Introduction
Heart failure occurs when the heart is unable to
maintain adequate blood circulation to meet the body's
requirements, and is involved in the development of cardiac
hypertrophy (1–3). Heart failure presents with an
elevation of catecholamine levels, which are responsible for the
functional uncoupling and downregulation of the adrenergic system
(4,5). The cardiomyocyte adrenergic receptor
(AR) subtypes β1 and β2 participate in the
catecholamine-mediated increase of cardiac inotropy or chronotropy
(6–8). The β1- and
β2ARs, which are homologous in structure, exert
different effects on cardiac function (9); these differences may be explained by
the distinct G-protein couplings associated with the βAR subtype
(10). In particular,
β2AR activates stimulatory guanine nucleotide-binding
proteins (Gs) and pertussis toxin-sensitive inhibitory guanine
nucleotide-binding protein (Gi) 2 and Gi3 signaling pathways,
whereas β1AR exclusively couples to the Gs/adenylyl
cyclase/cyclic AMP (cAMP)/protein kinase A (PKA) signaling cascade
(11). However, the differing
underlying mechanisms between β1AR and β2AR
remain unknown.
Notably, the vascular endothelial growth factor
(VEGF) family has been revealed to exhibit an ability to initiate
angiogenic cascades in the absence of ischemia or inflammation
(12,13). Furthermore, β1- and
β2ARs were demonstrated to mediate
norepinephrine-induced VEGF upregulation (12). Important roles for VEGF-A have been
suggested in the cardiovascular system, including angiogenesis and
vasodilation (13). The
transcription factor GATA4 has been reported to directly regulate
VEGF expression, via binding to the promoter of the VEGF-A gene;
GATA4 has been suggested to function as a stress-response
regulator, by coordinating angiogenetic processes following
hemodynamic load, through the modulation of non-hypoxic and
load-responsive mechanisms (14).
However, it is currently unclear whether distinct β1-
and β2AR-mediated signaling mechanisms are involved in
VEGF-A regulation in hemodynamically challenged hearts. The
molecular mechanisms underlying the differences between
β1- and β2AR signaling remain largely
unknown. Therefore, the present study aimed to compare the
expression of Gs, VEGF-A and their associated proteins, in hearts
from β1AR transgenic (TG), β2AR TG and
wild-type (WT) mice.
Materials and methods
TG mice
The generation of β1AR and
β2AR TG mice that overexpress human cardiac-specific
β1- or β2ARs was performed as previously
described (6,7). In brief, wild-type human
β1- and β2-AR cDNA was ligated into the
SalI site (exon 3) of the full-length 5.5-kb α-myosin heavy
chain (MHC) promoter, obtained from Dr. Arthur R. Struch, (Mayo
Clinic, Rochester, MN, USA). The linearized constructs were
injected into male pronuclei of fertilized FVB/N mouse oocytes and
implanted into pseudopregnant female oviducts (Taconic Biosciences,
Rensselaer, NY, USA). Genomic DNA from tail-cuts was screened for
the presence of transgenes, using targeted PCR with the following
primers: For β1AR, forward primer
5′-AGGACTTCACATAGAAGCCTAG-3′, located in the α-MHC promoter, and
reverse primer 5′-TGTCCACTGCTGAGACAGCG-3′, located in the
β1AR coding sequence. For β2AR, forward
primer 5′-GGAGCAGAGTGGATATCACG-3′, located in the open reading
frame, and reverse primer 5′-GTCACACCACAGAAGTAAGG-3′, located in
the SV40 polyadenylation region. A total of 39 male mice (n=13
mice/group) were housed in individual cages (temperature, 23°C;
humidity, 60%) under 12/12 h light/dark cycles, and provided with
commercial chow and water ad libitum. Mice were examined at
6 months of age, to ensure the presented phenotypes were
independent of the confounding effects of cardiac growth and
associated alterations to βAR functional coupling. Animal
procedures were approved by the University of Maryland Baltimore
Institutional Animal Care and Use Committee.
Physiological parameters
Physiological parameters were evaluated in an
isolated work-performing experiment, according to a previously
published procedure (7). Briefly,
mice (n=5/group; age, 6 months; weight, ~35 g) were anesthetized
via intraperitoneal injection of 100 mg/kg Nembutal and 1.5 units
of heparin to prevent microthrombosis, the heart and aorta were
attached to a 20-gauge cannula and subjected to temporary
retrograde perfusion with oxygenated Krebs-Henseleit solution (118
mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 0.5 mM Na-EDTA, 25 mM
NaHCO3, 1.2 mM KH2PO4, 11 mM
glucose; followed by saturation with 95% O2+5%
CO2). A polyethelene-50 catheter was inserted into the
apex of the left ventricle to measure intraventricular pressure.
