Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease characterized by inflammatory cell infiltration,
progressive destruction of cartilage and bone, and synovial cell
hyperplasia and hypertrophy (1).
RA patients typically experience joint pain, stiffness and
functional disability in the early morning h (2). Patients with chronic inflammatory
diseases exhibit disrupted circadian rhythms (3,4).
Several studies have reported a bi-directional
interaction between inflammation and the circadian clock (5–7).
Immune system performance is significantly affected by disruption
of the circadian clock (6), and
the cellular expression of core clock genes directly alters
inflammation (7). This phenomenon
may negatively impact the pathogenesis of RA. In addition,
disturbances in the circadian clock have serious effects on a
number of diseases, including immune-mediated disorders of the
brain (7), infections (8,9),
cardiovascular disease and sleep disorders (10). The molecular mechanisms underlying
circadian rhythm regulation involve an interplay between feedback
and feed-forward transcriptional loops including clock genes, such
as circadian locomotor output cycles kaput (CLOCK), brain
and muscle ARNT like-1 (BMAL1), rar-related orphan receptor
α, deleted in esophageal cancer-1 and -2, cryptochrome
(CRY)-1 and -2, nuclear receptor subfamily 1
group D member 1 and period (PER)-1, -2, and -3 (11–14),
which alter the expression of a number of clock-controlled genes
(15).
Amongst the clock genes, period 2 (PER2) is
located on the long arm of chromosome 2 at position 37.3, and
encompasses 25 exons encoding PER2 proteins, which are key
molecular components in controlling mammalian circadian rhythms at
the level of gene expression, physiology and pathology (16). PER2 inhibits transcriptional
activation of CLOCK/BMAL1 in vitro (17,18)
by binding to enhancer-box motifs in their respective promoters
(19). PER proteins are
phosphorylated by several isoforms of casein kinase 1 in a complex
manner, which regulates their degradation and nuclear trafficking
(20). In addition, PER1 and PER2
form stable complexes with the casein kinases and either of the CRY
proteins (17,21). PER proteins are the rate-limiting
component for this step, and are necessary for nuclear import of
the complex; they serve as shuttles for nuclear CRY proteins
(22). Nuclear CRY and PER
proteins inhibit the activity of the heterodimeric BMAL1-CLOCK
complex (BCC), potentially via different mechanisms (17), thereby terminating four negative
feedback loops and regulating the expression of CRY and
PER genes. PER2 serves a role in the positive
regulation of aryl hydrocarbon receptor nuclear translocator like
(ARNTL, also known as BMAL1) expression (23). In humans, a single mutation in
PER2 causes familial advanced sleep phase syndrome (24), and its loss causes arhythmicity in
mice (25,26). The behavioral phenotypes of
Per1-null mutant mice are similar to those of Per2
mutants; however, comparison of the molecular consequences of these
mutations revealed significant differences between the two.
Disruption of Per2 expression was reported to result in
reduced transcription levels of further clock genes, whereas
Per1 appeared to function predominantly at the
posttranscriptional level (26).
Previous studies have suggested that the circadian
rhythm is associated with cortisol levels; cortisol levels are
highest in the early morning immediately after awakening, whereas
they are low at around midnight (27,28).
In addition, the circadian clock gene PER2, which is
generated in the suprachiasmatic nucleus of the hypothalamus
(29), is associated with the
hypothalamic-pituitary-adrenal axis, stress (30), and neuroendocrine-immunologic
pathways, which are relevant to rheumatic diseases (30,31).
Based on these observations, the present study aimed to investigate
the association between polymorphisms in the PER2 gene in
Korean RA patients, and to determine the expression levels of PER2
in synovial RA cells during lipopolysaccharide (LPS)-induced
inflammation.
Materials and methods
Subjects
A case-control study was conducted to determine the
genetic association between PER2 single nucleotide
polymorphisms (SNPs) and RA. A total of 256 unrelated patients with
RA (age, 50.47±12.85 years; male/female, 47/209) were enrolled
between January and February 2008 from the rheumatic center of
Kyung Hee University Hospital (Seoul, Korea). Each patient was
diagnosed by a rheumatologist according to ACR 1987 Rheumatoid
Arthritis diagnostic criteria (32). A total of 499 control subjects
(age, 46.05±12.67; male/female, 215/284) that participated in a
general health checkup program of Kyung Hee University Hospital
were recruited. Patients with diabetes (fasting blood sugar >120
mg), hypertension (systolic blood pressure >140 mm Hg and/or
diastolic blood pressure >90 mm Hg), dyslipidemia (total
cholesterol >200 mg/dl and triglyceride levels >150 mg/dl),
obesity (body mass index (BMI) >30 kg/m2), smoking or
previous history of smoking 5 years ago, postmenopausal women,
evidence of cardio vascular disease or family history of coronary
heart disease were excluded from the present study. This study was
performed in accordance with the guidelines set forth by the
Declaration of Helsinki, and written informed consent was obtained
from all subjects. This study was approved by the ethics review
committee of the Medical Research Institute, School of Medicine,
Kyung Hee University. Demographic data were obtained from patient
medical records or through interviews at the time of enrollment.
