Introduction
Tubulointerstitial fibrosis is a final common
pathway that leads to chronic kidney disease (CKD) progression
(1). Renal fibrosis is associated
with the prognosis of several kidney diseases, and suppression of
fibrosis progression may protect renal function. Renal interstitial
fibrosis is characterized by inflammatory cell infiltration,
interstitial fibroblast proliferation, tubular atrophy and
excessive accumulation of extracellular matrix (2). Several drugs currently used for other
disorders may also exert antifibrotic activity. Blockers of the
renin-angiotensin-aldosterone system and inhibitors of
3-hydroxy-3-methyl-glutaryl coenzyme A reductase have been
demonstrated to prevent fibrosis in cardiovascular and renal
diseases (3). While specific
targeting of drugs to kidney cells would be desirable, this
technology only exists at the preclinical stage and with limited
success (4). Therefore, drug
repositioning, i.e., reinvestigation of existing drugs for novel
therapeutic indications, has been a trending topic in drug
development (5).
Colchicine has been traditionally used for the
prevention and treatment of gout and acute gout, respectively
(6). The drug is known to exert
anti-inflammatory action by inhibiting migration and chemotaxis of
neutrophils and other motile cells via inhibition of microtubule
polymerization (7). Colchicine is
also used to treat Behçet's syndrome, primary biliary cirrhosis and
familial Mediterranean fever (8).
It is also beneficial for the treatment or prevention of
cardiovascular diseases, including pericarditis, postsurgical
atrial fibrillation and acute cardiovascular syndromes (8). Furthermore, colchicine has been
demonstrated to possess antifibrotic properties mediated by
suppressing collagen synthesis and secretion in hepatic and
pulmonary fibrosis experimental models (9,10).
Previous studies have demonstrated that colchicine prevents renal
injury in animal models of chronic cyclosporine nephrotoxicity
(11), anti-glomerular basement
membrane glomerulonephritis (12),
diabetic nephropathy (13) and
hypertensive CKD (14). Therefore,
previous evidence suggests that colchicine may serve as a
repositioned drug for the treatment of kidney diseases.
Unilateral ureteral obstruction (UUO) is a
well-established experimental model of renal tubulointerstitial
fibrosis (15). Several factors
are important in the process of UUO-mediated fibrosis, including
macrophage inflammation (16),
pre-inflammatory cytokine/chemokine production (17), angiotensin II (18) and fibroblast activation (19). The present study aimed to
investigate whether colchicine attenuates tubulointerstitial
fibrosis in a mouse model of UUO, and whether colchicine has
potential as a novel repositioned drug for the treatment of renal
fibrosis.
Materials and methods
Animals
Male C57BL/6 mice were purchased from the Central
Laboratory for Experimental Animals, Inc. (Osaka, Japan) and housed
in a temperature (22±2°C) and humidity (40±20%)-controlled room
under a 12 h light-dark cycle. The mice were given free access to
tap water and standard laboratory chow (MF; Oriental Yeast Co.,
Ltd., Tokyo, Japan). All animals were cared for in accordance with
the guidelines set forth by the Guide for the Care and Use of
Laboratory Animals 8th Edition (National Institutes of Health,
Bethesda, MD, USA), and the present study was approved by the
ethics committee of the Kawasaki Medical School (Kurashiki, Japan;
approval no. 13-051).
Experimental groups and protocol
Male mice (age, 8 weeks; weight, 23–28 g) were
randomly divided into two groups, vehicle-treated (n=8) and
colchicine-treated (n=14). Colchicine (0.5 mg/kg/day; cat. no.
C9754; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was diluted
into saline and administered by ALZET® osmotic pumps
(model no. 1004; Durect Corporation, Cupertino, CA, USA) over 3
weeks, which were implanted under the skin of the dorsal region.
The UUO procedure was conducted 7 days following the pump
insertion, by ligation of the left ureter as previously described
(18). The right, non-UUO-operated
kidneys were used as control samples. A total of 14 days following
UUO, the body weight was recorded, the mice were sacrificed, and
their kidneys were carefully extracted and weighed. Blood samples
were obtained at the time of death for measurement of serum
creatinine and serum alanine aminotransferase. The levels of
alanine aminotransferase and creatinine were measured by a
commercial laboratory at SRL, Inc. (Tokyo, Japan) using an
automatic inspection device (DRI-CHEM, FDC-700; Fujifilm
Corporation, Tokyo, Japan).
Histology and
immunohistochemistry
Kidney tissues were fixed in 4% paraformaldehyde
overnight at room temperature. Then, tissues were dehydrated in
graded alcohols and embedded in paraffin using standard techniques.
Kidney sections (4 µm thick) were prepared from paraffin-embedded
tissues. The sections were de-waxed, re-hydrated by standard
techniques and stained with Masson's trichrome stain. Images were
then captured using a BZ-9000 fluorescence microscope (Keyence
Corporation, Osaka, Japan). For immunohistochemistry,
deparaffinized serial sections were rehydrated in PBS and subjected
to antigen retrieval by microwave heating (500 W, 15 min). Sections
were washed with PBS and incubated in 0.3% hydrogen peroxide (in 1X
PBS) to block endogenous peroxidase activity, prior to further
incubation with the primary antibodies for 1 h at 37°C in a
humidified chamber. Primary antibodies against vimentin (dilution,
1:500; rabbit polyclonal; cat. no. sc-7557R; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and α-smooth muscle actin
(α-SMA; dilution, 1:100; rabbit polyclonal; cat. no. ab5694; Abcam,
Cambridge, UK) were used and detected using the Histofine Simple
Stain MAX-PO kit (Nichirei Corporation, Tokyo, Japan) and
3,3′-diaminobenzidine (Sigma-Aldrich; Merck KGaA). The severity of
tubulointerstitial injury was evaluated by examining 10 optical
fields in randomly selected tissue samples. Images of scarred areas
(stained blue with Masson's trichrome) and areas with positive
staining for vimentin and α-SMA (brown) were quantified using a
color image analyzer (Win ROOF version 5.6; Mitani Co., Fukui-city,
Fukui, Japan). Glomeruli, tubules and blood vessels of the cortex
were excluded. Results were presented as a % of the relative volume
of the scanned interstitium.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction and RT-qPCR were performed as
described previously (20).
