Introduction
Neuropathic pain is a widespread health problem
(1). It is a complex disorder,
which leads to chronic illness. Although considerable progress has
been made, the mechanisms of neuropathic pain remain to be fully
elucidated (2). Accumulating
evidence indicates that neuroinflammation may be critical in the
initiation and maintenance of neuropathic pain, which is now
considered to be a neuroimmune disorder (3–6).
Studies have shown that the activation of glial cells, including
microglia and astrocytes, contributes to central nervous system
neuroinflammation and promotes central sensitization, promoting the
subsequent development and maintenance of neuropathic pain
(7–9). In addition, several studies have
shown that inhibiting microglial and astrocytic activation have
analgesic effects in neuropathy (10–12).
However, no effective drugs have been found to provide efficient
treatment.
Chinese herbs are an important resource for
potential novel drugs to develop safe and effective agents for the
management of neuropathic pain. Several plants have been found to
be effective for antagonizing chronic neuropathic pain (13–15).
Paeoniae alba Radix, the dried roots of Paeonia
lactiflora Pallas or Paeonia veitchii Lynch, is one of
the traditional Chinese crude drug herbs. It has been widely used
in traditional Chinese prescriptions to alleviate various issues,
such as blood extravasation, blood stagnation and female genital
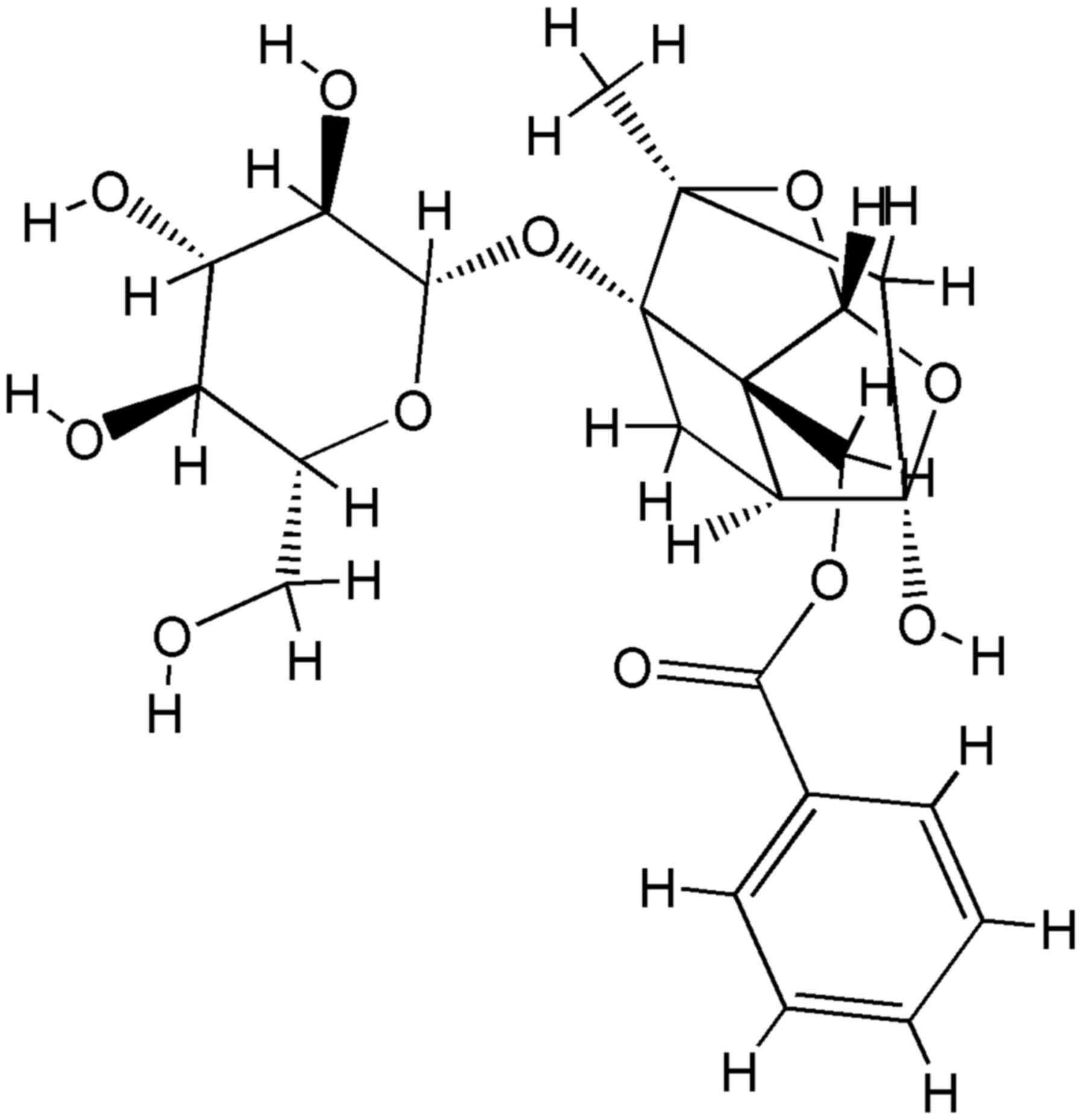
diseases (16). Paeoniflorin
(Fig. 1), a monoterpene glycoside,
is one of the principal active ingredients of Paeoniae alba
Radix, and has been reported to exhibit several pharmacological
effects, including anti-inflammatory, anti-oxidant and
neuroprotective effects (17–20).
In nervous disorders in particular, PF has been reported to exert
neuroprotective effects against Alzheimer's disease, cerebral
ischemia and Parkinson's disease in experimental models (18,21,22).
However, no there have been no reports on the analgesic properties
of PF in neuropathic pain.
Previous studies have suggested that PF has potent
neuroprotective effects by inhibiting inflammatory responses
(18,19,23,24).
As neuroinflammation is important in the initiation and maintenance
of neuropathic pain, the present study hypothesized that PF can
ameliorate neuropathic pain. Therefore, the present study aimed to
observed the antagonistic effect of PF on neuropathic pain in a rat
model of chronic constriction injury (CCI) and to examine its
underlying mechanism.
