Introduction
Silicosis, a form of occupational lung disease
caused by the inhalation of crystalline silica dust, is
characterized by silicotic nodule formation and pulmonary
interstitial fibrosis (1).
Occupational exposure to respirable crystalline silica dust
particles occurs in sand blasting, drilling, pulverizing, cutting
bricks and concrete blocks, grinding concrete and use of other
pneumatic equipment (2) every
year, therefore, silicosis is an occupational health concern in
developing and developed countries (3,4). In
China, ~20,000 cases of pneumoconiosis are diagnosed each year, and
silicosis is the most common, fastest progressing and serious type
(5). The pathological process of
silicosis includes progressive inflammation, fibroblast
proliferation and collagen deposition. In the initial inflammatory
responses, alveolar macrophages, the first cells responded to
stimuli of the body, are important. Following exposure to silica, a
number of macrophages undergo apoptosis, resulting in the
production of reactive oxygen species (ROS), which include hydroxyl
radicals, superoxide anions, hydrogen peroxide, singlet oxygen
(6,7) and nitric oxide (NO) (8). The generation of oxidants results in
cell and lung damage; increase the expression of inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, and transforming growth factor (TGF)-β; activate cell
signaling pathways, including the mitogen-activated protein kinase
pathways; and phosphorylate and activate specific transcription
factors, including nuclear factor (NF)-κB, which intensifies
chronic inflammation and promotes pulmonary fibroblasts to
proliferate and synthesize excess collagen (1).
Oleanolic acid (3β-hydroxyolean-12-en-28-oic acid;
OA) is a plant-derived pentacyclic terpenoid, which exists
naturally in vegetable oil, food and certain medicinal herbs either
as a free acid, or as an aglycone of triterpenoid saponins
(9). It has been shown to exhibit
numerous pharmacological properties, including hepatoprotective,
anti-oxidative, anti-inflammatory and anticancer activities.
Therefore, OA and its derivatives possess a wide range of
applications (10). It has been
reported that OA may exert beneficial effects on renal and liver
fibrosis by activating nuclear factor, erythroid 2 like 2 (11) and has been used as an oral
treatment for human liver dysfunction in China (10). However, the effectiveness of OA in
the treatment of fibrotic lung diseases, including silicosis,
remains to be elucidated. It has been reported that the
phosphatidylinositol-3-kinase (PI3K)/Akt pathway is the most
important pathway for the fibroblast to myofibroblast
differentiation of normal and diseased primary human lung
fibroblasts (12). The present
study aimed to investigate the protective effects of OA in an
experimental model of silica-induced inflammation and fibrosis by
examining the oxidation/antioxidant system, TNF-α, TGF-β1,
Akt/NF-κB and collagen.
Materials and methods
Materials
OA (purity >99.9%; cat. no. 110742-200513) was
purchased from the National Institutes for Food and Drug Control
(Beijing, China) and was suspended in 0.6% sodium carboxymethyl
cellulose for use. Crystalline SiO2 (~95%; 1–5 µm),
obtained from the National Institute of Occupational Health and
Poison Control, Chinese Center for Disease Control and Prevention
(Beijing, China), were subjected to grinding, and heating for at
180°C for 6 h, followed by dilution with sterile saline to a
concentration of 50 mg/ml in suspension, autoclaved and stored at
4°C. Enzyme-linked immunosorbent assay (ELISA) kits,
malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione
(GSH) test kits, and Masson's stain were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Phosphorylated
(p-)AKT1 (phospho S473; cat. no. ab81283) and AKT (cat. no.
ab28422) antibody were purchased from Abcam (Cambridge, MA, USA).
NF-κB antibody (cat. no. sc-8008) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Animals and treatment
A total of 96 male adult Wistar rats (aged 6–8 weeks
and weighing 180–200 g) were purchased from Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China). The animal
experiments were reviewed and approved by the Institutional Animal
Care and Use Committee at the North China University of Science and
Technology (Tangshan, China). The animals received food and water
according to guidelines set by the National Institutes of Health
(Bethesda, MD, USA) and were housed in an air-conditioned room with
a 12-h light/dark cycle at constant temperature (21°C) and 55%
humidity for at least 1 week prior to the experiments.
The rats were divided into four groups according to
the randomized block design, namely, a control group, model group,
solvent control group and OA group, with 24 rats in each group.
With the exception of the rats in the control group, the rats were
induced by intratracheal instillation of SiO2 (250
mg/kg). The rats in the OA group were intragastrically administered
with OA (60 mg/kg) daily from the second day following
SiO2 administration. The rats in the solvent control
group were gavaged daily with 0.6% sodium carboxymethyl cellulose
(10 ml/kg) solution, whereas the rats in the control group were
gavaged with physiological saline in the same conditions for 56
consecutive days. The rats (n=6/group) were sacrificed on days 7,
14, 28 and 56. Blood samples were collected into heparinized tubes
via the abdominal aorta at the four distinct time points. The blood
samples were immediately centrifuged at 3,000 × g for 10 min
at room temperature, and the serum was frozen at −80°C for
subsequent analyses. The lung tissues were immediately perfused
with physiological saline solution to removed blood cells. Then
right lower lobe tissues were removed and stored at −80°C for
western blot analysis, and the right upper lobes were used for the
determination of hydroxyproline. The left lung tissues were fixed
in paraformaldehyde for the assessment of morphological changes
using hematoxylin and eosin (HE) staining and Masson's staining
with immunohistochemical analysis.
Analysis of the oxidation/antioxidant
system
The oxidation/antioxidant system includes MDA, SOD
and GSH peroxidase (GHS-Px). The MDA content and activities of
SOD/GSH-Px in serum samples were assayed using MDA and SOD/GSH-Px
test kits according to the manufacturer's protocol (Nanjing
Jiancheng Bioengineering Institute). MDA, the end product of lipid
peroxidation of cells, condenses with thiobarbituric acid to form a
product with a maximum absorption at 532 nm, therefore, the MDA
content in the samples was determined by comparing the optical
density (O.D.) of the samples to the standard substance. SOD and
GSH-Px, two important antioxidant enzymes, can scavenge free
radicals and inhibit lipid peroxidation. The activities of SOD and
GSH-Px activity are expressed in units, with 1 unit defined as 50%
inhibition of nitrite formation. SOD can inhibit the hydroxylamines
of superoxide radical anions oxidized to form nitrite, and appear
violet with a color reagent; the change in absorbance was recorded
at 550 nm. The activity of GSH-Px was determined using the
disulfide-nitrobenzoic acid direct method and compared at the O.D.
at 412 nm with the standard substance.
