Introduction
It is assumed that most peripheral tissues have at
least a limited ability for self-repair however, the central
nervous system (CNS) is known to be relatively resistant to
regeneration (1). In general,
although neural stem cells (NSCs) have the potential to generate a
large number of specific neural phenotypes in vitro,
including neurons, neurogliocytes and Schwann cells, NSCs do not
participate in neuropathy plerosis (2). In addition, a small number of stem
cells have been demonstrated to reprogramme to an alternative
differential fate to become a neuron (3). The majority of differentiation
follows a unidirectional and irreversible route, therefore the
development of novel approaches to generate neurons from non-neural
lineages are required. This would have important implications for
the study of neural development, neurological disease modelling and
regenerative medicine.
A specialized quiescent population of outer root
sheath cells (ORSCs), residing in the hair follicle, has exhibited
pluripotency for differentiation into epithelial-mesenchymal
lineage cells (4). A previous
study indicated that human ORSCs have the potential for developing
non-invasive treatments for skin disorders (5). Due to improved culture techniques,
ORSCs can be induced to develop highly differentiated epidermal
equivalents (6). Notably, it has
been reported that implantation of multipotent nestin-positive hair
follicle stem cells promotes the repair of spinal cord and
peripheral nerves (7–9). However, little has been reported on
the isolation of nestin-negative human ORSCs and whether they can
successfully differentiate into nerve cells in vitro. The
aim of the present study was to isolate nestin-negative ORSCs from
adult healthy volunteers and investigate the neuronal
differentiation of these cells.
Materials and methods
Cell isolation and culture
The present study included a total of 10 individuals
(with a mean age of 45.2±9.8 years). All participants were
clinically free of disease, including hair and/or scalp disease,
for ≥6 months, as defined by medical history and physical
examination. The subjects were recruited from September 2014 to
January 2015 from the Shanghai Ninth People's Hospital, Shanghai
Jiao Tong University, School of Medicine, Shanghai, China. Human
scalp samples were obtained with written informed consent from all
participants. The study was approved by the Ethics Committee of the
Shanghai Ninth People's Hospital, Shanghai Jiao Tong University,
School of Medicine, Shanghai, China. Human scalp samples were
soaked in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 0.5%
penicillin and 0.5% streptomycin, for ≤6 h. The epidermis and
adipose tissue was then peeled away from the dermis, cut into small
fragments using scissors and treated with dispase (12.5 mg/ml;
Roche Applied Science, Penzberg, Germany) for 24 h at room
temperature. The suspension was digested with 0.1% trypsin for 30
min at 37°C and filtered through a cell strainer (40 µm diameter
holes). ORSCs were obtained by centrifugation at 1,000 × g for 5
min at 4°C. Subsequently, cells were cultured in medium containing
epidermal growth factor (20 ng/ml), basic fibroblast growth factor
(b-FGF; 40 ng/ml), and 2% B27 supplement (Gibco-BRL; Thermo Fisher
Scientific, Inc.) in DMEM/F12 (1:1; Gibco-BRL; Thermo Fisher
Scientific, Inc.) in 5% CO2 at 37°C for 2 weeks.
Human hair follicle ORSC passage
culture and neural differentiation
Following primary culture for 2 weeks, the spheres
were dissociated by pipetting, and the cells were subcultured for 5
days. For experiments, cells at the first subculture were used. A
three-step process of neuro-induction was performed on isolated
sphere-forming cells. First, 2×105 sphere-forming cells
were cultured in medium containing DMEM-high glucose (HG) medium
with 20% fetal bovine serum (FBS) and 10 ng/ml b-FGF (R&D
Systems, Inc., Minneapolis, MN, USA) for 24 h. The sphere-forming
cells were then washed with phosphate-buffered saline three times
and cultured in DMEM-HG medium containing 1 mM β-mercaptoethanol
(Sigma; Merck KGaA, Darmstadt, Germany) and 10 ng/ml neurotrophin
(NT)-3 (R&D Systems, Inc.) for 48 h. Following this, the
sphere-forming cells were cultured in DMEM-HG medium containing 10
ng/ml NT-3, 10 ng/ml nerve growth factor (NGF) and 50 ng/ml
brain-derived neurotrophic factor (BDNF; R&D Systems, Inc.) for
5 days. The total duration of neural differentiation was 8 days.