The pulmonary vein was connected to a second cannula and the
antegrade perfusion was initiated with a basal workload of 300 mmHg
ml/min (venous return, 6 ml; mean aortic pressure, 50 mmHg). Hearts
were allowed to equilibrate for 20 min. Atrial pressure was
monitored through a sidearm in the left atrial cannula (National
Instruments Corporation, Austin, Texas, US). The left ventricular
pressure signals were analyzed off-line using NI Biobench software
version 1.0 (National Instruments Corporation). The first positive
and negative derivatives of the left intraventricular pressure
curve [that is, the maximal rate pressure development (+dP/dt) and
the maximal rate pressure decline (−dP/dt)], the duration of
contraction and relaxation [that is, time to peak pressure (TPP)],
and the time to half relaxation (TR½) were calculated. TPP was
normalized with respect to peak systolic pressure, calculated as:
Systolic pressure-end diastolic pressure. TR½ was normalized with
respect to ½ relaxation pressure, calculated as: (Systolic
pressure-diastolic pressure)/2.
Hematoxylin and eosin (H&E)
staining
Mice (n=5/group) were anesthetized via
intraperitoneal injection of pentobarbital sodium (100 mg/kg).
Hearts were isolated and fixed with 10% buffered formalin for 2
days at room temperature. The heart/body weight ratio was
calculated according to the following formula: Heart/body weight
ratio=heart weight (mg)/body weight (g). Hearts were embedded in
paraffin and sectioned (6 µm). The sections were stained with
H&E, and digital images were captured using a light microscope
and analyzed using the digital imaging analysis software Leica
Steel Expert version 2.0 (Leica Microsystems GmbH, Wetzlar,
Germany). Cardiomyocyte diameter was determined as the shortest
distance across the nucleus in transverse cell sections.
Cardiomyocytes (n=100) from 5 randomly selected microscope fields
(x200 magnification) from the posterior wall of the left ventricle
were measured to represent the average cardiomyocyte diameter.
Western blotting
The expression of heart proteins was detected
according to previously published procedures (15). Briefly, proteins were extracted
from heart tissue (~50 mg tissue; n=3 mice/group) using
radioimmunoprecipitation assay buffer (1:4 w:v; R2002, Biosesang,
Inc., Soungnam, Korea) containing 150 mM NaCl, 1% Triton X-100, 1%
sodium deooxycholate, 50 mM Tris HCl (pH 7.5), 0.1% SDS, 2 mM EDTA
(pH 8.0) and phosphatase inhibitor cocktail for 10 min over ice.
Lysates were centrifuged at 19,000 × g for 10 min at 4°C and
supernatants were collected. Protein concentrations were determined
using a bicinchoninic acid assay kit. Equal amounts of extracted
protein samples (200 mg/ml) were subjected to Tris-Glycine-SDS
PAGE, transferred onto polyvinylidene fluoride membranes. Membranes
were blocked with 5% non-fat skim milk in TBS containing 0.1% Tween
20 for 2 h at room temperature and probed with the following
primary antibodies overnight at 4°C: Anti-Gs (1:500; cat no.
sc26766), anti-Gi2 (1:500; cat no. sc391), anti-Gi3 (1:500; cat no.
sc262), anti-G-protein-coupled receptor kinase 2 (GRK2; 1:500; cat
no. sc562), anti-GATA binding protein 4 (GATA4; 1:1,000; cat no.
sc1237) and anti-GAPDH (1:2,000; cat no. sc166574), obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA); anti-VEGF-A
(1:1,000; cat no. ab1316) and anti-phosphorylated (p)-GATA4 (1:500;
cat no. ab5245) obtained from Abcam (Cambridge, MA, USA). Membranes
were then incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat no.