Briefly, total RNA was isolated from kidneys using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), followed by digestion with DNase (Sigma-Aldrich;
Merck KGaA). cDNA was synthesized from total RNA (1 µg) using
Moloney murine leukemia virus reverse transcriptase (Thermo Fisher
Scientific, Inc.) with oligo (dT)12–18 as a primer
(Thermo Fisher Scientific, Inc.). Reverse transcription was
performed for 50 min at 37°C according to the manufacturer's
protocol (Thermo Fisher Scientific, Inc.). Primers and probes for
TaqMan analysis were designed using sequence information from
GenBank (National Institutes of Health) (21) and the Primer3 online software
(http://frodo.wi.mit.edu/primer3/;
accessed July 1, 2015). Primer and probe sequences are listed in
Table I. TaKaRa Premix Ex Taq
(Takara Bio, Inc., Otsu, Japan), with a final reaction volume of 20
ml, was used for the TaqMan probe-based RT-PCR reaction, which was
performed on a Applied Biosystems 7500 Fast Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following cycling conditions: 2 min at 50°C, 10 min at 95°C, 55
cycles of 15 sec at 95°C and 1 min at 60°C. The level of mRNA
expression in each sample was quantified using the absolute
quantification standard curve method (22). Plasmid cDNA of each gene was used
to prepare absolute standards. The concentration was measured using
the A260, which was converted to the number of copies
using the molecular weight of the DNA. Each mRNA expression levels
were normalized to those of the housekeeping 18S ribosomal RNA
gene.
 | Table I.Sequences of primers and probes used
for quantitative polymerase chain reaction. |
Table I.
Sequences of primers and probes used
for quantitative polymerase chain reaction.
| Gene | Accession number | Primer and TaqMan
probe sequences (5′-3′) |
|---|
| Fibronectin | NM_010233 | Forward primer:
ATGATGAGGTGCACGTGTGT |
|
|
| Reverse primer:
TGACGCTTGTGGAACGTGT |
|
|
| TaqMan probe:
FAM-TCGTGGAGAATGGGCATGCA-TAMRA |
| α1 type I
collagen | NM_007742 | Forward primer:
TGTGCGATGACGTGCAAT |
|
|
| Reverse primer:
TTGGGTCCCTCGACTCCTAC |
|
|
| TaqMan probe:
FAM-ACTGGACTGTCCCAACCCCCAAAG-TAMRA |
| E-cadherin | NM_010217 | Forward primer:
CGTGTACCCAGGTCTCAGAAG |
|
|
| Reverse primer:
TTGTTTCTTTGTCCCTGTTGG |
|
|
| TaqMan probe:
FAM-ACGAGACTGGGTCATCCCTCCCAT-TAMRA |
| 18S rRNA | NR_003278 | Forward primer:
CCTGCGGCTTAATTTGACTC |
|
|
| Reverse primer:
GACAAATCGCTCCACCAACT |
|
|
| TaqMan probe: FAM-
TCTTTCTCGATTCCGTGGGTGGTG -TAMRA |
Cell culture
NRK-49F cells, a fibroblastic clone of normal rat
kidney cells, were purchased from the Japanese Collection of
Research Bioresources Cell Bank (Osaka, Japan), The cells were
cultured in Dulbecco's modified Eagle's medium supplemented with 5%
fetal bovine serum (FBS), 1% non-essential amino acids and 1%
penicillin/streptomycin (all Sigma-Aldrich; Merck KGaA). Confluent
cells at passages 4–10 were used in subsequent experiments.
Cell viability and lactate
dehydrogenase (LDH) assays
Effects of colchicine on cell viability were
evaluated using the Premix
(2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
sodium salt (WST-1) Cell Proliferation Assay system (cat. no.
MK400; Takara Bio, Inc.). LDH levels (an indicator of cell injury)
were measured using the LDH Cytotoxicity Detection kit (cat. no.
MK401; Takara Bio, Inc.). These assays were conducted according to
the manufacturer's protocols. Cells (1×103 cells) were
seeded in 96-well plates and were co-incubated with 100 nM
angiotensin II (cat. no. A9525; Sigma-Aldrich; Merck KGaA) and 0–10
nM colchicine (cat. no. C9754; Sigma-Aldrich; Merck KGaA) for 24 h,
prior to performing the WST-1 or LDH assays.
