Introduction
Hepatic failure, a condition where the number of
functioning hepatocytes significantly decreases, is fatal (1). Transplantation of hepatocytes in
patients with liver failure could be a promising treatment. Human
induced pluripotent stem (iPS) cells are established with the
introduction of reprogramming factors (2,3). iPS
cells are an ideal source for the generation of hepatocytes. The
current protocols for generating hepatocytes from iPS cells consist
of sequential stimulation with growth factors and introduction of
transcription factors (4–9). The generated hepatocytes remain in an
immature state, namely hepatic progenitor-like cells (10).
Aspartate aminotransferase (AST) is expressed in the
cytosol (cytosolic AST) and mitochondria (mitochondrial AST) in
myocytes and hepatocytes (11).
AST catalyzes the interconversion of aspartate and α-ketoglutarate
to glutamate and oxaloacetate. Alanine aminotransferase (ALT) is
mainly expressed in hepatocytes and catalyzes the conversion of
pyruvate and glutamate to L-alanine and a-ketoglutarate (12). Glycogen synthase synthesizes
glycogen from glucose metabolites (13). If a cell expresses AST, ALT, and
glycogen synthase, it is considered to have a metabolic function
specific to that of hepatocytes.
Glucose is indispensable for the survival of cells.
Hepatocytes express galactokinase, an enzyme that catalyzes the
conversion of galactose to galactose-1-phosphate.
Galactose-1-phosphate enters the glycolytic pathway and is used as
a source of energy by the cells. Hepatocyte selection medium (HSM),
a medium that lacks glucose but contains galactose, is used to
eliminate iPS cells and specifically select for hepatocytes
(14). Hepatocytes survive in HSM
because they express galactokinase, allowing them to utilize the
galactose in the medium for their energy needs. Expression of
α-fetoprotein (AFP), a marker of immature hepatocytes, is increased
in iPS cells cultured in HSM (15), suggesting that glucose deprivation
and galactose supplementation promote hepatocyte differentiation of
iPS cells.
2-Deoxy-D-glucose (2DG) is an analogue of glucose
(16), which is taken up by cells
similar to glucose. However, unlike glucose, it is not metabolized
and it is accumulated in the cells. 2DG is used in positron
emission tomography for diagnostic imaging of cancer (17). It is hypothesized that if 2DG was
added in a medium, the cultured cells would take up 2DG similar to
glucose, but the cells would not be able to metabolize 2DG as a
source of energy. Such addition of 2DG in the medium would
therefore be similar to glucose deprivation. In the present study,
the effect of galactose and 2DG was examined on hepatocyte
differentiation of iPS cells, by evaluating the expression of AFP,
as a marker of immature hepatocytes. In addition, the expression
levels of AST, ALT, and glycogen synthase in iPS cells cultured in
HSM or hepatocyte differentiation inducer (HDI) were examined.
Materials and methods
Cell culture
A human iPS cell line, 201B7 (Riken BioResource
Center, Tsukuba, Japan), was cultured on 10 cm dishes coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) in ReproFF
medium (ReproCELL, Inc., Yokohama, Japan) at 37°C with 5% carbon
dioxide in a humidified chamber. Once confluent, the cells were
rinsed with PBS and harvested using Accutase (Innovative Cell
Technologies, Inc., San Diego, CA, USA). The cells were then
observed under a microscope (CKX41N-31 PHP; Olympus Corporation,
Tokyo, Japan).
Reagents
Non-essential amino acids solution (NEAA; 100x) and
sodium pyruvate (100x) were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The apoptosis inhibitor M50054
[2,2′-methylenebis (1,3-cyclohexanedione)] was purchased from Merck
KGaA (Darmstadt, Germany). FPH1 [2-(N-(5-chloro-2-methylphenyl)
methylsulfonamido)-N-(2,6-difluorophenyl) acetamide] was purchased
from Xcessbio Biosciences, Inc. (San Diego, CA, USA) (18). Galactose, ornithine, glycerol,
oncostatin M, nicotinamide, proline and L-glutamine were purchased
from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 2DG was
purchased from Sigma-Aldrich; Merck KGaA.
