Introduction
Transient forebrain ischemia induces the
damage/death of pyramidal neurons in the CA1 region of the
hippocampus (1–3). It has previously been reported that
aged animals are less vulnerable to ischemia, and ischemia-induced
neuronal degeneration occurs much later than in adult animals
(4–6).
Microglia, which are primary immune cells that are
located in the central nervous system, and astrocytes, which act as
important modulators of neuronal activity, are both involved in
maintaining homeostasis of the brain microenvironment (7). Microglia and astrocytes maintain a
resting phenotype under physiological conditions; however, in the
process of aging or pathological conditions, including
ischemia-reperfusion injury, they exhibit activation with
morphological and functional alterations, including hypertrophy and
the release of various factors, which have been reported to
modulate the injury process (8–10).
It is well known that glial cells serve complex roles in
neuroinflammation and in the regeneration of brain tissue following
ischemic insults (11,12).
In the case of stroke, exercise treatment has been
used in humans to aid the remaining functions (13). In experimental animals, exercise
reduces astrocyte and microglial activation in the acute phase
following transient focal ischemia in rats (14) and traumatic brain injury in mice
(15). The effects of exercise on
glial activation in animal models of brain injuries have previously
been investigated; however, long-term alterations to glial
activation in the ischemic hippocampus in aged animals have yet to
be fully elucidated. Therefore, the present study aimed to
investigate the effects of post-ischemic exercise on neuronal
damage and gliosis in the hippocampus following transient cerebral
ischemia in the aged gerbil, a useful animal model for transient
cerebral ischemia and aging research (16–19).
Materials and methods
Experimental animals
A total of 35 male Mongolian gerbils (Meriones
unguiculatus; age, 22–24 months; weight, 80–90 g) were supplied
by the Experimental Animal Center, Kangwon National University
(Chuncheon, South Korea). Gebrils were housed in a conventional
facility, at a temperature of 23±3°C and relative humidity of
55±5%, under 12/12 h light/dark cycles, and were allowed free
access to food and water. Animal handling and experimental
protocols were approved by the Institutional Animal Care and Use
Committee of Kangwon National University (approval no.
KW-130424-1). The gerbils were randomly divided into five groups:
i) Sham group (n=7), which underwent sham surgery; ii) ischemia
group (n=7), which underwent 5 min of transient forebrain ischemia;
iii) ischemia-SD4 group (n=7), which had a sedentary routine for 4
weeks (SD4) from 5 days post-ischemia; iv) ischemia-TR1 group
(n=7), which performed 1 week treadmill exercise (TR) from 5 days
post-ischemia; and, v) ischemia-TR4 group (n=7), which performed 4
weeks TR from 5 days post-ischemia. The animals were sacrificed 31
days following ischemia; at which point, the TR training was
concluded in the ischemia-TR4 group.
Induction of transient cerebral
ischemia
Following the method described in our previous study
(20), the gerbils were
anesthetized with a mixture of 2.5% isoflurane (Baxter Healthcare
Corporation, Deerfield, IL, USA) in 33% oxygen and 67% nitrous
oxide. After a sagittal ventral midline incision, common carotid
arteries were carefully separated from the respective vagal nerves
and were occluded for 5 min using nontraumatic aneurysm clips
(Yasargil FE 723K; Aesculap AG, Tuttlingen, Germany). Following
occlusion for 5 min, the clips were removed and the wounds were
sutured with wound clips (12022–09; Fine Science Tools, Inc.,
Foster City, CA, USA). Normothermic body (rectal) temperature
(37±0.5°C) was monitored until the animals completely recovered
from anesthesia. Sham surgery animals were subjected to the same
surgical procedures without the occlusion of the bilateral common
carotid arteries.
Treadmill exercise
The running speed and duration of treadmill exercise
was determined according to Sim's protocol (21–23),
with modification. Briefly, from 5 days post-ischemia, the gerbils
in the TR groups were forced to run on a motorized treadmill for 30
min/day and 5 days/week for 1 or 4 consecutive weeks. The exercise
workload consisted of running at a speed of 5 m/min for the first 5
min, 7 m/min for the next 5 min and then 10 m/min for the last 20
min with 0° inclination. The animals in the SD group were placed on
the treadmill for 30 min, without being induced to run.
Tissue processing for histology
Tissue processing was performed according to a
previously published procedure (20). Briefly, animals (n=7/group) were
anesthetized with sodium pentobarbital (40 mg/kg, i.p.; JW
Pharmaceutical Co., Ltd., Seoul, Korea) and perfused transcardially
with 4% paraformaldehyde. Brain tissues were serially sectioned
into 30 µm coronal sections.
Cresyl violet (CV) staining
To investigate morphological alterations, CV
staining was performed according to a previously published
procedure (24). Briefly, the
sections were stained with 1% CV acetate (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and immersed in serial ethanol baths.
CV-stained structures were observed under an AxioM1 light
microscope (Zeiss AG, Oberkochen, Germany) equipped with a camera
(Axiocam; Zeiss AG) and photomicrographs were captured. The
CV-stained structures were examined in a 250×250 µm area that
included the stratum pyramidale at the center of the hippocampal
CA1 region, or in the whole dentate gyrus, using the image analysis
system Optimas version 6.5 (CyberMetrics, Scottsdale, AZ, USA).
