Introduction
The periodontal ligament (PDL) is a complex, highly
specialized cellular connective tissue that supports the teeth by
surrounding and anchoring them to the alveolar bone. In addition,
periodontal ligament cells (PDLCs) facilitate the bone-regenerative
potential of periodontium (1). The
versatility of the PDL is primarily due to the ability of PDLCs to
adapt to various microenvironments, including inflammatory and
loading conditions (2).
An increasing number of patients with malocclusion
and malpositioned teeth are opting for orthodontic therapy to
correct these conditions and improve aesthetics and function. The
adult periodontium is exposed to a variety of physical forces,
including those caused by orthodontic therapy. Orthodontic force
serves as biomechanical stress that promotes the remodelling
process and metabolism of bone matrix (3). Earlier studies and models have
investigated the biochemical responses evoked by biomechanical
forces, including static compression (4), dynamic compression (5), centrifugal force (6) and tensile stress (7), on various oral tissues, including
PDLs.
It is important to understand the physiological and
homeostatic response of the periodontium to mechanical and physical
forces under various conditions, including an inflammatory
microenvironment. It is understood that periodontitis is associated
with an increase in pro-inflammatory cytokines, including
interleukin-1 beta (IL-1β) and tumour necrosis factor α (TNF-α)
(8,9). IL-1β and TNF-α mediate the
pathogenesis of periodontitis, and their expression promotes
periodontal tissue destruction (10,11).
Elevated IL-1β and TNF-α levels have previously been detected in
the serum and gingival tissues of patients with periodontitis
(12). Additionally, primary cells
cultured from inflammatory PDL tissues were demonstrated to secrete
IL-1β and TNF-α levels that were similar to those from healthy
donors stimulated with exogenous cytokines (13). These cytokines are primary chemical
mediators in the inflammatory microenvironment and serve as markers
of a large number of inflammatory diseases, including periodontitis
(14,15). Notably, there appears to be
ambiguity regarding the exact effects of these cytokines in
different cell types. For instance, studies on human mesenchymal
stem cells (hMSCs) revealed that expression of the master
transcription factor for osteoblast differentiation, runt-related
transcription factor 2 (RUNX2), was enhanced in the presence of
media pre-conditioned with increased pro-inflammatory cytokine
levels from classically activated monocytes (16,17).
By contrast, inflammatory stimuli additionally induced factors that
facilitate bone resorption, including matrix metalloproteinases
(MMPs) in PDL fibroblasts (18)
and the anterior cruciate ligament (19). In vitro (20) and in vivo (21,22)
studies have demonstrated that alveolar bone resorption is closely
associated with MMP production. Notably, inflammatory cytokines
appear to trigger cellular responses that facilitate bone
apposition and bone resorption. Evidence regarding the mechanisms
underlying the process of osteoblastogenesis in response to
physical forces in inflammatory microenvironments is beginning to
emerge. In particular, understanding the PDLC response to tensile
strength and inflammatory status is pivotal for comprehending tooth
movement in the inflammatory microenvironment.
Previously, two key factors important in PDL
osteogenesis, RUNX2 and type I collagen (COL-I), have obtained
increasing attention from researchers. RUNX2 is a transcription
factor that serves an important role in the transcription of many
osteogenesis regulating genes (23). COL-I is responsible for the
formation of the primary fibril-forming collagen in the PDL, forms
solid fibres located between the alveolar bone and the cementum,
and functions as a cushion mitigating masticatory and orthodontic
force loading (24,25). The present study investigated the
combined effects of tensile strength, IL-1β and TNF-α in PDLCs.
RUNX2 and COL-I mRNA and protein expression levels and alkaline
phosphatase (ALP) activity were measured. The results obtained from
this study will improve understanding of the risks associated with
periodontitis in adult orthodontic patients, and the association
between orthodontic force and periodontal inflammation.
Materials and methods
Isolation and identification of
PDLCs
The present study was approved by the Ethics
Committee of the School of Stomatology, Wenzhou Medical University
(Wenzhou, China; no. WYKQ2013003). A total of 6 teeth were obtained
from four systemically healthy patients (age, 18–28 years; two
males and two females) during extraction as part of orthodontic
management or tooth impaction treatment. Informed consent allowing
the use of these teeth to harvest PDLCs for the current study was
obtained from all patients. Extracted teeth were immediately
processed for PDLC isolation. Sterile 1X PBS was used to gently
clean the root surfaces of the extracted tooth and PDL tissues were
gently scraped off the middle third portion of the roots. PDL
tissues were digested with type I collagenase (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) solution (3 mg/ml dissolved in Hank's
balanced salt solution) for 45 min at 37°C and suspended in
α-minimum essential medium (α-MEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 100 U/ml penicillin
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml
streptomycin (Hyclone; GE Healthcare Life Sciences) and 100 M/ml
ascorbic acid (Sigma-Aldrich; Merck KGaA). The tissues were
incubated at 37°C in a humidified atmosphere of 5% CO2
for two weeks in 25 cm2 cell culture flasks (Corning
Incorporated, Corning, NY, USA). The culture medium was replaced
every three days and PDLCs were used for the study at passage 3 to
5.
To confirm their mesenchymal origin, PDLCs were
subjected to immunocytochemical staining for vimentin and
cytokeratin (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) according to methods previously described (26).
Cytokine treatment and application of
tensile strength
PDLCs were seeded onto COL-I-coated silicone
Bioflex® culture plates (Flexcell International
Corporation, Burlington, NC, USA) supplemented with α-MEM medium at
a density of 2×105 cells/well. When the cells reached
70–80% confluence, they were exposed to pro-inflammatory cytokines
and tensile strength. As previously described (18), PDLCs were treated with 5 ng/ml
IL-1β and 10 ng/ml TNF-α (Peprotec, Inc., Rocky Hill, NJ, USA), to
simulate an inflammatory microenvironment in vitro.
Following this, cells were subjected to uni-axial tensile strength
[12% elongation (27) sinusoidal
curve, 0.5 Hz (28)], using a
FX-5000T™ Flexercell Tension Plus™ system
(Flexcell International Corporation). Uni-axial tensile strength
was designed to simulate occlusion and orthodontic tooth movement.
The 12% tensile strength value was chosen according to finite
element model data (29).
