Introduction
Wound healing is an essential biological process in
the maintenance of tissue and organ homeostasis (1). Various events are regulated during
wound healing, including inflammation, angiogenesis, cell
proliferation, cell migration, cell death and the synthesis and
reorganization of extracellular matrix (ECM) (1,2).
Wound healing is delayed in chronic conditions such as diabetic
wounds, and defects in multiple processes associated with the wound
healing process are responsible for this delay (3). For example, angiogenesis and dermal
wound healing are dependent upon the proliferation and migration of
dermal cells and ECM accumulation, and these processes are severely
impaired in diabetic wound healing (3).
In response to various factors, endothelial
progenitor cells (EPCs) are mobilized and recruited by injured
tissues, where they differentiate into endothelial cells and induce
new blood vessel growth to accelerate wound healing and
regeneration (4–6). Compared with the normal injury
response, the mobilization and recruitment of EPCs are impaired in
diabetic wounds, and reduced levels of stromal cell-derived
factor-1 at the wound may be implicated in this impairment
(7,8).
The Hippo signaling pathway regulates various
important biological phenomena, including cell proliferation, cell
death, cell polarity and mechanotransduction (9,10).
The Yes-associated protein (YAP) is one of the terminal effectors
in the Hippo pathway and it regulates the transcription of target
genes in the nuclei by interacting with the transcriptional
enhancer associated domain family of transcription factors
(9). YAP activity is primarily
regulated by subcellular localization following phosphorylation
(11). When the Hippo signaling
pathway is activated, YAP is phosphorylated by upstream kinases,
large tumor suppressor kinase 1 (LATS1) and LATS2, and the
phosphorylated YAP is retained in the cytoplasm via physical
interaction with 14-3-3 proteins (11). However, unphosphorylated YAP enters
the nucleus and activates target genes that induce cell
proliferation (9–11). Wound healing requires YAP
activation in epithelial and dermal tissues (12).
Substance P (SP) is a peptide composed of 11 amino
acids that was identified as a neurotransmitter in the central
nervous system associated with pain sensation. It has been also
demonstrated that SP acts as an immune modulator and injury
messenger in various peripheral tissues (13). Furthermore, SP mobilizes
mesenchymal stem cells (13) and
EPCs (6) in the bone marrow, and
induces them to migrate into the injured peripheral tissues where
they are involved in regeneration. It has also been demonstrated
that SP accelerates the normal acute and chronic wound healing
processes (14–16). Notably, a previous study
demonstrated that subcutaneous administration of SP accelerates the
normal acute wound healing response via increased angiogenesis
resulting from SP-mediated EPC mobilization (17). By contrast, serum levels of SP are
decreased in diabetic patients (18), and the SP degradation activity of
neutral endopeptidase is increased in chronic diabetic wounds
(19). These results indicate that
the decrease in SP may be implicated in impaired diabetic wound
healing.
The present study used db/db type 2 diabetic (db/db)
mice to determine whether subcutaneous administration of SP
accelerates healing in an acute diabetic wound model. In addition,
the current study also investigated whether impaired EPC
mobilization in diabetic wounds could be rescued in db/db mice
through the subcutaneous administration of SP. Furthermore, the
present study investigated whether YAP activation was involved in
the SP-mediated acceleration of diabetic dermal wound healing.
Materials and methods
Mice
A total of 9 male db/db mice (7–17 weeks-old; 20–25
g) were purchased from Nara Biotech (Seoul, Korea) and were
maintained under a 12 h light:dark cycle at a controlled
temperature (25±2°C) in a humidified atmosphere (40–70%) with
unlimited access to food and water. All procedures were approved by
the Kyung Hee University Medical Center Institutional Animal Care
and Use Committee (Seoul, Korea). The mice were randomly divided
into two groups; 4 mice for a control group and 5 mice for the SP
treated group.
Wound healing model
After hyperglycemia was confirmed in 16-week-old
db/db mice (484–600 mg/dl), full-thickness skin wounds were
created. Mice were anesthetized using ketamine (8 mg/kg; Yuhan Co.,
Ltd., Seoul, Korea) and Rompun® (Xylazine; 5.6 mg/kg;
Bayer Korea Ltd.; Bayer AG, Leverkusen, Germany). The dorsal skin
was shaved and a wound was created using a 4 mm biopsy punch.