The primary culture of ORSCs following 2 days (passage 0, day 2;
P0D2) and the subculture sphere-forming cells prior neural
differentiation (P1D5) acted as control groups.
Immunofluorescence staining
Following dissociation of the sphere-forming cells,
the cells were fixed in 4% paraformaldehyde and rinsed with PBS
three times. Non-specific binding was blocked in PBS containing 5%
bovine serum albumin (Sigma) and 0.1% Triton X-100 at 37°C for 1 h.
A total of 1×105 cells were characterized
immunocytochemically using an anti-nestin antibody (catalog no.
sc-23927; 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-cytokeratin (CK) antibody (catalog no. M082101-2; 1:100; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA), anti-CK15
antibody (catalog no. ab52816; 1:100; Abcam, Cambridge, UK),
anti-CK18 antibody (catalog no. sc-70917; 1:50; Santa Cruz
Biotechnology, Inc.), anti-CK19 antibody (catalog no. MA5-13156;
1:150; NeoMarkers; Thermo Fisher Scientific, Inc.),
anti-follistatin (FST) antibody (catalog no. MAB669; 1:100; R&D
Systems, Inc.), anti-growth associated protein (GAP)-43 antibody
(catalog no. sc-33705; 1:50; Santa Cruz Biotechnology, Inc.),
anti-glial fibrillary acidic protein (GFAP) antibody (catalog no.
ab7260; 1:100; Abcam, Cambridge, UK), anti-neuronal nuclei (NeuN)
antibody (catalog no. ABN78; 1:50; Chemicon; EMD Millipore,
Billerica, MA, USA), anti-neurofilament medium (NF-M) antibody
(catalog no. sc-71688; 1:50; Santa Cruz Biotechnology, Inc.),
anti-neuron-specific enolase (NSE) antibody (catalog no. MAB324-K;
1:50; Chemicon; EMD Millipore), anti-neurotensin receptor-3 (NTR-3)
antibody (catalog no. sc-25055; 1:50; Santa Cruz Biotechnology,
Inc.), anti-p75 neurotrophin receptor (catalog no. ab8874;
P75NTR; 1:250; Abcam) and anti-S100 antibody (catalog
no. GA50461-2; 1:200; Dako; Agilent Technologies, Inc.). Following
washing with PBS containing 0.05% Tween-20 3 times for 3 min at
room temperature, CK, NSE, NF-M and GAP-43 were visualized using a
fluorescein isothiocyanate-conjugated goat anti-mouse IgG (catalog
no. F8264; 1:500; Sigma), and CK-19, GFAP, S100 and
p75NTR were visualized using rhodamine-conjugated goat
anti-rabbit IgG (catalog no. SAB3700846; 1:500; Invitrogen; Thermo
Fisher Scientific, Inc.) as secondary antibodies at 37°C for 1 h.
Nuclei were visualized with DAPI (0.1 mg/ml; catalog no. D8417;
Sigma). Mounting medium (containing 45% acrylic resin and 55%
xylenes; catalog no. 03989; Sigma) was used for microscopic
observation. The immunofluorescent signal was observed under a
fluorescence microscope (Leica DM2500; Leica, Wetzlar,
Germany).
Results
Nestin-negative ORSC separation,
identification and neural differentiation
Facial skin samples were obtained from 10 adult
healthy volunteers, and the epidermis, rich in hair follicles, was
peeled off from the dermis following dispase treatment.