HSM and HDI media
201B7 cells were cultured in 6-well plates coated
with Matrigel (BD Biosciences) in HSM or HDI for 2 days and then
subjected to reverse transcription-quantitative polymerase chain
reaction (RT-qPCR). HSM consisted of Leibovitz's-15 (L15) medium
(Thermo Fisher Scientific, Inc.), with the omission of arginine,
tyrosine, glucose, and sodium pyruvate and the addition of
galactose (900 mg/l), ornithine (1 mM), glycerol (5 mM), and
proline (260 mM) (14). Proline
(30 mg/l) was added because it is required for DNA synthesis
(19). Aspartic acid was not
included because it is one of the products of ornithine metabolism
and a substrate for arginine synthesis. KnockOut serum replacement
(Thermo Fisher Scientific, Inc.) was added at a final concentration
of 10% and was used instead of fetal calf serum to establish
xeno-free, defined conditions. HDI medium was prepared using
oncostatin M (20 ng/ml), FPH1 (20 µM), M50054 (100 mg/l), NEAA
(1x), sodium pyruvate (1x), nicotinamide (1.2 mg/ml), proline (30
ng/ml), and L-glutamine (0.3 mg/ml). Proline and nicotinamide are
required for proliferation of primary hepatocytes (19,20).
Conventional media
201B7 cells were harvested with Accutase, and
transferred to a 15 ml tube (Asahi Glass, Tokyo, Japan). The cells
were centrifuged at 100 × g for 3 min at 4°C. The cells were spread
onto 6-well plates (Asahi Glass) coated with Matrigel at the
density of 106 cells for each well and were cultured in
ReproFF, L15, William's E (WE) (Thermo Fisher Scientific, Inc.), or
Dulbecco's modified Eagle's medium/Ham's F-12 nutrient mixture
(DF12; Sigma-Aldrich; Merck KGaA). L15, WE, and DF12 were
supplemented with 1.2 mg/ml nicotinamide, 30 ng/ml proline, and 10%
Knockout serum replacement. Nicotinamide and proline were added
because they are required for proliferation of primary hepatocytes
(19,20). For some experiments, 2DG at a
concentration of 0, 0.1, 1 or 10 µM was added and galactose at a
concentration of 900 mg/ml was added, as indicated. The cells were
cultured for 7 days and then subjected to RT-qPCR.
RT-qPCR
Total RNA (5 µg), isolated with Isogen (Nippon Gene
Co., Ltd., Tokyo, Japan), was used for first-strand cDNA synthesis
with SuperScript III reverse transcriptase and oligo (dT) primers
(Thermo Fisher Scientific, Inc.), following the manufacturer's
instructions. Total RNA from human fetal liver and adult liver was
purchased from Clontech Laboratories, Inc. (Mountainview, CA, USA).
qPCR was performed in a volume of 20 µl for 40 cycles of two steps
consisting of a 5 sec denaturation step and a 5 sec
annealing-extension step, using Fast SYBR Green Master Mix (Thermo
Fisher Scientific, Inc.) and the results were analyzed using the
MiniOpticon Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The primer sequences are listed in Table I. RPL19 was used as an endogenous
reference control because it is a constitutively expressed
housekeeping gene (21). Gene
expression levels were analyzed automatically using the MiniOpticon
system based on the 2−ΔΔCq method (22). The relative expression was
calculated as the expression level of a specific gene divided by
that of RPL19.