Disease activity was determined on the basis of the following
biochemical parameters: C-reactive protein (CRP), erythrocyte
sedimentation rate (ESR) and titer of rheumatoid factor (RF).
X-rays of the hands and feet were obtained from all patients and
radiographic findings were used to classify patients with bone
erosion.
Human cartilage samples were obtained from 3 female
healthy individuals (age, 42.33±12.06 years; weight, 65.00±12.53
kg), 3 female patients with osteoarthritis (OA; age, 52.67±7.51
years; weight, 68.33±6.43 kg) and 3 female patients with RA (age,
49.66±7.64 years; weight, 60.00±9.85 kg) at the Soonchunhyang
University Hospital (Cheonan, Korea) between December 2011 and
November 2012. The study protocol was approved by the Institutional
Review Board of the Soonchunhyang University College of Medicine.
Written informed consent was obtained from all subjects prior to
enrollment.
SNP genotyping
PER2 SNPs were identified using National
Center for Biotechnology Information websites (www.ensembl.org; www.ncbi.nlm.nih.gov/SNP; and www.hapmap.org). A total of 3 PER2 SNPs were
selected for analysis, as previously described (33,34).
The three selected SNPs consisted of one nonsynonymous SNP
(rs934945) and two intronic SNPs (rs2304674 and rs6754875). Blood
samples were drawn from all subjects following overnight fasting.
DNA was isolated from whole blood samples of each subject using the
GenEx™ Blood kit (cat. no. 220-301; GeneAll Biotechnology, Co.,
Ltd., Seoul, Korea), according to the manufacturer's instructions.
PER2 SNPs were genotyped according to a previously described
method (35). Genomic DNA was
amplified by polymerase chain reaction (PCR) using primers for each
SNP. Oligonucleotide primers of PER2 were the following: rs934945,
sense 5′-GACTTCTGGGAGCACTG GG-3′, antisense
3′-CGTGTTAGCCAGGAAGGTCT-5′; rs6754875, sense
5′-TTGTCATGGCAGCTGTCTCT-3′, antisense 3′-TAGGGGAGAAAACCAGGAGA-5′;
and rs2304674, sense 5′-TTGTCATGGCAGCTGTCTCT-3′ and antisense
3′-TAGGGGAGAAAACCAGGAGA-5′. The PCR products were sequenced using
an ABI PRISM 3730xl DNA analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The sequence data were
analyzed using SeqManII software version 6.1 (DNASTAR, Inc.,
Madison, WI, USA).
Cell culture and treatment
Human articular cartilage was sliced and washed in
serum-free Dulbecco's modified Eagle's medium (DMEM; WELGENE, Inc.,
Gyeongsan, Korea) containing D-glucose, L-glutamine, sodium
pyruvate and sodium bicarbonate, prior to digestion with 0.1%
collagenase (Invitrogen; Thermo Fisher Scientific, Inc.) for 3 h at
37°C. Undigested fragments were removed by filtration of the
solution through a nylon mesh (70 µm mesh size; BD Biosciences,
Franklin Lakes, NJ, USA). Isolated cells were washed three times
with PBS (pH 7.4), centrifuged at 211 × g for 10 min at room
temperature and then resuspended in serum-free DMEM (WELGENE,
Inc.). Subsequently, cells were incubated in DMEM supplemented with
20% fetal bovine serum (FBS; WELGENE, Inc.) and containing
D-glucose, L-glutamine, sodium pyruvate, sodium bicarbonate, 100
U/ml penicillin and 100 µg/ml streptomycin (WELGENE, Inc.) for 4
days at 37°C in a 5% CO2 atmosphere, until they reached
70–80% confluency. The morphological features and the expression
levels of type II collagen and aggrecan were consistent with a
chondrocytic phenotype. The cells were passaged upon reaching
confluence by gentle trypsinization; cells were used for
experiments between passage 4 and 8. Following stimulation with LPS
(10 µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), the cells
were collected at 12 and 24 h.