Untreated PDLCs were incubated using the same apparatus and
cultured on the same Bioflex plates. PDLCs were divided into four
groups: Untreated cells [I(−)/T(−)]; cells treated with tensile
strength alone [I(−)/T(+)]; cells treated with IL-1β/TNF-α alone
[I(+)/T(−)]; and cells treated with tensile strength and
IL-1β/TNF-α [I(+)/T(+)].
ELISA
Protein levels of IL-1β and TNF-α in the cell
culture supernatants were compared using ELISA kits (cat. nos.
EK101B4 and EK1824, respectively; Sunny Elisa, Hangzhou, China),
according to the manufacturer's protocol. The absorbance was
measured at a wavelength of 405 nm using a microplate reader (Tecan
Group, Ltd., Mannedorf, Switzerland).
MTT assay
Proliferation of PDLCs following various treatments
was determined by MTT assay (Amresco, LLC, Solon, OH, USA). A total
of 320 µl MTT solution (5 g/l dissolved in ddH2O) was
added to each well of a 6-well plate and cells were incubated at
37°C for 4 h. The MTT solution and culture medium was removed and
3,000 µl dimethyl sulfoxide was added. Complete dissolution of each
sample was aided by 10 min of light-tight vibration. The absorbance
was measured at a wavelength of 490 nm using a spectrophotometer.
Pure culture medium without PDLCs was used as the blank
control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from PDLCs following
treatment using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). A PrimeScript™ RT reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China) was used to reverse
transcribe cDNA from cellular RNA. qPCR was performed using a
LightCycler® 480 Real-Time PCR Detection system (Roche
Diagnostics, Basel, Switzerland) and the LightCycler®
480 SYBR-Green I Master qPCR kit (Roche Diagnostics) using the
following parameters: 40 cycles of 10 sec at 95°C, 10 sec at 60°C
and 20 sec at 72°C. Primer sequences of osteogenic genes, including
RUNX2, COL-I and GAPDH were synthesized by Invitrogen; Thermo
Fisher Scientific, Inc. GAPDH was employed as an internal
reference. Primer sequences are listed in Table I.
 | Table I.Primers sequences. |
Table I.
Primers sequences.
| Gene | Sequence |
|---|
| RUNX2 | F:
5′-CCCGTGGCCTTCAAGGT-3′ |
|
| R:
5′-CGTTACCCGCCATGACAGTA-3′ |
| COL-I | F:
5′-CCAGAAGAACTGGTACATCAGCAA-3′ |
|
| R:
5′-CGCCATACTCGAACTGGAATC-3′ |
| GAPDH | F:
5′-GAAGGTGAAGGTCGGAGTC-3′ |
|
| R:
5′-GAGATGGTGATGGGATTTC-3′ |
Western blot analysis
PDLCs were washed three times with precooled PBS at
4°C and promptly lysed in high intensity radioimmunoprecipitation
assay lysis buffer with 1 mM phenylmethylsulfonyl fluoride, then
scraped from the Bioflex plates. The concentration of total protein
in the lysates was assayed using the BCA Protein Quantitation kit
(Fudebio, Hangzhou, China) according to the manufacturer's
protocol. Total protein from cell lysates (50 µg per lane) were
separated per lane by 8% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skimmed milk for 2 h and
subsequently incubated with primary antibodies against RUNX2
(1:1,000; cat. no. 8486; rabbit; Cell Signaling Technology, Inc.,
Danvers, MA, USA), COL-1 (1:10,000; cat. no. sc-8785; goat; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and GAPDH (1:10,000;
cat. no. KC-5G5; rabbit; KangChen Bio-tech, Inc., Shanghai, China)
overnight at 4°C. Following this, membranes were incubated for 2 h
at room temperature with the following horseradish peroxidase
(HRP)-conjugated secondary antibodies: Goat anti-rabbit IgG
(1:5,000; cat. no. AP132P; EMD Millipore) and rabbit anti-goat IgG
(1:10,000; cat. no. AP106P; EMD Millipore). Immunoreactive protein
bands were detected by using Immobilon Western Chemiluminescent HRP
Substrate (EMD Millipore). Protein levels were quantified using
ChemiDoc™ XRS+ (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc.).
ALP activity assay
To evaluate the impact of tensile strength and the
inflammatory microenvironment on ALP activity, an ALP assay kit
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was
used according to the manufacturer's protocol. The optical density
was measured at a wavelength of 405 nm using a microplate reader
(Tecan Group, Ltd.).
Mineralization assay
To investigate osteogenic differentiation potential,
cells were initially grown under the conditions described above for
48 h. Following this, cells were immediately cultured in commercial
osteogenic media supplemented with 50 µg/ml ascorbic acid, 0.1 µM
dexamethasone and 10 mM β-glycerophosphate (Cyagen Biosciences,
Santa Clara, CA, USA) for 14 days (13,30).
Cells were subsequently fixed in 4% formalin for 30 min at room
temperature and stained with alizarin red S (Cyagen Biosciences)
for 30 min. To quantify mineralization, the mineral stain was
solubilized in 5% SDS in 0.5 ml 0.5 N HCl for 30 min. The
absorbance was measured at a wavelength of 405 nm using a
microplate reader.