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
| Gene | Sequence (5′-3′) | Product size
(bp) | GenBank accession
no. |
|---|
| AFP |
F-ACACAAAAAGCCCACTCCAG | 147 | NM_001134 |
|
|
R-GGTGCATACAGGAAGGGATG |
|
|
| Cytosolic AST |
F-GATGGAGAAGATCGTGCGGAT | 133 | M37400 |
|
|
R-AATCCGGTCAGCCATTGTCTT |
|
|
| Mitochondrial
AST |
F-ACATGTAGTGACACAGGGCAG | 192 | M22632 |
|
|
R-CACGAGGAACCTGACACTTCA |
|
|
| ALT |
F-GAGCCCACTGTACTTGCTCTT | 113 | BC018207 |
|
|
R-TTTTCCTGGAGTCAGACTGCC |
|
|
| GS |
F-ATATCCCAGGCCTTCCTCAGT | 164 | S70004 |
|
|
R-CGTGGCTCAGTGAAAATGGTG |
|
|
| RPL19 |
F-CGAATGCCAGAGAAGGTCAC | 157 | BC095445 |
|
|
R-CCATGAGAATCCGCTTGTTT |
|
|
Statistical analysis
Relative expression levels of genes were analyzed by
one-factor analysis of variance using JMP version 5.0J software
(SAS Institute, Inc., Cary, NC, USA). Student's t-test was used as
a post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
To analyze the effect of culturing iPS cells in
different media on the expression levels of liver-specific genes,
201B7 cells were cultured in ReproFF, HSM, or HDI for 2 days. RNA
was isolated and subjected to RT-qPCR in order to determine the
mRNA expression levels of cytosolic AST (Fig. 1A), mitochondrial AST (Fig. 1B), ALT (Fig. 1C) and glycogen synthase (Fig. 1D). The mRNA expression levels of
mitochondrial AST, ALT, and glycogen synthase significantly
increased by culture in HSM and HDI compared with ReproFF media
(Fig. 1B-D). The expression level
of cytosolic AST significantly increased by culture in HDI compared