Subsequently, the epidermis was cut into small fragments, digested
with trypsin, and filtered through a 40-µm-pore cell strainer;
ORSCs were obtained by centrifuging the cell suspension. Of the
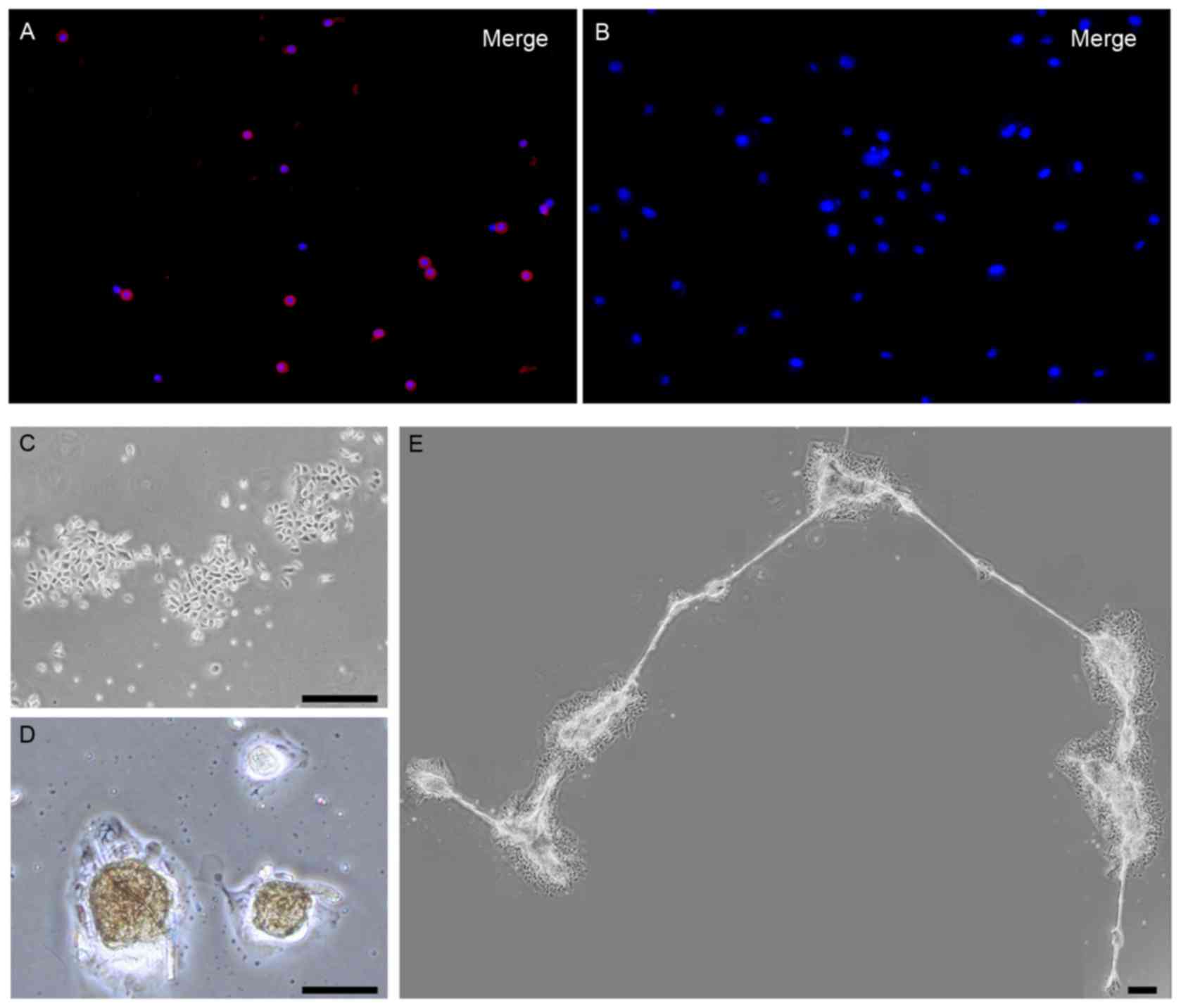
ORSCs dissociated from hair follicles in 48 h primary cultures,
75±4% of them were positive for nestin (red staining) as
demonstrated by immunofluorescence staining (Fig. 1A). However, the nestin-positive
ORSCs were reduced with prolonged incubation in vitro:
Following 9 days of primary culture, nestin expressing ORSCs
disappeared entirely in vitro, and ORSCs remained
nestin-negative following 5 days of subculture (Fig. 1B). Based on these results, ORSCs
were cultured in the presence of neurabasal medium containing
DMEM-HG, 20% FBS and 10 ng/ml b-FGF. The majority of the cells
exhibited colony growth (Fig. 1C),
and, except for nestin-negative expression, NTR-3, GAP-43, GFAP,
P75NTR, NSE, NF-M, NeuN, FST, CK15 and CK18 were
negatively expression in ORSCs. However, CK19, CK and S100 were
only expressed in a small number of ORSCs (Table I). The ORSCs started to form
spheres 5 days after the start of the subculture (first-generation)
in the presence of neurabasal medium (Fig. 1D), and numerous spheres were
connected by a filamentary structure (Fig. 1E).