Materials and methods
Drugs
PF was extracted from Paeoniae alba Radix
(Weifang Shengtai Pharmaceutical Industry Co., Ltd., Weifang,
China; cat. no. 20150301), and the preparative separation and
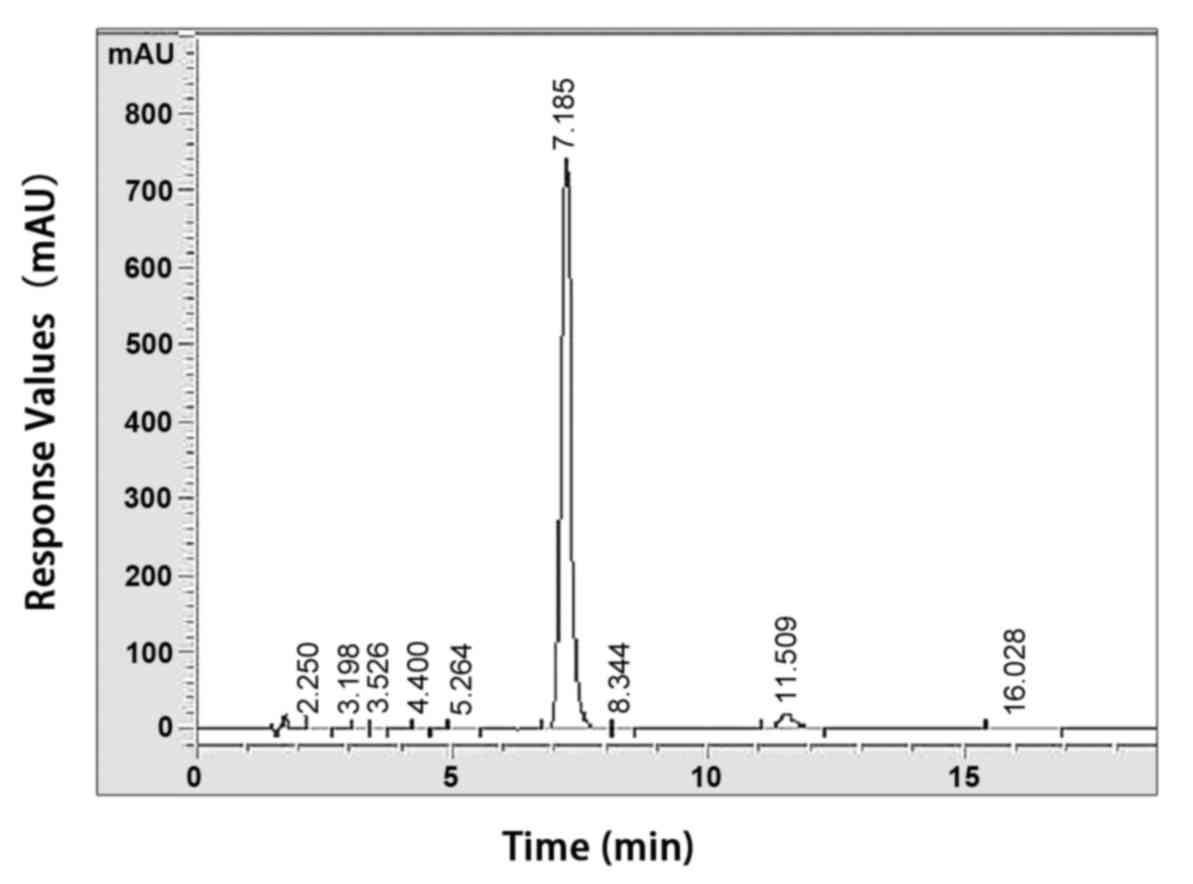
purification were performed as described previously (25). The purity of PF was determined to
be >98% using a high-performance liquid chromatographic assay
(Fig. 2). PF was dissolved in
normal saline solution (8 mg/ml).
Animals
A total of 30 male Wistar rats (7-week-old; 200–220
g) were obtained from SPF Biotechnology Co., Ltd. (Beijing, China).
The rats were housed in a 12-h light/dark schedule at a temperature
of 23±2°C and a humidity of 60±5% environment with free access to
food and water during the 1-week acclimatization period. All animal
experiments conformed to the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (Bethesda,
MD, USA). The use of the rats was reviewed and approved by the
animal care committee of Beijing University of Chinese Medicine
(Beijing, China).
CCI of the sciatic nerve
The animals were subjected to CCI, as previously
described by Bennett and Xie (26). In brief, the rats were anesthetized
with chloral hydrate (300 mg/kg intraperitoneal injection) and the
right sciatic nerve was exposed at the mid-thigh level. Proximal to
the sciatic trifurcation, adhering tissue was removed from ~7 mm of
the nerve and four ligatures (chromic catgut 4.0) were tied loosely
at 1.0 mm intervals. Sham surgery was performed by exposing the
right sciatic nerve without ligation.
Drug treatment
The rats were randomly divided into three groups (10
rats in each group): Sham surgery group (Sham), CCI group (CCI) and
CCI+50 mg/kg PF group. The optimal administration dosage of PF was
selected according to the results of preliminary experiments. The
PF or vehicle (10 ml/kg) was administered by intraperitoneal
injections once a day for 11 days, starting on the first day
post-CCI.
Mechanical withdrawal threshold
assessment
Mechanical allodynia was examined by assessing the
paw withdrawal threshold (PWT) in grams using calibrated Von Frey
filaments (North Coast Medical, Inc., Gilroy, CA, USA) as described
by Chaplan et al (27). The
rats were placed in transparent plexiglass cages on top of an
elevated metal mesh floor, and a series of von Frey filaments of
logarithmically incremental stiffness were applied using Chaplan's
up-down method at the central region of the plantar surface of the
right hindpaw, to identify the filament closest to the pain
response threshold. Each measurement was repeated three times at
intervals of 15 min, and the average force evoking reliable
withdrawal was determined as the threshold. This assessment was
performed 1 day prior to CCI surgery, and 3, 7 and 11 days post-CCI
surgery.