Western blot analysis
RA and normal synovial cells were cultured in 10-cm
culture dishes to ~80% confluence (1×106 cells/well) and
were serum-starved in DMEM without FBS for 24 h. The cells were
subsequently incubated for a further 12 or 24 h in the presence of
LPS. Cells were lysed in NP40 buffer (ELPIS-Biotech, Inc., Daejeon,
Korea) containing 1 mM PMSF protease inhibitor. Protein
concentration was measured using a colorimetric Bio-Rad Protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amounts of protein (50 µg) were separated by 12% SDS-PAGE, and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% skimmed
milk, membranes were probed with anti-PER2 (dilution, 1:1,000; cat.
no. ab64460; Abcam, Cambridge, UK) or anti-β-actin (dilution,
1:1,000; cat. no. sc-81178; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) antibodies overnight at 4°C. Subsequently, the membrane
was washed in TBS containing 0.1% Tween-20, and incubated with the
following horseradish peroxidase-conjugated secondary antibodies
for 1 h at room temperature: Anti-mouse immunoglobulin (Ig) G
(dilution 1:10,000; cat. no. A9044; Sigma-Aldrich; Merck KGaA) or
anti-rabbit IgG (dilution, 1:2,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.). Protein bands were visualized using the
WesternBright™ enhanced chemiluminescence kit (Advansta, Inc.,
Menlo Park, CA, USA). The images were captured using the ChemiDoc™
XRS+imaging system (Bio-Rad Laboratories, Inc.). Protein bands were
quantified using ImageJ image analysis software version, 1.40
(National Institutes of Health, Bethesda, MD, USA). Experiments
were performed in triplicate.
Statistical analysis
The Hardy-Weinberg equilibrium (HWE) was assessed
using SNPStats (https://www.snpstats.net/snpstats/start.htm?q=snpstats/start.htm).
SNPStats and SNPAnalyzer Pro version 1.0 (Istech Corp., Goyang,
Korea) were also used to evaluate the odds ratios (ORs), 95%
confidence intervals (CIs), and P-values. Multiple logistic
regression analysis, adjusted for age and gender as covariables,
was performed. In the logistic regression analysis for each SNP,
models were used that assumed the following: Co-dominant
inheritance, in which the relative hazard differs between subjects
with 1 minor allele and those with 2 minor alleles; dominant
inheritance, in which subjects with 1 or 2 minor alleles have the
same relative hazard; or recessive inheritance, in which subjects
with 2 minor alleles are at increased risk for the disease.
Bonferroni correction (Pc) was applied by multiplying
the P-values by the number of SNPs (n=3). The χ2 test
was used to compare allele frequencies between groups. To avoid
coincidental findings due to multiple testing, a Bonferroni
correction was applied by decreasing the significance level to
P=0.01 (P=0.05/5) for each of the three SNPs. Western blotting
results are presented as the mean ± standard deviation and/or
standard error of the mean. Differences between groups were
compared using the Student's t-test. Statistical analysis of
western blotting results was performed using IBM SPSS software
version, 19.0 (IBM SPSS, Armonk, NY USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Subject characteristics
The clinical and demographic characteristics of the
RA patients and control subjects are presented in Table I. The mean age (± standard
deviation) of the RA patients and the control subjects was
50.47±12.85 and 46.05±12.67 years, respectively. There were 47 male
and 209 female (n=256) RA patients and 215 male and 284 female
(n=499) control subjects. RA patients were classified into clinical
subgroups according to ESR level (≥30 or <30 mm/h), CRP level
(≥0.5 or <0.5 mg/dl), and the presence or absence of RF and bone
erosion. A total of 160 RA patients (62.5%) presented with ESR
levels of ≥30 mm/h and 96 (37.5%) with an ESR level of <30 mm/h.
A total of 182 patients with RA (71.09%) exhibited CRP levels of
≥0.5 mg/dl and 74 (28.91%) patients displayed a CRP level of
<0.5 mg/dl. There were 219 RA patients (85.54%) with and 37
(14.46%) without RF. Bone erosion was present in 134 (52.34%) and
absent in 122 (47.66%) RA patients.
 | Table I.Clinical and demographic features of
the RA and control subjects. |
Table I.
Clinical and demographic features of
the RA and control subjects.
| Characteristic | No. of
patients |
|---|
| RA subjects | 256 |
| Age
(years, mean ± SD) | 50.47±12.85 |
| Gender
(male/female) |
47/209 |
| ESR
(mm/h, mean ± SD) | 42.98±29.19 |
| ESR
(≥30/<30 mm/h) | 160/96 |
| CRP
(mg/dl, mean ± SD) | 2.41±5.21 |
| CRP
(≥0.5/<0.5 mg/dl) | 182/74 |
| RF
(positive/negative) | 219/37 |
| Bone
erosion (positive/negative) |
134/122 |
| Control
subjects | 499 |
| Age
(years, mean ± SD) | 46.05±12.67 |
| Gender
(male/female) |
215/284 |
SNP genotype distributions
The genotype distributions of all SNPs were in HWE
(P>0.05). As shown in Table
II, out of the 3 SNPs, rs2304674 alone was statistically
associated with RA in the codominant [OR=0.68 (0.47), 95% CI:
0.48–0.96 (0.25–0.87), P=0.0089, Pc=0.0267] and dominant
model (OR=0.63, 95% CI: 0.46–0.87, P=0.0044, Pc=0.0132)
after Bonferroni correction. In the codominant model, the
respective TT and CC genotype frequencies were 55.3 and 10.1% in
the control group and 66.1 and 5.7% in the RA group (Table III). The CC genotype was
associated with a decreased risk of RA (Table II). In the dominant model,
genotypes containing the C allele (CC/TC) and not containing the C
allele (TT) made up 44.7 and 55.3% in the control group, and 33.9
and 66.1% in the RA group, respectively (Table III). The rs2304674 allele was
significantly associated with RA (OR=2.02, 95% CI: 1.55–2.63,
P<0.001, Pc<0.001; Table II). The rs2304674 T allele
frequency was higher in the RA (80.2%) when compared with the
control group (66.8%; Table
III). The frequency of the rs6754875 allele was loosely
associated with the development of RA. The C allele of rs6754875
was less prevalent in the RA group (24.8%) than in the control
group (30.1%; Table III);
however, the difference was not significant following Bonferroni
correction (Table II). The
association between the 3 SNPs and the clinical characteristics of
the RA patients was then assessed and included ESR, CRP, RF and
bone erosion parameters. However, no significant differences were
observed in these factors among the subgroups (data not shown).