SC-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h
at room temperature. Protein bands were visualized using Amersham
ECL Western Blotting Detection Reagent (GE Healthcare Life
Sciences, Chalfont, UK) and the signals were acquired using a
LAS-3000 charged coupled device camera (Fujifilm Holdings
Corporation, Tokyo, Japan). Blots were semi-quantified using
densitometric analysis and protein expression was normalized to
GAPDH. Semi-quantification was performed using the Scion Image
software version 4.0.3.2 (Scion Corporation, Walkersville, MD,
USA). Experiments were performed in triplicate.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Differences of the means amongst the groups were
estimated by one-way analysis of variance followed by a post hoc
Bonferroni test for multiple comparisons. All analyses were
performed using SPSS version 20.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Physiological parameters
The cardiac function of β1AR TG and
β2AR TG mice was enhanced, and the cardiac
contractility/relaxation and heart rate of β1- and
β2AR TG mice were significantly increased compared with
the WT mice (Table I). No
significant differences between the β1AR and
β2AR TG mouse hearts were identified; however, the
clinical parameters, including left ventricular systolic pressure
(SP), left ventricular diastolic pressure (DP), left ventricular
end-diastolic pressure (EDP) and TPP in β2AR TG mice appeared to be
slightly increased compared with in β1AR TG mice.
 | Table I.Physiological parameters in the
isolated work-performing hearts of the WT, β1AR TG and
β2AR TG mice. |
Table I.
Physiological parameters in the
isolated work-performing hearts of the WT, β1AR TG and
β2AR TG mice.
| Physiological
parameter | WT | β1AR
TG | β2AR
TG |
|---|
| Heart/body weight
ratio (mg/g) | 3.61±0.03 |
3.91±0.07a |
3.78±0.06a |
| SP (mmHg) | 133.4±1.7 |
159.4±3.5a |