Fluoro-Jade B (F-J B)
histofluorescence staining
Histofluorescence staining was performed according
to a previously published procedure (25). F-J B (high-affinity fluorescent
marker for the localization of neuronal degeneration)
histofluorescence staining was performed to examine neuronal
degeneration. Briefly, the sections were immersed in a solution
containing 1% sodium hydroxide in 80% alcohol, transferred to a
solution containing 0.06% potassium permanganate diluted in water,
then transferred to an aquaeous solution containing 0.0004% F-J B
(Histo-Chem, Inc., Jefferson, AR, USA). After washing 3 times in
water, the sections were placed on a slide warmer (~50°C) and
examined using an epifluorescent microscope (Zeiss AG) with blue
(450–490 nm) excitation source and a barrier filter.
Immunohistochemistry
Immunohistochemistry was performed according to our
previously published procedure (24). Briefly, immunostaining was
performed using mouse anti-glial fibrillary acidic protein (GFAP;
1:800; cat no. MAB360; EMD Millipore, Billerica, MA, USA) for
astrocytes and rabbit anti-ionized calcium binding adaptor molecule
1 (Iba-1; 1:800; cat no. 019-19741; Wako Pure Chemical Industries,
Ltd., Osaka, Japan) for microglia overnight at 4°C. Subsequently,
samples were incubated with biotinylated horse anti-mouse
immunoglobulin G (1:250; cat no. BA2000; Vector Laboratories, Inc.,
Burlingame, CA, USA) or goat anti-rabbit antibodies (1:250; cat no.
BA1000; Vector Laboratories, Inc.) for 2 h at room temperature, and
streptavidin peroxidase complex (1:200; Vector Laboratories, Inc.)
for 1 h at room temperature. To establish the specificity of the
immunostaining, a negative control test was performed and resulted
in the absence of immunoreactivity in all structures.
Data analysis
In order to quantitatively analyze the number of F-J
B-positive cells, digital images from seven sections per animal
were captured using a light microscope (AxioM1; Zeiss AG) equipped
with a digital camera (Axiocam; Zeiss AG) and connected to a PC
monitor. The number of F-J B-positive cells was counted in a
250×250 µm square including the stratum pyramidale at the center of
the hippocampal CA1 region or in the whole dentate gyrus using the
image analysis system Optimas version 6.5 (CyberMetrics). Cell
counts were carried out by averaging the counts from each
animal.
To quantitatively analyze the density of GFAP- and
Iba-1-immunoreactive structures, the corresponding hippocampal