Analysis of the levels of TNF-α and
TGF-β1
The serum contents of TNF-α and TGF-β1 were detected
using ELISA, according to the manufacturer's protocol (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). The anti-rat TNF-α
(cat. no. EK0526) or TGF-β1 (cat. no. EK0514) specific antibody was
coated on the ELISA plates. The standards and samples were pipetted
into the wells and the TNF-α/TGF-β1 present in the sample bound to
the wells by the immobilized antibody. The liquid was removed from
the wells and biotinylated anti-rat TNF-α or TGF-β1 antibody (1:100
dilution, at 37°C for 60 min) was added. Following washing away
unbound biotinylated antibody, HRP-conjugated streptavidin was
pipetted into the wells for 30 min at 37°C. The wells were again
washed, and TMB color liquid was added to the wells for 20 min at
37°C, with color developing in proportion to the level of TNF-α
(TGF-β1) bound. The stop solution alters the color from blue to
yellow, and the intensity of the color was measured at 450 nm. The
placental TNF-α/TGF-β1 levels were calculated as pg/ml using the
standard curve.
Determination of collagen content
The content of collagen in the right upper lobe lung
tissue was determined using a hydroxyproline assay according to the
manufacturer's protocol (Nianjing Jiancheng Bioengineering
Institute.). Briefly, the lung tissue samples (80 mg) were
hydrolyzed in boiling water for 5 h. Following addition of the
immobilized reagents, the mixture was placed in 60°C water for 15
min and centrifuged at 3,000 × g for 15 min at room temperature
after cooled down. The hydroxyproline content of the supernatant
was quantified by spectrophotometry at 550 nm. The data are
expressed as collagen (µg)/wet weight (mg).
Immunohistochemical analysis of
AKT1-phospho S473, NF-κB, and collagen types I and II
The immunohistochemistry used
Streptavidin-peroxidase Histostain TM-Plus kits (cat. no. SP-9000;
OriGene Technologies, Inc., Beijing, China). The important stages
were as follows: Paraffin-embedded sections (5 µm) were
deparaffinized, rehydrated and underwent removal of endogenous
peroxidase with 3% H2O2. Following incubation with goat
serum working solution, the working solution was discarded and the
tissue sections were incubated with primary antibodies against
AKT1-phospho S473 (1:200; Abcam), NF-κB (1:200; Santa Cruz
Biotechnology, Inc.), and collagen type I (cat. no. BA0325) and III
(cat. no. BA0326; 1:200; Wuhan Boster Biological Technology, Ltd.)
overnight at 4°C, followed by the biotinylated secondary antibody
at 37°C for 15 min and streptavidin-peroxidase at 37°C for 15 min.
Immunoreactivity was visualized with DAB (Fuzhou Maixin Biotech.
Co., Ltd., Fuzhou, China). Brown color staining was considered a
positive result. Sections were counterstained with hematoxylin and
images were captured from six separate randomly selected fields
using Olympus FV1000 (magnification ×400; Olympus Inc., Center
Valley, PA, USA). Quantitative analysis was performed in a
blinded-manner using an automatic image analysis system at Beijing
University of Aeronautics and Astronautics (Beijing, China), with
the average O.D. values as quantitative indicators.
Western blot analysis of AKT1 and
AKT1-phospho S473
The middle lobe of the right lung (100 mg) was lysed
with RIPA lysis buffer (1 ml), and then centrifuged at 10,000 × g
for 15 min at 4°C. The supernatant was collected and protein
content was determined using a protein assay kit (Beyotime
Institute of Biotechnology, Tianjin, China). The proteins (10 µg)
were separated by 10% SDS-PAGE under a constant voltage of 120 V
for 2 h, and then transferred onto a polyvinylidene fluoride
membrane at a constant electric current of 250 mA for 30 min
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following blocking
with 5% non-fat dry milk, the membranes were incubated at 4°C
overnight with primary antibodies at the following dilution ratios:
GAPDH antibody (cat. no. sc-25778, 1:2,000; Santa Cruz
Biotechnology, Inc.); anti-AKT1-phospho S473 antibody (1:5,000;
Abcam); anti-AKT1 antibody (1:5,000; Abcam). The membranes were
then washed three times with PBST and incubated with HRP-conjugated
anti-rabbit IgG antibody (cat. no. 074-1506, 1:5,000; KPL, Inc.,
Gaithersburg, MD, USA) for 2 h at room temperature, followed by
washing with PBST. The proteins were visualized using
chemiluminescence (ECL; Beyotime Institute of Biotechnology).