Ras homolog gene family member A
(RhoA) activity assay
RhoA activity was assessed using the RhoA G-LISA
Activation Assay Biochem kit (cat. no. BK121; Cytoskeleton, Inc.,
Denver, CO, USA) according to the manufacturer's protocol. Cells
were incubated with 0 to 10 nM colchicine at 37°C for 30 min,
followed by treatment with 100 nM angiotensin II. The cells were
collected for analysis 12 min post-stimulation. Cells were lysed
with lysis buffer containing Tris pH 7.5, MgCl2, NaCl,
nonionic detergent and SDS. Cell lysates were then incubated in a
Rho-GTP affinity plate with binding buffer for 30 min at 4°C prior
to further incubation with the anti-RhoA primary antibody for 45
min at room temperature. Following plate washing with kit wash
buffer, the appropriate secondary antibody was added and incubated
at room temperature for 45 min. Following incubation, the plate was
washed with kit wash buffer again then the mixed horseradish
peroxidase detection reagent was added into each well. The
luminescence signals were detected using a microplate luminescence
reader (23). All antibodies and
reagents used for the RhoA activity assay were provided by the
Cytoskeleton, Inc. kit.
Wound-healing assay
Cells were grown to confluence in 10 cm plates, and
a sterile 200 µl pipette tip was used to scratch the cell layer.
The cells were washed twice with PBS, after which medium
supplemented with 100 nM angiotensin II, 0.5% FBS and 0–10 nM
colchicine was added. An inverted microscope (IX81N; Olympus
Corporation, Tokyo, Japan) was used to assess the cells at 0 and 20
h. Cell migration rates (%) were calculated using the following
formula: [(0 h scratch area-20 h scratch area)/0 h scratch
area]x100.
Actin fiber staining
Actin fibers were stained with Acti-stain 555
phalloidin (cat. no. PHDH1-A; Cytoskeleton, Inc.). Fibroblasts were
cultured as aforementioned prior to the wound-healing assay, and
actin staining was performed according to the manufacturer's
protocol. Briefly, the culture medium was removed, and the cells
were gently washed with PBS prior to fixation with paraformaldehyde
(3.7%) for 10 min. The cells were washed again with PBS,
permeabilized with 0.5% Triton X-100 for 5 min, washed, and stained
with phalloidin for 30 min in the dark at room temperature. Nuclei
were stained with Hoechst 33342 (cat. no. H3570; Thermo Fisher
Scientific, Inc.), and images of the cells were captured using a
BZ-9000 fluorescence microscope (Keyence Corporation).
Statistical analysis
In vitro experiments were performed in
duplicate and independently repeated three times. Data are
expressed as the mean ± standard error of the mean. Statistical
significance was evaluated using a two-tailed unpaired Student's
t-test for comparisons between two groups, or one-way analysis of
variance with Tukey-Kramer post hoc tests for comparisons among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Colchicine treatment attenuates
fibrosis in UUO kidneys
No significant renal or liver dysfunction was
observed in the UUO-operated animals, as indicated by measurements
of serum creatinine and alanine aminotransferase, respectively
(Table II). In the UUO kidneys,
fibrosis was observed mainly in the interstitium surrounding
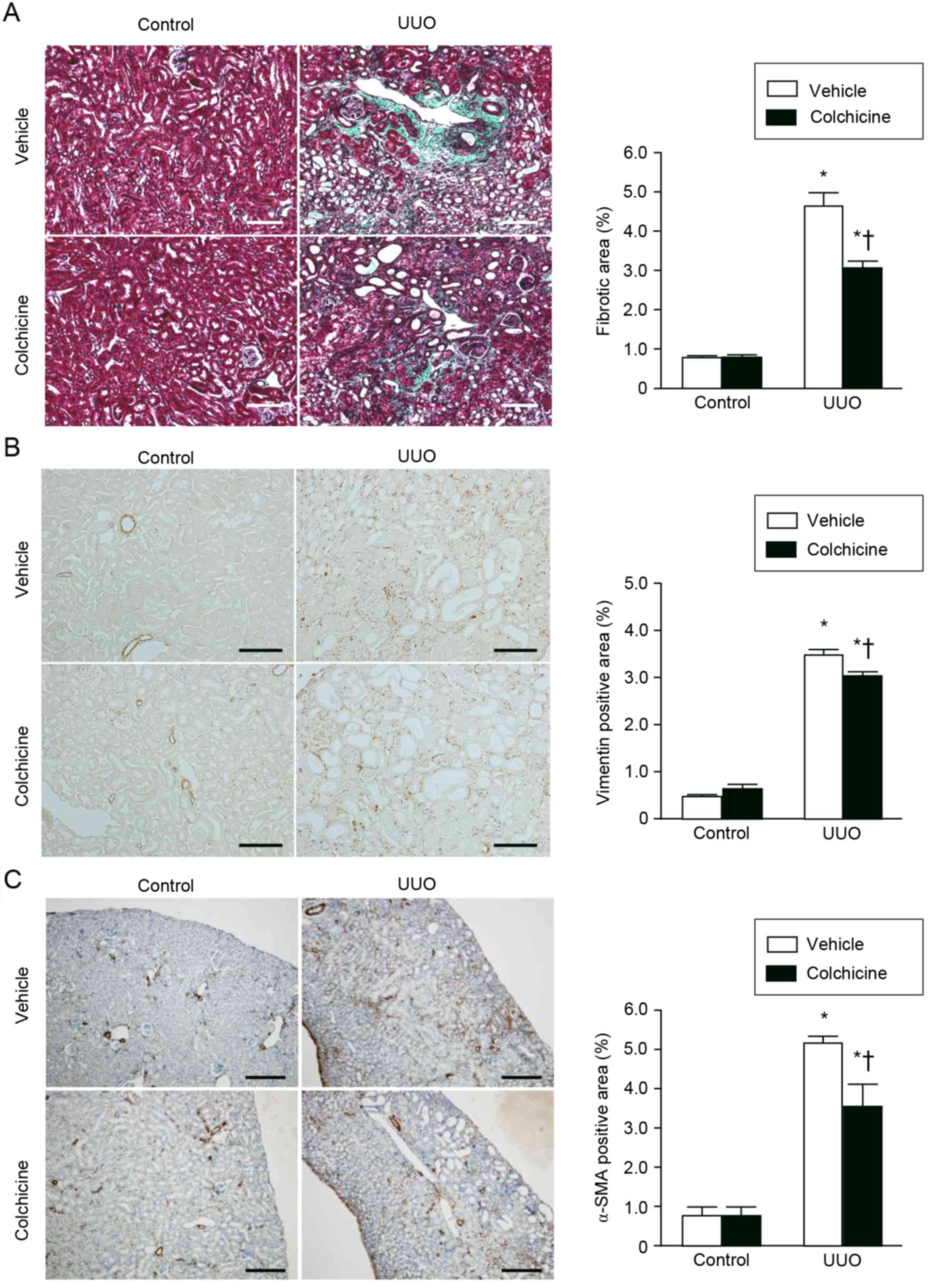
atrophic or dilated tubules (Fig.