 | Table II.Genetic models of three SNPs
associated with RA. |
Table II.
Genetic models of three SNPs
associated with RA.
| A, rs934945 (AA, AG
and GG alleles) |
|---|
|
|---|
| Model | OR (95% CI) | P-value |
Pc-value |
|---|
| Co-dominant | 1 | 0.17 | 0.51 |
|
| 1.18
(0.85–1.64) |
|
|
|
| 1.57
(0.98–2.52) |
|
|
| Dominant | 1.26
(0.93–1.72) | 0.14 | 0.42 |
| Recessive | 1.45
(0.93–2.26) | 0.11 | 0.33 |
| Overdominant | 1.06
(0.78–1.45) | 0.69 | 1 |
| A or G alleles | 0.80
(0.64–1.00) | 0.05 | 0.16 |
|
| B, rs6754875 (AA,
AC and CC alleles) |
|
| Model | OR (95% CI) | P-value |
Pc-value |
|
| Co-dominant | 1 | 0.09 | 0.26 |
|
| 0.71
(0.51–0.99) |
|
|
|
| 0.69
(0.40–1.20) |
|
|
| Dominant | 0.70
(0.52–0.96) | 0.03 | 0.08 |
| Recessive | 0.79
(0.46–1.35) | 0.39 | 1 |
| Overdominant | 0.75
(0.62–0.99) | 0.04 | 0.12 |
| A or C alleles | 1.31
(1.02–1.67) | 0.03 | 0.10 |
|
| C, rs2304674 (TT,
TC and CC alleles) |
|
| Model | OR (95% CI) | P-value |
Pc-value |
|
| Co-dominant | 1 | 0.01 | 0.03 |
|
| 0.68
(0.48–0.96) |
|
|
|
| 0.47
(0.25–0.87) |
|
|
| Dominant | 0.63
(0.46–0.87) | 0.004 | 0.01 |
| Recessive | 0.53
(0.29–0.98) | 0.04 | 0.11 |
| Overdominant | 0.74
(0.53–1.04) | 0.08 | 0.23 |
| T or C alleles | 2.02
(1.55–2.63) | <0.001 | <0.001 |
 | Table III.Genotype and allele frequencies of
PER2 SNPs identified in RA and control subjects. |
Table III.
Genotype and allele frequencies of
PER2 SNPs identified in RA and control subjects.
| A, rs934945 |
|---|
|
|---|
|