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
used to perform statistical analyses. Multiple group comparisons
were performed using one-way analysis of variance followed by
pair-wise comparison with the Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of OA on histopathologic
changes in the lungs
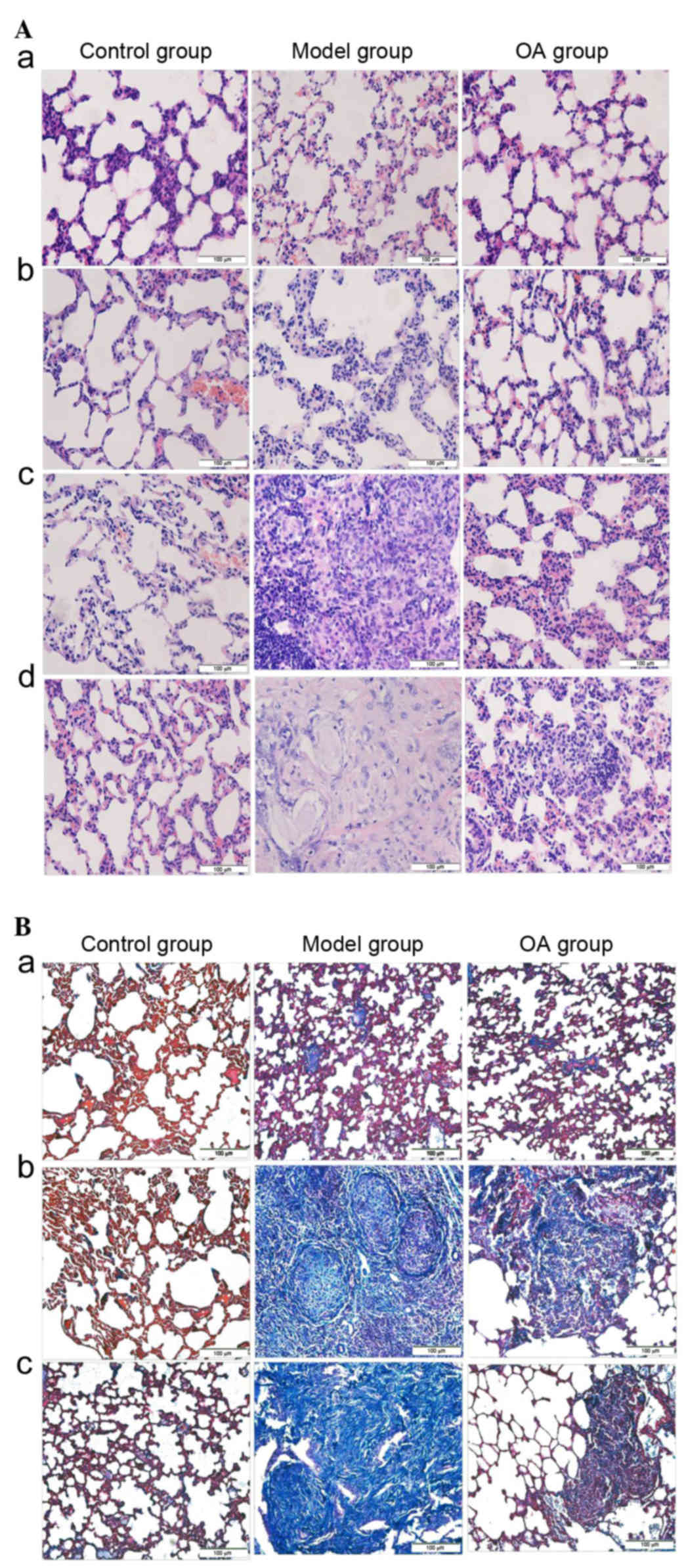
Pathological changes in the lung tissues of the rats
were observed by light microscopy with HE and Masson's staining. As
shown in Fig. 1Aa-d, the lungs of
the rats in the control group, which received physiological saline,
had a thin alveolar septum without significant inflammation and no
obvious abnormalities shown by the HE stain. However, in the model
group and solvent control group, at day 14 post-instillation, there
was marked infiltration of inflammatory cells and alveolar septum
thickening in the lungs, with occasional small numbers of cellular
nodules (stage I; and Table I). At
28 days, primarily cellular nodules (stage I+) and fibrotic
cellular nodules (stage II+) were observed. At 56 days, fibrous
nodules were integrated with each other, there were more fibrotic
cellular nodules (stages II+ and III). By contrast, OA treatment
significantly reduced inflammatory cell infiltration and alveolar
septum thickening at 14 days; the size and number of cellular
nodules (stage I+) were decreased at 28 and 56 days. Masson's
staining (Fig. 1Ba-c) showed: blue
collagen fibers, red muscle fibers, cytoplasm and red blood cells,
and brown nuclei. In the control group, there was a small quantity
of blue collagen fiber around the bronchial and alveolar septum
area during the investigation. In the model group and solvent
control group, diffuse collagen fibers were increased and arranged
irregularly in the nodules at 28 days; increased collagen
deposition was present, which was arranged in concentric circles,
at 56 days; lung fibrosis was aggravated. OA treatment
significantly reduced collagen fibers, in small sections or small
bundles. These results indicated that the silicosis model was
successfully constructed and that OA exerted a significant
protective effect.
 | Table I.Silicotic nodule grades in the lungs
of rats in each group (n=6). |
Table I.
Silicotic nodule grades in the lungs
of rats in each group (n=6).
|
| Nodule grade
following instillation |
|---|
|
|
|
|---|
| Group | 7 days | 14 days | 28 days | 56 days |
|---|
| Control | 0 | 0 | 0 | 0 |
| Silicosis | 0 | I | I+-II+ | II+-III |
| Solvent | 0 | I | I+-II+ | II+-III |
| OA | 0 | 0-I | I+ | I+ |
Effect of OA on oxidative stress in
the lungs
As shown in Table
II, compared with the control group, the content of MDA in sera
of the model group and solvent control group increased, peaking at
14 days, followed by a marginal decrease, although significant
differences were found in the statistical analysis (P<0.05).
However, OA treatment significantly decreased the content of MDA,
compared with the content in the model group and solvent control
groups at corresponding time points (P<0.05).
 | Table II.Effect of OA on oxidative stress in
sera of the rats with silicosis. |
Table II.
Effect of OA on oxidative stress in
sera of the rats with silicosis.
|
| MDA content
(µmol/l) | SOD activity
(U/ml) | GSH-Px activity
(U/ml) |
|---|
|
|
|
|
|
|---|
| Group | 7 d | 14 d | 28 d | 56 d | 7 d | 14 d | 28 d | 56 d | 7 d | 14 d | 28 d | 56 d |
|---|
| Control | 3.42±0.14 | 3.68±0.23 | 3.74±0.06 | 3.52±0.04 | 100.94±11.08 | 101.42±13.35 | 101.89±13.09 | 102.46±14.35 | 4.78±0.58 | 4.46±0.21 | 4.42±0.10 | 4.12±0.33 |
| Silicosis |
8.12±0.10a |
18.42±0.08a |
17.97±0.08a |
16.62±1.06a |
127.63±12.07a | 142.59±8.47a |
157.63±8.67a |
160.47±16.78a |
10.68±0.78a |
13.32±0.93a |
15.43±0.49a |
16.42±1.47a |
| Solvent |
8.42±0.08a |
18.39±0.40a |
17.42±0.08a |
16.36±0.99a |
129.42±9.45a |
143.69±20.11a |
159.42±8.46a |
160.76±19.13a |
10.34±0.39a |
13.68±0.92a |
15.76±0.25a |
16.78±0.54a |
| OA |
4.62±0.4b,c |
5.68±0.26b,c |
7.78±0.21b,c |
8.94±0.06b,c |
156.43±21.25b,c |
166.57±22.15b,c |
180.43±21.40b,c |
189.97±16.16b,c |
14.43±0.05b,c |
16.62±0.57b,c |
18.43±0.54b,c |
21.43±0.16b,c |
The activities of SOD/GSH-Px increased in the sera
from the model group and solvent control group, compared with the
control group at corresponding time points (P<0.05), however,
the activities of SOD/GSH-Px increased with time in the sera from
the model group and solvent control group. Compared with the model
group and solvent control group, the activities of SOD/GSH-Px were
significantly increased in the OA group at the corresponding time
points (P<0.05).