1A). Masson's trichrome staining demonstrated that colchicine
treatment significantly suppressed renal interstitial fibrosis
compared with the vehicle treatment (Fig. 1A). The number of interstitial cells
expressing vimentin, a mesenchymal marker, was significantly
increased in the UUO kidneys compared with the control kidneys;
however, this effect was attenuated by colchicine treatment
(Fig. 1B). Expression of the
myofibroblast marker α-SMA was also significantly increased in the
UUO kidneys compared with the control kidneys, and this
UUO-mediated increase was also attenuated by colchicine treatment
(Fig. 1C).
 | Table II.Physical and biochemical
characteristics of mice on day 14 following UUO. |
Table II.
Physical and biochemical
characteristics of mice on day 14 following UUO.
| Group | UUO+Vehicle
(n=8) | UUO+Colchicine
(n=14) | P-value |
|---|
| Body weight (g) | 26.0±0.6 | 25.2±0.5 | 0.32 |
| Serum ALT (IU/l) | 20.1±0.7 | 21.6±3.0 | 0.70 |
| Serum Cr
(µmol/l) | 11.9±0.6 | 12.4±0.5 | 0.60 |
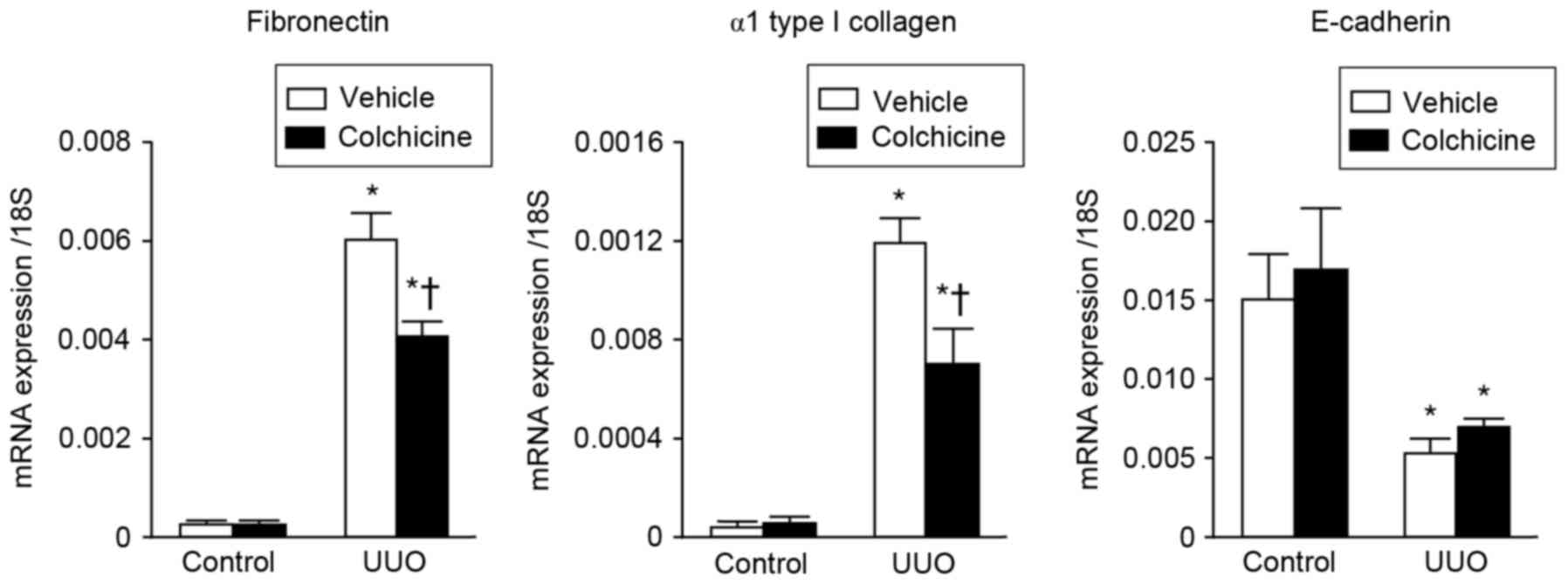
The effects of colchicine treatment on the mRNA
expression levels of profibrotic genes fibronectin and α1 type I
collagen were examined in the UUO and control kidneys. The results
demonstrated that the expression levels of fibronectin and α1 type
I collagen were higher in the UUO kidneys compared with the control
kidneys (Fig. 2). However,
expression of these genes in the UUO kidneys was significantly
attenuated by colchicine treatment compared with the vehicle
treatment (Fig. 2). In addition,
the mRNA expression levels of the epithelial adhesion molecule
E-cadherin were decreased in the UUO kidneys compared with the
control kidneys; however, its expression was not affected by
colchicine treatment (Fig. 2).