 | Table I.Differentiation marker expressions of
passage 1 outer root sheath cells prior to and following neurogenic
induction. |
Table I.
Differentiation marker expressions of
passage 1 outer root sheath cells prior to and following neurogenic
induction.
| A, Neural
markers |
|---|
|
|---|
| Marker | P0 D2 | P1: Prior to NI | P1: Following NI |
|---|
| Nestin | Positive | Negative | Positive |
| NTR-3 | Negative | Negative | Positive |
| GAP-43 | Positive | Negative | Positive |
|
P75NTR | Positive | Negative | Positive |
| GFAP | Positive | Negative | Positive |
| S100 | Rare positive | Rare positive | Positive |
| NSE | Negative | Negative | Negative |
| NF-M | Negative | Negative | Negative |
| NeuN | Negative | Negative | Negative |
|
| B, Cytokeratin and
ORS markers |
|
| CK | Rare positive | A few positive | Positive |
| CK19 | Positive | A few positive | Positive |
| CK15 | A few positive | Negative | Negative |
| CK18 | Negative | Negative | Negative |
| FST | A few positive | Negative | Negative |
Immunofluorescent staining of markers
for P1 ORSCs following neuro-induction
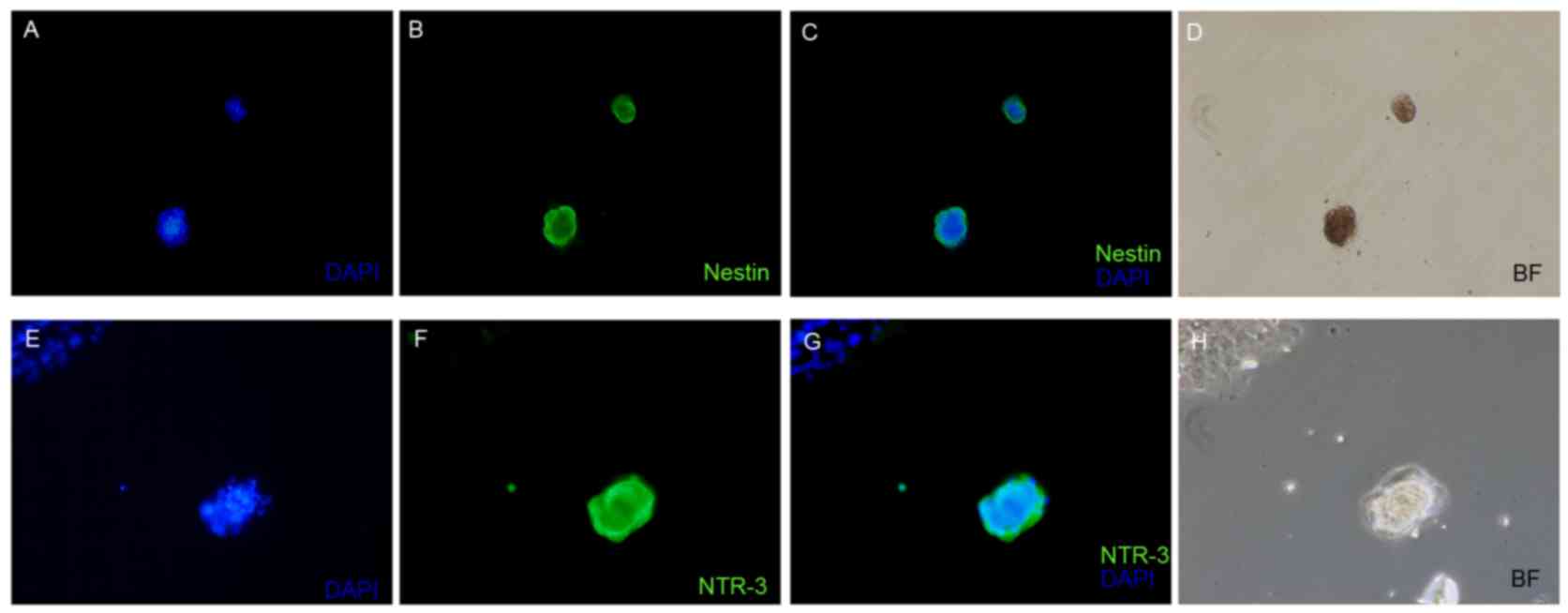
Eight days after neuroinduction sphere-forming cells
were derived from ORSCs, and the single cells were cultured in
medium containing DMEM-HG and NT-3 (10 ng/ml), NGF (10 ng/ml) and
BDNF (50 ng/ml). Notably, the expression of nestin was identified