Effect of OA on collagen changes in
the lungs
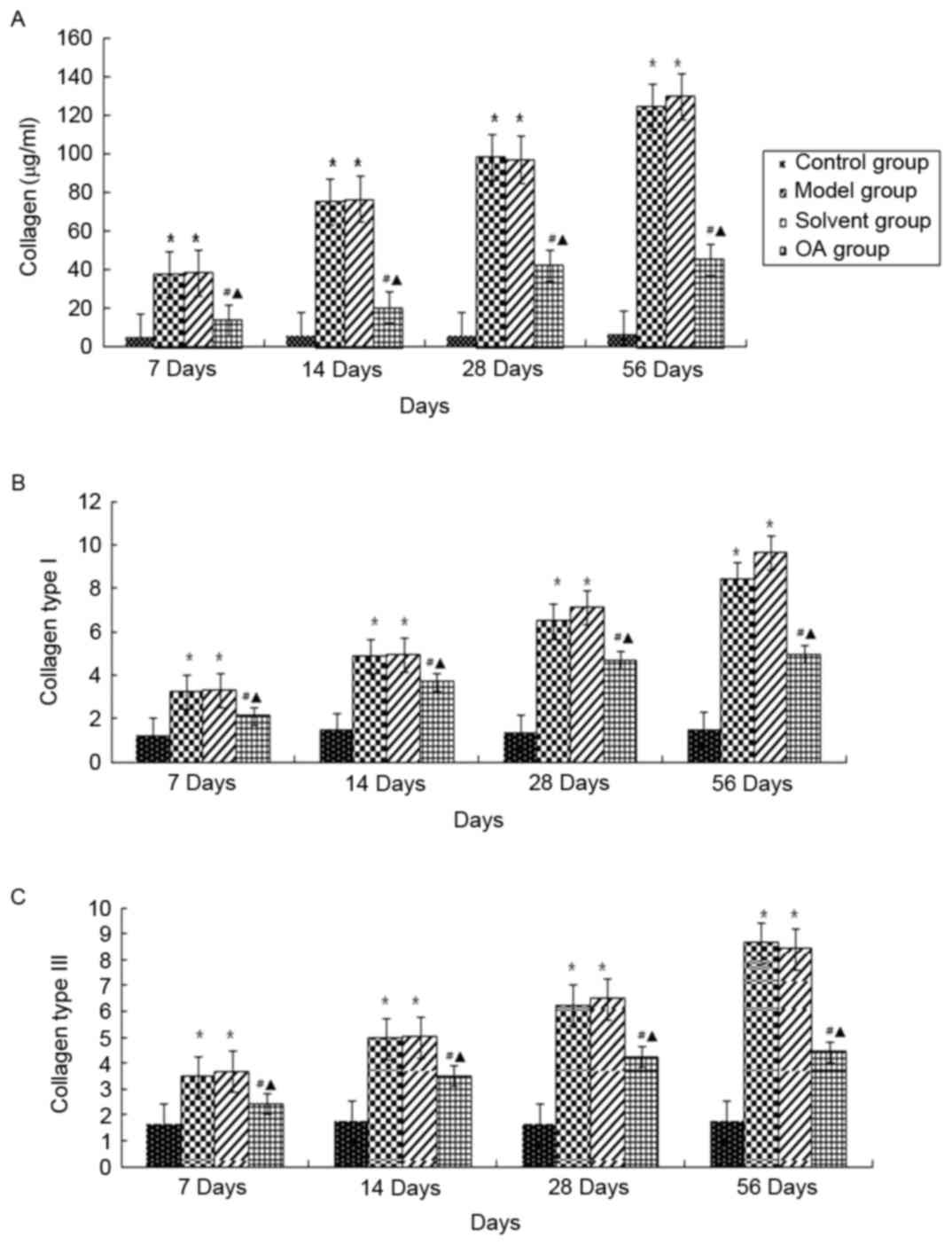
Hydroxyproline content is an important indicator of
total collagen in lung fibrosis, which are predominantly composed
of collagen types I and III. In the present study, we detected the
content of hydroxyproline using a hydroxyproline kit, and the
expression levels of collagen types I and III using
immunohistochemistry (Fig. 2A-C).
No significant differences in the hydroxyproline content or levels
of collagen types I and III were found in the control group during
the investigation. However, the total collagen/hydroxyproline
content and the expression levels of collagen I and III in the
model group and solvent control group were significantly higher,
compared with those in the control group at corresponding time
points (P<0.05). As expected, OA treatment significantly reduced
these levels, compared with those in the model group and solvent
control group (P<0.05), but remained higher, compared with those
in the control group (P<0.05).