Genotype/allele | Control frequency
(%) | RA frequency
(%) |
|---|
| AA | 55 (11.1) | 38 (15.3) |
| AG | 206 (41.6) | 107 (43.1) |
| GG | 234 (47.3) | 103 (41.5) |
| Total A | 316 (31.9) | 184 (36.9) |
| Total G | 674 (68.1) | 314 (63.1) |
|
| B, rs6754875 |
|
|
Genotype/allele | Control frequency
(%) | RA frequency
(%) |
|
| AA | 235 (50.2) | 146 (58.9) |
| AC | 184 (39.3) | 81 (32.7) |
| CC | 49 (10.5) | 21 (8.5) |
| Total A | 654 (69.9) | 373 (75.2) |
| Total C | 282 (30.1) | 123 (24.8) |
|
| C, rs2304674 |
|
|
Genotype/allele | Control frequency
(%) | RA frequency
(%) |
|
| TT | 268 (55.3) | 164 (66.1) |
| TC | 168 (34.6) | 70 (28.2) |
| CC | 49 (10.1) | 14 (5.7) |
| Total T | 536 (66.8) | 398 (80.2) |
| Total C | 266 (33.2) | 98 (19.8) |
Expression of PER2 in RA synovial
cells during LPS-induced inflammatory response
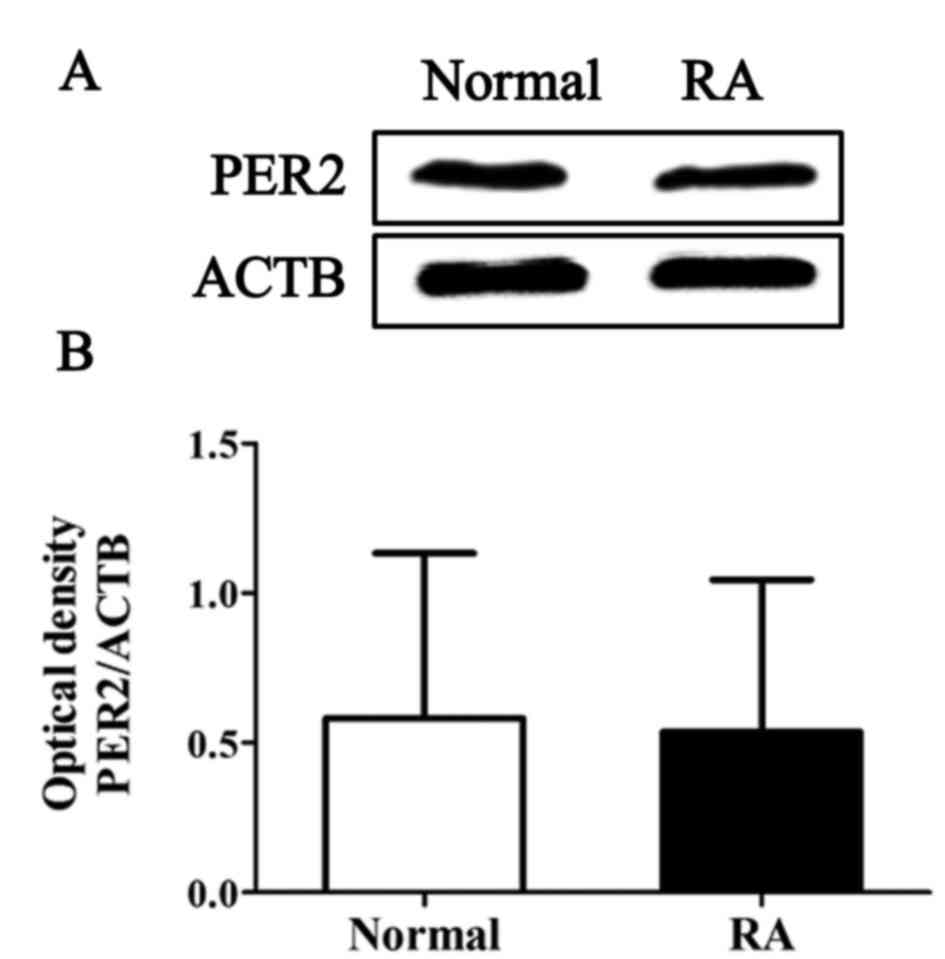
The protein expression levels of PER2 in normal and
RA synovial cells were examined by western blot analysis. The
results demonstrated that the expression levels of PER2 were not
significantly altered in RA synovial cells when compared with
control cells under normal physiological conditions (Fig. 1). By contrast, the expression of
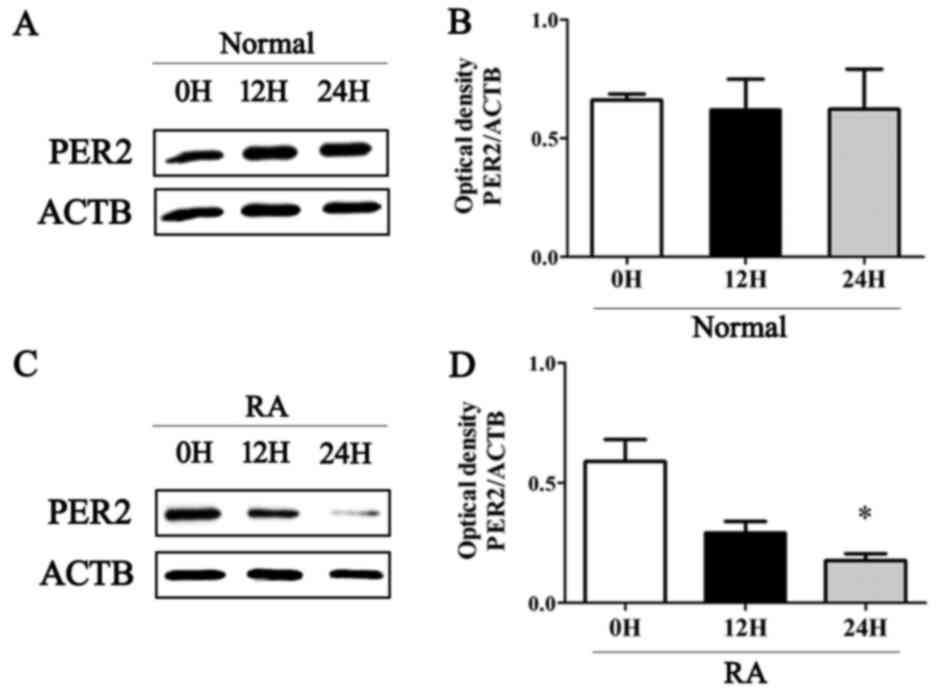
PER2 was significantly decreased in RA synoviocytes following LPS
stimulation for 24 h, whereas normal synovial cells were unaffected
(Fig. 1). The protein levels of
PER2 decreased in a time-dependent manner in RA synovial cells
(Fig. 2).