Colchicine treatment reduces RhoA
activity in NRK-49F cells
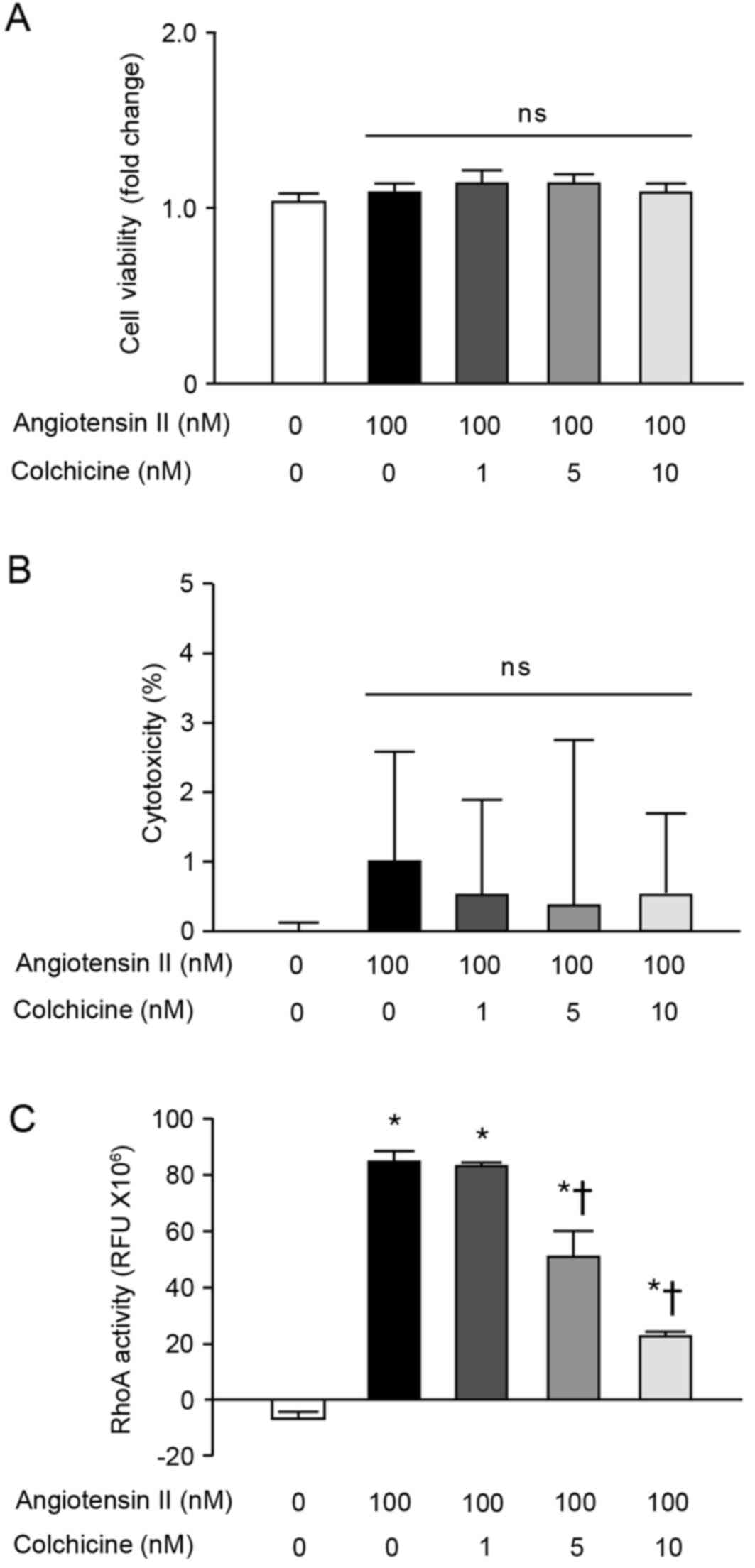
The effects of colchicine on normal fibroblast
activities were further examined in vitro, by measuring the
cell viability and RhoA activity of NRK-49F normal rat kidney
fibroblast cells. Angiotensin II, colchicine, or co-stimulation
with both, had no significant effects on the viability (Fig. 3A) or death (Fig. 3B) of NRK-49F cells. RhoA activity
in NRK-49F cells was significantly increased by angiotensin II
stimulation (Fig. 3C); however,
colchicine treatment significantly suppressed this
angiotensin-II-mediated RhoA activity in a dose-dependent manner
(Fig. 3C).