with ReproFF media, but not in HSM media (Fig. 1A). These results suggest that the
absence of glucose or arginine and the addition of galactose and
ornithine increased the expression levels of liver-specific genes
and glycogen synthase. Expression levels of each gene were analyzed
with fetal and adult liver to compare with those of HSM and HDI and
identify whether iPS cells acquired expression of liver-specific
genes. Cytosolic and mitochondrial AST were expressed in HSM and
HDI at comparable levels to fetal and adult liver.
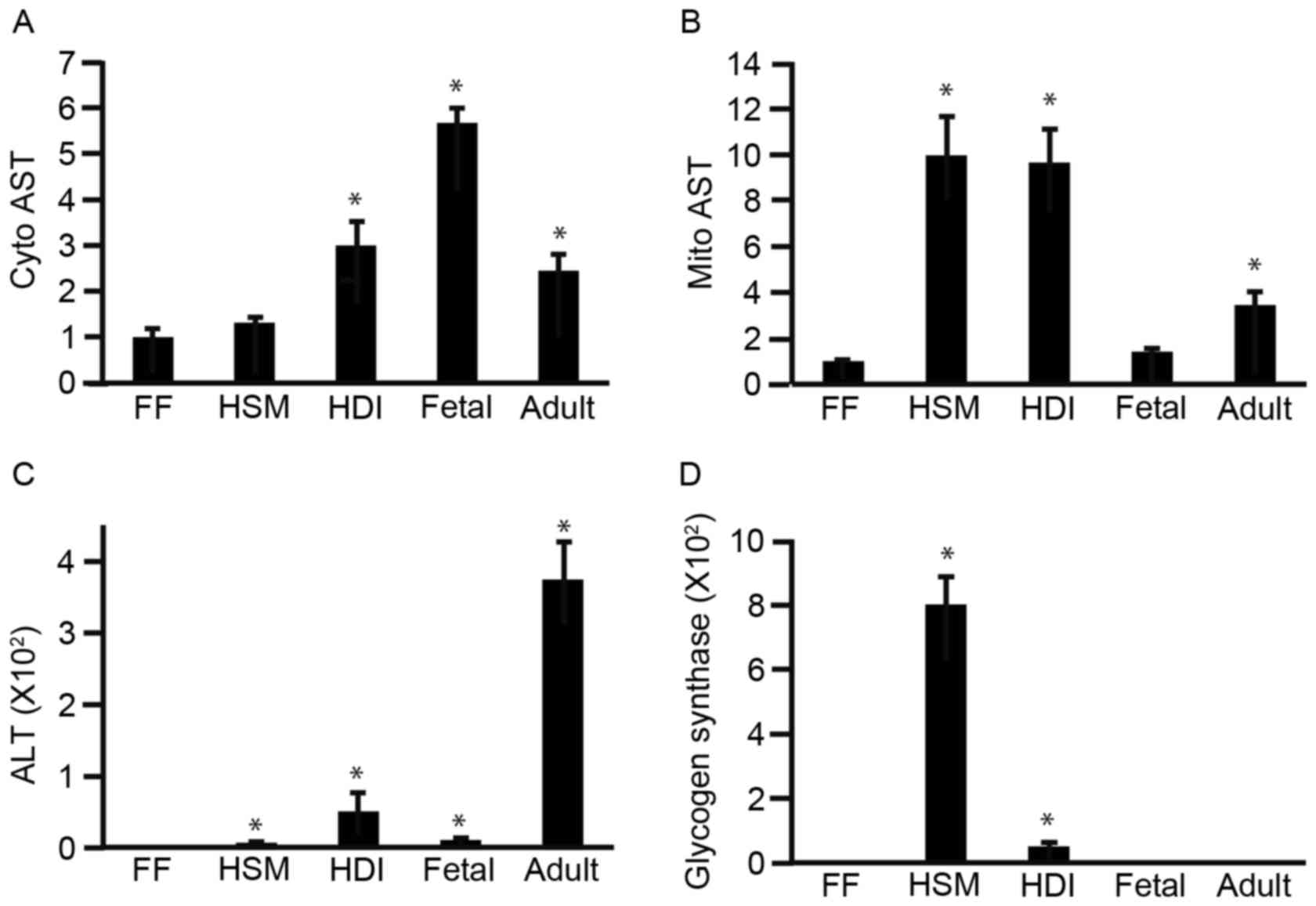 | Figure 1.Effect of different culture media on
the expression of liver-specific genes. 201B7 cells were cultured
in FF, HSM, or HDI media for 2 days. The cells were then subjected
to reverse transcription-quantitative polymerase chain reaction to
analyze the mRNA expression levels of (A) Cyto AST, (B) Mito AST,
(C) ALT and (D) glycogen synthase. *P<0.05 compared with FF
(n=3). FF, ReproFF; HSM, hepatocyte selection medium; HDI,
hepatocyte differentiation inducer; Cyto, cytosolic; AST, aspartate
aminotransferase; Mito, mitochondrial; ALT, alanine
aminotransferase; Fetal, fetal liver total RNA; Adult, adult liver
total RNA. |
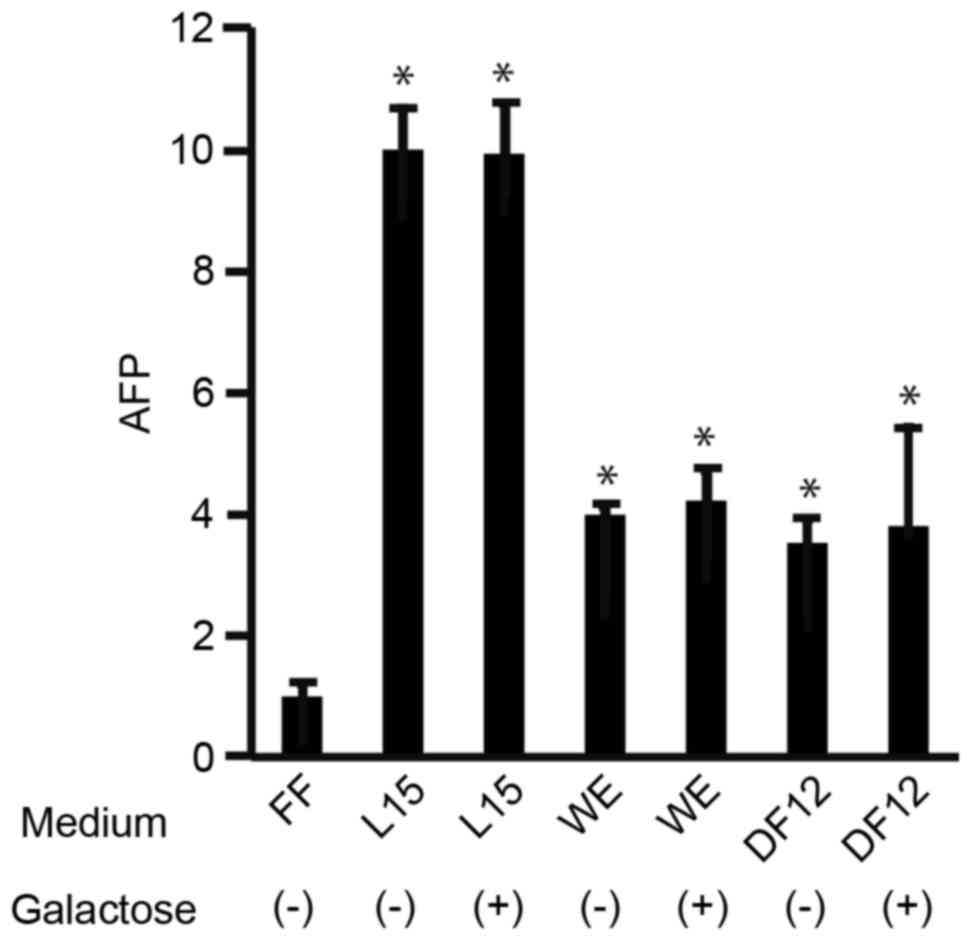
To address the possibility that galactose affected
hepatocyte differentiation of 201B7 cells, the cells were cultured
in L15, WE, and DF12 media in the absence or presence of galactose
and then total RNA was isolated and subjected to RT-qPCR (Fig. 2). The mRNA expression levels of