Effect of OA on cytokines in the
lungs
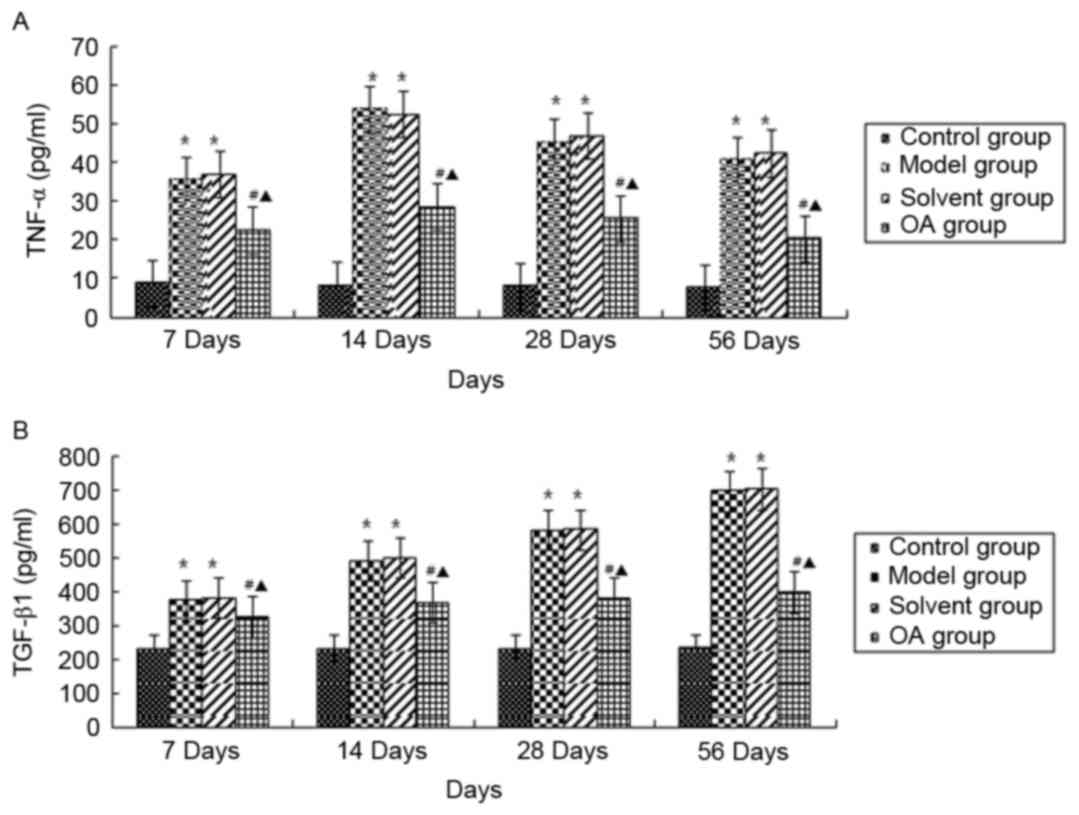
TNF-α and TGF-β1 are important cytokines and are
involved in inflammation and pulmonary fibrosis. As shown in
Fig. 3A and B, no differences in
the serum contents of TNF-α and TGF-β1 were found in rats of the
control group at the four time points (P>0.05). The serum
contents of TNF-α in the model group and solvent control group were
significantly increased, peaking at day 14 day post-instillation,
with a subsequent marginal decrease, but with statistically
significant differences at each time point, compared with those in
the control group (P<0.05), whereas TGF-β1 increased following
instillation (P<0.05). No significant differences between the
model group and solvent control group were found at different time
points. OA treatment had inhibitory effects on the contents of
TNF-α and TGF-β1, compared with the model group and solvent group
at the corresponding time points (P<0.05).
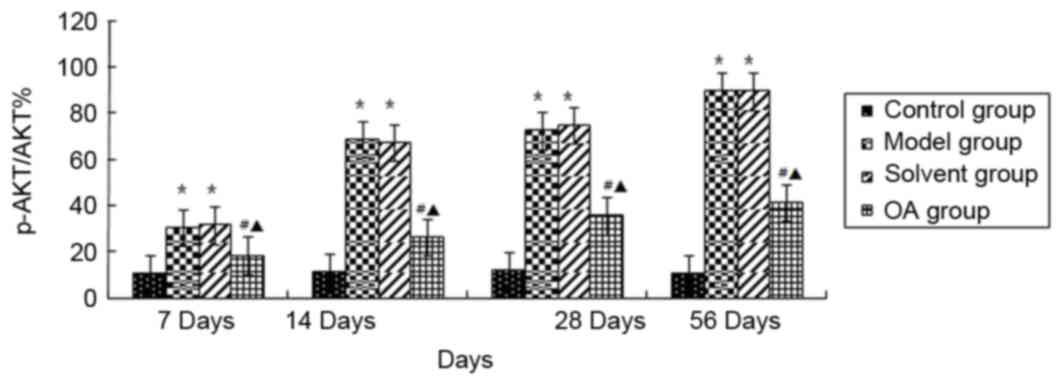
Effect of OA on p-AKT1/NF-κB in the
lungs
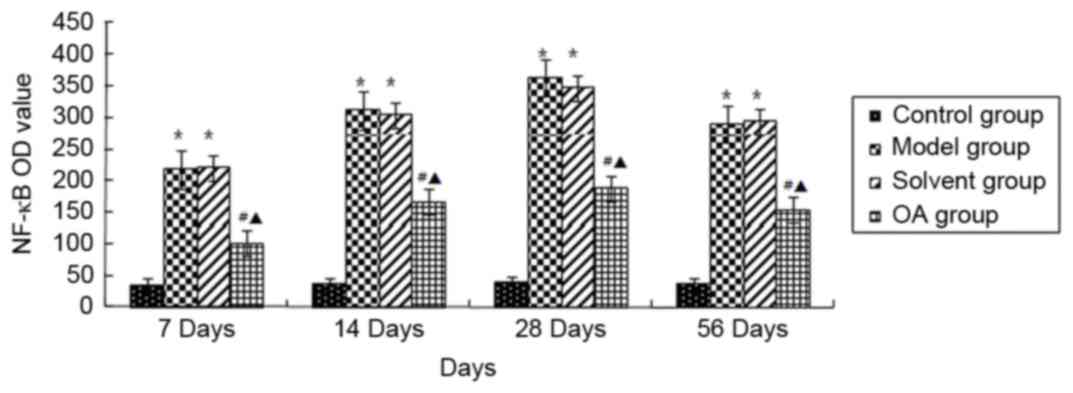
Immuno-histochemical methods were used to observe
the expression of p-AKT1/NF-κB in the rat lung tissues. As shown in
Fig. 4, the levels of NF-κB p65 in
the nuclear fractions were significantly increased in the model
group and solvent control group, compared with that in the control
group (P<0.05); whereas positive staining for p-AKT1 (Fig. 5) was primarily present in the
nuclei of interstitial cells in the lung tissues of the model group
and solvent control group, but not in the control group. OA
treatment markedly reduced this positive staining (P<0.05). The
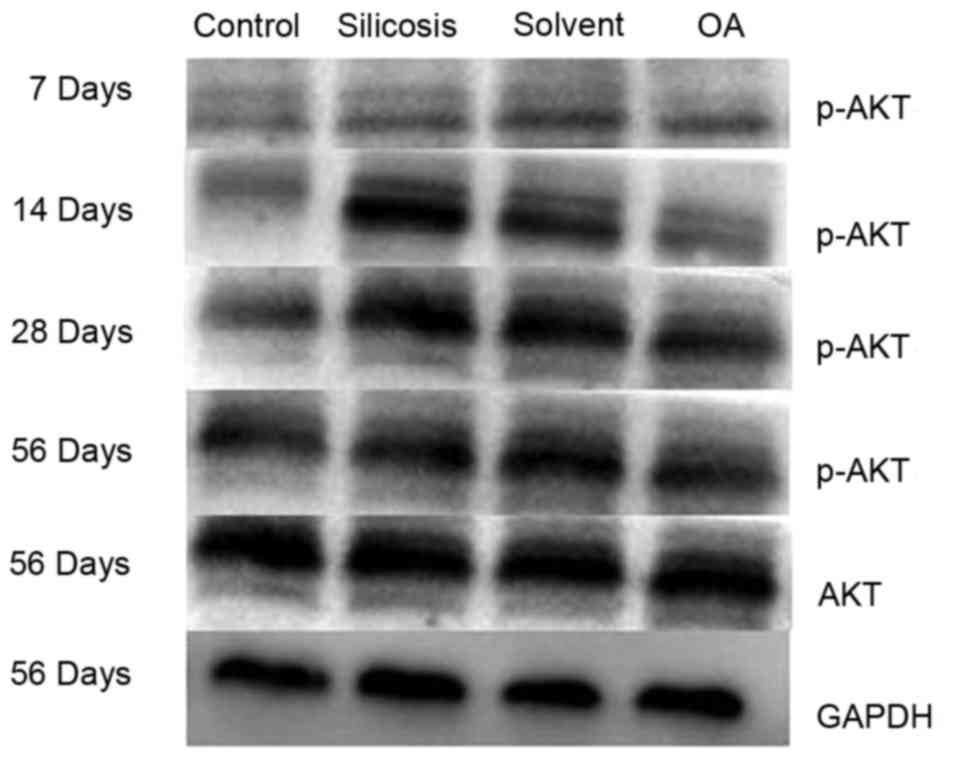
immunohistochemical results were further confirmed using western
blot analysis (Fig. 6), which
showed that the model group and solvent control groups exhibited
increased expression levels of ph-AKT1, compared with levels in the
control group at the corresponding time points (P<0.05) and that
OA treatment significantly weakened the expression of p-AKT1 in the
rat lungs, compared with levels in the model group and solvent
control group at the corresponding time points (P<0.05).
Discussion
In the present study, the effects of OA on
silica-induced lung injury and fibrosis were examined for the first
time, to the best of our knowledge. Firstly, successful
establishment of the silicosis model in rats was confirmed by
observing morphology and pathological changes in lung tissues with
HE and Masson's staining. Subsequently, biochemical indicators,
including changes in cytokines, collagen and the AKT/NF-κB pathway
were measured in the pathogenesis of silicosis, and the effects of
OA on these changes were determined. The resulting data suggested
that OA possessed protective effects against silica-induced lung