Hydrogel (Pluronic F127; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was applied to the wounds. Subsequently, the wounds were
dressed with Mepitel® (Mölnlycke Health Care,
Gothenburg, Sweden) and Tegaderm™ (3M; Maplewood, MN, USA).
Substance P (10 nM/kg; EMD Millipore, Billerica, MA, USA) was
subcutaneously injected once a day into the flanks of the mice for
2 consecutive days after wound creation. Digital images of the
wounds were obtained at 7 days and wound size was analyzed using
Adobe Photoshop CS6 software (Adobe Systems, Inc., San Jose, CA,
USA). The animals were sacrificed by cervical dislocation and the
wound tissues were harvested for further analysis.
Tissue processing and histological
analysis
Harvested wound tissues were collected and fixed
overnight in 4% paraformaldehyde (PFA; 3 M) at 4°C. The fixed
tissues were embedded in paraffin according to standard procedures
and the tissue paraffin blocks were sectioned at a thickness of 4
µm. To perform histological analysis, the sections were stained
with hematoxylin and eosin (Sigma-Aldrich; Merck KGaA). Images were
collected using an Olympus BX41 light microscope (Olympus
Corporation, Tokyo, Japan).
Immunohistochemistry
Immunostaining against α-smooth muscle actin (αSMA),
proliferating cell nuclear antigen (PCNA) and YAP was performed
according to standard procedures. Briefly, paraffin sections (4 µm)
were deparaffinized and dehydrated. The dehydrated sections were
subsequently incubated in peroxidase blocking solution (0.5%
H2O2 in methanol) to inhibit endogenous
peroxidase activity and antigen retrieval was performed by boiling
in sodium citrate buffer (10 mM; pH 6.0). The sections were blocked
with 5% nonfat milk (BD Biosciences, Franklin Lakes, NJ, USA) in
phosphate-buffered saline containing 0.2% Tween-20 (PBST) for 30
min at room temperature. Then, the sections were incubated in PBST
containing 5% nonfat milk for 1.5 h using the following primary
antibodies: Anti-αSMA (M0851; 1:200; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA), anti-PCNA (sc-56; 1:50; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-YAP (14,074; 1:100;
Cell Signaling Technology, Inc., Danvers, MA, USA). Non-fluorescent
staining was performed using the VECTASTAIN®
Elite® ABC kit (Vector Laboratories, Inc., Burlingame,
CA, USA) and the DAB peroxidase (HRP) substrate kit (Vector
Laboratories, Inc.), according to the manufacturer's protocols.
Nuclei were stained in pink with Vector® Nuclear Fast
Red (Vector Laboratories, Inc.) or purple with hematoxylin. Images
were collected using an Olympus BX41 light microscope (Olympus
Corporation, Tokyo, Japan). If required, the total tissue area in
each image was measured using Adobe Photoshop CS6 software (Adobe
Systems, Inc.).
In vivo Matrigel plug assay and
EPC-colony forming unit (CFU) assay
Following anesthetization, 0.2 ml growth
factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
combined with heparin (40 U/ml; Sigma-Aldrich; Merck KGaA) and
murine basic fibroblast growth factor (0.5 µg/ml; R&D Systems,
Inc., Minneapolis, MN, USA) was injected subcutaneously into the
dorsal skin of 11-week-old db/db mice (301–458 mg/dl). For mice
assigned to the SP-treatment group, SP (10 nM/kg) was injected
subcutaneously into the flank for 2 days after injection of the
Matrigel mixture. Matrigel plugs were harvested 7 days after
injection and fixed in 4% PFA for 4 h on ice. After preparing
cryosections (12 µm), the sections were blocked with 5% nonfat milk
(BD Biosciences) in PBST for 30 min at room temperature. Then the
sections were treated with the secondary antibody (goat anti-rat
Alexa Fluor® 488-conjugated IgG; A-11006; 1:500;
Invitrogen; Thermo Fisher Scientific, Inc.) for 45 min at room
temperature following the incubation with primary antibody (rat
anti-CD31 monoclonal antibody; CBL1337; 1:100; Chemicon; EMD
Millipore, Billerica, MA, USA) for 2 h at room temperature to
analyze endothelial cells in the sections. Images were captured
using a Zeiss LSM 700 confocal microscope (Zeiss AG, Oberkochen,
Germany). Following retrieval of the Matrigel plugs, bone marrow
cells obtained from the femur of each mouse were flushed with
α-minimal essential media (Sigma-Aldrich; Merck KGaA) and 20%
heat-inactivated fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) (20), and a
cell suspension (1×105 cells/ml) was made with medium
prepared using the endothelial Cell Growth Medium EGM™-2 Bulletkit
(Lonza Group AG, Basel, Switzerland). Cells were seeded on 35 mm
tissue culture dishes at a density of 1×104 cells/dish
using MethoCult™ media (Stemcell Technologies, Inc., Vancouver, BC,
Canada) and three dishes were prepared for each mouse. At 9 days
after seeding, the cell culture media were carefully washed out and
the number of adherent colonies was analyzed using a Nikon ECLIPSE
TS 100 inverted microscope (Nikon Corporation, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-test was used to evaluate differences between sample
means. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA,
USA.).