areas were measured from seven sections per animal. Images of all
GFAP- and Iba-1-immunoreactive structures were captured through an
AxioM1 light microscope (Zeiss AG) equipped with a camera (Axiocam;
Zeiss AG) and connected to a PC monitor. Densities of GFAP- and
Iba-1-immunoreactive structures were evaluated on the basis of
optical density (OD), obtained following the transformation of the
mean gray level using the formula: OD=log (256/mean gray level).
The background was subtracted and the OD ratio for each image was
calibrated as % relative optical density (ROD) using Adobe
Photoshop version 8.0 (Adobe Systems, San Jose, CA, USA) and ImageJ
software version 1.49 (National Institutes of Health, Bethesda, MD,
USA). The mean value of the OD of the sham group was designated as
100% and the ROD in each group was calibrated and expressed as a
percentage of the sham group.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean of at least 2 independent experiments. Data from F-J B
immunofluorescence and immunohistochemical staining were analyzed
using one-way analysis of variance, followed by a post hoc
Bonferroni-Dunn Test using SPSS version 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
CV-positive cells
Sham group
CV staining is presented in Fig. 1. CV-positive cells were detected
throughout the hippocampus; in particular, they were aggregated in
the stratum pyramidale of the hippocampus proper (CA1-3 regions)
and the granular cell layer of the dentate gyrus (Fig. 1Aa-d).
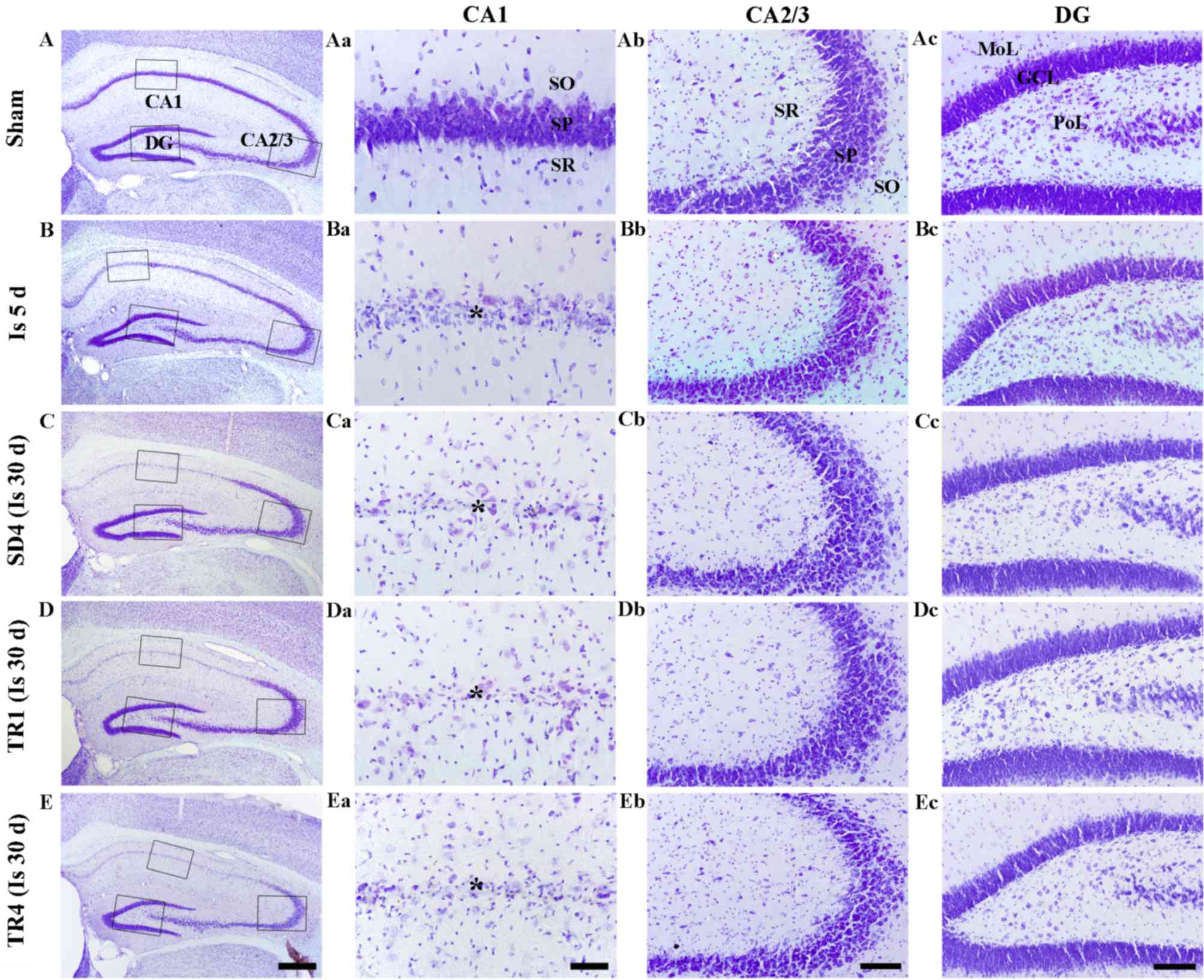 | Figure 1.CV staining in the (A) sham, (B and
C) ischemia and (Da and E) TR groups. Only a small number of
CV-positive cells were detected in the SP (asterisk) of the CA1
region 5 days post-ischemia. In the SD4, TR1 and TR4 groups, the
pattern of CV-positive cells distribution was similar to the
ischemia group at 5 days post-ischemia. Scale bar: (Aa-Ea) 400 µm,
(Ab-Eb) 40 µm and (Ac-Ec and Ad-Ed) 100 µm. CV, cresyl violet; TR,
treadmill exercise; SD, sedentary routine; DG, dentate gyrus; GCL,
granule cell layer; MoL, molecular layer; PoL, polymorphic layer;
SO, stratum oriens; SR, stratum radiatum; SP, stratum
pyramidale. |
Ischemia groups
CV-positive cells were markedly decreased in the CA1
stratum pyramidale, but not in the other subregions, 5 days
post-ischemia (Fig. 1Ba-d). In the
SD4 group, the distribution pattern of CV-positive cells was
similar to the ischemia group at 5 days post-ischemia (Fig. 1Ca-d).
TR-groups
In the TR1 and TR4 groups, the distribution pattern