Thermal withdrawal latency
assessment
Thermal hyperalgesia was measured using a BME-410C
thermal pain stimulator (Institute of Medical Biology, Chinese
Academy Of Medical Sciences, Beijing, China) as described
previously by Hargreaves et al (28). The rats were placed in transparent
plexiglass cages on top of an elevated glass platform, and
appropriate intensity radiant heat (55±0.5°C) was applied from
underneath the platform to the plantar surface of the hindpaw until
the rats showed positive signs of pain (licking or withdrawing the
paw). The time taken for the rat to lick or withdraw its paw was
recorded and defined as the paw withdrawal latency (PWL). A cut-off
time of 25 sec was used to prevent tissue damage. Each measurement
was repeated three times at intervals of 15 min, and the average
force evoking reliable withdrawal was determined as the threshold.
This assessment was performed 1 day prior to CCI surgery, and 1, 3,
7 and 11 days post-CCI surgery.
Enzyme-linked immunosorbent assay
(ELISA)
On the 11th day following CCI surgery, 60 min
following the final drug administration (PF or vehicle), the rats
were sacrificed by overdose with chloral hydrate (350 mg/kg
intraperitoneal). The L4-L5 spinal cords ipsilateral to the nerve
injury were removed, frozen in liquid nitrogen and then stored at
−80°C until further processing. The frozen spinal cords were
homogenized in cold phosphate-buffered solution (10 µl/mg tissue).
Following centrifugation at 10,000 × g for 15 min, the supernatant
was used for ELISA. The expression levels of the TNF-α and IL-1β
cytokines were measured using ELISA kits (Cusabio Biotech Co.,
Ltd., Wuhan, China) according to the manufacturer's protocol.
Immunostaining
The L4-L5 spinal cord ipsilateral to the nerve
injury was fixed with 4% paraformaldehyde for 24 h and
paraffin-embedded and cut into 5 µm thick sections. The spinal cord
sections were deparaffinized following routine methods. Sections
were then subjected to antigen retrieval (pH 6.0, citric acid
antigen repair buffer) and heated in microwave for 15 min (8 min at
mid-range and 7 min at low-grade). The sections were blocked using
3% BSA (cat. no. A8020; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) for 30 min at room temperature. The
sections were then incubated with goat polyclonal anti-ionized
calcium-binding adapter molecule-1 (Iba-1) antibody (cat. no.
ab5076; 1:100; Abcam, Cambridge UK) or rabbit polyclonal anti-glial
fibrillary acidic protein (GFAP; cat. no. BA0056; 1:100; Boster
Systems, Inc., Pleasanton, CA, USA) at 4°C overnight. Following
extensive washing in PBS, the sections were incubated with
appropriate biotinylated secondary antibodies (cat. nos. GB23204
and GB23303; 1:200 in PBS; Goodbio technology Co., Ltd., Wuhan,
China) at room temperature for 1 h, followed by incubation using an
SABC immunohistochemistry kit (Goodbio Technology Co., Ltd.)
according to the manufacturer's protocol. The microglia and
astrocytes were stained and observed using an Image-Pro Plus 6.0
imaging analysis system (Media Cybernetics, Inc., Rockville, MD,
USA).
Western blot analysis
The protein was extracted by RIPA lysis buffer (cat.
no. P0013C; Beyotime Institute of Biotechnology, Haimen, China).