Results
SP accelerates wound closing in db/db
type 2 diabetic mice
A previous study demonstrated that subcutaneous
injection of SP accelerates the wound healing process in normal
mice (17). Therefore, the present
study investigated whether subcutaneous administration of SP (10
nM/kg) also affects wound healing in a db/db type 2 diabetic mouse
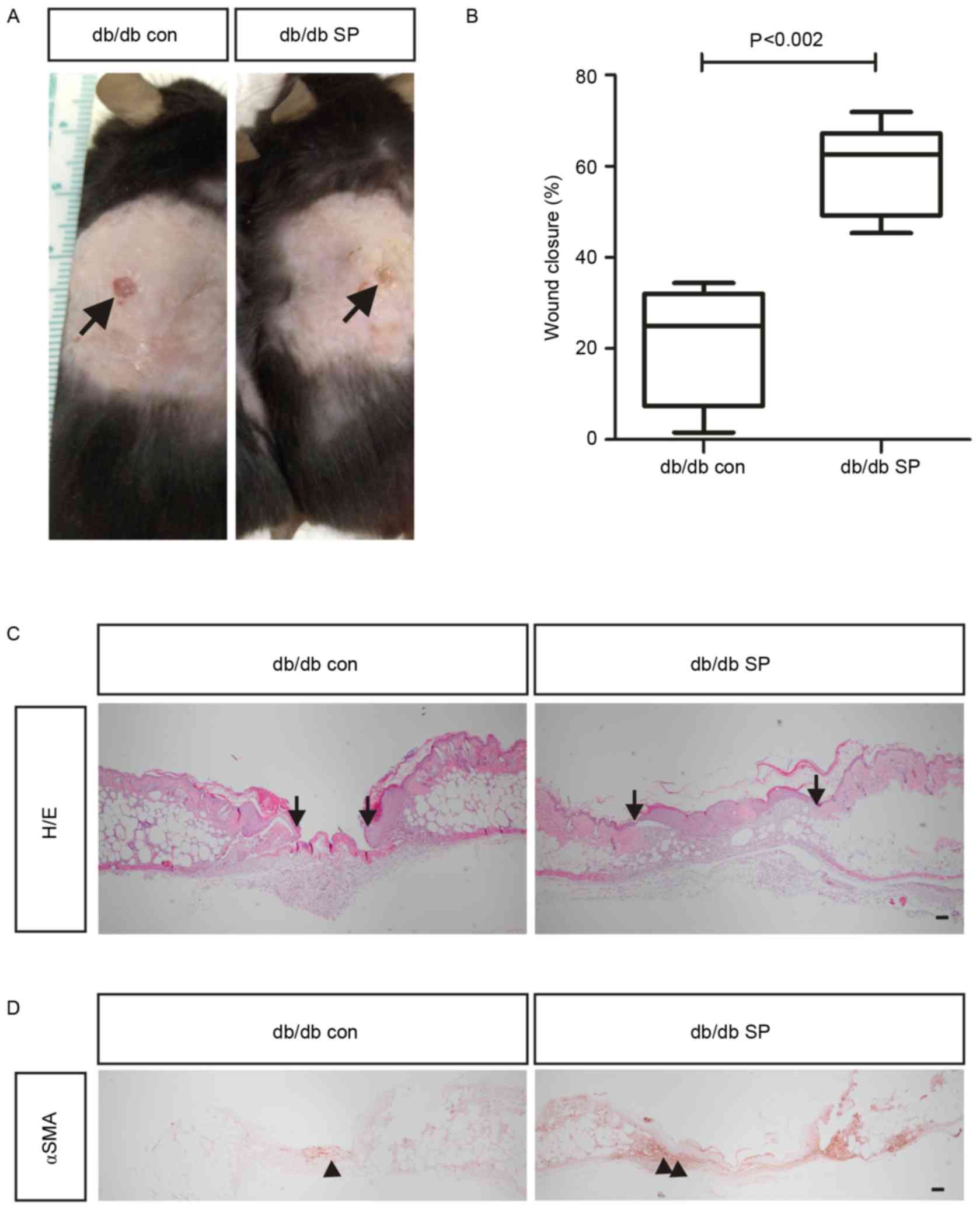
model. At 7 days after wound creation, full-thickness closure of
the excision wound was significantly enhanced in db/db mice with SP
treatment (59.06±18.62%; n=6) compared with control mice
(21.48±6.99%; n=4; Fig. 1A and B).