Statistical analysis
All experiments were performed at least three times
and all data are expressed as the mean ± standard deviation. The
Student's t-test was used for comparison between two groups and
one-way analysis of variance was used for multiple comparisons,
followed by Tukey's post hoc test. Statistical analysis was
performed using SPSS 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
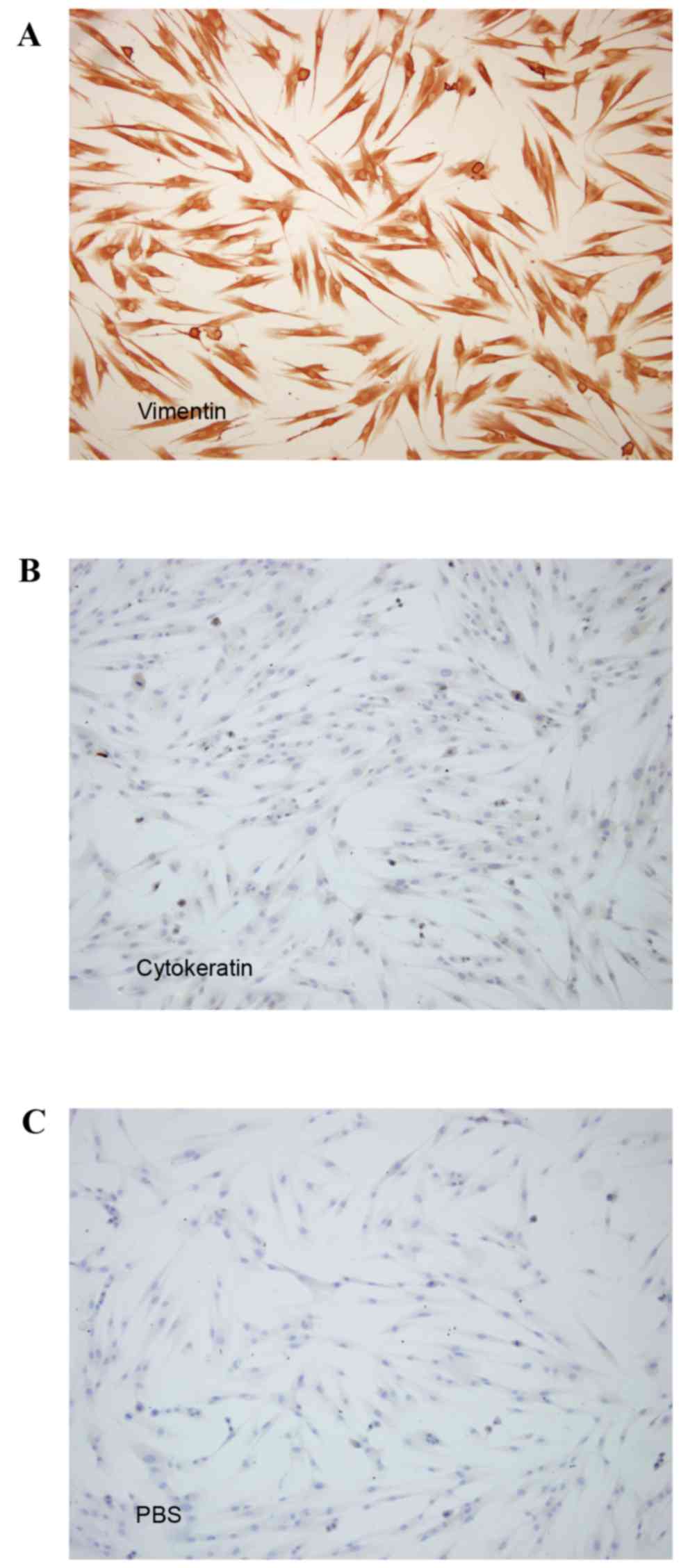
Morphology and phenotype of PDLCs
To confirm that cells derived from the PDL were of
mesodermal origin, the presence of vimentin and cytokeratin was
detected by staining (28). The
observation that the cells were positive for vimentin (Fig. 1A) but negative for cytokeratin
(Fig. 1B) was consistent with a
mesodermal origin. Cells in PBS served as the negative control
(Fig. 1C). PDLCs exhibited no
particular morphological alterations and remained shuttle-shaped
following stimulation with IL-1β+, TNF-α and/or tensile
strength.
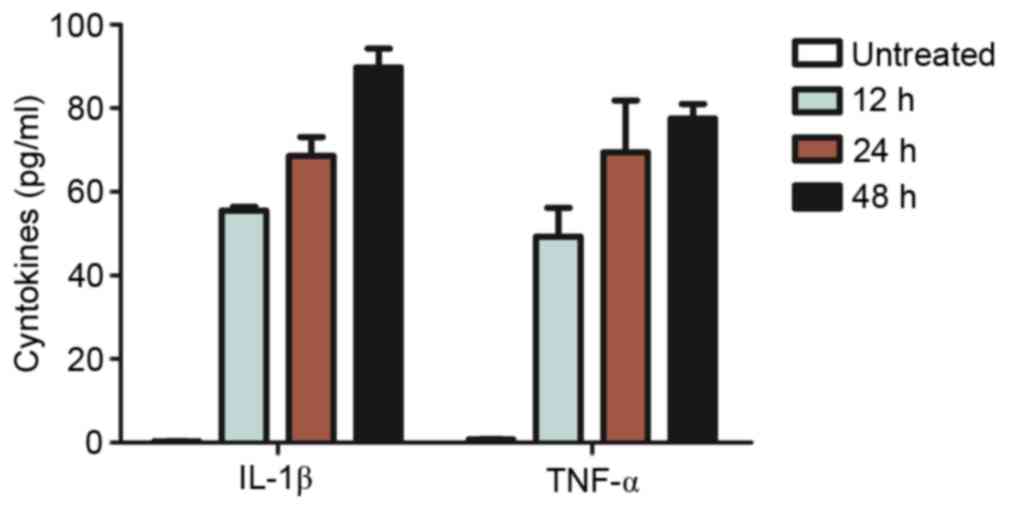
Tensile strength induces
pro-inflammatory secretion with minimally increased
proliferation
Tensile strength induced the secretion of
pro-inflammatory cytokines in a time-dependent manner (Fig. 2) with a minimal increase in
proliferation (Fig. 3). Continuous
tensile strength up to 48 h led to the secretion of IL-1β
(89.76±4.56 pg/ml) and TNF-α (77.52±3.51 pg/ml; Fig. 2). PDLCs treated with 5 ng/ml IL-1β
and 10 ng/ml TNF-α exhibited a significant decrease in
proliferative capacity compared with untreated controls. Tensile
strength was demonstrated to have minimal influence on
proliferation of PDLCs alone; however, it enhanced pro-inflammatory
cytokine inhibition of PDLC proliferation (Fig. 3).