in ORSCs following a three-step process of neuroinduction (Fig. 2A-D). NTR-3 is present in the brain,
particularly in the hippocampus, dentate gyrus and cerebral cortex,
indicating that it may have a functional significance in
metabolically active brain regions (10). During embryonic brain development,
NTR3 mRNA is expressed in all brain areas from E9.5 to E13.5, at
which point, dividing cells lining the neural canal and the
ventricles, as well as cells already differentiating, express NTR3
(10). The present study observed
that NTR-3 was detected in ORSCs following neuroinduction by
immunofluorescent staining (Fig.
2E-H). In addition, CK, CK-19 and S100 were expressed within a
subset of ORSCs, which were located in an island-shaped structure
at the base of the sphere-forming structure (Fig. 3B, C and M, respectively). In
addition, NSE (Fig. 3G), NF-M
(Fig. 3L) were not detected in
ORSCs following neuroinduction, nor were NeuN, FST, CK15 and CK18
(Table I). Furthermore, GFAP
(Fig. 3H and I), GAP-43 (Fig. 3Q and S) and P75NTR
(Fig. 3R and S) were detected in
the majority of the sphere-forming cells. Similar results were
obtained in the control group (P0D2), however; NTR-3 was expressed
in very low levels (Fig. 4). In
P1D5 control group, the immunofluorescent staining revealed that
there were low expression levels of CK15, CK19 and S100 in ORSCs,
however, no other expression was observed in ORSCs (Table I).