In addition to wound contraction, increased re-epithelialization
was observed in the SP-treated mice compared with control mice
(Fig. 1C). αSMA-positive
myofibroblasts have an important role in wound contracture
(21), and the detection of
αSMA-positive cells is delayed in diabetic wounds compared with
non-diabetic wounds (22).
Notably, SP injection increased the number of αSMA-positive cells
in wounds of SP-treated mice compared with the control group
(Fig. 1D), thereby enhancing wound
contraction. Collectively, the results indicate that subcutaneous
injection of SP may accelerate wound closure by increasing the
number of αSMA-expressing myofibroblasts in a db/db mouse model of
type 2 diabetes.
SP enhances cellular proliferation and
YAP activation in the wound dermis
YAP is one of the key effectors in the Hippo
signaling pathway that controls various important biological
phenomena, including cellular proliferation, cell survival and
organ size determination (9,23).
Notably, it has been previously demonstrated that YAP controls skin
wound healing (12).
Post-translational stability and nuclear localization of YAP are
required for it to function as a transcriptional activator that
increases cellular proliferation (9). YAP is also required for skin wound
healing in normal mice (12).
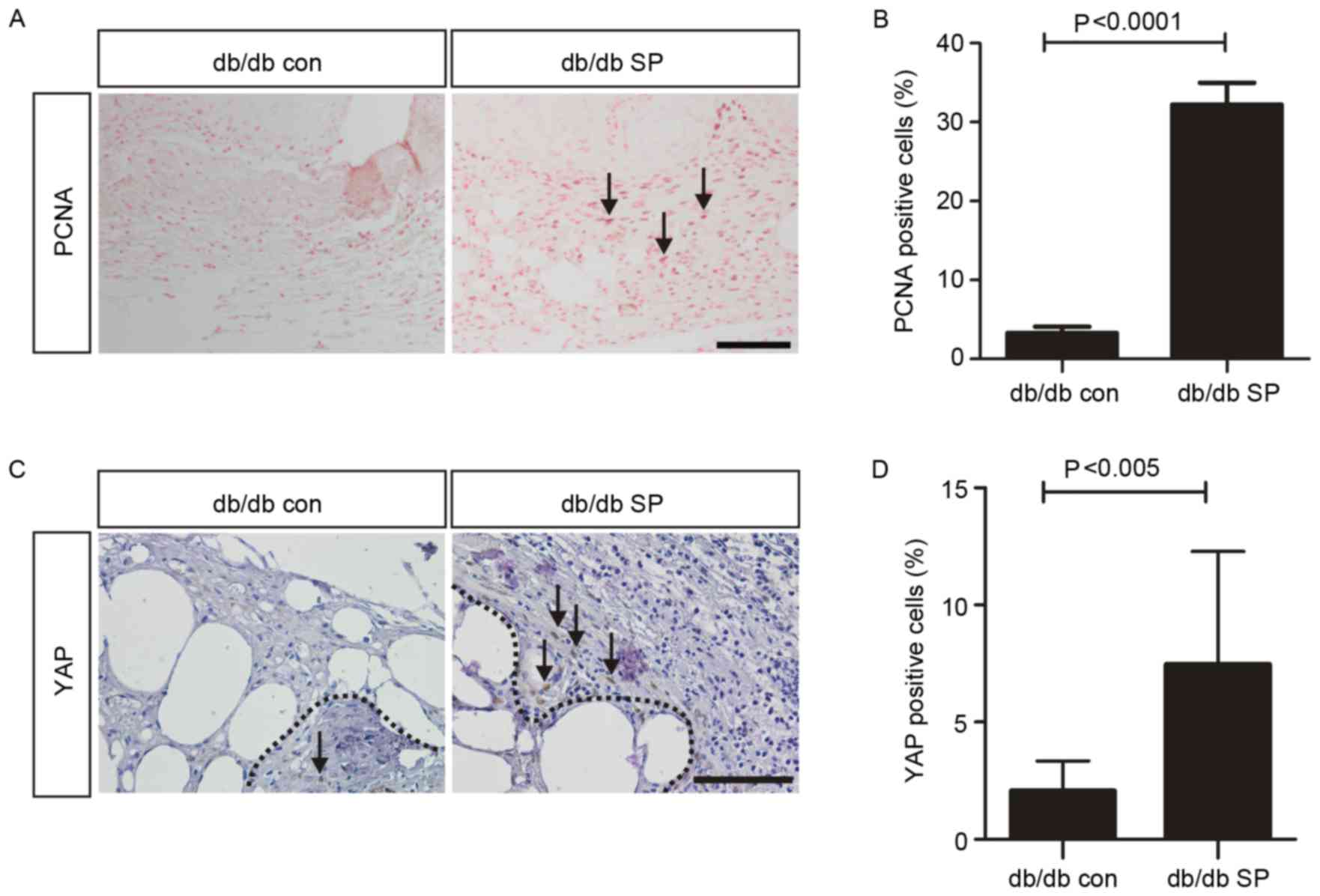
Therefore, the current study investigated the effects of SP