Discussion
The purpose of the present study was to evaluate the
association between genetic polymorphisms in PER2 and
susceptibility to RA, and to compare the protein expression levels
of PER2 in normal and RA synoviocytes. An association between
specific SNPs of the PER2 gene and RA was observed; the
rs2304674 SNP of the PER2 gene was associated with RA, with
the CC genotype associated with a decreased risk of RA. In
addition, immunoblotting was performed to assess PER2 expression in
RA synovial cells compared with normal cells. The results revealed
that PER2 expression decreased following LPS treatment for 12 and
24 h in RA cells, whereas no significant effect of LPS treatment on
PER2 expression in control cells was observed.
Previous studies have demonstrated that LPS modifies
the biological clock (5,6) via alterations in the expression of
several circadian clock genes, including Per2 (36). It has been suggested that LPS
impacts on the timing of the circadian rhythm by altering the
levels of proinflammatory cytokines in the brain (36), and thus affects rheumatic diseases
(37). Immune signaling molecules
affect circadian rhythms; however, the circadian system in turn
regulates the immune system. A number of immune markers have been
implicated in circadian regulation, including interleukin (IL)-2,
IL-10, granulocyte-macrophage colony stimulating factor, C-C motif
chemokine receptor 2, IL-6, IL-1β, tumor necrosis factor (TNF)-α,
monocyte chemotacticprotein-1, interferon (IFN)-γ and IFN receptors
(38,39). In addition, genetic manipulations
of circadian timing modulate innate immunity. A previous study
demonstrated that the daily rhythm of IFN-γ mRNA and protein
expression was absent in Per2-mutant mice (40). Furthermore, these mice were
deficient in their ability to produce IL-10 and IFN-γ in response
to LPS (41). Notably, macrophages
display endogenous rhythms in clock gene expression (39,42),
phagocytosis (43) and LPS
sensitivity (44).
In a previous study, Hashiramoto et al
(43) investigated the association
between mammalian clock genes and arthritis using knockout animals
and collagen-induced arthritis animal models. The authors examined
whether the daily expression of clock genes in the synovial cells
of foot joints was altered by the induction of arthritis using a
mixture of anti-type II collagen monoclonal antibodies and LPS. In
naive C57/BL6 mouse joints, daily expression of nuclear PER2 was
lower during the daylight (8:00 a.m.) and higher at night (8:00
p.m.), whereas in arthritic joints, PER2 was expressed even during
daylight (8:00 a.m.). Induction of arthritis resulted in a 6 h
retrograde shift in Per1/2 mRNA expression. The authors
suggested that normal circadian gene expression profiles are
significantly disturbed in arthritic conditions (43). In the study, an influence of
arthritis on clock gene expression was reported in wild-type mice
that were administered with an anti-collagen antibody and LPS,
following the assessment of PER2 protein levels in the synovium
(42). They found that PER2 is
usually expressed at night; however, in the arthritis model PER2
was highly expressed in the morning. In addition, the phase of
Per1 and Per2 mRNA expression in spleen lymphocytes
was shifted back ~6 h, and overall Bmal1, Per1, and
Per2 mRNA expression levels were reduced. Furthermore, the
authors observed that TNF-α inhibited the expression of PER2 in RA
fibroblast-like synoviocytes, and it was suggested that the onset
of arthritis may impact on the expression of clock genes in
vivo (44). Decreased
expression of PER2 by TNF-α may additionally contribute to the
resistance of synovial cells to apoptosis, and may contribute to
tumor-like growth of the synovium. In agreement with previous
studies, the expression of PER2 observed in the present study was
similar between control and RA synoviocytes under normal
conditions; however, expression was decreased in RA synoviocytes
following induction of the inflammatory response.
The present study was the first to investigate the
potential effect of PER2 SNPs and PER2 expression in RA. The
results indicate that PER2 polymorphisms may contribute to
increased RA susceptibility via alterations in PER2 protein
expression. PER2 may be one of several genes that serve a
role in polygenic susceptibility to RA. Due to the relatively small
number of subjects in the current study, these findings must be
validated by future studies using larger sample sizes. In addition,
a substantial difference was present in the sex ratio of the study
population. Sex and age were adjusted for during all statistical
analyses; however, this inconsistency is a limitation of the
current study and further validation is required. Future
investigations should employ in vitro or animal models to
further elucidate the role of PER2 in RA.