AFP, a marker of immature hepatocytes, were significantly higher in
the L15, WE and DF12 media compared with the ReproFF media
(P<0.05; Fig. 2). However, for
each medium, mRNA expression levels of AFP were similar in the
presence or absence of galactose (Fig.
2). These results suggest that galactose had no effect on the
differentiation of 201B7 cells to the hepatocyte lineage.
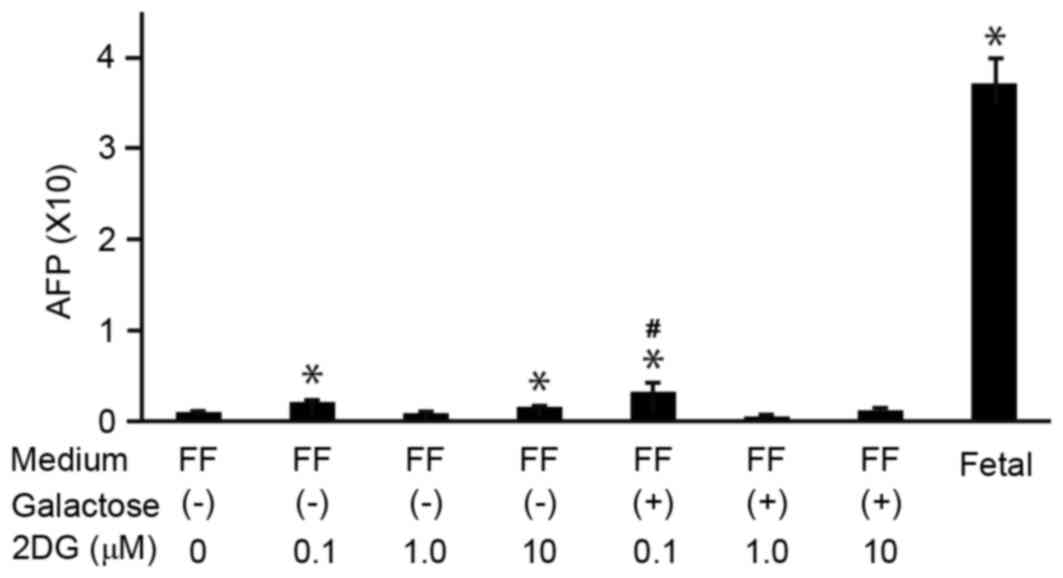
To analyze the effect of the glucose analogue 2DG on
hepatocyte differentiation of 201B7 cells, the cells were cultured
in ReproFF medium in the absence or presence of 2DG and/or
galactose. Total RNA was then isolated and subjected to RT-qPCR to
determine the mRNA expression levels of AFP (Fig. 3). The mRNA expression levels of AFP
significantly increased with the addition of 0.1 µM 2DG (Fig. 3). In the absence of galactose, AFP
was upregulated with 0.1 and 10 µM but not with 1.0 µM of 2DG. It
was not clear why expression level of AFP with 1.0 µM of 2DG did
not increase. The expression levels of AFP increased at 0.1 mM of
2DG with galactose more than without it. Again, the reason was not
clear, but it may be speculated that mechanism of galactose
metabolism is involved with hepatocyte differentiation. Addition of
galactose acted synergistically with the 0.1 µM 2DG treatment to
further increase mRNA expression of AFP in 201B7 cells (Fig. 3). These results suggested that 2DG
and galactose promoted hepatocyte differentiation of 201B7
cells.