administration on YAP expression in wounds. SP significantly
increased the number of PCNA-positive proliferating cells in the
dermis of db/db mice compared with control mice (32.3±1.60% vs.
3.30±0.46%; Fig. 2A and B). In
addition, YAP was detected in a significantly higher percentage of
dermal cell nuclei in the SP-treated mice compared with control
mice (7.47±1.52% vs. 2.06±0.43%; Fig.
2C and D). Overall, the results indicate that subcutaneous
administration of SP may promote accelerated wound healing by
inducing YAP-mediated cellular proliferation.
SP enhances angiogenesis in dermal
wounds and induces mobilization of EPCs
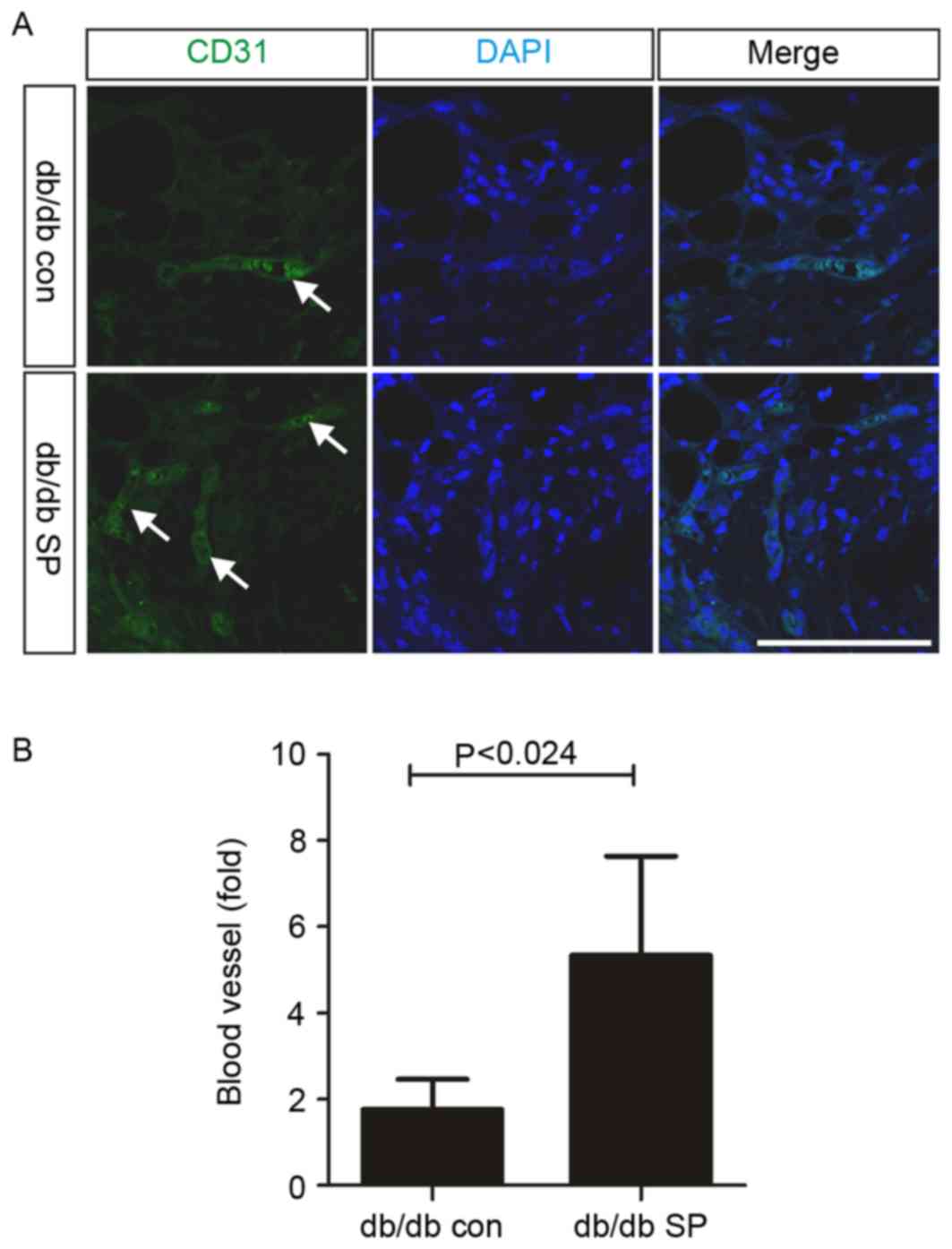
A previous study demonstrated that subcutaneous SP
treatment increases angiogenesis and the mobilization of EPCs in
normal mice (17). In the present
study, subcutaneous SP injection significantly increased the
frequency of CD31-positive cells in the wounded dermis of db/db
mice, compared with the control (control, 1.77±0.35, n=4 vs.
SP-treated, 5.33±1.20, n=4; Fig, 3A
and B). EPC mobilization is important for angiogenesis and
wound healing (8,24), and its function is impaired in
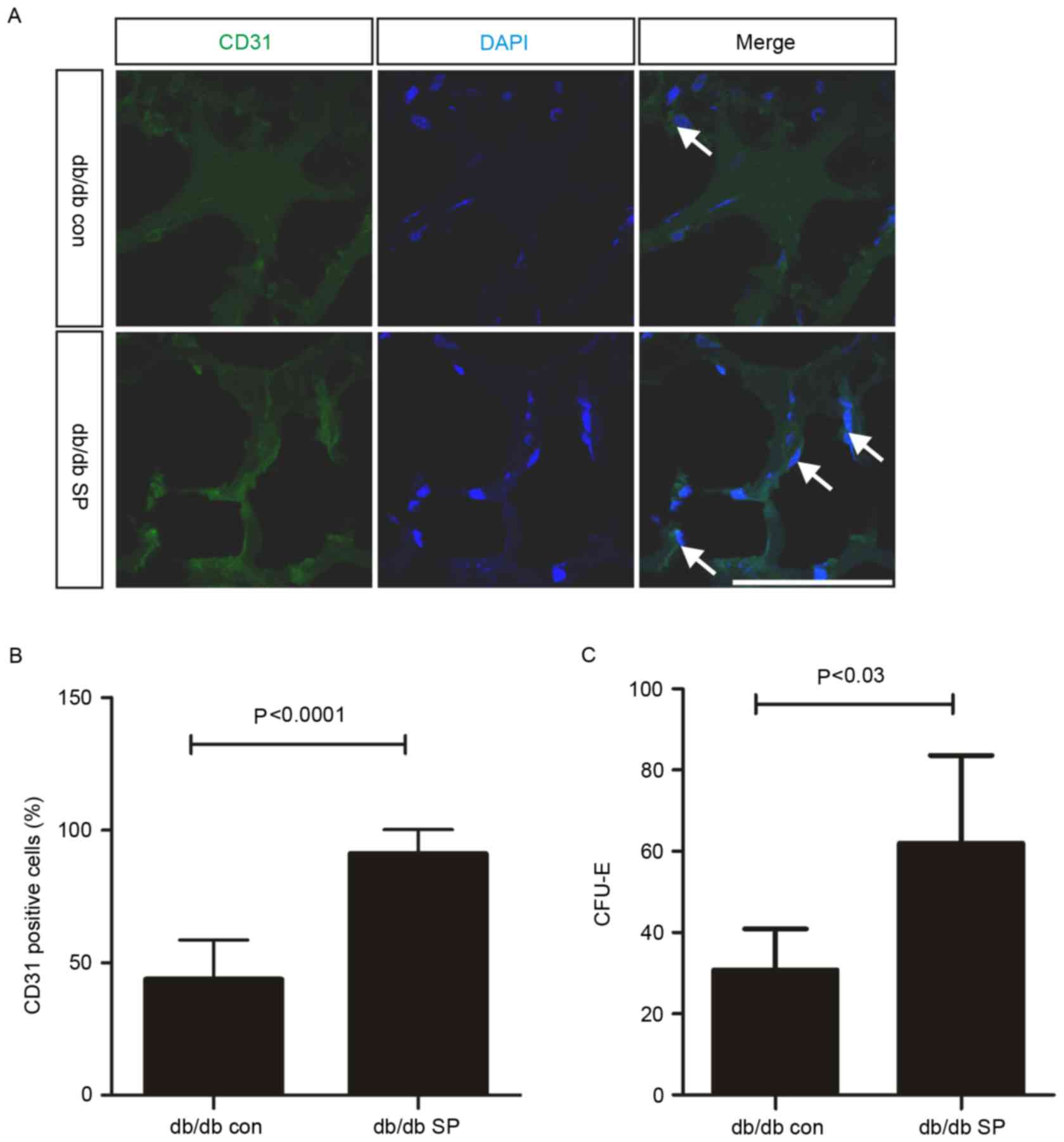
diabetes (8). In order to confirm
the mobilization of EPCs, Matrigel plug assays were performed in
uninjured db/db mice, and subcutaneous SP treatment significantly
increased the number of CD31-positive cells in uninjured db/db mice
