano
GM, Casati MZ, Sallum EA, Nociti FH and Silvério KG:
Characterization of highly osteoblast/cementoblast cell clones from
a CD105-enriched periodontal ligament progenitor cell population. J
Periodontol. 85:e205–e211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu YY, Xiao E and Graves DT: Diabetes
mellitus related bone metabolism and periodontal disease. Int J
Oral Sci. 7:63–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krakauer JC, McKenna MJ, Buderer NF, Rao
DS, Whitehouse FW and Parfitt AM: Bone loss and bone turnover in
diabetes. Diabetes. 44:775–782. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karima M, Kantarci A, Ohira T, Hasturk H,
Jones VL, Nam BH, Malabanan A, Trackman PC, Badwey JA and Van Dyke
TE: Enhanced superoxide release and elevated protein kinase C
activity in neutrophils from diabetic patients: Association with
periodontitis. J Leukoc Biol. 78:862–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ko KI, Coimbra LS, Tian C, Alblowi J,
Kayal RA, Einhorn TA, Gerstenfeld LC, Pignolo RJ and Graves DT:
Diabetes reduces mesenchymal stem cells in fracture healing through
a TNFα-mediated mechanism. Diabetologia. 58:633–642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calabrese N, D'Aiuto F, Calabrese A, Patel
K, Calabrese G and Massi-Benedetti M: Effects of periodontal
therapy on glucose management in people with diabetes mellitus.
Diabetes Metab. 37:456–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han X and Amar S: Identification of genes
differentially expressed in cultured human periodontal ligament
fibroblasts vs. human gingival fibroblasts by DNA microarray
analysis. J Dent Res. 81:399–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolton K, Segal D, McMillan J, Sanigorski
A, Collier G and Walder K: Identification of secreted proteins
associated with obesity and type 2 diabetes in Psammomys obesus.
Int J Obes (Lond). 33:1153–1165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heo JS, Lee SY and Lee JC: Wnt/β-catenin
signaling enhances osteoblastogenic differentiation from human
periodontal ligament fibroblasts. Mol Cells. 30:449–454. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu
N, Ding B, Liu W, Liu Y, Shi H, et al: High levels of β-catenin
signaling reduce osteogenic differentiation of stem cells in
inflammatory microenvironments through inhibition of the
noncanonical Wnt pathway. J Bone Miner Res. 26:2082–2095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu W, Konermann A, Guo T, Jäger A, Zhang
L and Jin Y: Canonical Wnt signaling differently modulates
osteogenic differentiation of mesenchymal stem cells derived from
bone marrow and from periodontal ligament under inflammatory
conditions. Biochim Biophys Acta. 1840:1125–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Q, Hu CH, Zhou CH, Cui XX, Yang K,
Deng C, Xia JJ, Wu Y, Liu LC and Jin Y: DKK1 rescues osteogenic
differentiation of mesenchymal stem cells isolated from periodontal
ligaments of patients with diabetes mellitus induced periodontitis.
Sci Rep. 5:131422015. View Article : Google Scholar : PubMed/NCBI
|