injury and fibrosis.
It is known that silicosis is a preventable
occupational disease with no effective treatments available. In
silica-induced inflammation and fibrosis, various mediators,
including ROS, cytokines and growth factors released from activated
alveolar macrophages, are key in the development and progression of
the disease (13–15). ROS, which include hydroxyl
radicals, superoxide anions, hydrogen peroxide and singlet oxygen,
are generated not only at the particle surface, but also by
phagocytic cells attempting to digest the silica particle.
Particle-derived ROS can also react with cell-derived ROS and RNS,
yielding novel toxic moieties, including peroxynitrite from NO and
superoxide anions (O2•) (16). Therefore, oxidative stress is
caused by an imbalance between the production of ROS and the
ability of the biological system to repair the resulting damage. It
has been demonstrated that oxidative stress is involved in the
development of silica-induced pulmonary disease in rats (8). In humans, silica exposure also
activates oxidative stress in the development and progression of
silicosis (17). The
quantification of oxidative stress can also be assessed by the
measurement of aldehydes, including MDA, the end product of lipid
peroxidation of cells. The plasma levels of MDA are correlated with
the severity of silicosis (18).
Oxidative stress is also evidenced by increased the expression of
antioxidant enzymes, including SOD and GSH-Px. SOD, a superoxide
anion radical scavenger, can inhibit the peroxidation of free
radicals and indirectly reflect the level of free radicals
scavenged. GSH-Px can promote the removal of hydrogen peroxide
(H2O2) and free radicals by GSH, and is
important in the integrity of cell membrane structure and function.
This was supported by a study by Zhang et al (19), which found that the mean serum
levels of GSH and MDA, and activity of SOD in the silicosis group
were significantly higher, compared with those in control subjects
without silicosis (P<0.05). Consistent with these results, the
present study found that MDA content and the activity of SOD/GSH-Px
in the sera of the model group and solvent control group were
increased, however, OA modulated these changes by downregulating
MDA content and increasing the activity of SOD/GSH-Px, which
suggested that OA may have an antioxidant role in the development
and progression of silicosis.
Intracellular ROS has a fundamental role in the
silica-induced transduction pathway leading to the production of
TNF-α. Scarfì et al found that H2O2
and OH• radicals were key signals in the enhanced production of
TNF-α in QA-stimulated RAW 264.7 murine macrophages (20). TNF-α is a pleiotropic cytokine,
shown to be involved in inflammation and fibrosis (21,22).