Western blot analysis was used to quantify the protein expression
of NF-κBp65 in the nucleus and the total protein expression of
phosphorylated (p-) p38 mitogen-activated protein kinase
(MAPK)/p38MAPK in the total protein extracted from the spinal cord.
The concentration of the protein was examined by BCA method using
protein quantitative kit (cat. no. P0012; Beyotime Institute of
Biotechnology) An equal quantity of protein (50 µg) was loaded and
separated by 12% SDS-PAGE. The resolved proteins were transferred
onto nitrocellulose membranes (EMD Millipore, Billerica, MA, USA).
The membranes were then blocked in 5% nonfat milk for 2 h at room
temperature and incubated overnight at 4°C with mouse anti-NF-κBp65
(cat. no. CST6956; 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-p-p38MAPK (cat. no. CST4631;
1:1,000; Cell Signaling Technology, Inc.) and rabbit anti-p38MAPK
(cat. no. CST8690; 1:1,000; Cell Signaling Technology, Inc.)
respectively. The blots were then incubated with goat anti-mouse
and goat anti-rabbit secondary antibodies (horseradish peroxidase;
Goodbio Technology Co., Ltd.) conjugated with horseradish
peroxidase (1:500, ZSGB-BIO, Beijing, China) for 1 h at room
temperature. The protein bands were visualized with
chemiluminescence reagent (Engreen Biosystem, Ltd., Beijing,
China). Western blot densitometry analysis of signal intensity was
performed using Image-Pro Plus imaging analysis system (version,
6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The effect of each treatment was analyzed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). All data are
expressed as the mean ± standard error of the mean. Statistical
analysis was performed using one-way analysis of variance, followed
by the least-significant difference post hoc test or Dunnett's T3
test for comparison of multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
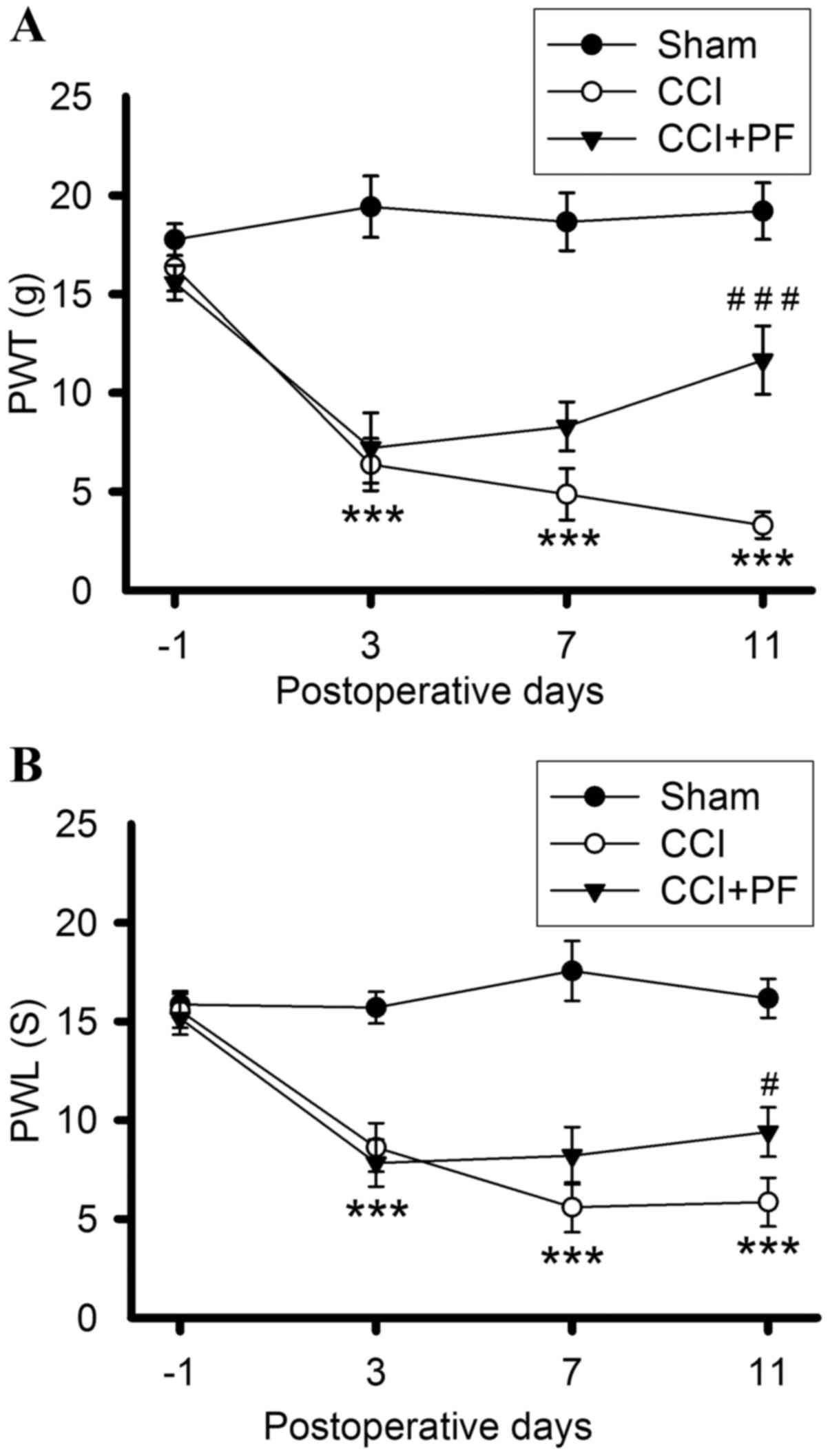
PF attenuates increased PWT and PWL in
CCI rats
The effects of PF on PWT and PWL in CCI rats are
shown in Fig. 3A and B. Prior to
CCI surgery, no significant differences were found in the baseline
PWL and PWT among all groups. PWT and PWL in the CCI groups
decreased markedly 3, 7 and 11 days post-CCI (P<0.001), compared
with those in the sham groups, indicating that CCI induced
long-lasting thermal hyperalgesia and mechanical allodynia.
Following PF administration, the PWL and PWT were significantly
increased in the rats, compared with those in the CCI group, on day
11 (PWT; P<0.001; PWL, P<0.05). These results demonstrated
that PF produced an antinociceptive effect on CCI-induced pain,
including mechanical and thermal hyperalgesia, 11 days following
CCI.
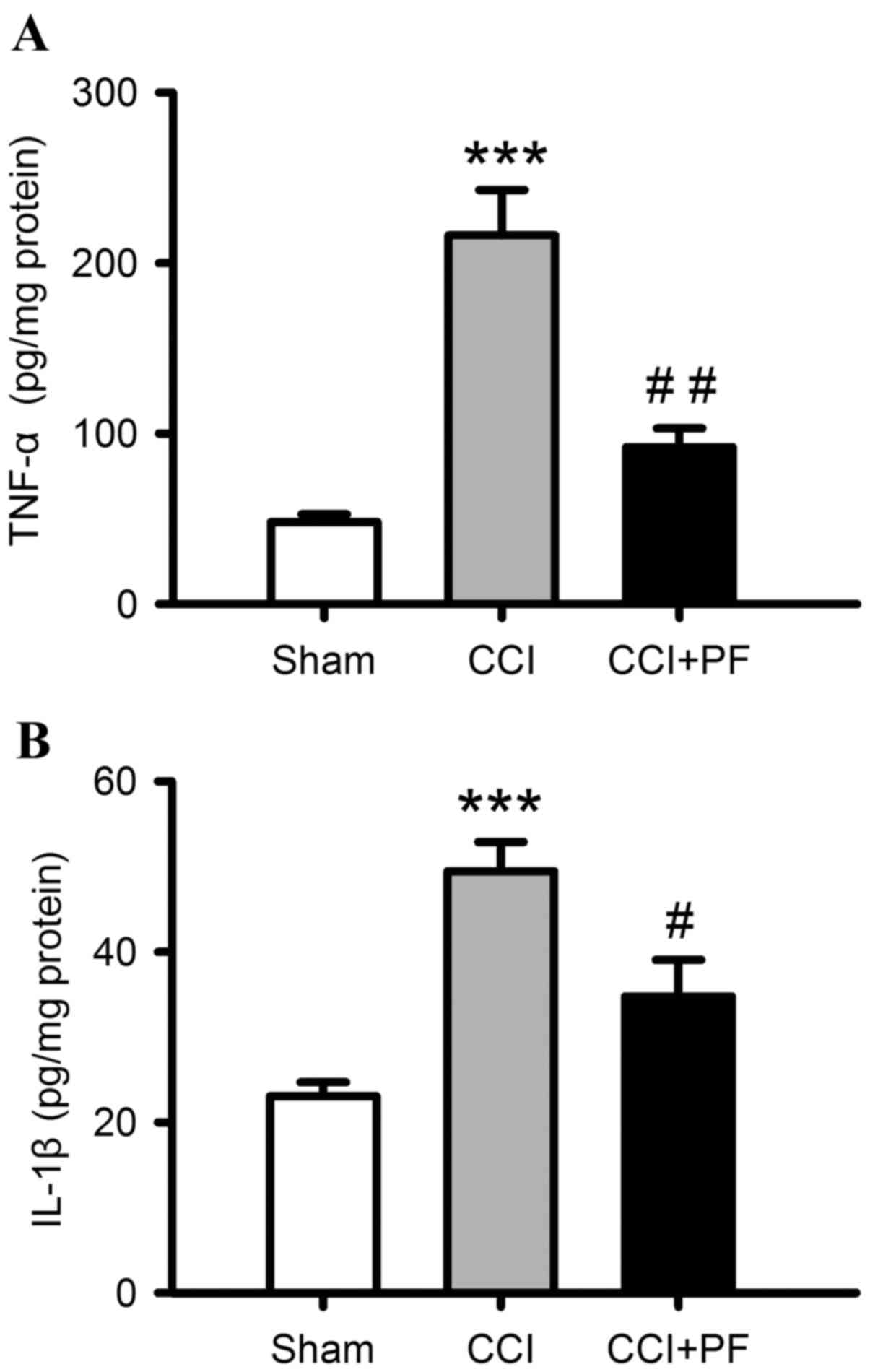
Anti-inflammatory effects of PF in CCI
rats
To investigate the effects of PF on CCI-induced
neuroinflammation, the levels of proinflammatory cytokines, IL-1β
and TNF-α, in spinal cord samples were determined. As shown in
Fig. 4A and B, compared with the
sham group, the levels of IL-1β, and TNF-α were markedly increased
in the spinal cord of the CCI rats (P<0.001). PF treatment
significantly decreased these elevated proinflammatory cytokine
levels, compared with the levels in the CCI groups (TNF-α,
P<0.01; IL-1β, P<0.05).