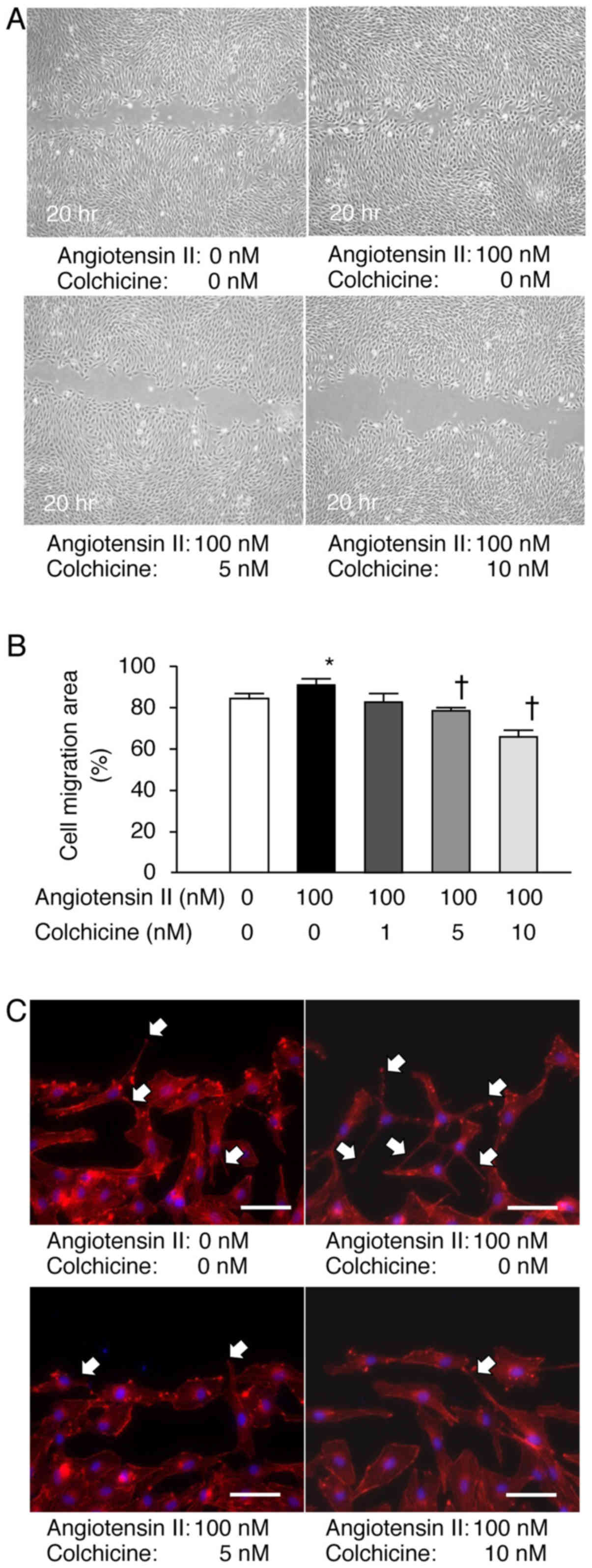
Colchicine treatment suppresses
angiotensin II-induced NRK-49F cell migration
Cells were stimulated with angiotensin II in the
presence or absence of colchicine and migration was examined in
vitro 20 h after wounding. Colchicine treatment inhibited the
NRK-49F cell migration in a concentration-dependent manner
(Fig. 4A and B). When cells were
stained with fluorescent phalloidin for visualization of actin
fibers, formation of filopodia was upregulated in the NRK-49F cells
treated with angiotensin II, whereas colchicine treatment
suppressed this effect (Fig.
4C).
Discussion
The present study investigated the therapeutic
potential of colchicine in UUO-induced renal fibrosis and
demonstrated that colchicine treatment ameliorated renal fibrosis
and fibrogenic gene expression in mouse UUO kidneys. In addition,
in in vitro experiments colchicine treatment inhibited
angiotensin II-induced RhoA activation and reduced fibroblast
migration. These findings indicated that colchicine may provide a
protective effect against kidney fibrosis by suppressing migration
of renal fibroblasts.
Mechanisms of existing drugs are continuously being
investigated at a molecular level, and identification of novel uses
for existing drugs (drug repositioning) has gained popularity
(5). Colchicine is a
microtubule-depolymerizing drug that has been used to treat
gout-associated arthritis (6),
Behçet's syndrome (24), primary
biliary cirrhosis (25) and
familial Mediterranean fever (26). In the present study, colchicine
pretreatment was effective for the prevention of renal fibrosis in
mice, suggesting that colchicine may be used in the future as a
repositioned drug for the treatment of kidney diseases. In a
clinical situation however, given the well-known toxicity of
colchicine, it is unlikely that colchicine administration will be
considered preventively. Therefore, further studies are required to
investigate whether colchicine treatment is also effective for the
treatment of renal fibrosis following UUO.
Clinically, appropriate blood concentrations for
treatment with colchicine range between 0.5 and 3.0 ng/ml (27). The therapeutic range is narrow, and
poisoning is dose-dependent. Gastrointestinal symptoms, including
diarrhea, nausea or abdominal pain, are frequently observed in
patients, and liver dysfunction or bone marrow suppression are less
frequent but severe side effects. In the present study, liver
dysfunction and renal damage were not observed in the mice
following colchicine administration. The colchicine concentrations
used in the in vitro experiments were based on the safe
blood concentrations, and the LDH assay revealed that the drug was
not cytotoxic in NRK-49F normal renal fibroblasts at these
concentrations. The present findings suggested that the beneficial
effects of colchicine could be expected even when used at low
doses.
In the present study, colchicine was demonstrated to
inhibit fibroblast migration in vitro without affecting cell
viability, and the inhibitory effect observed was postulated to be
due to the suppression of RhoA activity. RhoA is a small GTPase
that regulates the formation of stress fibers through Rho kinase
(28). Stress fibers create
cellular tension and induce rear retraction in cells, a necessary
step of cell migration (29).
Inhibition of stress fiber formation therefore abolishes the
migratory capacity of cells. Both inhibition or activation of Rho
decrease cell migration (30),
indicating that dynamic Rho regulation is important for cell
migration. An association between Rho/Rho-kinase signaling and
renal fibrosis has previously been reported (31), and colchicine has been demonstrated
to attenuate renal fibrosis via RhoA inhibition in a 5/6
nephrectomized rat model of hypertensive CKD (14). In the present study, the inhibitory
effects of colchicine on RhoA/Rho-kinase activation observed in
renal fibroblasts further confirmed that this signaling pathway may
be important for attenuation of renal fibrosis.