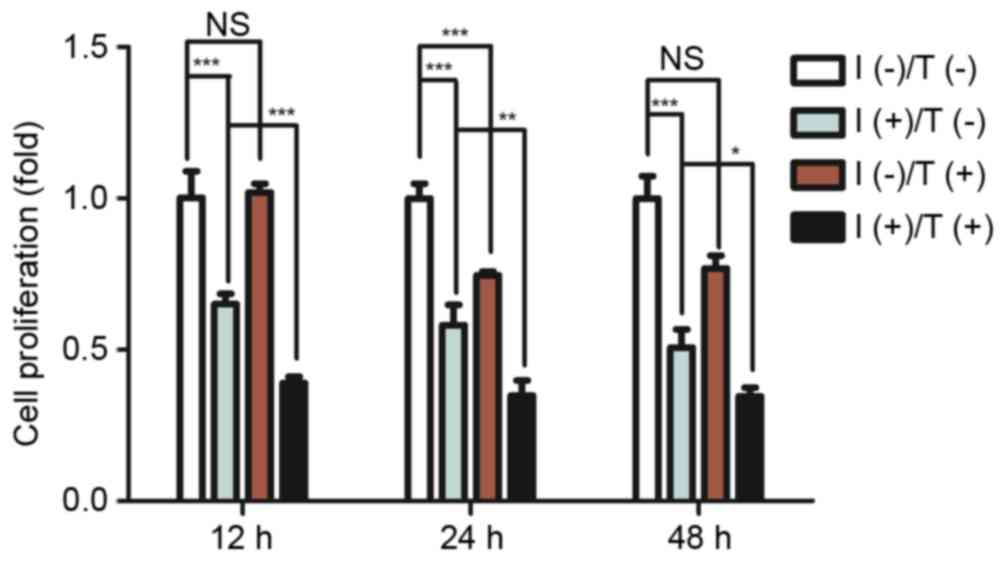 | Figure 3.Effect of pro-inflammatory cytokines
and/or tensile strength on proliferation of PDLCs. Cell
proliferation was determined by MTT assay after 12, 24 or 48 h
exposure to 5 ng/ml IL-1β and 10 ng/ml TNF-α and tensile strength.
Data are expressed as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01, ***P<0.001. I(−)/T(−), untreated cells;
I(−)/T(+), cells treated with tensile strength alone; I(+)/T(−),
cells treated with IL-1β/TNF-α alone; I(+)/T(+), cells treated with
tensile strength and IL-1β/TNF-α at each time point. PDLCs,
periodontal ligament cells; IL-1β, interleukin-1β; TNF-α, tumor
necrosis factor-α. |
Tensile strength and a
pro-inflammatory environment alters the osteogenic and matrix
deposition potential of PDLCs
To investigate the osteogenic potential of PDLCs,
the expression of osteogenic markers including RUNX2 and COL-I were
determined. Tensile strength did not alter RUNX2 mRNA (Fig. 4A) or protein (Fig. 4B) expression levels in PDLCs.
However, COL-I mRNA (Fig. 4C) and
protein (Fig. 4D) expression
levels were significantly reduced following tensile strength.
Treatment with pro-inflammatory cytokines significantly increased
mRNA and protein expression levels of RUNX2; however, decreased
those of COL-I. The increase in RUNX2 induced by pro-inflammatory
cytokines was markedly inhibited by tensile strength and was
reduced below the levels observed in untreated controls. Similarly,
downregulation of COL-I mRNA and protein expression levels induced
by pro-inflammatory cytokines was accentuated by tensile strength.
Combining tensile strength with pro-inflammatory cytokine exposure
decreased COL-I mRNA and protein expression levels to markedly
reduced levels compared with untreated PDLCs. Representative
western blot images of COL-I and RUNX2 protein expression levels
are presented in Fig. 4E.
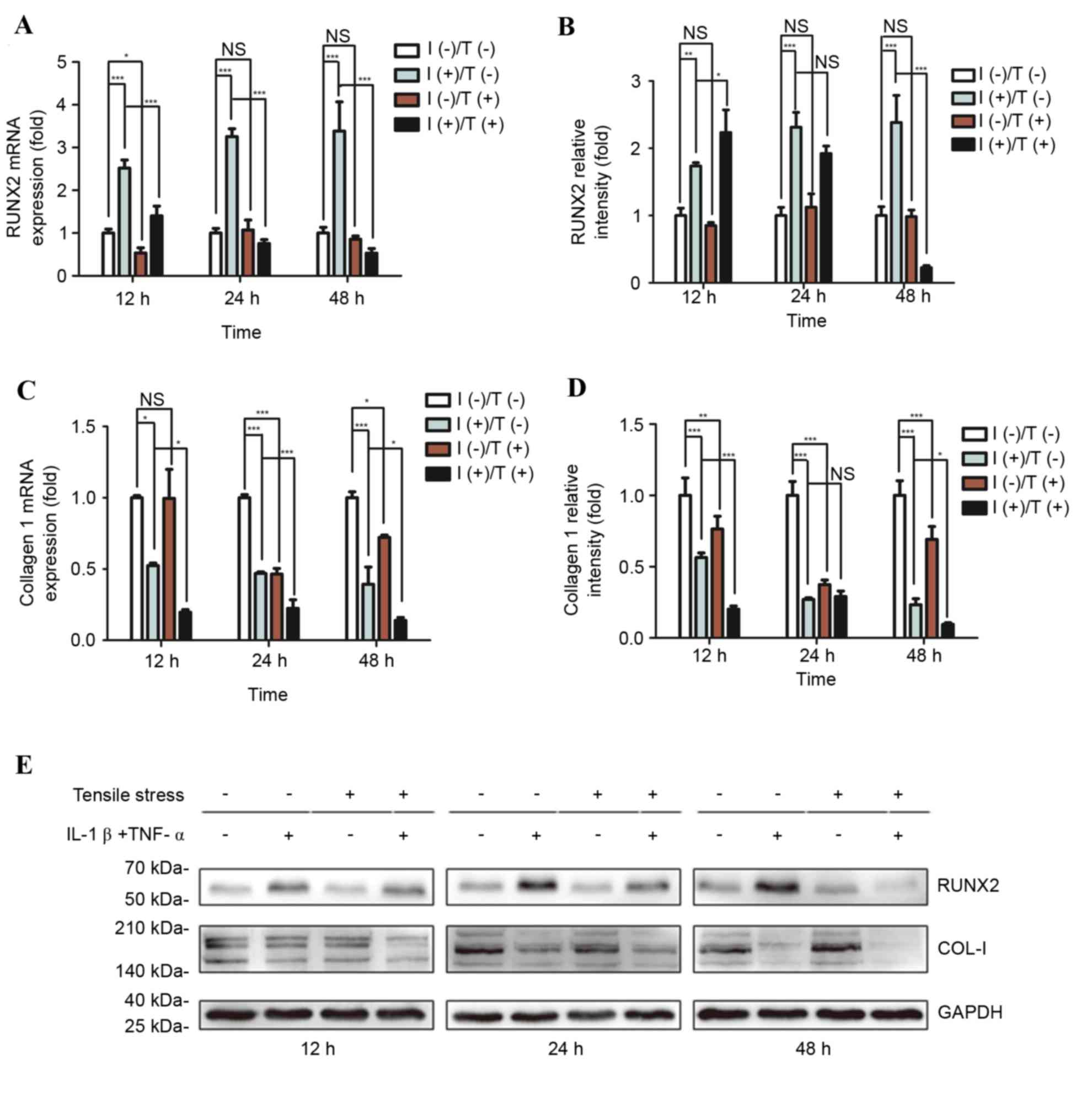 | Figure 4.Differential expression of RUNX2 and
COL-I in response to pro-inflammatory stimuli and/or tensile
strength. Relative osteogenic and matrix gene expression and