of CV-positive cells in the hippocampus was also similar to the SD4
group (Fig. 1Da-d and Ea-d).
F-J B-positive cells
Sham group
F-J B staining is presented in Fig. 2. F-J B-positive cells were not
detected in any layers of the hippocampus proper and the dentate
gyrus (Fig. 2Aa-d).
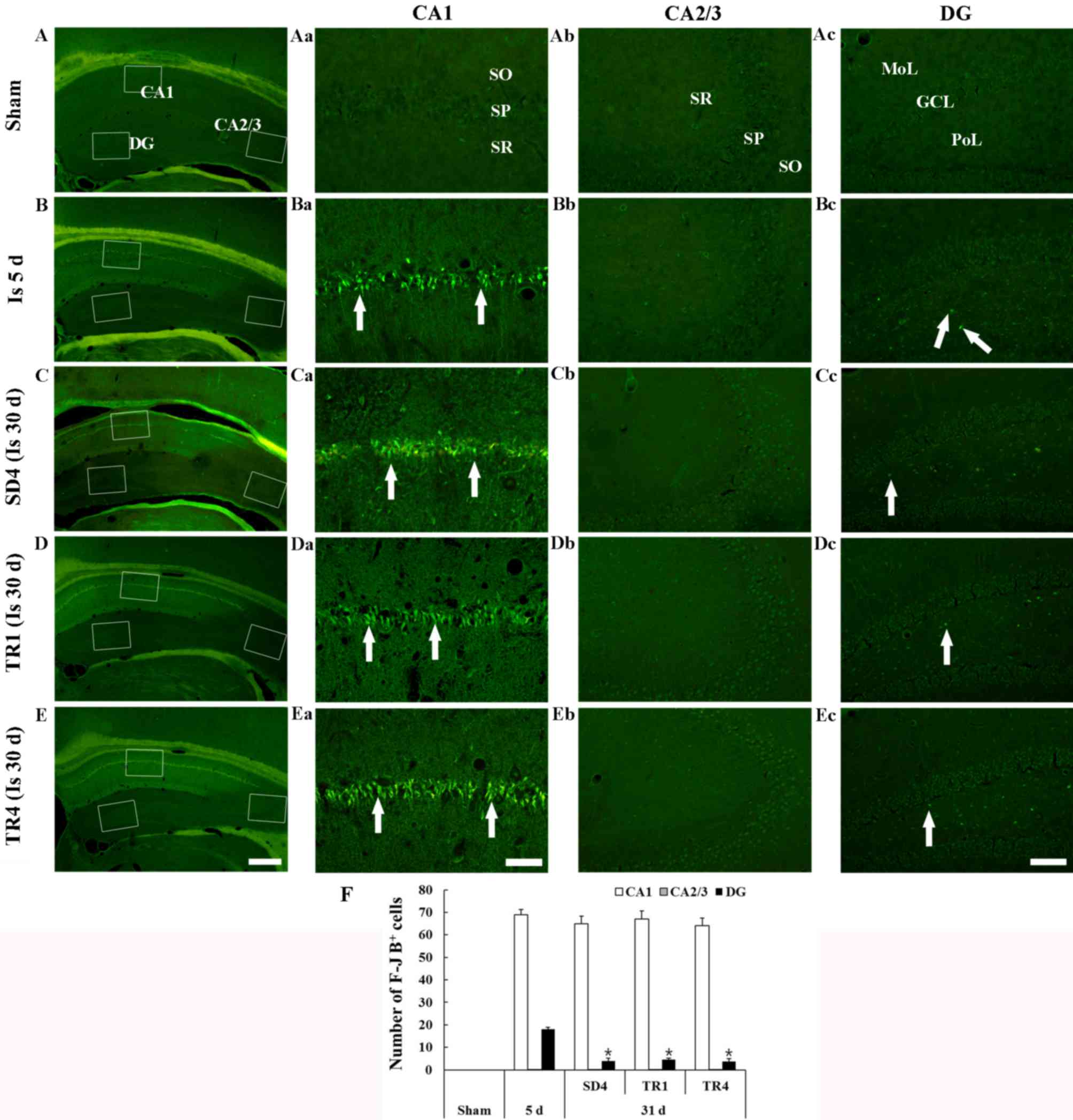 | Figure 2.F-J B histofluorescence staining in
the (A) sham, (B and C) ischemia and (D and E) TR groups. In the
sham group, no F-J B-positive cells were detected. However, at 5
days post-ischemia, numerous F-J B-positive neurons (arrows) were
detected in the SP of the CA1 region and in the PoL of the DG. In
the SD4, TR1 and TR4 groups, the number of F-J B-positive neurons
(arrows) in the SP of the CA1 region and in the PoL of the DG was
similar between the groups. Scale bar: (Aa-Ea) 400 µm, (Ab-Eb) 40
µm and (Ac-Ec and Ad-Ed) 100 µm. (F) Number of F-J B-positive cells
in the SP of the CA1 and CA2/3 regions and in the PoL of the DG
(n=7/group). Data are presented as the mean ± standard error of
mean. *P<0.05 vs. ischemia group at 5 days post-ischemia. F-J B,
Fluoro-Jade B; TR, treadmill exercise; SD, sedentary routine; DG,
dentate gyrus; GCL, granule cell layer; MoL, molecular layer; PoL,
polymorphic layer; SO, stratum oriens; SR, stratum radiatum; SP,
stratum pyramidale. |
Ischemia groups
A total of 5 days post-ischemia, numerous F-J
B-positive cells were detected in the stratum pyramidale of the CA1
region and a small number of F-J B-positive cells were detected in
the polymorphic layer of the dentate gyrus (Fig. 2Ba-d and F). In the SD4 group, the
distribution pattern and number of F-J B-positive cells in the CA1
stratum pyramidale was similar to the ischemia-group at 5 days
post-ischemia; however, the number of F-J B-positive cells was
significantly decreased in the polymorphic layer of the dentate
gyrus (Fig. 2Ca-d and F).
TR-groups
In the TR1 and TR4 groups, the number of F-J
B-positive cells in the CA1 stratum pyramidale and in the
polymorphic layer of the dentate gyrus was similar to the SD4 group
and no significant difference in the number of F-J B-positive cells
was observed between the TR1 and TR4 groups (Fig. 2Da-d, Ea-d and F).
GFAP-immunoreactive astrocytes
Sham group
GFAP staining is presented in Fig. 3. GFAP-immunoreactive astrocytes in
the sham group were easily detected in all layers of the
hippocampus proper and the dentate gyrus. The astrocytes appeared
to be at resting form and had a small body with thread-like thin
processes (Fig. 3Aa-d).