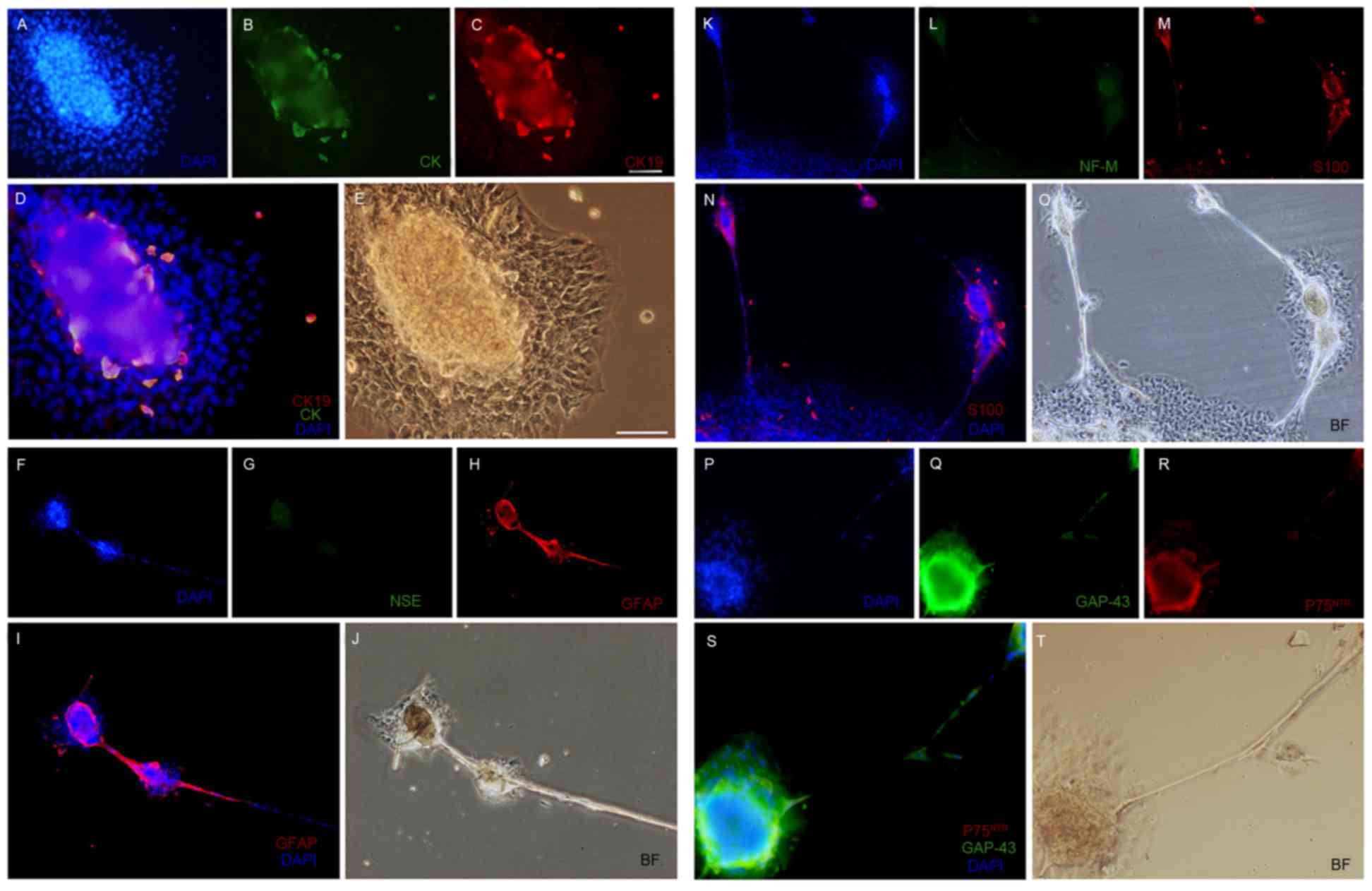 | Figure 3.Immunofluorescent staining for (A)
nuclei (blue), (B) CK (green) and (C) CK19 (red), (D) merge picture
for CK and CK19 and (E) BF control in sphere-forming cells.
Immunofluorescent staining for (F) nuclei (blue), (G) NSE (green)
and (H) GFAP (red), (I) merge picture for GFAP and (J) BF control
in the sphere-forming cells. Immunofluorescent staining for (K)
nuclei (blue), (L) NF-M (green) and (M) S100 (red), (N) merge
picture for S100 and (O) BF control in the sphere-forming cells.