by ~2-fold (control, 43.84±5.57% vs. SP-treated, 91.20±2.97%;
Fig. 4A and B). In addition to the
SP-mediated increase in EPC mobilization, SP treatment also
resulted in a 2-fold increase in the number of EPC-CFUs in the bone
marrow of db/db mice (control, 30.75±5.07, n=2 vs. SP-treated,
62.00±8.82, n=3; Fig. 4C).
Therefore, subcutaneous treatment with SP may increase EPC
mobilization, which may contribute to enhanced angiogenesis in the
wound dermis and an increase in the EPC population within the bone
marrow of db/db mice.
Discussion
The present study demonstrated that subcutaneous
administration of SP accelerated diabetic wound healing.
Furthermore, it was also observed that subcutaneous injection of SP
increased EPC mobilization in diabetes and increased the activation
of YAP in the wound dermis of db/db mice. Increased EPC
mobilization and YAP activation may contribute to accelerated
diabetic wound healing through enhanced angiogenesis and increased
fibroblast proliferation, respectively. The results of a previous
study (17) and the current study
demonstrated that systemic SP treatment by subcutaneous injection
induced EPC mobilization in normal and diabetic acute wounds.
Notably, diabetic mice treated with SP exhibited a similar rate of
wound closure compared with the normal wound healing process in
normal mice (data not shown). However, the efficiency of EPC
mobilization mediated by subcutaneous SP injection in the diabetic
and normal wound environments was not compared. Furthermore, the
mechanisms responsible for SP-mediated mobilization of EPCs remain
undefined, and it is not established whether EPC migration in db/db
mice is mediated by the same mechanisms involved in normal acute
wound healing. Further studies are required to address these
questions. In the diabetic wound dermis, subcutaneous treatment
with SP not only increased the number of cells expressing nuclear
YAP, but also increased the number of proliferating cells. In
addition, the number of αSMA-positive cells was increased in the
diabetic wound dermis. Collectively, YAP activation, as a result of
subcutaneous administration of SP, may lead to the upregulation of
αSMA-positive cell proliferation, which may subsequently accelerate
wound contracture. Notably, primary cultured dermal fibroblasts of
db/db mice expressed lower levels of YAP mRNA compared with normal
mice (data not shown). Therefore, it is important to determine
whether SP controls only YAP activation through post-translational
regulatory processes, or if it influences YAP activation and its
transcriptional level activity.
Acknowledgements
The current study was supported by Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
2015R1D1A1A09057839), the Korean Health Technology R&D Project
(Ministry of Health & Welfare, Republic of Korea; grant no.