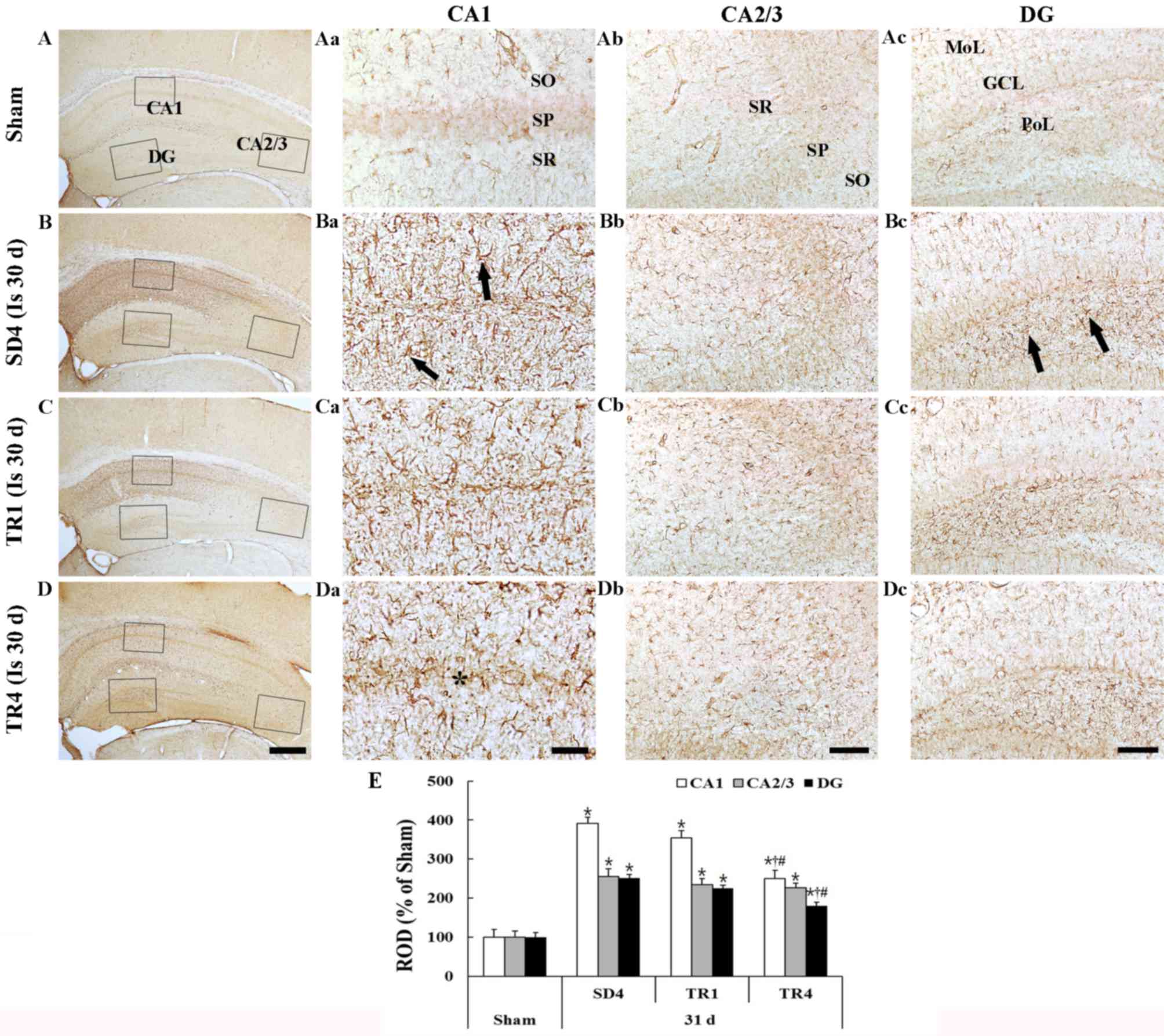 | Figure 3.GFAP immunohistochemistry in the (A)
sham, (B) SD4, (C) TR1 and (D) TR4 groups. In the SD4 group,
GFAP-immunoreactive astrocytes (arrows) were activated in the CA1
region and in the PoL of the DG. In the TR1 group, the activation
was similar to the SD4 group; however, in the TR4 group, the
activation was significantly decreased compared with the SD4 group.
Scale bar: (Aa-Da) 400 µm, (Ab-Db) 40 µm and (Ac-Dc and Ad-Dd) 100
µm. (E) ROD expressed as a percentage of GFAP-immunoreactive
structures (n=7/group). Data are presented as the mean ± standard
error of mean. *P<0.05 vs. sham group; †P<0.05 vs.
SD4 group; #P<0.05 vs. TR1 group. GFAP, glial
fibrillary acidic protein; SD, sedentary routine; TR, treadmill
exercise; DG, dentate gyrus; GCL, granule cell layer; MoL,
molecular layer; PoL, polymorphic layer; SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum; ROD, relative optical
density. |
Ischemia group
In the SD4 group, numerous GFAP-immunoreactive
astrocytes demonstrated a typical activated form that had a
punctuated cytosol with thick processes (Fig. 3Ba-d). The density of the
GFAP-immunoreactive structures (ROD) was significantly increased in
all subregions compared with in the sham group (P<0.05; Fig. 3E); in particular, the activation
was marked in the CA1 region and in the polymorphic layer of the
dentate gyrus.
TR groups
In the TR1 group, the morphology of
GFAP-immunoreactive astrocytes in the hippocampus proper and the
dentate gyrus was similar to the SD4 group (Fig. 3Ca-d) and the ROD of
GFAP-immunoreactive structures was not significantly different
compared with the SD4 group (Fig.
3E). However, in the TR4 group the ROD was significantly
decreased (P<0.05) compared with in the SD4 and TR1 groups
(Fig. 3Da-d and E).
Iba-1-immunoreactive microglia
Sham group
Iba-1 staining is presented in Fig. 4. Iba-1-immunoreactive microglia
were evenly distributed throughout the hippocampus. The microglia
appeared to be at resting form and exhibited fine processes with
web-like network characteristics (Fig.
4Aa-d).