168.1±4.8a |
| DP (mmHg) | −8.1±1.36 |
−33.7±1.3a |
−37.7±3.4a |
| EDP (mmHg) | 7.7±0.9 |
2.1±0.5a |
3.9±0.8a |
| +dP/dt
(mmHg/s) | 3961±41 |
5927±187a |
5775±342a |
| −dP/dt
(mmHg/s) | 2763±130 |
5179±206a |
5270±156a |
| HR
(beats/minute) | 247±3.9 |
334±4.3a |
316±0.9a |
| TPP
(msec/mmHg) | 0.40±0.01 |
0.25±0.02a |
0.31±0.03a |
| TR1/2
(msec/mmHg) | 0.63±0.02 |
0.40±0.03a |
0.47±0.03a |
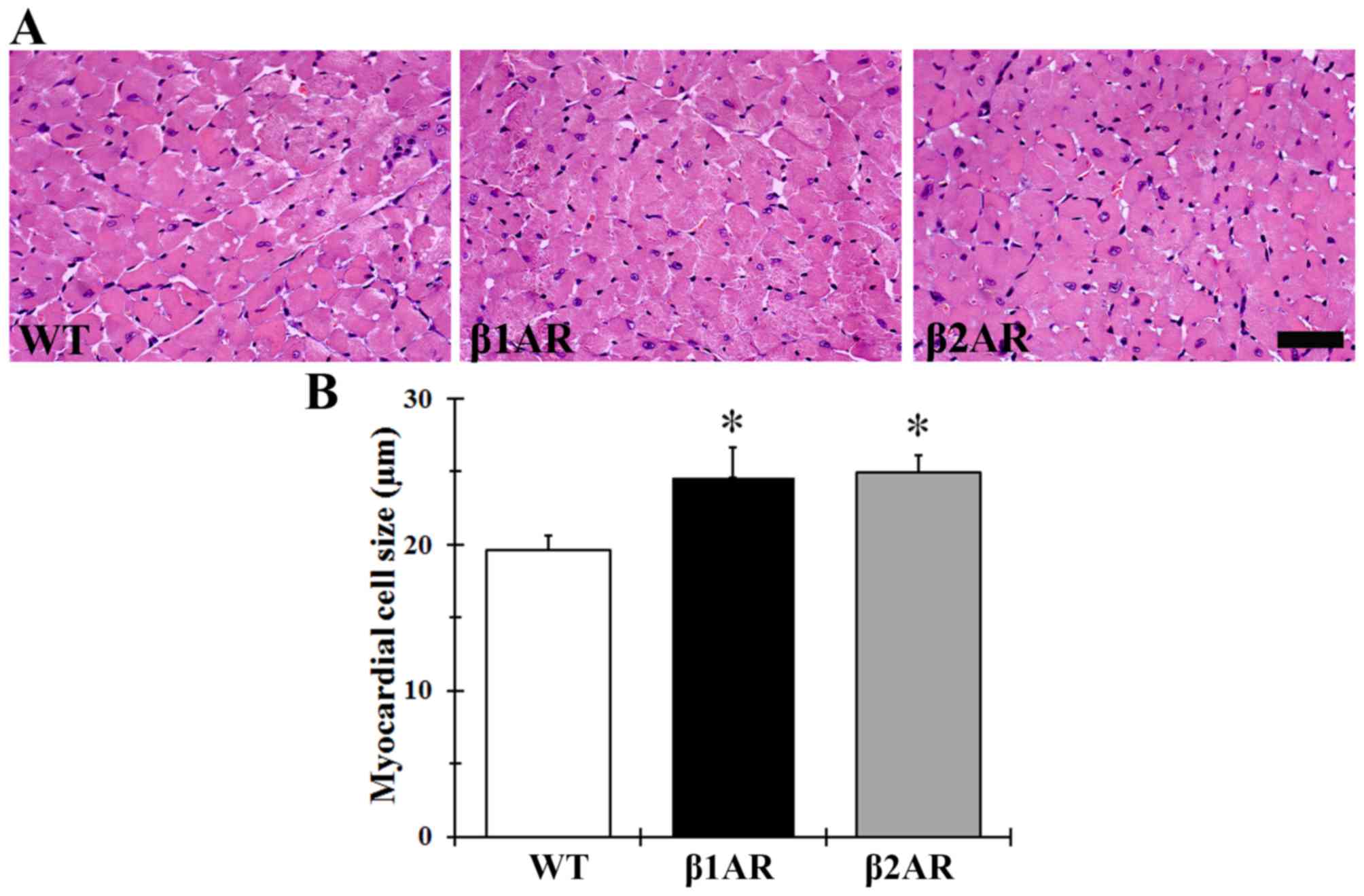
Myocardial hypertrophy
Heart/body weight ratio was significantly greater in
β1AR and β2AR TG mice compared with in WT
mice (3.91±0.07 and 3.78±0.06 mg/g vs. 3.61±0.03 mg/g,
respectively; P<0.05); however, no statistical significance was
identified between the two transgenic groups. The diameter of the
cardiomyocytes demonstrated a similar trend to the heart/body
weight ratios; cell diameters were greater in βAR TG mice compared
with in the WT mice, however, no significant differences in
cardiomyocyte size were detected between the two transgenic groups
(Fig. 1).
Expression levels of G proteins and
GRK2
Protein expression levels of GRK2, Gs, Gi2 and Gi3
were examined by western blot analysis (Fig. 2). Gs protein expression level was
significantly decreased, whereas Gi2, Gi3 and GRK2 expressions were
significantly increased, in β1AR and β2AR TG
mice compared with WT mice (Fig.
2). The upregulation of Gi2 was greatest in the β2AR
TG mice compared with the other two groups.
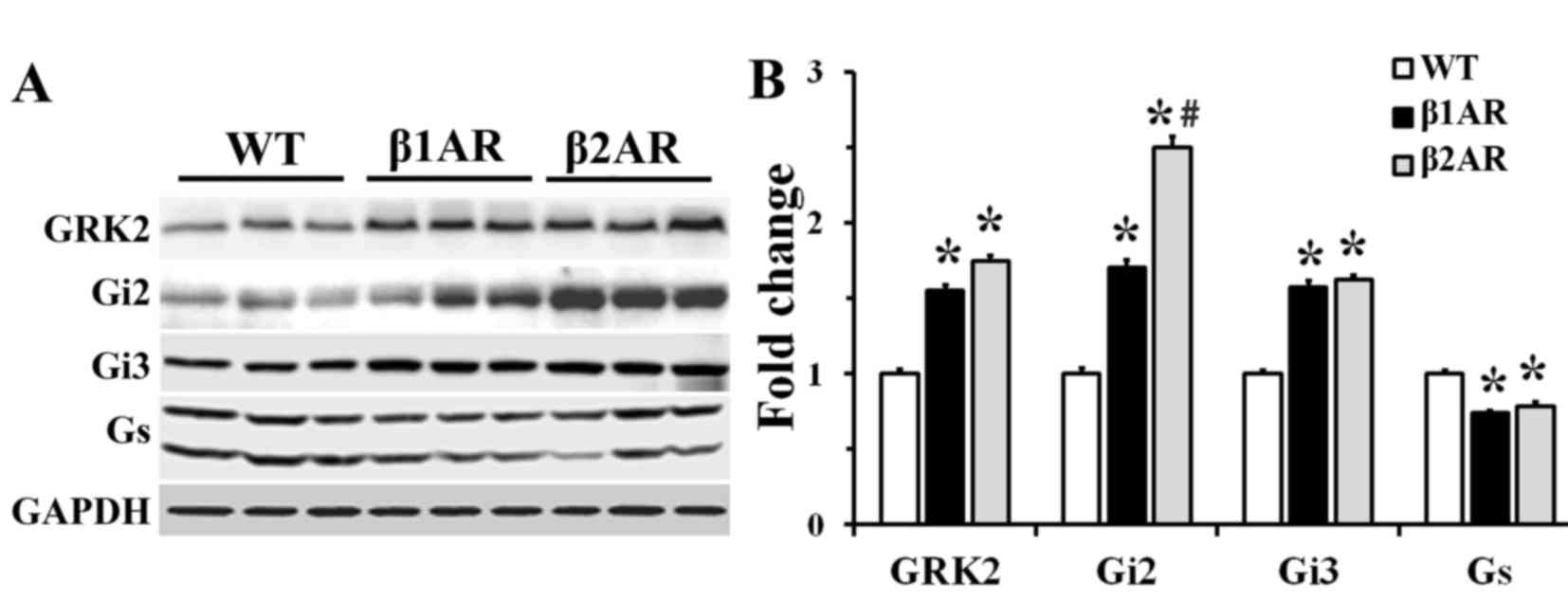 | Figure 2.GRK2, Gi2, Gi3 and Gs protein
expression in cardiac homogenates from WT, β1AR TG and
β2AR TG mouse hearts. (A) Western blot analysis of GRK2,
Gi2, Gi3 and Gs. (B) Quantification of western blot analysis;
protein expressions are normalized to GAPDH. Values are the mean ±
standard error of the mean. N=3 mice/group. *P<0.05, vs. WT
mice. #P<0.05 vs. β1AR TG mice. AR,
adrenergic receptor; Gi, inhibitory guanine nucleotide-binding