Immunofluorescent staining for (P) nuclei (blue), (Q) GAP-43
(green) and (R) P75NTR (red), (S) merge picture for
GAP-43 and P75NTR and (T) BF control in the
sphere-forming cells. Magnification, ×400. DAPI,
4′,6-diamidino-2-phenylindole; CK, cytokeratin; BF, brightfield;
NSE, neuron-specific enolase; GFAP, glial fibrillary acidic
protein; NF-M, neurofilament medium; GAP-43, growth associated
protein-43; P75NTR, p75 neurotrophin receptor. |
 | Figure 4.Immunofluorescent staining for neural
differentiation markers in the control primary culture of outer
root sheath cells at 2 days (P0D2). Magnification, ×50. DAPI,
4′,6-diamidino-2-phenylindole; GAP-43, growth associated
protein-43; GFAP, glial fibrillary acidic protein; CK, cytokeratin;
NeuN, neuronal nuclei; P75NTR, p75 neurotrophin
receptor; NF-M, neurofilament medium; FST, follistatin; NTR-3,
neurotensin receptor-3; BF, brightfield. |
Discussion
Nestin is an intermediate filament protein that is
expressed in a variety of tissues, including those in the CNS and
the peripheral nervous system. Nestin is downregulated and replaced
by tissue-specific intermediate filament proteins in the
progression of tissue differentiation (11,12).
Previous studies have demonstrated that nestin is expressed in
bulge-area stem cells of the hair follicle, and multipotent
nestin-positive hair-follicle bulge stem cells can form neurons
(4,8). In non-balding human scalp skin,
nestin was detected by immunohistochemical analysis in the
epidermis and the upper two-thirds of the hair follicle, however,
not in the lower third of the follicle (13). Transfection of nestin-expressing
hair-follicle cells into nude-mouse skin, has revealed that they
are interconnected by an nestin-driven GFP (ND-GFP)-labeled dermal
vascular network, which developed into an extensively branched
network, appearing to anastomose with existing vessels in the
recipient nude mice. In addition, the nestin-expressing follicle
cells contributed to wound repair as well as skin transplant
survival (14,15). These results indicated that nestin
is present in ORSCs, and that ORSCs are significant in the periodic
cycle of hair follicle and wound repair in epidermal tissue.
Nervous tissue wound repair and regeneration are the most complex
problems in regenerative medicine. However, the use of hair
follicle-derived cells to develop nerve regeneration and functional
recovery may be a suitable treatment strategy for neurodegenerative
diseases and may possess an important value in regenerative
medicine.
The present study demonstrated that the isolation of
nestin-negative ORSCs derived from the human hair follicle
exhibited the neuronal differentiation of these cells. The isolated
cells exhibited sphere-forming ability, and nestin was identified
in ORSCs following a three-step process of neuroinduction.
Similarly, Amoh et al (16)
demonstrated that ND-GFP hair-follicle stem cells differentiate
into neurons, glia, keratinocytes, smooth muscle cells and
melanocytes in vitro, and that ND-GFP-expressing stem cells
extensively differentiate into neurons following transplantation to
the subcutis of nude mice. In the present study, neurodevelopmental
markers were detected in the ORSC-derived nestin-positive spherical
cell mass. The neuronal specific markers, GAP-43, NTR-3 and
P75NTR were induced, and the gliocyte markers GFAP and
S100 were detected in these sphere-forming cells, however, the
mature neuron-associated markers, NF-M, NeuN and NSE were not
expressed, suggesting that sphere-forming cells may preferentially
differentiate into neural stem cell-like cells and not into the
mature neurons or neurogliocytes.
Hair follicle stem cells serve a vital role in
neurodegenerative diseases and regenerative medicine. However, to
date, no biochemical markers have been identified that serve as
indicators of hair follicle stem cell differentiation, therefore,
it is difficult to separate and culture these cells. However, it is
established that the multifunctional hair follicle stem cells can
be isolated from hair follicle ORSCs and are involved in wound
repair and neuroinduction (4,8,17).
Therefore, hair follicle ORSCs represent a variety of novel
approaches to treat neurodegenerative diseases and regenerative
medicine. The present study did not verify the value of neural
differentiation into sphere-forming cells for therapeutic
application, however, the differentiation of nestin-negative human
hair follicle ORSCs into neurons in vitro may serve as a
theoretical underpinning for neurodegenerative diseases and
regenerative medicine.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81272109).