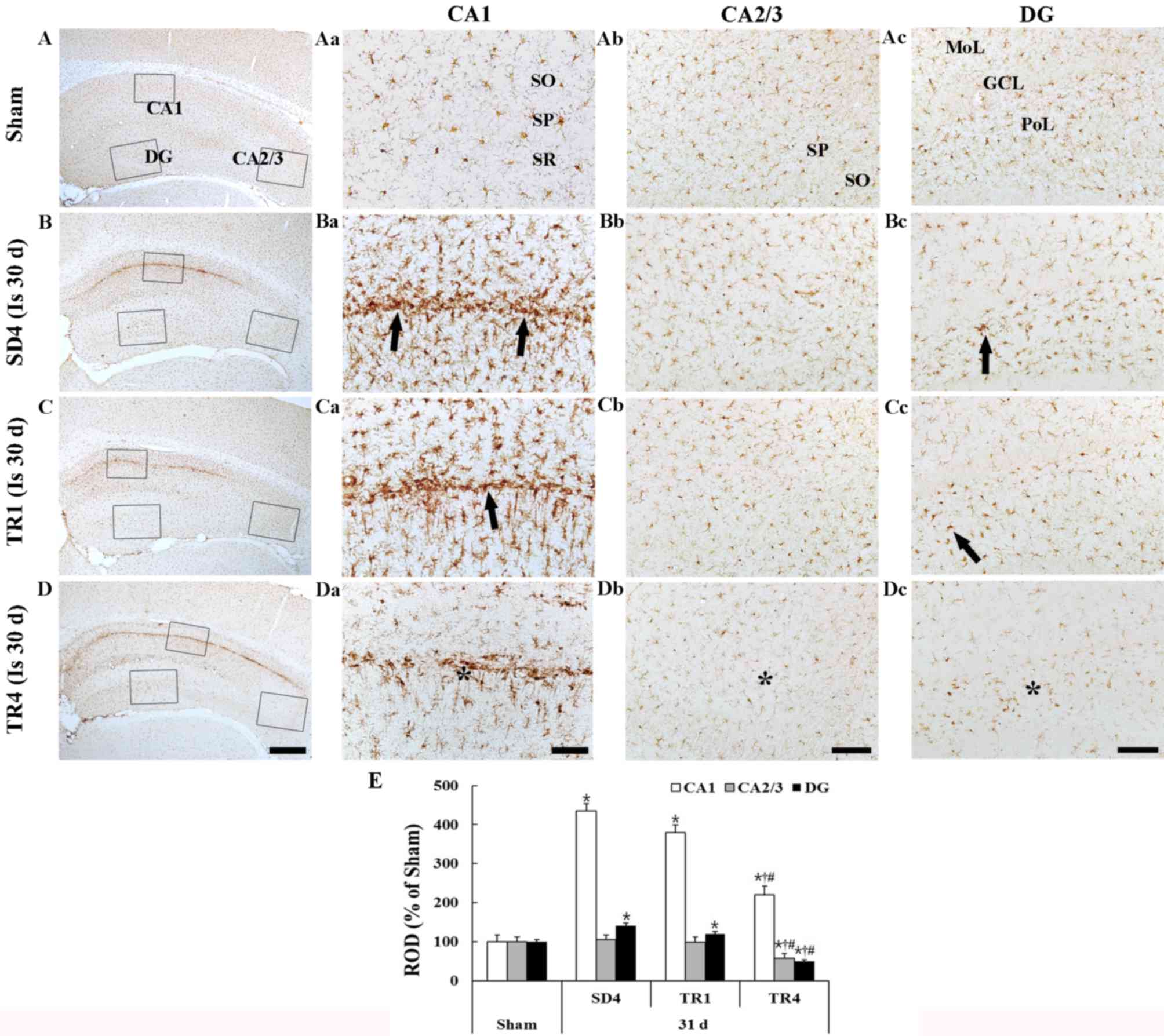 | Figure 4.Iba-1 immunohistochemistry in the (A)
sham, (B) SD4, (C) TR1 and (D) TR4 groups. Iba-1-immunoreactive
microglia were activated (arrows) in the CA1 region and the PoL of
the DG in the SD4 group. In the TR4 group, the activation of
Iba-1-immunoreactive microglia was significantly decreased
(asterisks) although the activation in the TR1 group was similar to
the SD4 group. Scale bar: (Aa-Da) 400 µm, (Ab-Db) 40 µm and (Ac-Dc
and Ad-Dd) 100 µm. (E) ROD expressed as a percentage of Iba-1
immunoreactive structures (n=7/group). Data are presented as the
mean ± standard error of the mean. *P<0.05 vs. the sham group;
†P<0.05 vs. the SD4 group; #P<0.05 vs.
the TR1 group). Iba-1, ionized calcium binding adaptor molecule 1;
SD, sedentary routine; TR, treadmill exercise; DG, dentate gyrus;
GCL, granule cell layer; MoL, molecular layer; SO, stratum oriens;
SP, stratum pyramidale; SR, stratum radiatum; ROD, relative optical
density. |
Ischemia group
In the SD4 group, Iba-1-immunoreactive microglia
were markedly altered in the CA1 region and in the polymorphic
layer of the dentate gyrus; they exhibited bulky cytoplasm with
short and thickened processes, which represents the activated form
(Fig. 4Ba-d and E). In particular,
activated Iba-1-immunoreactive microglia were aggregated in the
stratum pyramidale of the CA1 region. The ROD of the
Iba-1-immunoreactive structures was significantly increased
(P<0.05) in the CA1 region and the polymorphic layer of the
dentate gyrus compared with in the sham group (Fig. 4E).
TR groups
In the TR1 group, the distribution pattern of
Iba-1-immunoreactive microglia in the hippocampus was similar to
the SD4 group; however, the activation of Iba-1-immunoreactive
microglia was slightly decreased in the CA1 region and the dentate
gyrus (Fig. 4Ca-d). In the TR4
group, the ROD of activated Iba-1-immunoreactive microglia was
significantly decreased (P<0.05) in the CA1 region and the
dentate gyrus compared with in the SD4 and TR1 groups (Fig. 4Da-c and E).
Discussion
Ischemic brain damage can lead to the development of
neuronal damage and gliosis (26),
and result in long-term functional disability (27,28).
The present study investigated the effects of long- and short-term
post-ischemic treadmill exercise on neuronal death and glial
activation in the aged gerbil hippocampus induced by 5 min of
transient cerebral ischemia.