protein; GRK2, G-protein coupled receptor kinase 2; Gs, stimulatory
guanine nucleotide-binding protein; TG, transgenic; WT, wild
type. |
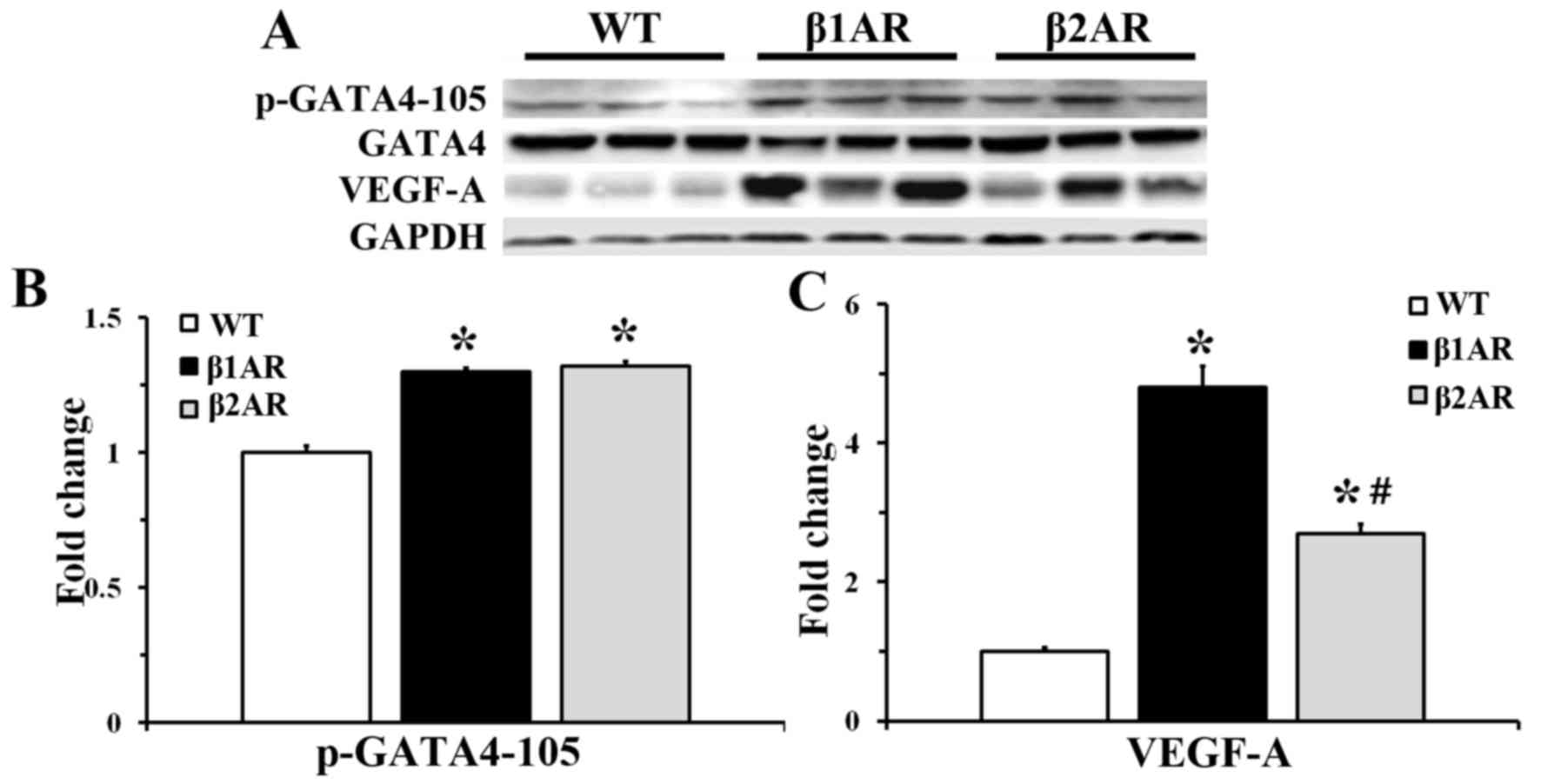
Expression levels of VEGF-A and
GATA4
Upregulation of VEGF-A expression was detected in
both β1AR and β2AR TG mice; however, the
β2AR TG mice exhibited a smaller increase compared with
β1AR TG mice. Furthermore, GATA4 serine 105
phosphorylation (p-GATA4) was similarly increased in both
β1 and β2AR TG mice, compared with the WT
group. Phosphorylation on serine 105 is an event known to augment
GATA4 activity. No significant difference in pGATA4 expression was
observed between the β1AR and β2AR TG mice
(Fig. 3).
Discussion
The present study examined physiological parameters
in an isolated work-performing heart experiment of β1AR
TG and β2AR TG mice. The results indicated that cardiac
contractility/relaxation and heart rate were stronger in the
β2AR TG mice, compared with the β1AR TG mice;
however, this difference was not significant. The reduced
hemodynamic functional response in the β1AR TG mouse
heart may reduce cardiac cellular stress and metabolic
expenditure.
The diameter of the cardiomyocytes was greater in
the βAR TG mice compared with the WT mice, indicating the presence
of hypertrophy. Previous studies reported that βAR signaling
induces cardiomyocyte apoptosis through a Gs-mediated PKA-dependent
mechanism (16–18), and β1- and
β2AR may exert different effects on cardiac apoptosis
(19,20), which may be due to the distinct
G-protein couplings of βAR subtypes (11). In the present study, GRK2, Gi2 and
Gi3 protein expression levels were significantly increased, whereas
Gs expression levels were significantly decreased in
β1AR and β2AR TG mice compared with WT.
Furthermore, Gi2 expression was significantly higher in the
β2AR TG mice, compared with the β1AR TG mice.