protein levels of RUNX2 and COL-I in PDLCs were assessed. (A) mRNA
and (B) protein expression levels of RUNX2, and (C) mRNA and (D)
protein expression levels of COL-I were determined following
treatment for 12, 24 or 48 h. (E) Representative western blot
images of RUNX2 and COL-I protein expression levels. GAPDH served
as an internal control. Data are expressed as the mean ± standard
deviation (n=3). *P<0.05, **P<0.01, ***P<0.001. I(−)/T(−),
untreated cells; I(−)/T(+), cells treated with tensile strength
alone; I(+)/T(−), cells treated with IL-1β/TNF-α alone; I(+)/T(+),
cells treated with tensile strength and IL-1β/TNF-α at each time
point. PDLCs, periodontal ligament cells; IL-1β, interleukin-1β;
TNF-α, tumor necrosis factor-α; NS, non-significant; RUNX2,
runt-related transcription factor 2; COL-I, type I collagen. |
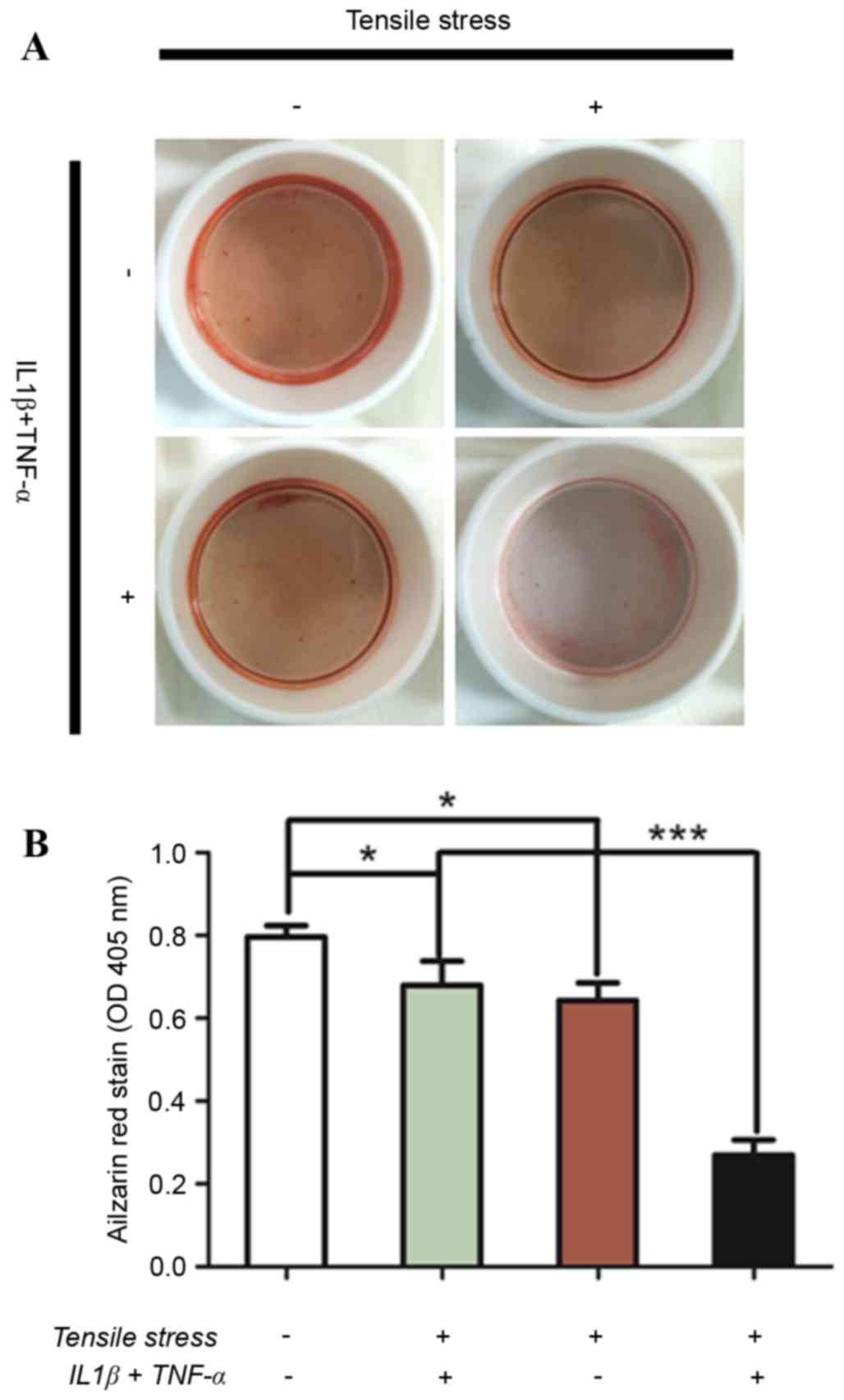
ALP activity and mineralized nodule
formation is decreased by tensile strength in an inflammatory
environment in PDLCs
To determine the osteogenic and mineralization
potential of PDLCs, the osteogenesis marker ALP and mineralization
status were analysed. Cells were subjected to the conditions
described above for 12, 24 or 48 h. ALP activity was reduced
following pro-inflammatory cytokine treatment or tensile strength
exposure. The greatest decrease in ALP activity was detected in
cells treated with combined tensile strength and pro-inflammatory
cytokines. The difference in ALP activity between the treatment
groups was most marked 48 h post-exposure (Fig. 5). Increased mineralization in
response to strength-induced osteogenic differentiation in the
inflammatory microenvironment was measured using Alizarin Red S
staining in PDLCs. A significant decrease in mineralization was
observed in PDLCs treated with either pro-inflammatory cytokines or
tensile strength followed by culturing in osteo-inductive medium
for 14 days (Fig. 6A). Notably, a
markedly significant decrease in Alizarin Red S staining was
observed in cells exposed to the combination of pro-inflammatory
cytokines and tensile strength (Fig.