In the present study, at 5 days post-ischemia, a
distinct neuronal loss was observed in the CA1 stratum pyramidale
and in the polymorphic layer of the dentate gyrus in the aged
gerbil hippocampus, as determined using CV and F-J B staining. This
result is consistent with our previous findings, which demonstrated
that a significant neuronal loss in the aged gerbil hippocampus was
detected in the CA1 stratum pyramidale (5) and in the polymorphic layer of the
dentate gyrus (29) 5 days after
transient ischemia. At 31 days post-ischemia in the SD4 group, the
number of F-J B-positive cells (dead neurons) in the CA1 region was
similar to that at 5 days post-ischemia. Furthermore, the present
study is the first, to the best of our knowledge, to report that
short- and long-term post-ischemic treadmill exercise did not
exhibit any neuroprotection in the TR1 and TR4 groups; the numbers
of F-J B-positive neurons in the CA1 region and the dentate gyrus
were no different compared with the SD4 group. It has previously
been reported that short- and long-term treadmill exercise,
initiated prior to ischemic neuronal death, exerted a
neuroprotective effect by suppressing transient cerebral
ischemia-induced apoptosis of the neurons in the CA1 region
(21–23). Based on the findings of the present
study and previous studies, it may be concluded that treadmill
exercise begun after transient cerebral ischemia-induced neuronal
degeneration cannot protect neurons in the aged hippocampus.
In the present study, the significant activation of
GFAP-immunoreactive astrocytes and Iba-1-immunoreactive microglia
was observed in the CA1 region and the dentate gyrus of the SD4
group, and their ROD was significantly increased compared with in
the sham group. However, 4 weeks of post-ischemic treadmill
exercise significantly reduced the number of activated astrocytes
and microglia in the CA1 region and in the dentate gyrus compared
with the sedentary control (SD4 group). Conversely, 1 week of
treadmill exercise did not effectively decrease their activation in
the ischemic hippocampus. It is well known that ischemic
hippocampus pathology is closely associated with an acute and
prolonged inflammatory response, which is characterized by the
production of inflammatory cytokines and the activation of resident
glial cells (30,31). In this regard, previous studies
have demonstrated that wheel-running exercise attenuated
age-related astrocyte hypertrophy (32) and microglial proliferation
(33). In addition, chronic
exercise inhibited the activation of astrocytes and microglia, and
other inflammatory-related factors, including inducible nitric
oxide synthase, in murine models of Alzheimer's and Parkinson's
diseases (34,35). The present results, along with the
aforementioned findings, indicated that long-term treadmill
exercise may alleviate increased neuroinflammation in the aged
gerbil hippocampus induced by transient cerebral ischemia.
In conclusion, the present study suggested that 4
weeks of treadmill exercise, initiated after neuronal death, cannot
influence neuronal protection; however, the exercise can
effectively alleviate transient cerebral ischemia-induced gliosis
in the hippocampus of aged gerbils.
Acknowledgements
The present study was supported by grants from the
Osong Innovation Center funded by the Ministry of Health &
Welfare, Republic of Korea (grant no. HO14C0001) and the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education (grant no.
NRF-2014R1A1A3051721).
References
|
1
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CS, Polsky K, Nadler JV and Crain BJ:
Selective neocortical and thalamic cell death in the gerbil after
transient ischemia. Neuroscience. 35:289–299. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petito CK, Torres-Munoz J, Roberts B,
Olarte JP, Nowak TS Jr and Pulsinelli WA: DNA fragmentation follows
delayed neuronal death in CA1 neurons exposed to transient global
ischemia in the rat. J Cereb Blood Flow Metab. 17:967–976. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horn M and Schlote W: Delayed neuronal
death and delayed neuronal recovery in the human brain following
global ischemia. Acta Neuropathol. 85:79–87. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee CH, Yoo KY, Choi JH, Park OK, Hwang
IK, Kim SK, Kang IJ, Kim YM and Won MH: Neuronal damage is much
delayed and microgliosis is more severe in the aged hippocampus
induced by transient cerebral ischemia compared to the adult
hippocampus. J Neurol Sci. 294:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamagaki C, Murata A, Asai S, Takase K,
Gonno K, Sakata T and Kinoshita T: Age-related changes of cornu
ammonis 1 pyramidal neurons in gerbil transient ischemia.
Neuropathology. 20:221–227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernal GM and Peterson DA: Phenotypic and
gene expression modification with normal brain aging in
GFAP-positive astrocytes and neural stem cells. Aging cell.
10:466–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gehrmann J, Bonnekoh P, Miyazawa T,
Hossmann KA and Kreutzberg GW: Immunocytochemical study of an early
microglial activation in ischemia. J Cereb Blood Flow Metab.
12:257–269. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Norden DM and Godbout JP: Review:
Microglia of the aged brain: Primed to be activated and resistant
to regulation. Neuropathol Appl Neurobiol. 39:19–34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi JH and Won MH: Microglia in the
normally aged hippocampus. Lab Anim Res. 27:181–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nedergaard M and Dirnagl U: Role of glial
cells in cerebral ischemia. Glia. 50:281–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai AY and Todd KG: Microglia in cerebral
ischemia: Molecular actions and interactions. Can J Physiol
Pharmacol. 84:49–59. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwakkel G, Van Peppen R, Wagenaar RC,
Dauphinee S Wood, Richards C, Ashburn A, Miller K, Lincoln N,
Partridge C, Wellwood I and Langhorne P: Effects of augmented
exercise therapy time after stroke: a meta-analysis. Stroke.
35:2529–2539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Zhang Q, Pu H, Wu Y, Bai Y,
Vosler PS, Chen J, Shi H, Gao Y and Hu Y: Very early-initiated
physical rehabilitation protects against ischemic brain injury.