Foerster et al (21)
reported that, unlike β1AR, the effects of
β2AR overexpression varied at the level of Gi2 and Gi3
proteins; β2AR was associated with more prominent Gi2
upregulation and suggested that Gi2 may contribute to a longer
survival and delayed cardiac pathology in β2AR TG mice.
Furthermore, Gi2 upregulation may reduce the deleterious effects of
catecholamine signaling, contribute to several protective aspects
ascribed to β2AR/Gi coupling and reduce cardiac
responsiveness against a number of Gαq-protein-related pro-growth
factors (22). Based on these
data, it may be hypothesized that the coupling of β2AR
and Gi may provide negative feedback to the
β2AR/Gs-mediated cAMP signal, thus resulting in reduced
cardiac inotropy.
Previous research has indicated that the declined
hemodynamic response in the β2AR TG mouse heart would
result in reduced capillary growth (23). Furthermore, it has been suggested
that regulatory circuits might exist between catecholamine-induced
inotropy and VEGF expression in the heart, which adjusts
hemodynamic load to myocardial blood supply (12). Heineke et al demonstrated
that GATA4 directly regulates VEGF expression by binding to the
VEGF-A gene promoter and functions as a stress-responsive regulator
that coordinates angiogenesis following alterations to hemodynamic
load via non-hypoxic and load-responsive mechanisms (14). Based upon these findings, the
present study examined the expression levels of GATA4 and VEGF-A in
both βAR TG mouse hearts. Although cardiac contractility/relaxation
and heart rate were similarly increased in β1 and
β2AR TG mice, VEGF-A protein expression levels were
significantly upregulated in myocardial tissue isolated from
β1AR TG mice compared with in tissue from
β2AR TG mice. In addition, p-GATA4 protein expression
was similarly increased in myocardial samples from β1
and β2AR TG mice.