Acknowledgements
The present study was supported by the Cooperative
Research Program for Agriculture Science and Technology Development
(project no. PJ011582) of the Rural Development Administration of
Korea.
References
|
1
|
Lee DM and Weinblatt ME: Rheumatoid
arthritis. Lancet. 358:903–911. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haas S and Straub RH: Disruption of
rhythms of molecular clocks in primary synovial fibroblasts of
patients with osteoarthritis and rheumatoid arthritis, role of
IL-1β/TNF. Arthritis Res Ther. 14:R1222012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Straub RH and Cutolo M: Circadian rhythms
in rheumatoid arthritis: Implications for pathophysiology and
therapeutic management. Arthritis Rheum. 56:399–408. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gibbs JE and Ray DW: The role of the
circadian clock in rheumatoid arthritis. Arthritis Res Ther.
15:2052013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keller M, Mazuch J, Abraham U, Eom GD,
Herzog ED, Volk HD, Kramer A and Maier B: A circadian clock in
macrophages controls inflammatory immune responses. Proc Natl Acad
Sci USA. 106:21407–21412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castanon-Cervantes O, Wu M, Ehlen JC, Paul
K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT and
Davidson AJ: Dysregulation of inflammatory responses by chronic
circadian disruption. J Immunol. 185:5796–5805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coogan AN and Wyse CA: Neuroimmunology of
the circadian clock. Brain Res. 1232:104–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Segall LA, Perrin JS, Walker CD, Stewart J
and Amir S: Glucocorticoid rhythms control the rhythm of expression
of the clock protein, Period2, in oval nucleus of the bed nucleus
of the stria terminalis and central nucleus of the amygdala in
rats. Neuroscience. 140:753–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burioka N, Fukuoka Y, Takata M, Endo M,
Miyata M, Chikumi H, Tomita K, Kodani M, Touge H, Takeda K, et al:
Circadian rhythms in the CNS and peripheral clock disorders:
Function of clock genes: Influence of medication for bronchial
asthma on circadian gene. J Pharmacol Sci. 103:144–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Young ME: The circadian clock within the
heart: Potential influence on myocardial gene expression,
metabolism, and function. Am J Physiol Heart Circ Physiol.
290:H1–H16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reppert SM and Weaver DR: Coordination of
circadian timing in mammals. Nature. 418:935–941. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roenneberg T and Merrow M: The network of
time: Understanding the molecular circadian system. Curr Biol.
13:R198–R207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunlap JC: Molecular bases for circadian
clocks. Cell. 96:271–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young MW and Kay SA: Time zones: A
comparative genetics of circadian clocks. Nat Rev Genet. 2:702–715.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto T, Nakahata Y, Soma H, Akashi M,
Mamine T and Takumi T: Transcriptional oscillation of canonical
clock genes in mouse peripheral tissues. BMC Mol Biol. 5:182004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vukolic A, Antic V, Van Vliet BN, Yang Z,
Albrecht U and Montani JP: Role of mutation of the circadian clock
gene PER2 in cardiovascular circadian rhythms. Am J Physiol Regul
Integr Comp Physiol. 298:R627–R634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akashi M, Tsuchiya Y, Yoshino T and
Nishida E: Control of intracellular dynamics of mammalian period
proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in
cultured cells. Mol Cell Biol. 22:1693–1703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin X, Shearman LP, Weaver DR, Zylka MJ,
de Vries GJ and Reppert SM: A molecular mechanism regulating
rhythmic output from the suprachiasmatic circadian clock. Cell.
96:57–68. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cavadini G, Petrzilka S, Kohler P, Jud C,
Tobler I, Birchler T and Fontana A: TNF-alpha suppresses the
expression of clock genes by interfering with E-box-mediated
transcription. Proc Natl Acad Sci USA. 104:12843–12848. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vanselow K, Vanselow JT, Westermark PO,
Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A and
Kramer A: Differential effects of PER2 phosphorylation: Molecular
basis for the human familial advanced sleep phase syndrome (FASPS).
Genes. 20:2660–2672. 2006. View Article : Google Scholar
|
|
21
|
Dunlap JC, Loros JJ and DeCoursey PT:
Biological timekeeping 1st edition. Sunderland (Massachusetts):
Sinauer Associates, Chronobiology; pp. 4062004
|
|
22
|
Lee C, Etchegaray JP, Cagapang FR, Loudon
AS and Reppert SM: Posttranslational mechanisms regulate the
mammalian circadian clock. Cell. 107:855–867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reppert SM and Weaver DR: Molecular
analysis of mammalian circadian rhythms. Annu Rev Physiol.
63:647–676. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toh KL, Jones CR, He Y, Eide EJ, Hinz WA,
Virshup DM, Ptácek LJ and Fu YH: An hPer2 phosphorylation site
mutation in familial advanced sleep phase syndrome. Science.