References
|
1
|
Zietlow R, Lane EL, Dunnett SB and Rosser
AE: Human stem cells for CNS repair. Cell Tissue Res. 331:301–322.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ninomiya M, Yamashita T, Araki N, Okano H
and Sawamoto K: Enhanced neurogenesis in the ischemic striatum
following EGF-induced expansion of transit-amplifying cells in the
subventricular zone. Neurosci Lett. 403:63–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Batista CE, Mariano ED, Marie SK, Teixeira
MJ, Morgalla M, Tatagiba M, Li J and Lepski G: Stem cells in
neurology-current perspectives. Arq Neuropsiquiatr. 72:457–465.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanno H, Kubo A, Yoshizumi T, Mikami T and
Maegawa J: Isolation of multipotent nestin-expressing stem cells
derived from the epidermis of elderly humans and TAT-VHL
peptide-mediated neuronal differentiation of these cells. Int J Mol
Sci. 14:9604–9617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Savkovic V, Flämig F, Schneider M, Sülflow
K, Loth T, Lohrenz A, Hacker MC, Schulz-Siegmund M and Simon JC:
Polycaprolactone fiber meshes provide a 3D environment suitable for
cultivation and differentiation of melanocytes from the outer root
sheath of hair follicle. J Biomed Mater Res A. 104:26–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Limat A and Hunziker T: Use of epidermal
equivalents generated from follicular outer root sheath cells in
vitro and for autologous grafting of chronic wounds. Cells Tissues
Organs. 172:79–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Uchugonova A, Kimura H, Zhang C,
Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, et al: The
bulge area is the major hair follicle source of nestin-expressing
pluripotent stem cells which can repair the spinal cord compared to
the dermal papilla. Cell Cycle. 10:830–839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amoh Y, Li L, Campillo R, Kawahara K,
Katsuoka K, Penman S and Hoffman RM: Implanted hair follicle stem
cells form Schwann cells that support repair of severed peripheral
nerves. Proc Natl Acad Sci USA. 102:17734–17738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amoh Y, Kanoh M, Niiyama S, Hamada Y,
Kawahara K, Sato Y, Hoffman RM and Katsuoka K: Human hair follicle
pluripotent stem (hfPS) cells promote regeneration of
peripheral-nerve injury: An advantageous alternative to ES and iPS
cells. J Cell Biochem. 107:1016–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazella J: Sortilin/neurotensin
receptor-3: A new tool to investigate neurotensin signaling and
cellular trafficking? Cell Signal. 13:1–6. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michalczyk K and Ziman M: Nestin structure
and predicted function in cellular cytoskeletal organisation.
Histol Histopathol. 20:665–671. 2005.PubMed/NCBI
|
|
12
|
Yay A, Ozdamar S, Canoz O, Baran M, Tucer
B and Sonmez MF: Intermediate filament protein nestin is expressed
in developing meninges. Bratisl Lek Listy. 115:718–722.
2014.PubMed/NCBI
|
|
13
|
Wang Y, Zhang Y, Zeng Y, Zheng Y, Fu G,
Cui Z and Yang T: Patterns of nestin expression in human skin. Cell
Biol Int. 30:144–148. 2006.PubMed/NCBI
|
|
14
|
Amoh Y, Li L, Yang M, Moossa AR, Katsuoka
K, Penman S and Hoffman RM: Nascent blood vessels in the skin arise
from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA.
101:13291–13295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang H, Tian L, Son YJ, Zuo Y, Procaccino
D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, et al:
Regulation of the intermediate filament protein nestin at rodent
neuromuscular junctions by innervation and activity. J Neurosci.
27:5948–5957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amoh Y, Li L, Katsuoka K, Penman S and
Hoffman RM: Multipotent nestin-positive, keratin-negative
hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci
USA. 102:5530–5534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu H, Fang D, Kumar SM, Li L, Nguyen TK,
Acs G, Herlyn M and Xu X: Isolation of a novel population of
multipotent adult stem cells from human hair follicles. Am J
Pathol. 168:1879–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|