6B).
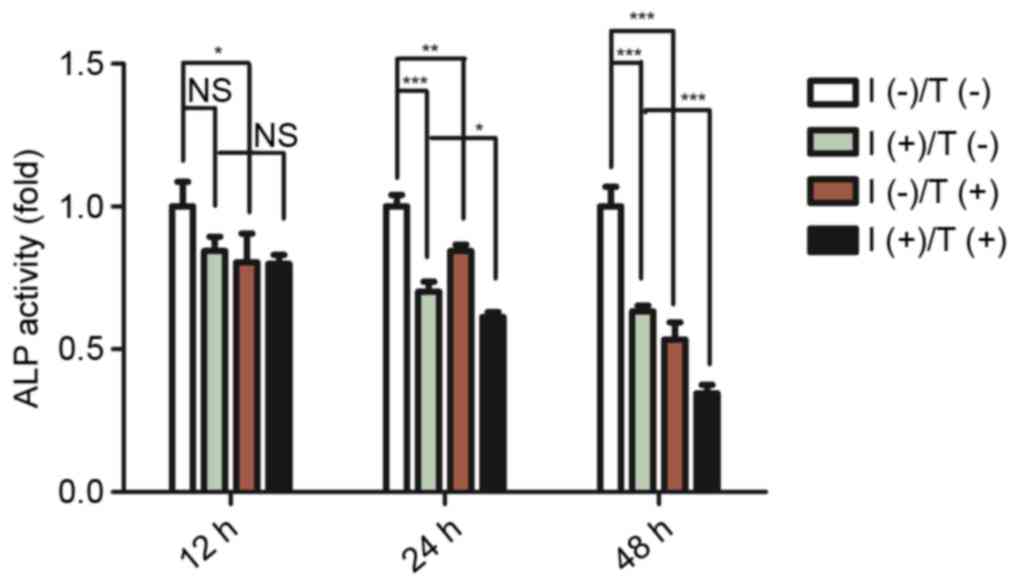 | Figure 5.Effect of pro-inflammatory cytokines
and/or tensile strength on ALP activity in PDLCs. ALP activity was
assessed in PDLCs treated for 12, 24 or 48 h. Data are expressed as
the mean ± standard deviation (n=3). *P<0.05, **P<0.01,
***P<0.001. I(−)/T(−), untreated cells; I(−)/T(+), cells treated
with tensile strength alone; I(+)/T(−), cells treated with
IL-1β/TNF-α alone; I(+)/T(+), cells treated with tensile strength
and IL-1β/TNF-α at each time point. PDLCs, periodontal ligament
cells; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; NS,
non-significant; ALP, alkaline phosphatase. |
Discussion
The demand for adult orthodontic treatment has grown
exponentially in recent years (31). This increased demand has resulted
in a proportional increase in the number of challenging cases,
particularly for orthodontists providing services to patients with
periodontal diseases (32,33). Unfortunately, the risks involved in
orthodontic therapy in patients with periodontal disease remain to
be fully elucidated. The present study demonstrated alterations in
the osteogenic potential of PDLCs in response to tensile strength
in inflammatory environments.
Cellular behaviour, including proliferation, is
highly dependent on biochemical and physical stimuli in the
extracellular environment. The present study revealed PDLCs were
sensitive to inflammatory mediators but insensitive to tensile
strength, which was consistent with previous studies. A previous
study demonstrated that conditioned medium from macrophages with
Porphyromonas gingivalis-lipopolysaccharide (LPS)
stimulation reduced the proliferation of osteoblasts (34). Another study demonstrated that
tensile force loading did not influence the viability of PDLCs
(35). However, the present study
revealed that suppression of PDLC proliferation was enhanced
following exposure to a combination of inflammatory and tensile
loading microenvironments. These findings indicated that mechanical
strength may amplify the inhibitory effects of inflammatory
mediators.
Orthodontic tooth movement causes bone remodelling
in areas under tensile strength, and this process is coupled with
an inflammatory reaction (36).
Previous in vivo studies have demonstrated that orthodontic
tooth movement resulted in increased levels of pro-inflammatory
cytokines in periodontal tissues (37,38).
The present study confirmed an increase in IL-1β and TNF-α levels
following exposure to tensile strength.
These results indicated that combined IL-1β and
TNF-α exposure and tensile force loading significantly decreased
RUNX2 expression in PDLCs. This result is consistent with a
previous PDLC study that reported that combined IL-1β and tensile
strain loading decreased RUNX2 expression (39). In the present study, combined IL-1β
and TNF-α exposure upregulated RUNX2 in PDLCs. The differences
between this study and previous investigations may be due to the
presence of TNF-α. Studies in hMSCs using LPS-stimulated
conditioned media reported high levels of secreted TNF-α and a
robust enhancement of RUNX2 expression (16). There is evidence that activated
monocytes and T lymphocytes produce inflammatory cytokines that
serve an essential role in the process of osteogenic
differentiation of mesenchymal tissue-derived cells (40,41).
Notably, IL-1β and TNF-α exposure robustly enhanced
RUNX2 and suppressed COL-I expression in PDLCs. Inflammatory
cytokines, including IL-1β and TNF-α are involved in the
up-regulation of MMPs (18,19).
MMPs mediate connective tissue breakdown (11) and the degradation of numerous
extracellular matrix proteins (42). The results of the present study are
consistent with those of a previous study that demonstrated that
the expression of COL-I was inhibited in various cell types
cultured in inflammatory media (13). These results demonstrated that
COL-I expression by PDLCs was markedly suppressed following
combined exposure to IL-1β, TNF-α and tensile force loading.