Therefore, the present study hypothesized that
VEGF-A-induced variation in angiogenesis may be associated with one
of the mechanisms that halts hypertrophic remodeling of the heart
in the β2AR TG mice. Notably, Tirziu et al
(13) reported that increased
endothelial cell mass and endothelial cell-cardiomyocyte
interactions stimulated hypertrophic growth, via releasing
paracrine factors, such as VEGF, from the vascular endothelium.
In conclusion, the present study demonstrated that
cardiac contractility/relaxation and heart rate was increased in
β1AR and β2AR TG mice, and that these
increases may be due to β2AR-mediated upregulation of Gi
protein expression and reduced upregulation of VEGF-A, in
comparison with β1AR TG mice.
Acknowledgements
The authors would like to thank Mr Seung Uk Lee
(Department of Neurobiology, School of Medicine, Kangwon National
University, Chuncheon, Republic of Korea) for his technical help in
this study. The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), the Ministry of Education (grant no. NRF-2014R1A1A2057263),
the Priority Research Centers Program (grant no. NRF-2009-0093812),
the National Research Foundation of Korea, the Ministry of Science,
ICT & Future Planning and the National Institutes of Health
(grant no. HL077101).
References
|
1
|
Cao S, Zeng Z, Wang X, Bin J, Xu D and
Liao Y: Pravastatin slows the progression of heart failure by
inhibiting the c-Jun N-terminal kinase-mediated intrinsic apoptotic
signaling pathway. Mol Med Rep. 8:1163–1168. 2013.
|
|
2
|
Sun JM, Wang CM, Guo Z, Hao YY, Xie YJ, Gu
J and Wang AL: Reduction of isoproterenol-induced cardiac
hypertrophy and modulation of myocardial connexin43 by a KATP
channel agonist. Mol Med Rep. 11:1845–1850. 2015.
|
|
3
|
Wang N, Cao Y and Zhu Y: Netrin-1 prevents
the development of cardiac hypertrophy and heart failure. Mol Med
Rep. 13:2175–2181. 2016.
|
|
4
|
Pereira SB, Velloso MW, Chermont S,
Quintão MM, Abdhala R Nunes, Giro C, Oliveira E, Alves T, Camacho
V, De Fátima Maia Contarato L, Pena FM, et al: β-adrenergic
receptor polymorphisms in susceptibility, response to treatment and
prognosis in heart failure: Implication of ethnicity. Mol Med Rep.
7:259–265. 2013.
|
|
5
|
Yu J, Yang S, Wang X and Gan R: Matrine
improved the function of heart failure in rats via inhibiting
apoptosis and blocking β3adrenoreceptor/endothelial nitric oxide
synthase pathway. Mol Med Rep. 10:3199–3204. 2014.
|
|
6
|
Liggett SB, Tepe NM, Lorenz JN, Canning
AM, Jantz TD, Mitarai S, Yatani A and Dorn GW II: Early and delayed
consequences of beta(2)-adrenergic receptor overexpression in mouse
hearts: Critical role for expression level. Circulation.
101:1707–1714. 2000. View Article : Google Scholar
|
|
7
|
Perez J Mialet, Rathz DA, Petrashevskaya
NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, Liggett SB, et al:
Beta 1-adrenergic receptor polymorphisms confer differential
function and predisposition to heart failure. Nat Med. 9:1300–1305.
2003. View Article : Google Scholar
|
|
8
|
Xiao RP, Zhu W, Zheng M, Chakir K, Bond R,
Lakatta EG and Cheng H: Subtype-specific beta-adrenoceptor
signaling pathways in the heart and their potential clinical
implications. Trends Pharmacol Sci. 25:358–365. 2004. View Article : Google Scholar
|
|
9
|
Zaugg M, Xu W, Lucchinetti E, Shafiq SA,
Jamali NZ and Siddiqui MA: Beta-adrenergic receptor subtypes
differentially affect apoptosis in adult rat ventricular myocytes.