291:1040–1043. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng B, Larkin DW, Albrecht U, Sun ZS,
Sage M, Eichele G, Lee CC and Bradley A: The mPer2 gene encodes a
functional component of the mammalian circadian clock. Nature.
400:169–173. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bae K, Jin X, Maywood ES, Hastings MH,
Reppert SM and Weaver DR: Differential functions of mPer1, mPer2,
and mPer3 in the SCN circadian clock. Neuron. 30:525–536. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mottonen T, Hannonen P, Leirisalo-Repo M,
Nissilä M, Kautiainen H, Korpela M, Laasonen L, Julkunen H,
Luukkainen R, Vuori K, et al: Comparison of combination therapy
with single-drug therapy in early rheumatoid arthritis: A
randomised trial. FIN-RACo trial group. Lancet. 353:1568–1573.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreland LW, Baumgartner SW, Schiff MH,
Tindall EA, Fleischmann RM, Weaver AL, Ettlinger RE, Cohen S,
Koopman WJ, Mohler K, et al: Treatment of rheumatoid arthritis with
a recombinant human tumor necrosis factor receptor (p75)-Fc fusion
protein. N Engl J Med. 337:141–147. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nader N, Chrousos GP and Kino T:
Interactions of the circadian CLOCK system and the HPA axis. Trends
Endocrinol Metab. 21:277–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kowanko IC, Knapp MS, Pownall R and
Swannell AJ: Domiciliary self-measurement in the rheumatoid
arthritis and the demonstration of circadian rhythmicity. Ann Rheum
Dis. 41:453–455. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cutolo M and Straub RH: Circadian rhythms
in arthritis: Hormonal effects on the immune/inflammatory reaction.
Autoimmu Rev. 7:223–228. 2008. View Article : Google Scholar
|
|
32
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Forbes EE, Dahl RE, Almeida JR, Ferrell
RE, Nimgaonkar VL, Mansour H, Sciarrillo SR, Holm SM, Rodriguez EE
and Phillips ML: PER2 rs2304672 polymorphism moderates
circadian-relevant reward circuitry activity in adolescents. Biol
Psychiatry. 71:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HJ, Kim L, Kang SG, Yoon HK, Choi JE,
Park YM, Kim SJ and Kripke DF: PER2 variation is associated with
diurnal preference in a Korean young population. Behav Genet.
41:273–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HK, Lee WY, Kwon JT, Sohn DR, Hong SJ
and Kim HJ: Association of ultraviolet radiation
resistance-associated gene polymorphisms with rheumatoid arthritis.
Biomed Rep. 2:117–121. 2013.PubMed/NCBI
|
|
36
|
Kwak Y, Lundkvist GB, Brask J, Davidson A,
Menaker M, Kristensson K and Block GD: Interferon-gamma alters
electrical activity and clock gene expression in suprachiasmatic
nucleus neurons. J Biol Rhythms. 23:150–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davis MC, Zautra AJ, Younger J, Motivala
SJ, Attrep J and Irwin MR: Chronic stress and regulation of
cellular markers of inflammation in rheumatoid arthritis:
Implications for fatigue. Brain Behav Immun. 22:24–32. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takane H, Ohdo S, Baba R, Koyanagi S,
Yukawa E and Higuchi S: Relationship between 24-hour rhythm in
antiviral effect of interferon-beta and interferon-alpha/beta
receptor expression in mice. Jpn J Pharmacol. 90:304–312. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayashi M, Shimba S and Tezuka M:
Characterization of the molecular clock in mouse peritoneal
macrophages. Biol Pharm Bull. 30:621–626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arjona A and Sarkar DK: The circadian gene
mPer2 regulates the daily rhythm of IFN-gamma. J Interferon
Cytokine Res. 26:645–649. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Malkani G, Shi X, Meyer M,
Cunningham-Runddles S, Ma X and Sun ZS: The circadian clock Period
2 gene regulates gamma interferon production of NK cells in host
response to lipopolysaccharide-induced endotoxic shock. Infect
Immun. 74:4750–4756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Keller M, Mazuch J, Abraham U, Eom GD,
Herzog ED, Volk HD, Kramer A and Maier B: A circadian clock in
macrophages controls inflammatory immune responses. Proc Natl Acad
Sci USA. 106:21407–21412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashiramoto A, Yamane T, Tsumiyama K,
Yoshida K, Komai K, Yamada H, Yamazaki F, Doi M, Okamura H and
Shiozawa S: Mammalian clock gene Cryptochrome regulates arthritis
via proinflammatory cytokine TNF-alpha. J Immunol. 184:1560–1565.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yoshida K, Hashimoto T, Sakai Y and
Hashiramoto A: Involvement of the circadian rhythm and inflammatory
cytokines in the pathogenesis of rheumatoid arthritis. J Immunol
Res. 2014:2824952014. View Article : Google Scholar : PubMed/NCBI
|