Cytokine exposure exhibited an inhibitory effect on COL-I
expression, and a stimulatory effect on RUNX2 expression. This may
explain the different patterns of bone formation and matrix
deposition in PDL tissue. Biomechanical strength imposed on PDLCs
in an inflammatory microenvironment appears to be detrimental and
results in progressive downregulation of RUNX2 and COL-I.
As biomechanical strength is involved in decreased
bone formation and matrix deposition under inflammatory conditions,
it was hypothesized that biomechanical strength may have a negative
effect on ALP activity and the mineralization process. It was
observed that ALP activity was significantly suppressed in PDLCs
that were exposed to tensile strength combined with
pro-inflammatory cytokines. Evidence from in vitro studies
revealed that PDLCs and MSCs interact and respond in different ways
to cytokines or biomechanical strength. For instance, increased ALP
activity in response to IL-1β and TNF-α or biomechanical strength
was observed in hMSCs (43,44).
On the other hand, decreased ALP activity in response to cytokines
or biomechanical strength in PDLCs has been reported by numerous
studies (13,15,35).
ALP activity and mineralization are interdependent and associated
with osteoblast differentiation. The present study revealed that
the level of mineralization in the I(+)/T(+) group was
significantly reduced compared with the I(+)/T(−) group. This
result suggested that biomechanical strength may serve as a key
stimulus influencing PDLC tolerance of inflammatory
microenvironments.
In conclusion, the present study demonstrated that
mechanical stresses, including tensile strength, enhanced the
suppression of osteogenesis, matrix deposition and mineralization
in PDLCs that is induced by an inflammatory microenvironment.
Therefore, it is important to assess the periodontal health status
of adults prior to orthodontic therapy.
Acknowledgements
The authors of the present study would like to thank
Dr Gerald Volière from the School and Hospital of Stomatology,
Wenzhou Medical University (Wenzhou, China) for reviewing and
editing this manuscript. The present study was supported by grants
from the National Natural Science Foundation of China (grant no.
81200795), the Zhejiang Provincial Natural Science Foundation of
China (grant nos. Y207360 & LY12H14003), and the Medical and
Health Science and Technology Plan of Zhejiang Province (grant no.
2015KYA149).
References
|
1
|
Ivanovski S, Gronthos S, Shi S and Bartold
PM: Stem cells in the periodontal ligament. Oral Dis. 12:358–363.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agarwal S, Long P, Seyedain A, Piesco N,
Shree A and Gassner R: A central role for the nuclear factor-kappaB
pathway in anti-inflammatory and proinflammatory actions of
mechanical strain. FASEB J. 17:899–901. 2003.PubMed/NCBI
|
|
3
|
Liu M, Dai J, Lin Y, Yang L, Dong H, Li Y,
Ding Y and Duan Y: Effect of the cyclic stretch on the expression
of osteogenesis genes in human periodontal ligament cells. Gene.
491:187–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YH, Nahm DS, Jung YK, Choi JY and Kim
SG, Cho M, Kim MH, Chae CH and Kim SG: Differential gene expression
of periodontal ligament cells after loading of static compressive
force. J Periodontol. 78:446–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakao K, Goto T, Gunjigake KK, Konoo T,
Kobayashi S and Yamaguchi K: Intermittent force induces high RANKL
expression in human periodontal ligament cells. J Dent Res.
86:623–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Jiang L, Liao G, Chen G, Liu Y, Wang
J, Zheng Y, Luo S and Zhao Z: Centrifugal forces within
usually-used magnitude elicited a transitory and reversible change
in proliferation and gene expression of osteoblastic cells UMR-106.
Mol Biol Rep. 36:299–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YQ, Li XT, Rabie AB, Fu MK and Zhang
D: Human periodontal ligament cells express osteoblastic phenotypes
under intermittent force loading in vitro. Front Biosci.
11:776–781. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okada H and Murakami S: Cytokine
expression in periodontal health and disease. Crit Rev Oral Biol
Med. 9:248–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gamonal J, Acevedo A, Bascones A, Jorge O
and Silva A: Levels of interleukin-1 beta, -8 and -10 and RANTES in
gingival crevicular fluid and cell populations in adult
periodontitis patients and the effect of periodontal treatment. J
Periodontol. 71:1535–1545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delima AJ, Oates T, Assuma R, Schwartz Z,
Cochran D, Amar S and Graves DT: Soluble antagonists to
interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss
of tissue attachment in experimental periodontitis. J Clin
Periodontol. 28:233–240. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graves DT and Cochran D: The contribution
of interleukin-1 and tumor necrosis factor to periodontal tissue
destruction. J Periodontol. 74:391–401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Górska R, Gregorek H, Kowalski J,
Laskus-Perendyk A, Syczewska M and Madalinski K: Relationship
between clinical parameters and cytokine profiles in inflamed
gingival tissue and serum samples from patients with chronic
periodontitis. J Clin Periodontol. 30:1046–1052. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu
N, Ding B, Liu W, Liu Y, Shi H, et al: High levels of β-catenin
signaling reduce osteogenic differentiation of stem cells in
inflammatory microenvironments through inhibition of the
noncanonical Wnt pathway. J Bone Miner Res. 26:2082–2095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YS, Pi SH, Lee YM, Lee SI and Kim EC:
The anti-inflammatory role of heme oxygenase-1 in
lipopolysaccharide and cytokine-stimulated inducible nitric oxide
synthase and nitric oxide production in human periodontal ligament
cells. J Periodontol. 80:2045–2055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou
J, Jin Y and Chen FM: Comparison of mesenchymal stem cells derived
from gingival tissue and periodontal ligament in different
incubation conditions. Biomaterials. 34:7033–7047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omar OM, Granéli C, Ekström K, Karlsson C,
Johansson A, Lausmaa J, Wexell CL and Thomsen P: The stimulation of
an osteogenic response by classical monocyte activation.
Biomaterials. 32:8190–8204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ekström K, Omar O, Granéli C, Wang X,
Vazirisani F and Thomsen P: Monocyte exosomes stimulate the
osteogenic gene expression of mesenchymal stem cells. PLoS One.