Circulation. 102:344–350. 2000. View Article : Google Scholar
|
|
10
|
Xiao RP, Cheng H, Zhou YY, Kuschel M and
Lakatta EG: Recent advances in cardiac beta(2)-adrenergic signal
transduction. Circ Res. 85:1092–1100. 1999. View Article : Google Scholar
|
|
11
|
Hanft LM and McDonald KS: Sarcomere length
dependence of power output is increased after PKA treatment in rat
cardiac myocytes. Am J Physiol Heart Circ Physiol. 296:H1524–H1531.
2009. View Article : Google Scholar :
|
|
12
|
Fredriksson JM, Lindquist JM, Bronnikov GE
and Nedergaard J: Norepinephrine induces vascular endothelial
growth factor gene expression in brown adipocytes through a
beta-adrenoreceptor/cAMP/protein kinase a pathway involving src but
independently of Erk1/2. J Biol Chem. 275:13802–13811. 2000.
View Article : Google Scholar
|
|
13
|
Tirziu D, Chorianopoulos E, Moodie KL,
Palac RT, Zhuang ZW, Tjwa M, Roncal C, Eriksson U, Fu Q, Elfenbein
A, et al: Myocardial hypertrophy in the absence of external stimuli
is induced by angiogenesis in mice. J Clin Invest. 117:3188–3197.
2007. View
Article : Google Scholar :
|
|
14
|
Heineke J, Auger-Messier M, Xu J, Oka T,
Sargent MA, York A, Klevitsky R, Vaikunth S, Duncan SA, Aronow BJ,
et al: Cardiomyocyte GATA4 functions as a stress-responsive
regulator of angiogenesis in the murine heart. J Clin Invest.
117:3198–3210. 2007. View
Article : Google Scholar :
|
|
15
|
Lee JC, Kim IH, Cho GS, Park JH, Ahn JH,
Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, et al: Ischemic
preconditioning-induced neuroprotection against transient cerebral
ischemic damage via attenuating ubiquitin aggregation. J Neurol
Sci. 336:74–82. 2014. View Article : Google Scholar
|
|
16
|
Dorn GW II, Tepe NM, Lorenz JN, Koch WJ
and Liggett SB: Low- and high-level transgenic expression of
beta2-adrenergic receptors differentially affect cardiac
hypertrophy and function in Galphaq-overexpressing mice. Proc Natl
Acad Sci USA. 96:pp. 6400–6405. 1999; View Article : Google Scholar :
|
|
17
|
Du XJ, Autelitano DJ, Dilley RJ, Wang B,
Dart AM and Woodcock EA: beta(2)-adrenergic receptor overexpression
exacerbates development of heart failure after aortic stenosis.
Circulation. 101:71–77. 2000. View Article : Google Scholar
|
|
18
|
Du XJ, Gao XM, Jennings GL, Dart AM and
Woodcock EA: Preserved ventricular contractility in infarcted mouse
heart overexpressing beta (2)-adrenergic receptors. Am J Physiol
Heart Circ Physiol. 279:H2456–H2463. 2000.
|
|
19
|
Endoh M: Force-frequency relationship in
intact mammalian ventricular myocardium: Physiological and
pathophysiological relevance. Eur J Pharmacol. 500:73–86. 2004.
View Article : Google Scholar
|
|
20
|
Engelhardt S, Hein L, Dyachenkow V,
Kranias EG, Isenberg G and Lohse MJ: Altered calcium handling is
critically involved in the cardiotoxic effects of chronic
beta-adrenergic stimulation. Circulation. 109:1154–1160. 2004.
View Article : Google Scholar
|
|
21
|
Foerster K, Groner F, Matthes J, Koch WJ,
Birnbaumer L and Herzig S: Cardioprotection specific for the G
protein Gi2 in chronic adrenergic signaling through beta
2-adrenoceptors. Proc Natl Acad Sci USA. 100:pp. 14475–14480. 2003;
View Article : Google Scholar :
|
|
22
|
Petrashevskaya N, Gaume BR, Mihlbachler
KA, Dorn GW II and Liggett SB: Bitransgenesis with
beta(2)-adrenergic receptors or adenylyl cyclase fails to improve
beta(1)-adrenergic receptor cardiomyopathy. Clin Transl Sci.
1:221–227. 2008. View Article : Google Scholar
|
|
23
|
Xu Q, Jennings NL, Sim K, Chang L, Gao XM,
Kiriazis H, Lee YY, Nguyen MN, Woodcock EA, Zhang YY, et al:
Pathological hypertrophy reverses β2-adrenergic receptor-induced
angiogenesis in mouse heart. Physiol Rep. 3:pii: e123402015.
View Article : Google Scholar
|