PF inhibits the activation of
microglia in CCI rats
To examine the possible mechanisms underlying the
protective effects of PF on neuropathic pain in rats, the
activation of astrocytes and microglia were monitored. As shown in
Fig. 5A-D, compared with the sham
group, the expression levels of the astrocyte marker (GFAP) and
microglial marker (Iba-1) were increased significantly in the CCI
rats (P<0.05). However, compared with the CCI group, the
expression of Iba-1 (P<0.05), but not GFAP (P>0.05) decreased
significantly in the CCI+PF group, suggesting that PF inhibited the
activation of microglia but not astrocytes.
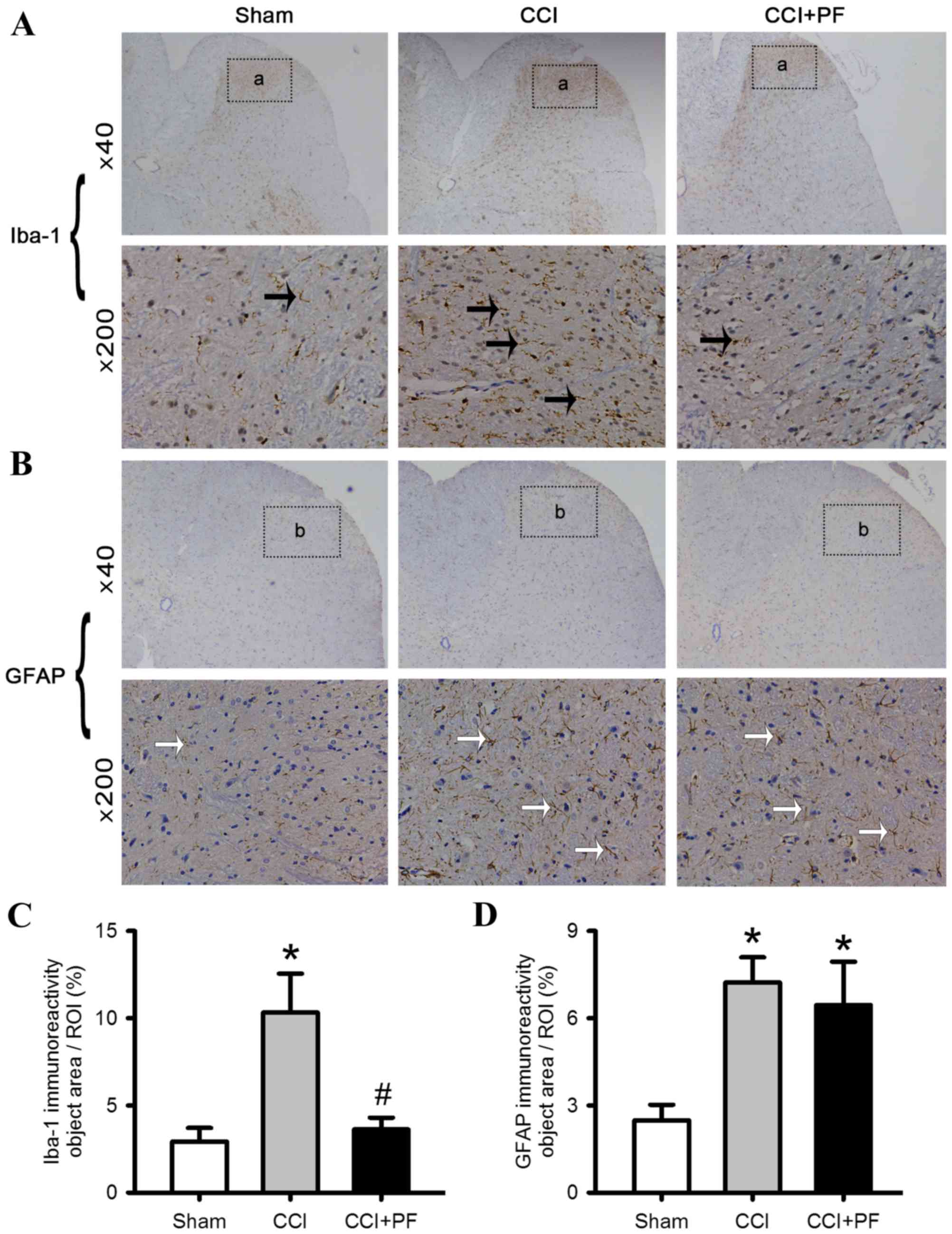 | Figure 5.Effects of PF on the activation of
microglia and astrocytes in the L4-L5 lumbar segment of CCI rats.
Representative images of spinal cord sections stained with
astrocyte marker, GFAP, and microglial marker, Iba-1, antibodies.