Discussion
Glucose is an indispensable component for cell
survival. During somatic cell reprogramming, energy production
shifts from an oxidative state to a glycolytic state (23). The source of energy depends on
glycolysis in iPS cells (24).
Galactose is metabolized through glycolysis following conversion to
galactose-1-phosphate by galactokinase. iPS cells do not survive in
either HSM or HDI media. In the present study, culturing the iPS
cell line 201B7 in either HSM or HDI media resulted in an increase
of the expression levels of AFP, suggesting the initiation of iPS
cell differentiation to the hepatocyte lineage (15,25).
AST, ALT, and glycogen synthase are markers of hepatic function
(26). In the present study, the
expression levels of AST, ALT, and glycogen synthase increased by
culture of 201B7 cells in HSM and HDI media, confirming that
hepatocyte differentiation was initiated in iPS cells by these
media. Tomizawa et al and Phillips et al (25,26)
have established a medium based on L15 that lacks glucose and
contains galactose, that promotes the differentiation of iPS cells
to the hepatocyte lineage. These previous reports, together with
the present results, suggest that hepatocyte differentiation is
initiated in iPS cells cultured in media lacking glucose and
supplemented with galactose. The detailed mechanism of this,
however, remains unclear.
Next, the aim was to identify whether glucose
deprivation or galactose supplementation was responsible for the
initiation of hepatocyte differentiation of iPS cells. The present
results demonstrated that galactose supplementation did not affect
the expression level of AFP. This suggests that galactose
supplementation was not involved in hepatocyte differentiation of
iPS cells.
A major problem with HSM and HDI media is that iPS
cells do not survive for >3 and >7 days, respectively. To
overcome this limitation, 2DG was used instead of glucose
deprivation. The results demonstrated that 2DG supplementation in
the media resulted in a significant increase in the mRNA expression
levels of AFP in 201B7 cells. Moreover, galactose supplementation
enhanced the 2DG-initiated AFP upregulation. These data suggest
that the addition of 2DG and galactose in the media initiated
hepatocyte differentiation of iPS cells.
A limitation of the present study was that analysis
of hepatocyte differentiation was mainly based on determination of
mRNA expression of only one hepatocyte marker, namely AFP. Further
studies with immunostaining for hepatocyte-specific markers, such
as AFP and albumin, and with functional analysis such as
indocyanine green uptake, will be required in the future to further
confirm the present results.
In conclusion, the present study demonstrated that
hepatocyte differentiation was initiated in iPS cells by culturing
in HSM and HDI media. The present results suggest that 2DG may be
used as a supplement instead of glucose deprivation to initiate
hepatocyte differentiation in iPS cells.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research from the Japan Society for the Promotion of
Science (grant no. 15K09032).
References
|
1
|
Sugawara K, Nakayama N and Mochida S:
Acute liver failure in Japan: Definition, classification, and
prediction of the outcome. J Gastroenterol. 47:849–861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirschi KK, Li S and Roy K: Induced
pluripotent stem cells for regenerative medicine. Annu Rev Biomed
Eng. 16:277–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeLaForest A, Nagaoka M, Si-Tayeb K, Noto
FK, Konopka G, Battle MA and Duncan SA: HNF4A is essential for
specification of hepatic progenitors from human pluripotent stem
cells. Development. 138:4143–4153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Si-Tayeb K, Noto FK, Nagaoka M, Li J,
Battle MA, Duris C, North PE, Dalton S and Duncan SA: Highly
efficient generation of human hepatocyte-like cells from induced
pluripotent stem cells. Hepatology. 51:297–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo
S, Song X, Guo Y, Zhao Y, Qin H, et al: Efficient generation of
hepatocyte-like cells from human induced pluripotent stem cells.
Cell Res. 19:1233–1242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayama K, Inamura M, Kawabata K,
Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T,
Furue Kusuda M and Mizuguchi H: Efficient generation of functional
hepatocytes from human embryonic stem cells and induced pluripotent
stem cells by HNF4α transduction. Mol Ther. 20:127–137. 2012.