Interstitial recruitment of myofibroblasts, which
are responsible for the excessive production and deposition of
extracellular matrix during renal fibrosis, occurs at an early
stage in UUO kidneys (32).
Activated fibroblasts or myofibroblasts have diverse origins,
including residential fibroblasts, vascular pericytes, bone marrow
cells, and epithelial-mesenchymal transition (EMT) (33). Loss of E-cadherin expression is one
of the markers of EMT (34). In
the present study, E-cadherin mRNA expression was decreased in UUO
kidneys compared with in control kidneys; however, colchicine
treatment did not reverse this effect. Therefore, these results
indicated that colchicine may have no effect on EMT following
UUO.
Although the UUO model is an established renal
fibrosis model and suitable for testing the antifibrotic activity
of novel compounds, the efficacy of colchicine in other renal
fibrosis models needs to be investigated further. In addition, a
limitation of the present study is that it did not assess whether
colchicine may attenuate fibrosis following UUO (as it was
administered in the mice as a pretreatment); therefore, it remains
unclear whether colchicine treatment may be effective against
advanced fibrosis. Further studies are required to evaluate the
efficacy of colchicine in other systems and the effects of
colchicine administration following UUO surgery. Although several
aspects require further investigation, the present study provides
valuable information regarding the effects of colchicine therapy on
CKD progression toward fibrosis. The recent results may be used as
the basis for developing more effective therapies against renal
fibrosis in the future.
Acknowledgements
The present study was supported in part by a
Research Project Grant from Kawasaki Medical School (grant no. 27
Dai-7) and by scholarship donations from Pfizer Academic
Contributions, Astellas Research Support and Chugai Pharmaceutical.
The authors would like to thank Ms. Etsuko Yorimasa and Ms. Tomoko
Taira (Kawasaki Medical School) for animal care and Ms. Satomi
Hanada, Ms. Keiko Satoh and Ms. Yoshiko Shirakiya (Kawasaki Medical
School) for help with in vitro experiments. This manuscript
has been edited and corrected by an experienced proofreader who is
a native speaker of English and who is under direct supervision of
Honyaku Center, Inc. (Osaka, Japan).
Glossary
Abbreviations
Abbreviations:
|
CKD
|
chronic kidney disease
|
|
UUO
|
unilateral ureteral obstruction
|
|
α-SMA
|
α-smooth muscle actin
|
|
LDH
|
lactate dehydrogenase
|
|
RhoA
|
Ras homolog gene family member A
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar :
|
|
2
|
Zeisberg M and Neilson EG: Mechanisms of
tubulointerstitial fibrosis. J Am Soc Nephrol. 21:1819–1834. 2010.
View Article : Google Scholar
|
|
3
|
Boor P, Sebekova K, Ostendorf T and Floege
J: Treatment targets in renal fibrosis. Nephrol Dial Transplant.
22:3391–3407. 2007. View Article : Google Scholar
|
|
4
|
Ramos AM, Gonzalez-Guerrero C, Sanz A,
Sanchez-Niño MD, Rodríguez-Osorio L, Martín-Cleary C,
Fernández-Fernández B, Ruiz-Ortega M and Ortiz A: Designing drugs
that combat kidney damage. Expert Opin Drug Discov. 10:541–556.
2015. View Article : Google Scholar
|
|
5
|
Ashburn TT and Thor KB: Drug
repositioning: Identifying and developing new uses for existing
drugs. Nat Rev Drug Discov. 3:673–683. 2004. View Article : Google Scholar
|
|
6
|
Nuki G: Colchicine: Its mechanism of
action and efficacy in crystal-induced inflammation. Curr Rheumatol
Rep. 10:218–227. 2008. View Article : Google Scholar
|
|
7
|
Hastie SB: Interactions of colchicine with
tubulin. Pharmacol Ther. 51:377–401. 1991. View Article : Google Scholar
|
|
8
|
Slobodnick A, Shah B, Pillinger MH and
Krasnokutsky S: Colchicine: Old and new. Am J Med. 128:461–470.
2015. View Article : Google Scholar
|
|
9
|
Rodríguez L, Cerbón-Ambriz J and Muñoz ML:
Effects of colchicine and colchiceine in a biochemical model of
liver injury and fibrosis. Arch Med Res. 29:109–116. 1998.
|
|
10
|
Ledwozyw A: The effect of colchicine and
vinblastine on bleomycin-induced lung fibrosis in rats. Acta
Physiol Hung. 82:383–389. 1994.
|
|
11
|
Disel U, Paydas S, Dogan A, Gulfiliz G and
Yavuz S: Effect of colchicine on cyclosporine nephrotoxicity,
reduction of TGF-beta overexpression, apoptosis, and oxidative
damage: An experimental animal study. Transplant Proc.
36:1372–1376. 2004. View Article : Google Scholar
|
|
12
|
McClurkin C Jr, Phan SH, Hsu CH, Patel SR,
Spicker JK, Kshirsagar AM, Yuan WY and Wiggins RC: Moderate
protection of renal function and reduction of fibrosis by
colchicine in a model of anti-GBM disease in the rabbit. J Am Soc
Nephrol. 1:257–265. 1990.
|
|
13
|
Li JJ, Lee SH, Kim DK, Jin R, Jung DS,
Kwak SJ, Kim SH, Han SH, Lee JE, Moon SJ, et al: Colchicine
attenuates inflammatory cell infiltration and extracellular matrix
accumulation in diabetic nephropathy. Am J Physiol Renal Physiol.