In several studies, serum levels of TNF-α in silicosis groups were
reported to be significantly higher, compared with those in control
groups (23,24). The released TNF-α increases the
function of neutrophils and eosinophils, which generates increased
superoxide and lysosomal enzyme release, and produces toxic effects
to surrounding tissues to further increase the inflammatory
response. TNF-α can also aggregation secrete abundant human TGF-β1,
promotes the proliferation of fibroblasts and secretes increased
collagen (25). The results of the
present study suggested that TNF-α was produced at an early stage
of the inflammatory process in the silicosis model, peaked 14 days
following instillation and remained at a higher level, compared
with levels in the control group, which was consistent with the
literature. Of note, OA treatment inhibited the levels of TNF-α,
compared with those in the model group and solvent group at the
corresponding time points.
TGF-β1, a multifunctional cytokine, regulates the
proliferation and differentiation of cells (26) and is known to promote the
pathogenesis of lung fibrosis. The mRNA and protein expression of
TGF-β1 have been shown to increase in the lungs of the silicotic
animals (27,28). Consistent with these results, the
present study found that, 56 days post-SiO2
instillation, the pulmonary expression of TGF-β1 increased almost
2.13-fold, compared with that in the control group, which was
markedly inhibited by chronic administration of OA. These data
suggested that OA suppressed the expression of TNF-α and TGF-β1,
accordingly reducing the rate of the development and progression of
silicosis.
As is already known, NF-κB, a critical transcription
factor in modifying the production of inflammatory cytokines,
growth factors and ROS, is activated by silica in macrophages and
other types of lung cells, and is important in the initiation and
progression of silica-induced pulmonary fibrosis (29–32).
Several studies have confirmed that the PI3K/Akt signaling pathway
can be an upstream activator of the NF-κB signaling cascade
(33) and adjust cells
physiological function (34). AKT,
a serine/threonine protein kinase, is activated via the PI3K
pathway. Activated AKT can promote the transcriptional activity of
NF-κB by accelerating the degradation of IKB and phosphorylating
NF-κB/p65 (35). OA has been
reported to inhibit the activation and nuclear translocation of
NF-κB, resulting in suppression of the TNF-α-induced inflammatory
response (36). In the present
study, the data showed that levels of p-Akt Ser473 and NF-κB/p65
were increased in the progression of silica-induced pulmonary
fibrosis in rats, and that OA treatment downregulated the
phosphorylation of AKT-Ser473 and decreased the level of NF-κB/p65.
These results indicated that OA inhibited silica-induced pulmonary
inflammation and fibrosis in rats, possibly through regulating the
AKT/NF-κB pathway.
In conclusion, these results of the present study
suggested that OA restored the oxidant/antioxidant balance, and
decreased pulmonary cytokines and collagen through regulating the
AKT/NF-κB pathway in silica-induced lung injury and fibrosis. The
protective effects of OA in silicosis may be relevant, not only
during the first steps of the lung inflammatory response, but also
subsequently. OA, a pentacyclic triterpenoid extensively found in a
variety of plants and medicinal herbs, may be an effective option
for treating silicosis, for which there are no other specific
treatment options.
Acknowledgements
This study was supported by the Tangshan Science and
Technology Bureau Foundation of China (grant no. 14130262B).
References
|
1
|
Rimal B, Greenberg AK and Rom WN: Basic
pathogenetic mechanisms in silicosis: Current understanding. Curr
Opin Pulm Med. 11:169–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flynn MR and Susi P: Engineering controls
for selected silica and dust exposures in the construction
industry-a review. Appl Occup Environ Hyg. 18:268–277. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leung CC, Yu IT and Chen W: Silicosis.
Lancet. 379:2008–2018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas CR and Kelley TR: A Brief Review of
silicosis in the United states. Environ Health Insights. 4:21–26.
2010.PubMed/NCBI
|
|
5
|
Occupational Health Technical Service
Network: Silicosis. http://www.zybw.com/zybw_list.aspx?c=31&a=60&t=xq&m=502Accessed.
January 5–2014.
|
|
6
|
Hamilton RF Jr, Thakur SA and Holian A:
Silica binding and toxicity in alveolar macrophages. Free Radic
Biol Med. 44:1246–1258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, He YL, Li QZ, Hao XH, Zhang ZF,
Yuan JX, Bai YP, Jin YL, Liu N and Chen G: N-acetylcysteine
alleviated silica-induced lung fibrosis in rats by down-regulation
of ROS and mitochondrial apoptosis signaling. Toxicol Mech Methods.
24:212–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Porter DW, Millecchia LL, Willard P,
Robinson VA, Ramsey D, McLaurin J, Khan A, Brumbaugh K, Beighley
CM, Teass A and Castranova V: Nitric oxide and reactive oxygen
species production causes progressive damage in rats after
cessation of silica inhalation. Toxicol Sci. 90:188–197. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J: Oleanolic acid and ursolic acid:
Research perspectives. J Ethnopharmacol. 100:92–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pollier J and Goossens A: Oleanolic acid.
Phytochemistry. 77:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung S, Yoon HE, Kim SJ, Kim SJ, Koh ES,
Hong YA, Park CW, Chang YS and Shin SJ: Oleanolic acid attenuates
renal fibrosis in mice with unilateral ureteral obstruction via
facilitating nuclear translocation of Nrf2. Nutr Metab (Lond).
11:22014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kulkarni AA, Thatcher TH, Olsen KC,
Maggirwar SB, Phipps RP and Sime PJ: PPAR-γ Ligands Repress
TGFb-induced myofibroblast differentiation by targeting the
PI3K/Akt pathway: Implications for therapy of fibrosis. PLoS One.
6:e159092011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thakur SA, Beamer CA, Migliaccio CT and
Holian A: Critical role of MARCO in crystalline silica-induced
pulmonary inflammation. Toxicol Sci. 108:462–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim Y, Kim JH, Kim KA, Chang HS, Park YM,
Ahn BY and Phee YG: Silica-induced apoptosis in vitro and in vivo.