View Article : Google Scholar
|
|
8
|
Zaret KS, Watts J, Xu J, Wandzioch E,
Smale ST and Sekiya T: Pioneer factors, genetic competence and
inductive signaling: Programming liver and pancreas progenitors
from the endoderm. Cold Spring Harb Symp Quant Biol. 73:119–126.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inamura M, Kawabata K, Takayama K, Tashiro
K, Sakurai F, Katayama K, Toyoda M, Akutsu H, Miyagawa Y, Okita H,
et al: Efficient generation of hepatoblasts from human ES cells and
IPS cells by transient overexpression of homeobox gene HEX. Mol
Ther. 19:400–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Single-step protocol for the
differentiation of human-induced pluripotent stem cells into
hepatic progenitor-like cells. Biomed Rep. 1:18–22. 2013.PubMed/NCBI
|
|
11
|
Pol S, Bousquet-Lemercier B, Pave-Preux M,
Pawlak A, Nalpas B, Berthelot P, Hanoune J and Barouki R:
Nucleotide sequence and tissue distribution of the human
mitochondrial aspartate aminotransferase mRNA. Biochem Biophys Res
Commun. 157:1309–1315. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ellinger JJ, Lewis IA and Markley JL: Role
of aminotransferases in glutamate metabolism of human erythrocytes.
J Biomol NMR. 49:221–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rines AK, Sharabi K, Tavares CD and
Puigserver P: Targeting hepatic glucose metabolism in the treatment
of type 2 diabetes. Nat Rev Drug Discov. 15:786–804. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8:e718972013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: An optimal medium
supplementation regimen for initiation of hepatocyte
differentiation in human induced pluripotent stem cells. J Cell
Biochem. 116:1479–1489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Li J, Wang F, Hu J, Wang S and
Sun Y: 2-Deoxy-D-glucose targeting of glucose metabolism in cancer
cells as a potential therapy. Cancer Lett. 355:176–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brito AF, Mendes M, Abrantes AM, Tralhão
JG and Botelho MF: Positron emission tomography diagnostic imaging
in multidrug-resistant hepatocellular carcinoma: Focus on
2-deoxy-2-(18F)fluoro-D-glucose. Mol Diagn Ther. 18:495–504. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan J, Schwartz RE, Ross NT, Logan DJ,
Thomas D, Duncan SA, North TE, Goessling W, Carpenter AE and Bhatia
SN: Identification of small molecules for human hepatocyte
expansion and iPS differentiation. Nat Chem Biol. 9:514–520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura T, Teramoto H, Tomita Y and
Ichihara A: L-proline is an essential amino acid for hepatocyte
growth in culture. Biochem Biophys Res Commun. 122:884–891. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitaka T, Sattler CA, Sattler GL, Sargent
LM and Pitot HC: Multiple cell cycles occur in rat hepatocytes
cultured in the presence of nicotinamide and epidermal growth
factor. Hepatology. 13:21–30. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tam S, Clavijo A, Engelhard EK and
Thurmond MC: Fluorescence-based multiplex real-time RT-PCR arrays
for the detection and serotype determination of foot-and-mouth
disease virus. J Virol Methods. 161:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panopoulos AD, Yanes O, Ruiz S, Kida YS,
Diep D, Tautenhahn R, Herrerías A, Batchelder EM, Plongthongkum N,
Lutz M, et al: The metabolome of induced pluripotent stem cells
reveals metabolic changes occurring in somatic cell reprogramming.
Cell Res. 22:168–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Madonna R, Görbe A, Ferdinandy P and De
Caterina R: Glucose metabolism, hyperosmotic stress, and
reprogramming of somatic cells. Mol Biotechnol. 55:169–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Hepatocyte selection medium
eliminating induced pluripotent stem cells among primary human
hepatocytes. World J Methodol. 5:108–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phillips JW, Jones ME and Berry MN:
Implications of the simultaneous occurrence of hepatic glycolysis
from glucose and gluconeogenesis from glycerol. Eur J Biochem.
269:792–797. 2002. View Article : Google Scholar : PubMed/NCBI
|