297:F200–F209. 2009. View Article : Google Scholar
|
|
14
|
Guan T, Gao B, Chen G, Chen X, Janssen M,
Uttarwar L, Ingram AJ and Krepinsky JC: Colchicine attenuates renal
injury in a model of hypertensive chronic kidney disease. Am J
Physiol Renal Physiol. 305:F1466–F1476. 2013. View Article : Google Scholar
|
|
15
|
Klahr S and Pukerson ML: The
pathophysiology of obstructive nephropathy: The role of vasoactive
compounds in the hemodynamic and structural abnormalities of the
obstructed kidney. Am J Kidney Dis. 23:219–223. 1994. View Article : Google Scholar
|
|
16
|
Diamond JR, Kees-Folts D, Ding G, Frye JE
and Restrepo NC: Macrophages, monocyte chemoattractant peptide-1
and TGF-beta 1 in experimental hydronephrosis. Am J Physiol.
266:F926–F933. 1994.
|
|
17
|
Kaneto H, Morrissey J and Klahr S:
Increased expression of TGF-beta 1 mRNA in the obstructed kidney of
rats with unilateral ureteral ligation. Kidney Int. 44:313–321.
1993. View Article : Google Scholar
|
|
18
|
Satoh M, Kashihara N, Yamasaki Y, Maruyama
K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K and
Makino H: Renal interstitial fibrosis is reduced in angiotensin II
type 1a receptor-deficient mice. J Am Soc Nephrol. 12:317–325.
2001.
|
|
19
|
Du F, Li S, Wang T, Zhang HY, Li DT, Du ZX
and Wang HQ: Implication of Bcl-2-associated athanogene 3 in
fibroblast growth factor-2-mediated epithelial-mesenchymal
transition in renal epithelial cells. Exp Biol Med (Maywood).
240:566–575. 2015. View Article : Google Scholar :
|
|
20
|
Satoh M, Fujimoto S, Horike H, Ozeki M,
Nagasu H, Tomita N, Sasaki T and Kashihara N: Mitochondrial
damage-induced impairment of angiogenesis in the aging rat kidney.
Lab Invest. 91:190–202. 2011. View Article : Google Scholar
|
|
21
|
Clark K, Karsch-Mizrachi I, Lipman DJ,
Ostell J and Sayers EW: GenBank. Nucleic Acids Res. 44:D67–D72.
2016. View Article : Google Scholar
|
|
22
|
Dhanasekaran S, Doherty TM and Kenneth J;
TB Trials Study Group, : Comparison of different standards for
real-time PCR-based absolute quantification. J Immunol Methods.
354:34–39. 2010. View Article : Google Scholar
|
|
23
|
Bolick DT, Whetzel AM, Skaflen M, Deem TL,
Lee J and Hedrick CC: Absence of the G protein-coupled receptor G2A
in mice promotes monocyte/endothelial interactions in aorta. Circ
Res. 100:572–580. 2007. View Article : Google Scholar
|
|
24
|
Yurdakul S, Mat C, Tüzün Y, Ozyazgan Y,
Hamuryudan V, Uysal O, Senocak M and Yazici H: A double-blind trial
of colchicine in Behçet's syndrome. Arthritis Rheum. 44:2686–2692.
2001. View Article : Google Scholar
|
|
25
|
Kaplan MM and Gershwin ME: Primary biliary
cirrhosis. N Engl J Med. 353:1261–1273. 2005. View Article : Google Scholar
|
|
26
|
La Regina M, Ben-Chetrit E, Gasparyan AY,
Livneh A, Ozdogan H and Manna R: Current trends in colchicine
treatment in familial Mediterranean fever. Clin Exp Rheumatol. 31(3
Suppl 77): S41–S46. 2013.
|
|
27
|
Deftereos S, Giannopoulos G, Papoutsidakis
N, Panagopoulou V, Kossyvakis C, Raisakis K, Cleman MW and
Stefanadis C: Colchicine and the heart: Pushing the envelope. J Am
Coll Cardiol. 62:1817–1825. 2013. View Article : Google Scholar
|
|
28
|
Murali A and Rajalingam K: Small Rho
GTPases in the control of cell shape and mobility. Cell Mol Life
Sci. 71:1703–1721. 2014. View Article : Google Scholar
|
|
29
|
Pellegrin S and Mellor H: Actin stress
fibres. J Cell Sci. 120:3491–3499. 2007. View Article : Google Scholar
|
|
30
|
Allen WE, Zicha D, Ridley AJ and Jones GE:
A role for Cdc42 in macrophage chemotaxis. J Cell Biol.
141:1147–1157. 1998. View Article : Google Scholar :
|
|
31
|
Takeda Y, Nishikimi T, Akimoto K, Matsuoka
H and Ishimitsu T: Beneficial effects of a combination of
Rho-kinase inhibitor and ACE inhibitor on tubulointerstitial
fibrosis induced by unilateral ureteral obstruction. Hypertens Res.
33:965–973. 2010. View Article : Google Scholar
|
|
32
|
Picard N, Baum O, Vogetseder A, Kaissling
B and Le Hir M: Origin of renal myofibroblasts in the model of
unilateral ureter obstruction in the rat. Histochem Cell Biol.
130:141–155. 2008. View Article : Google Scholar :
|
|
33
|
Mack M and Yanagita M: Origin of
myofibroblasts and cellular events triggering fibrosis. Kidney Int.
87:297–307. 2015. View Article : Google Scholar
|
|
34
|
Zeisberg M and Kalluri R: The role of
epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med
(Berl). 82:175–181. 2004. View Article : Google Scholar
|