8:e752272013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossa C Jr, Liu M, Patil C and Kirkwood
KL: MKK3/6-p38 MAPK negatively regulates murine MMP-13 gene
expression induced by IL-1beta and TNF-alpha in immortalized
periodontal ligament fibroblasts. Matrix Biol. 24:478–488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Tang Z, Xue R, Singh GK, Shi K, Lv
Y and Yang L: Combined effects of TNF-α, IL-1β and HIF-1α on MMP-2
production in ACL fibroblasts under mechanical stretch: An in vitro
study. J Orthop Res. 29:1008–1014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuji K, Uno K, Zhang GX and Tamura M:
Periodontal ligament cells under intermittent tensile stress
regulate mRNA expression of osteoprotegerin and tissue inhibitor of
matrix metalloprotease-1 and -2. J Bone Miner Metab. 22:94–103.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kusano K, Miyaura C, Inada M, Tamura T,
Ito A, Nagase H, Kamoi K and Suda T: Regulation of matrix
metalloproteinases (MMP-2, -3, -9 and -13) by interleukin-1 and
interleukin-6 in mouse calvaria: Association of MMP induction with
bone resorption. Endocrinology. 139:1338–1345. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J, Kitti U, Teronen O, Sorsa T, Husa V,
Laine P, Rönkä H, Salo T, Lindqvist C and Konttinen YT:
Collagenases in different categories of peri-implant vertical bone
loss. J Dent Res. 79:1870–1873. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lian JB and Stein GS: Runx2/Cbfa1: A
multifunctional regulator of bone formation. Curr Pharm Des.
9:2677–2685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda N, Yokoyama K, Takeshita S and
Watanabe M: Role of epidermal growth factor and its receptor in
mechanical stress-induced differentiation of human periodontal
ligament cells in vitro. Arch Oral Biol. 43:987–997. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawarizadeh A, Bourauel C, Götz W and
Jäger A: Early responses of periodontal ligament cells to
mechanical stimulus in vivo. J Dent Res. 84:902–906. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen B, Sun HH, Wang HG, Kong H, Chen FM
and Yu Q: The effects of human platelet lysate on dental pulp stem
cells derived from impacted human third molars. Biomaterials.
33:5023–5035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wescott DC, Pinkerton MN, Gaffey BJ, Beggs
KT, Milne TJ and Meikle MC: Osteogenic gene expression by human
periodontal ligament cells under cyclic tension. J Dent Res.
86:1212–1216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han LM, Li S, Wang L and Xu Y: Cyclic
tensile stress during physiological occlusal force enhances
osteogenic differentiation of human periodontal ligament cells via
ERK1/2-Elk1 MAPK pathway. DNA Cell Biol. 32:488–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Natali AN, Pavan PG and Scarpa C:
Numerical analysis of tooth mobility: Formulation of a non-linear
constitutive law for the periodontal ligament. Dent Mater.
20:623–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeh Y, Yang Y and Yuan K: Importance of
CD44 in the proliferation and mineralization of periodontal
ligament cells. J Periodontal Res. 49:827–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pabari S, Moles DR and Cunningham SJ:
Assessment of motivation and psychological characteristics of adult
orthodontic patients. Am J Orthod Dentofacial Orthop.
140:e263–e272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hajishengallis G: Aging and its impact on
innate immunity and inflammation: Implications for periodontitis. J
Oral Biosci. 56:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eke PI, Dye BA, Wei L, Thornton-Evans GO
and Genco RJ: CDC Periodontal Disease Surveillance workgroup: James
Beck (University of North Carolina, Chapel Hill, USA), Gordon
Douglass (Past President, American Academy of Periodontology), Roy
Page (University of Washin): Prevalence of periodontitis in adults
in the United States: 2009 and 2010. J Dent Res. 91:914–920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Wang H, Ye Q, Ye J, Xu C, Lin L,
Deng H and Hu R: Co-regulation of LPS and tensile strain
downregulating osteogenicity via c-fos expression. Life Sci.
93:38–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamaguchi M, Shimizu N, Shibata Y and
Abiko Y: Effects of different magnitudes of tension-force on
alkaline phosphatase activity in periodontal ligament cells. J Dent
Res. 75:889–894. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Long P, Hu J, Piesco N, Buckley M and
Agarwal S: Low magnitude of tensile strain inhibits
IL-1beta-dependent induction of pro-inflammatory cytokines and
induces synthesis of IL-10 in human periodontal ligament cells in
vitro. J Dent Res. 80:1416–1420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baba S, Kuroda N, Arai C, Nakamura Y and
Sato T: Immunocompetent cells and cytokine expression in the rat
periodontal ligament at the initial stage of orthodontic tooth
movement. Arch Oral Biol. 56:466–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bletsa A, Berggreen E and Brudvik P:
Interleukin-1alpha and tumor necrosis factor-alpha expression
during the early phases of orthodontic tooth movement in rats. Eur
J Oral Sci. 114:423–429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nokhbehsaim M, Deschner B, Winter J,
Reimann S, Bourauel C, Jepsen S, Jäger A and Deschner J:
Contribution of orthodontic load to inflammation-mediated
periodontal destruction. J Orofac Orthop. 71:390–402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rifas L: T-cell cytokine induction of
BMP-2 regulates human mesenchymal stromal cell differentiation and
mineralization. J Cell Biochem. 98:706–714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rifas L, Arackal S and Weitzmann MN:
Inflammatory T cells rapidly induce differentiation of human bone
marrow stromal cells into mature osteoblasts. J Cell Biochem.
88:650–659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nemoto T, Kajiya H, Tsuzuki T, Takahashi Y
and Okabe K: Differential induction of collagens by mechanical
stress in human periodontal ligament cells. Arch Oral Biol.
55:981–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding J, Ghali O, Lencel P, Broux O,
Chauveau C, Devedjian JC, Hardouin P and Magne D: TNF-alpha and
IL-1beta inhibit RUNX2 and collagen expression but increase
alkaline phosphatase activity and mineralization in human
mesenchymal stem cells. Life Sci. 84:499–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang P, Wu Y, Jiang Z, Jiang L and Fang
B: Osteogenic response of mesenchymal stem cells to continuous
mechanical strain is dependent on ERK1/2-Runx2 signaling. Int J Mol
Med. 29:1083–1089. 2012.PubMed/NCBI
|