(A) Iba-1 staining in the spinal card; black arrows indicate
Iba-1-positive microglia; (a) regions selected for quantitative
analysis of microglial marker activation. (B) GFAP staining in the
spinal cord; white arrows indicate GFAP-positive astrocytes; (b)
regions selected for quantitative analysis of astrocyte marker
activation. (C) Quantification of the effect of PF on the
microglial marker Iba-1. (D) Quantification of the effect of PF on
the astrocyte marker GFAP. Data are expressed as the mean ±
standard error of the mean (n=3). *P<0.05, compared with the
Sham group; #P<0.05, compared with the CCI group.
CCI, chronic constriction injury; PF, peaoniflorin; GFAP, glial
fibrillary acidic protein; Iba-1, ionized calcium-binding adapter
molecule-1. |
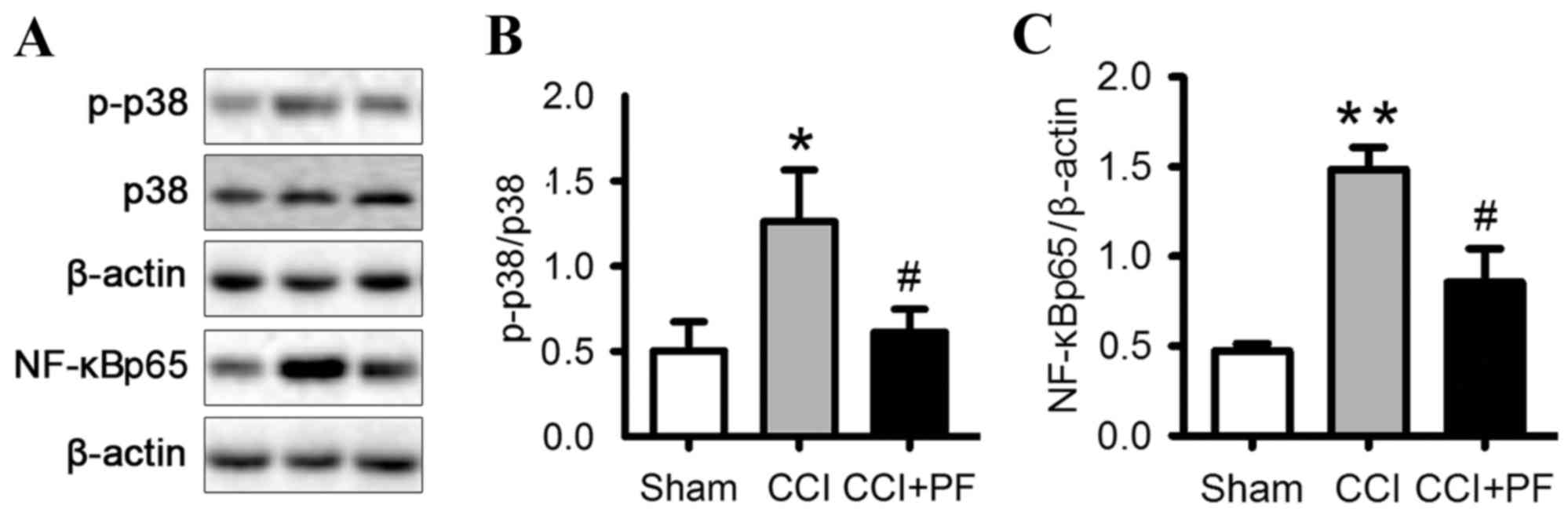
Effects of PF on the activation of
p38MAPK in the spinal cord of CCI rats
To further examine the mechanisms underlying the
effect of PF, the present study investigated the expression of
p-p38 in the spinal cord. As shown in Fig. 6A and B, the expression level of
p-p38 was significantly increased in the CCI group, compared with
that in the sham group (P<0.05). PF treatment markedly decreased
the elevated level of p-p38 observed in the CCI group (P<0.05).
These results demonstrated that PF inhibited the p38MAPK
pathway.
Effects of PF on the activation of
NF-κB in the spinal cord of CCI rats
NF-κB is a crucial transcription factor complex
controlling the expression of proinflammatory and pain mediators.
To investigate the mechanism underlying the analgesic effect of PF,
the present study monitored the expression of NF-κBp65, a nuclear
protein associated with the NF-κB signaling pathway, in the spinal
cord of the CCI rats. As shown in Fig.
6C, compared with the sham group, the expression level of
nuclear NF-κBp65 was significantly increased by CCI (P<0.01). PF
significantly decreased the protein expression of NF-κBp65 in the
spinal cord, compared with that in the CCI group (P<0.05). These
results indicated that PF inhibited the NF-κB pathway.
Discussion
The present study demonstrated for the first time,
to the best of our knowledge, that PF alleviated the neuropathic
pain induced by CCI in rats. It was found that PF attenuated
CCI-induced neuropathic pain, including mechanical and thermal
hyperalgesia, and decreased expression levels of the TNF-α and
IL-1β proinflammatory cytokines in the spinal cord. In addition, PF
inhibited the activation of microglia and reduced the elevated
expression of p-p38 MAPK/NF-κB in the spinal cord induced by CCI.
These results suggested that PF offers potential for use as a
therapeutic agent for neuropathic pain.
The CCI model is a widely used rodent model to
investigate neuropathic pain mechanisms (29–31).
This model successfully produces long-lasting thermal hyperalgesia
and mechanical allodynia, similar to human behavioral responses
(32,33). In the present study, it was found
that CCI produced marked mechanical allodynia and thermal
hyperalgesia in rats. However, administration of PF for 11 days
attenuated mechanical allodynia and thermal hyperalgesia,
suggesting the possible therapeutic efficacy of PF. In addition, PF
was observed to have a more marked analgesic effect in thermal
hyperalgesia, compared with mechanical allodynia. Further
investigations are required to examine the underlying
mechanism.
Increasing evidence has indicated that
proinflammatory cytokines are a critical factor in the initiation
and maintenance of hyperalgesia in animal models of neuropathic
pain (4). The proinflammatory
cytokine-mediated process during neuroinflammation can be induced
by nerve injury (5). The CCI model
induces the upregulation of proinflammatory cytokines, including
IL-1β, IL-6 and TNF-α, in the spinal cord (34,35).