Front Biosci (Elite Ed). 4:2476–2489. 2012.PubMed/NCBI
|
|
15
|
Chen MF, Huang TY, Kuo YM, Yu L, Chen HI
and Jen CJ: Early postinjury exercise reverses memory deficits and
retards the progression of closed-head injury in mice. J Physiol.
591:985–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang KM, Cheng FC, Huang YL, Chung SY,
Jian ZY and Lin MC: Trace element, antioxidant activity, and lipid
peroxidation levels in brain cortex of gerbils after cerebral
ischemic injury. Biol Trace Elem Res. 152:66–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YR, Lei RY, Wang CE, Zhang BA, Lu H,
Zhu HC and Zhang GB: Effects of catalpol on ATPase and amino acids
in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci.
35:1229–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shcherbak NS, Galagudza MM, Ovchinnikov
DA, Kuzmenkov AN, Yukina GY, Barantsevich ER, Tomson VV and
Shlyakhto EV: Activity of succinate dehydrogenase in the neocortex
and hippocampus of Mongolian gerbils with ischemic and reperfusion
brain injury. Bull Exp Biol Med. 155:14–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Wang T, Feng WY, Wang ZY, Cheng MS
and Wang YJ: Ecdysterone protects gerbil brain from temporal global
cerebral ischemia/reperfusion injury via preventing neuron
apoptosis and deactivating astrocytes and microglia cells. Neurosci
Res. 81(82): 21–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn JH, Choi JH, Park JH, Kim IH, Cho JH,
Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al: Long-term
exercise improves memory deficits via restoration of myelin and
microvessel damage, and enhancement of neurogenesis in the aged
gerbil hippocampus after ischemic stroke. Neurorehabil Neural
Repair. 30:894–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sim YJ, Kim H, Kim JY, Yoon SJ, Kim SS,
Chang HK, Lee TH, Lee HH, Shin MC, Shin MS and Kim CJ: Long-term
treadmill exercise overcomes ischemia-induced apoptotic neuronal
cell death in gerbils. Physiol Behav. 84:733–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sim YJ, Kim SS, Kim JY, Shin MS and Kim
CJ: Treadmill exercise improves short-term memory by suppressing
ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci
Lett. 372:256–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee MH, Kim H, Kim SS, Lee TH, Lim BV,
Chang HK, Jang MH, Shin MC, Shin MS and Kim CJ: Treadmill exercise
suppresses ischemia-induced increment in apoptosis and cell
proliferation in hippocampal dentate gyrus of gerbils. Life Sci.
73:2455–2465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn JH, Choi JH, Kim JS, Lee HJ, Lee CH,
Yoo KY, Hwang IK, Lee YL, Shin HC and Won MH: Comparison of
immunoreactivities in 4-HNE and superoxide dismutases in the
cervical and the lumbar spinal cord between adult and aged dogs.
Exp Gerontol. 46:703–708. 2011.PubMed/NCBI
|
|
25
|
Candelario-Jalil E, Alvarez D, Merino N
and León OS: Delayed treatment with nimesulide reduces measures of
oxidative stress following global ischemic brain injury in gerbils.
Neurosci Res. 47:245–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugawara T, Lewén A, Noshita N, Gasche Y
and Chan PH: Effects of global ischemia duration on neuronal,
astroglial, oligodendroglial, and microglial reactions in the
vulnerable hippocampal CA1 subregion in rats. J Neurotrauma.
19:85–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li DQ, Bao YM, Li Y, Wang CF, Liu Y and An
LJ: Catalpol modulates the expressions of Bcl-2 and Bax and
attenuates apoptosis in gerbils after ischemic injury. Brain Res.
1115:179–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takagi N: Pathology and strategies for the
treatment of ischemic brain injury. Yakugaku Zasshi. 129:1215–1219.
2009.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn JH, Shin BN, Park JH, Kim IH, Cho JH,
Chen B, Lee TK, Tae HJ, Lee JC, Cho JH, et al: Long-term
observation of neuronal degeneration and microgliosis in the gerbil
dentate gyrus after transient cerebral ischemia. J Neurol Sci.
363:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Danton GH and Dietrich WD: Inflammatory
mechanisms after ischemia and stroke. J Neuropathol Exp Neurol.
62:127–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stoll G and Jander S: The role of
microglia and macrophages in the pathophysiology of the CNS. Prog
Neurobiol. 58:233–247. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Latimer CS, Searcy JL, Bridges MT, Brewer
LD, Popović J, Blalock EM, Landfield PW, Thibault O and Porter NM:
Reversal of glial and neurovascular markers of unhealthy brain
aging by exercise in middle-aged female mice. PLoS One.
6:e268122011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kohman RA, DeYoung EK, Bhattacharya TK,
Peterson LN and Rhodes JS: Wheel running attenuates microglia
proliferation and increases expression of a proneurogenic phenotype
in the hippocampus of aged mice. Brain Behav Immun. 26:803–810.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sung YH, Kim SC, Hong HP, Park CY, Shin
MS, Kim CJ, Seo JH, Kim DY, Kim DJ and Cho HJ: Treadmill exercise
ameliorates dopaminergic neuronal loss through suppressing
microglial activation in Parkinson's disease mice. Life Sci.
91:1309–1316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leem YH, Lee YI, Son HJ and Lee SH:
Chronic exercise ameliorates the neuroinflammation in mice carrying
NSE/htau23. Biochem Biophys Res Commun. 406:359–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|


