Toxicol Lett. 108:335–339. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Yang F, Yan J, Li Q, Wei Z, Feng H,
Wang R, Zhang L and Zhang X: New anti-fibrotic mechanisms of
n-acetyl-seryl-aspartyl-lysyl-proline in silicon dioxide-induced
silicosis. Life Sci. 87:232–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fubini B and Hubbard A: Reactive Oxygen
Species (ROS) and reactive nitrogen species (RNS) generation by
silica in inflammation and fibrosis. Free Radic Biol Med.
34:1507–1516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palabiyik SS, Girgin G, Tutkun E, Yilmaz
OH and Baydar T: Immunomodulation and oxidative stress in denim
sandblasting workers: Changes caused by silica exposure. Arh Hig
Rada Toksikol. 64:431–437. 2013.PubMed/NCBI
|
|
18
|
Pelclová D, Fenclová Z, Syslová K, Vlčková
S, Lebedová J, Pecha O, Běláček J, Navrátil T, Kuzma M and Kačer P:
Oxidative stress markers in exhaled breath condensate in lung
fibroses are not significantly affected by systemic diseases. Ind
Health. 49:746–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JW, Lv GC, Yao JM and Hong XP:
Assessment of serum antioxidant status in patients with silicosis.
J Int Med Res. 38:884–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scarfì S, Magnone M, Ferraris C, Pozzolini
M, Benvenuto F, Benatti U and Giovine M: Ascorbic acid pre-treated
quartz stimulates TNF-alpha release in RAW264.7 murine macrophages
through ROS production and membrane lipid peroxidation. Respir Res.
10:252009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang ZY, Zou L, Shi SS, Lu YR, Dong J,
Yang CH, Lu YC and Dai GK: Effects of curcumin on TNF-alpha and
TGF-beta1 in serum and lung tissue of SiO2-induced fibrosis in
mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 25:399–401. 2009.(In
Chinese). PubMed/NCBI
|
|
22
|
Li Z, Xue J, Yan S, Chen P and Chen L:
Association between tumor necrosis factor-α 308G/A gene
polymorphism and silicosis susceptibility: A meta-analysis. PLoS
One. 8:e766142013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao RM, Zhang XT, Yan YL, He EQ, Guo P,
Zhang YY, Zhao DK, Yang ZG, Chen J, Yao MY, et al: Change of serum
TGF-beta1 and TNF-alpha in silicosis patients. Zhonghua Lao Dong
Wei Sheng Zhi Ye Bing Za Zhi. 29:606–607. 2011.(In Chinese).
PubMed/NCBI
|
|
24
|
Slavov E, Miteva L, Prakova G, Gidikova P
and Stanilova S: Correlation between TNF-alpha and
IL-12p40-containing cytokines in silicosis. Toxicol Ind Health.
26:479–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ortiz LA, Lasky J, Gozal E, Ruiz V,
Lungarella G, Cavarra E, Brody AR, Friedman M, Pardo A and Selman
M: Tumor necrosis factor receptor deficiency alters matrix
metalloproteinase 13/tissue inhibitor of metalloproteinase 1
expression in murine silicosis. Am J Respir Crit Care Med.
163:244–252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu H, Yang F, Sun Y, Yuan Y, Cheng H, Wei
Z, Li S, Cheng T, Brann D and Wang R: A New Antifibrotic Target of
Ac-SDKP: Inhibition of Myofibroblast Differentiation in Rat Lung
with Silicosis. PLoS One. 7:e403012012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan LH, Liu TF, Guo M, Liu ML, Wang ZP and
Si SJ: Effect of schisandrin B on lung mRNA expression of
transforming growth factor-beta1 signal transduction molecule in
rat lungs exposed to silica. Zhonghua Lao Dong Wei Sheng Zhi Ye
Bing Za Zhi. 29:255–259. 2011.(In Chinese). PubMed/NCBI
|
|
28
|
Maron-Gutierrez T, Castiglione RC, Xisto
DG, Oliveira MG, Cruz FF, Peçanha R, Carreira-Junior H, Ornellas
DS, Moraes MO, Takiya CM, et al: Bone marrow-derived mononuclear
cell therapy attenuates silica-induced lung fibrosis. Eur Respir J.
37:1217–1225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Porter DW, Ye J, Ma J, Barger M, Robinson
VA, Ramsey D, McLaurin J, Khan A, Landsittel D, Teass A and
Castranova V: Time course of pulmonary response of rats to
inhalation of crystalline silica: NF-kappa B activation,
inflammation, cytokine production, and damage. Inhal Toxicol.
14:349–367. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Giuseppe M, Gambelli F, Hoyle GW,
Lungarella G, Studer SM, Richards T, Yousem S, McCurry K, Dauber J,
Kaminski N, et al: Systemic Inhibition of NF-kappaB activation
protects from silicosis. PLoS One. 4:e56892009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen F and Shi X: NF-kappaB, a pivotal
transcription factor in silica-induced diseases. Mol Cell Biochem.
234–235:169–176. 2002. View Article : Google Scholar
|
|
32
|
Hubbard AK, Timblin CR, Shukla A, Rincón M
and Mossman BT: Activation of NF-kappaB-dependent gene expression
by silica in lungs of luciferase reporter mice. Am J Physiol Lung
Cell Mol Physiol. 282:L968–L975. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Venkatesan B, Valente AJ, Prabhu SD,
Shanmugam P, Delafontaine P and Chandrasekar B: EMMPRIN activates
multiple transcription factors in cardiomyocytes, and induces
interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB
andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 49:655–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jing Y, Liu LZ, Jiang Y, Zhu Y, Guo NL,
Barnett J, Rojanasakul Y, Agani F and Jiang BH: Cadmium Increases
HIF-1 and VEGF Expression through ROS, ERK, and AKT signaling
pathways and induces malignant transformation of human bronchial
epithelial cells. Toxicol Sci. 125:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yasuda T: Activation of Akt leading to
NF-κB up-regulation in chondrocytes stimulated with fibronectin
fragment. Biomed Res. 32:209–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takada K, Nakane T, Masuda K and Ishii H:
Ursolic acid and oleanolic acid, members of pentacyclic
triterpenoid acids, suppress TNF-α-induced E-selectin expression by
cultured umbilical vein endothelial cells. Phytomedicine.
17:1114–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|