HI13C1479) and a grant from Kyung Hee University in 2016 (grant no.
KHU-20160701).
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
EPC
|
endothelial progenitor cell
|
|
EPC-CFU
|
endothelial progenitor cell-colony
forming unit
|
|
αSMA
|
α-smooth muscle actin
|
|
SP
|
substance P
|
|
YAP
|
Yes-associated protein
|
References
|
1
|
Shaw TJ and Martin P: Wound repair at a
glance. J Cell Sci. 122:3209–3213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brem H and Tomic-Canic M: Cellular and
molecular basis of wound healing in diabetes. J Clin Invest.
117:1219–1222. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Velazquez OC: Angiogenesis and
vasculogenesis: Inducing the growth of new blood vessels and wound
healing by stimulation of bone marrow-derived progenitor cell
mobilization and homing. J Vasc Surg. 45:(Suppl A). A39–A47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amadesi S, Reni C, Katare R, Meloni M,
Oikawa A, Beltrami AP, Avolio E, Cesselli D, Fortunato O, Spinetti
G, et al: Role for substance p-based nociceptive signaling in
progenitor cell activation and angiogenesis during ischemia in mice
and in human subjects. Circulation. 125:1774–1786, S1-S19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fadini GP, Miorin M, Facco M, Bonamico S,
Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A,
Agostini C and Avogaro A: Circulating endothelial progenitor cells
are reduced in peripheral vascular complications of type 2 diabetes
mellitus. J Am Coll Cardiol. 45:1449–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gallagher KA, Liu ZJ, Xiao M, Chen H,
Goldstein LJ, Buerk DG, Nedeau A, Thom SR and Velazquez OC:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu FX and Guan KL: The Hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gumbiner BM and Kim NG: The Hippo-YAP
signaling pathway and contact inhibition of growth. J Cell Sci.
127:709–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen CG, Moroishi T and Guan KL: YAP and
TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol.
25:499–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee MJ, Byun Ran M, Furutani-Seiki M, Hong
JH and Jung HS: YAP and TAZ regulate skin wound healing. J Invest
Dermatol. 134:518–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn
W, Jiang MH, Kim JC and Son Y: A new role of substance P as an
injury-inducible messenger for mobilization of CD29(+) stromal-like
cells. Nat Med. 15:425–435. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kant V, Kumar D, Kumar D, Prasad R, Gopal
A, Pathak NN, Kumar P and Tandan SK: Topical application of
substance P promotes wound healing in streptozotocin-induced
diabetic rats. Cytokine. 73:144–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leal EC, Carvalho E, Tellechea A, Kafanas
A, Tecilazich F, Kearney C, Kuchibhotla S, Auster ME, Kokkotou E,
Mooney DJ, et al: Substance p promotes wound healing in diabetes by
modulating inflammation and macrophage phenotype. Am J Pathol.
185:1638–1648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Di G, Qi X, Qu M, Wang Y, Duan H,
Danielson P, Xie L and Zhou Q: Substance P promotes diabetic
corneal epithelial wound healing through molecular mechanisms
mediated via the neurokinin-1 receptor. Diabetes. 63:4262–4274.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Um J, Jung N, Chin S, Cho Y, Choi S and
Park KS: Substance P enhances EPC mobilization for accelerated
wound healing. Wound Repair Regen. 24:402–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang LH, Zhou SX, Li RC, Zheng LR, Zhu JH,
Hu SJ and Sun YL: Serum levels of calcitonin gene-related peptide
and substance P are decreased in patients with diabetes mellitus
and coronary artery disease. J Int Med Res. 40:134–140. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antezana M, Sullivan SR, Usui M, Gibran N,
Spenny M, Larsen J, Ansel J, Bunnett N and Olerud J: Neutral
endopeptidase activity is increased in the skin of subjects with
diabetic ulcers. J Invest Dermatol. 119:1400–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baustian C, Hanley S and Ceredig R:
Isolation, selection and culture methods to enhance clonogenicity
of mouse bone marrow derived mesenchymal stromal cell precursors.
Stem Cell Res Ther. 6:1512015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hinz B: Formation and function of the
myofibroblast during tissue repair. J Invest Dermatol. 127:526–537.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Darby IA, Bisucci T, Hewitson TD and
MacLellan DG: Apoptosis is increased in a model of
diabetes-impaired wound healing in genetically diabetic mice. Int J
Biochem Cell Biol. 29:191–200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|