The increase of proinflammatory cytokines in the spinal cord
promotes the transduction of detrimental signals by increasing
excitatory synaptic transmission and decreasing inhibitory synaptic
transmission (36). In the present
study, it was found that PF significantly inhibited the
overexpression of spinal IL-1β and TNF-α in the CCI rat model.
These results indicated PF as a potential candidate to control
neuroinflammation-induced pain.
Following peripheral nerve injury, sensitized
primary afferent terminals release nociceptive neurotransmitters
and mediators, including glutamate, substance P and fractalkine,
which activate spinal microglia and astrocytes (37). Activated microglia and astrocytes
contribute to neuroinflammation, accelerate facilitatory pain
transmission, and contribute to the subsequent development and
maintenance of neuropathic pain (7–9). The
results of the present study showed that spinal astroglia and
microglia were markedly activated in the CCI injury model, whereas
administration of PF for 11 days inhibited the activation of
microglia, suggesting that the activities of PF involved regulation
of the spinal glial-neuroimmune system.
MAPKs are a crucial molecules in cell signaling,
which consist of p38MAPK, extracellular signal-related kinase 1/2
and c-Jun amino terminal kinase 1/2 (38). Emerging evidence has indicates that
nerve injury results in the activation of p38MAPK in the spinal
cord, and p38MAPK regulates the production of proinflammatory
cytokines to promote the development of neuropathic pain (39–41).
In addition, several studies have suggested that p38MAPK is
critical in microglial signaling under neuropathic pain conditions
and represents a valuable therapeutic target for neuropathic pain
(42,43). The results of the present study
indicated that PF treatment prevented the CCI-induced upregulation
in the protein level of p-p38 when measured 11 days post-nerve
injury. The same trend was observed in the effect of PF on the
inhibition of CCI-induced activation and release of proinflammatory
cytokines. These results suggested that PF-mediated inhibition of
CCI-induced p38MAPK activation is a possible mechanism underlying
its inhibitory action on neuropathic pain.
NF-κB, a pleiotropic factor, which regulates several
physiological processes and is important in regulating the immune
response (44). Previous studies
have demonstrated that the activation of NF-κB occurs in the spinal
cord, which is involved in the transmission and processing of
nociceptive information. Following activation, NF-κB transfers into
the nucleus and regulates the synthesis and release of
proinflammatory cytokines, including IL-1β, IL-6 and TNF-α, which
may be crucial in neuroinflammation (45,46).
It has been reported that the administration of NF-κB inhibitors
exerts an analgesic effect in various animal pain models (47,48).
In the present study, it was found that PF reduced the CCI-elevated
expression of NF-κB in the spinal cord. In addition, PF inhibited
the activation of NF-κB and the subsequent expression of
proinflammatory cytokines, which may be beneficial for reducing
neuropathic pain.
In conclusion, the results of the present study
demonstrated that PF produced a significant analgesic action in
CCI-injury rats and that this activity was associated with the
modulation of neuroinflammation in the spinal cord. These results
suggested that PF is a potential therapeutic agent for neuropathic
pain, which merits further investigation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81473370 and 81173569).
References
|
1
|
Gilron I, Watson CP, Cahill CM and Moulin
DE: Neuropathic pain: A practical guide for the clinician. CMAJ.
175:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cornelius VR, Sauzet O, Williams JE, Ayis
S, Farquhar-Smith P, Ross JR, Branford RA and Peacock JL: Adverse
event reporting in randomised controlled trials of neuropathic
pain: Considerations for future practice. Pain. 154:213–220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streit WJ, Mrak RE and Griffin WS:
Microglia and neuroinflammation: A pathological perspective. J
Neuroinflammation. 1:142004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moalem G and Tracey DJ: Immune and
inflammatory mechanisms in neuropathic pain. Brain Res Rev.
51:240–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Myers RR, Campana WM and Shubayev VI: The
role of neuroinflammation in neuropathic pain: Mechanisms and
therapeutic targets. Drug Discov Today. 11:8–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wahba M and Waln O: Asterixis related to
gabapentin intake: A case report and review. Postgrad Med.
125:139–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marchand F, Perretti M and McMahon SB:
Role of the immune system in chronic pain. Nat Rev Neurosci.
6:521–532. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Milligan ED and Watkins LR: Pathological
and protective roles of glia in chronic pain. Nat Rev Neurosci.
10:23–36. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bradesi S: Role of spinal cord glia in the
central processing of peripheral pain perception.
Neurogastroenterol Motil. 22:499–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sweitzer SM, Schubert P and DeLeo JA:
Propentofylline, a glial modulating agent, exhibits antiallodynic
properties in a rat model of neuropathic pain. J Pharmacol Exp
Ther. 297:1210–1217. 2001.PubMed/NCBI
|
|
11
|
Jean YH, Chen WF, Sung CS, Duh CY, Huang
SY, Lin CS, Tai MH, Tzeng SF and Wen ZH: Capnellene, a natural
marine compound derived from soft coral, attenuates chronic
constriction injury-induced neuropathic pain in rats. Br J
Pharmacol. 158:713–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YC, Huang SY, Jean YH, Chen WF, Sung
CS, Kao ES, Wang HM, Chakraborty C, Duh CY and Wen ZH: Intrathecal
lemnalol, a natural marine compound obtained from Formosan soft
coral, attenuates nociceptive responses and the activity of spinal
glial cells in neuropathic rats. Behav Pharmacol. 22:739–750. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YS, Park HJ, Kim TK, Moon DE and Lee
HJ: The effects of Ginkgo biloba extract EGb 761 on mechanical and
cold allodynia in a rat model of neuropathic pain. Anesth Analg.
108:1958–1963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao T, Hao J, Wiesenfeld-Hallin Z, Wang DQ
and Xu XJ: Analgesic effect of sinomenine in rodents after
inflammation and nerve injury. Eur J Pharmacol. 721:5–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou X, Cheng H, Xu D, Yin Q, Cheng L,
Wang L, Song S and Zhang M: Attenuation of neuropathic pain by
saikosaponin a in a rat model of chronic constriction injury.
Neurochem Res. 39:2136–2142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu SH, Wu DG and Chen YW: Chemical
constituents and bioactivities of plants from the genus
Paeonia. Chem Biodivers. 7:90–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong SZ, Ge QH, Li Q, Qu R and Ma SP:
Peoniflorin attentuates Abeta(1–42)-mediated neurotoxicity by
regulating calcium homeostasis and ameliorating oxidative stress in
hippocampus of rats. J Neurol Sci. 280:71–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nam KN, Yae CG, Hong JW, Cho DH, Lee JH
and Lee EH: Paeoniflorin, a monoterpene glycoside, attenuates
lipopolysaccharide-induced neuronal injury and brain microglial
inflammatory response. Biotechnol Lett. 35:1183–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu YM, Jin R, Yang L, Zhang J, Yang Q, Guo
YY, Li XB, Liu SB, Luo XX and Zhao MG: Phosphatidylinositol 3
kinase/protein kinase B is responsible for the protection of
paeoniflorin upon H2O2-induced neural
progenitor cell injury. Neuroscience. 240:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu HQ, Zhang WY, Luo XT, Ye Y and Zhu XZ:
Paeoniflorin attenuates neuroinflammation and dopaminergic
neurodegeneration in the MPTP model of Parkinson's disease by
activation of adenosine A1 receptor. Br J Pharmacol. 148:314–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang HR, Peng JH, Cheng XB, Shi BZ, Zhang
MY and Xu RX: Paeoniflorin atttenuates amyloidogenesis and the
inflammatory responses in a transgenic mouse model of Alzheimer's
disease. Neurochem Res. 40:1583–1592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong H, Li R, Yu C, Xu T, Zhang X and Dong
M: Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis
in PC12 cells via suppressing reactive oxygen species-mediated
PKCδ/NF-κB pathway. Neuroscience. 285:70–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Wang J, Wang J, Wang P and Xue Y:
Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis
of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling
pathways. Brain Res. 1618:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen F, Lu HT and Jiang Y: Purification of
paeoniflorin from Paeonia lactiflora Pall. By high-speed
counter-current chromatography. J Chromatogr A. 1040:205–208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vierck CJ, Acosta-Rua AJ and Johnson RD:
Bilateral chronic constriction of the sciatic nerve: A model of
long-term cold hyperalgesia. J Pain. 6:507–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaggi AS, Jain V and Singh N: Animal
models of neuropathic pain. Fundam Clin Pharmacol. 25:1–28. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu LW, Chen JY, Yu KL, Cheng KI, Wu PC
and Wu BN: Neuroprotective and anti-inflammatory activities of
atorvastatin in a rat chronic constriction injury model. Int J
Immunopathol Pharmacol. 25:219–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LX and Wang ZJ: Animal and cellular
models of chronic pain. Adv Drug Deliv Rev. 55:949–965. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Qiu HQ, Liu H, Liu M, Huang ZY, Yang
J, Su YP and Yu CX: Effects of koumine, an alkaloid of Gelsemium
elegans Benth., on inflammatory and neuropathic pain models and
possible mechanism with allopregnanolone. Pharmacol Biochem Behav.
101:504–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Costa B, Trovato AE, Colleoni M, Giagnoni
G, Zarini E and Croci T: Effect of the cannabinoid CB1 receptor
antagonist, SR141716, on nociceptive response and nerve
demyelination in rodents with chronic constriction injury of the
sciatic nerve. Pain. 116:52–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kiguchi N, Kobayashi Y and Kishioka S:
Chemokines and cytokines in neuroinflammation leading to
neuropathic pain. Curr Opin Pharmacol. 12:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawasaki Y, Zhang L, Cheng JK and Ji RR:
Cytokine mechanisms of central sensitization: Distinct and
overlapping role of interleukin-1beta, interleukin-6, and tumor
necrosis factor-alpha in regulating synaptic and neuronal activity
in the superficial spinal cord. J Neurosci. 28:5189–5194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao H and Zhang YQ: Spinal glial
activation contributes to pathological pain states. Neurosci
Biobehav Rev. 32:972–983. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Obata K, Yamanaka H, Kobayashi K, Dai Y,
Mizushima T, Katsura H, Fukuoka T, Tokunaga A and Noguchi K: Role
of mitogen-activated protein kinase activation in injured and
intact primary afferent neurons for mechanical and heat
hypersensitivity after spinal nerve ligation. J Neurosci.
24:10211–10222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu JT, Xin WJ, Wei XH, Wu CY, Ge YX, Liu
YL, Zang Y, Zhang T, Li YY and Liu XG: p38 activation in uninjured
primary afferent neurons and in spinal microglia contributes to the
development of neuropathic pain induced by selective motor fiber
injury. Exp Neurol. 204:355–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu L, Huang Y, Yu X, Yue J, Yang N and Zuo
P: The influence of p38 mitogen-activated protein kinase inhibitor
on synthesis of inflammatory cytokine tumor necrosis factor alpha
in spinal cord of rats with chronic constriction injury. Anesth
Analg. 105:1838–1844, table of contents. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji RR and Suter MR: p38 MAPK, microglial
signaling, and neuropathic pain. Mol Pain. 3:332007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rojewska E, Popiolek-Barczyk K, Jurga AM,
Makuch W, Przewlocka B and Mika J: Involvement of pro- and
antinociceptive factors in minocycline analgesia in rat neuropathic
pain model. J Neuroimmunol. 277:57–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM and
Yao SL: Alleviation of neuropathic pain by intrathecal injection of
antisense oligonucleotides to p65 subunit of NF-kappaB. Br J
Anaesth. 97:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Makarov SS: NF-kappaB as a therapeutic
target in chronic inflammation: Recent advances. Mol Med Today.
6:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Niederberger E and Geisslinger G: The
IKK-NF-kappaB pathway: A source for novel molecular drug targets in
pain therapy. FASEB J. 22:3432–3442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Laughlin TM, Bethea JR, Yezierski RP and
Wilcox GL: Cytokine involvement in dynorphin-induced allodynia.
Pain. 84:159–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei XH, Yang T, Wu Q, Xin WJ, Wu JL, Wang
YQ, Zang Y, Wang J, Li YY and Liu XG: Peri-sciatic administration
of recombinant rat IL-1β induces mechanical allodynia by activation
of src-family kinases in spinal microglia in rats. Exp Neurol.
234:389–397. 2012. View Article : Google Scholar : PubMed/NCBI
|