Introduction
Streptococcus mutans (S. mutans) is
the most well recognized causative agent implicated in the
pathogenesis of dental caries (tooth decay), which is an infectious
disease of human dentition still exhibiting high global prevalence
(1–3). Virulence of this bacterium arises
from its ability to form a biofilm on teeth and produce organic
acids (acidogenicity) from dietary sucrose within the biofilm,
causing tooth decay (4,5). The structural matrix of the biofilm
consists of water-insoluble glucans synthesized from sucrose by
several isoforms of the glucosyltransferase (Gtf) enzyme present in
S. mutans bacteria (5). In
this respect, S. mutans produces water-insoluble and partly
water-soluble glucans using GtfB and GtfC enzymes encoded by
gtfB and gtfC genes, respectively. The synthesized
insoluble glucans possess a capacity to concentrate protons
generated by the proton-extruding F-type ATPase (F-ATPase), thereby
retaining acidogenicity of S. mutans biofilm (6). In addition, it has been reported that
biofilm bacteria have up to a 1,000-fold more tolerance to
antimicrobials than planktonic bacteria (7,8).
Therefore, it is important to develop novel pharmaceuticals in
order to inhibit S. mutans biofilm formation and its
acidogenicity.
Natural products have been optimized to interact
with biological systems through a long natural selection process
(9), and because of this, nature
is considered the best concocter of medicines and has been a source
of medicines for millennia (10–13).
During the last two decades, various plants have been tested for
their antimicrobial activity and many of them exhibit significant
antibacterial activity against Streptococcus species (14). In this context, Rhus coriaria
L. (sumac) fruits contain many of the bioactive compounds that
were characterized in detail by using high-performance liquid
chromatography-diode array detector-hyphenated with tandem mass
spectrometry (HPLC-DAD-ESI-MS/MS) in the investigation performed by
Abu-Reidah et al (15).
Among these bioactive components, methyl gallate (MG) has
antibacterial and anti-biofilm effects for S. mutans
bacteria, as reported by Kang et al (16). However, currently available studies
did not provide sufficient data whether MG can suppress the
development of S. mutans biofilm on polystyrene and glass
surfaces as well as inhibit acidogenicity. In the scientific report
of Kang et al (16),
colorimetric assay was applied for testing the effect of MG on
S. mutans adherence. Colorimetric assay is considered a
well-established method for quantification of the biofilm biomass
(17,18). It could be used as a pre-test for
checking potential existence of the biofilm. Quantitative
assessment of the developed biofilm should be further performed
applying an optical profilometry assay, by testing the effect on
both surface roughness and thickness parameters of the biofilm. To
our best of knowledge, this is the first article reporting the
effect of sumac methanolic extract and its constituent MG
(bioactive phytochemical that was isolated from the crude extract
and quantitated by HPLC) on biofilm formation by using an optical
profilometry assay.
Materials and methods
Materials
Rhus coriaria L. was purchased from Al Alim
Ltd. (Medicinal Herb Center, Zippori, Israel), and the fruits were
ground to yield a reddish-purple powder. MG (analytical standard)
was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
Plant extraction
The air-dried, powdered fruit of sumac plant (25 g)
was packed in an Erlenmeyer flask and extracted with 250 ml
methanol (MeOH), sonicated for 2 h at 45°C, then left in a dark
glass bottle at room temperature for 24 h for complete extraction.
The methanolic extract was filtered and evaporated to dryness with
a rotary vacuum evaporator.
Instruments
A Waters Alliance e2695 separations module, 2998
photo diode array (PDA) and Empower version 3 software was used
(Waters, Eschborn, Germany). The preparative HPLC system consisted
of a 3535-quaternary gradient module and a 996 PDA detector.
1H-NMR and 13C-NMR
measurements of isolated MG from sumac was carried out on a Bruker
Avance II 500 spectrometer, which was equipped with a 5 mm indirect
detection probe with Z gradient.
The UHPLC system (Accela; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was equipped with an XBridge ODS-column
(150×2.1 mm i.d.), 3.5-µm particle size (Waters, Milford, MA, USA)
and a mobile phase containing 0.1% formic acid (FA) as (eluent A)
and MeOH containing 0.1% FA as (eluent B). Linear gradient elution
was used starting with 95% A and continuously increased to 100% B
in 20 min. The UHPLC system was coupled to LTQ Orbitrap XL system
(Thermo Fisher Scientific, Inc.) equipped with an electrospray
ionization (ESI) source. The positive ionization mode was used at a
scan range of m/z 100–1000.
Chromatographic conditions
MG quantitation was run on a Waters HPLC ODS Column
(XBridge, 4.6 ID ×150 mm, 5 µm) with a guard column (Xbridge ODS,
20×4.6 mm ID, 5 µm). The mobile phase consisted of water (labeled
A) and acetonitrile (labeled B) solvent mixture. The gradient was
as follows: 95% A and 5% B at 0 min, held there for 2 min, then
raised to 50% A and 50% B over 13 min, then to 10% A and 90% B over
1 min, held there for 3 min, and finally returned to 95% A, 5% B
over 1 min. All of the samples were filtered with a 0.45 µm
micro-porous filter. The PDA wavelengths ranged from 210 to 500 nm,
and the monitoring wavelength of MG was 272 nm. The flow rate was 1
ml/min. The injection volume was 10 µl, and the column temperature
was at room temperature.
The UHPLC was equipped with an XBridge ODS-column
(150×2.1 mm i.d.), 3.5 µm particle size (Waters) and a mobile phase
containing 0.1% formic acid (FA) as (eluent A) and MeOH containing
0.1% FA as (eluent B). Linear gradient elution was used starting
with 95% A and continuously increased to 100% B in 20 min.
The prep-HPLC experiments were run on an ODS column
(Agilent PrepHT C18, 22.2×250 mm, 10 µm). The linear gradient
started at 98% A, where it stayed for 3 min; it was then raised to
5% A over 15 min, where it remained for another 4 min and was then
returned to 98% A over 2 min. The flow rate used was 15 ml/min, the
injection volume was 1,000 µl.
Methyl gallate standards
preparation
Five different concentration levels of standards for
MG were prepared: 10, 50, 100, 250 and 500 ppm in methanol
diluents. These standards were used to construct a calibration
curve to quantitate MG in sumac.
Sumac sample preparation for the
preparative HPLC injections
Crude sumac was prepared by dissolving ~3,000 mg in
15 ml of methanol, sonicated for 3 min and then filtered via a 0.45
µm membrane filter before injection. The concentration of the final
red solution was 200 mg/ml. This solution (1 ml) was injected into
the preparative HPLC Chromatograph and six fractions were
collected.
Bacterial strain and culture
conditions
Streptococcus mutans UA159 (700610; American
Type Culture Collection, Manassas, VA, USA) was selected for this
study because it preferably colonizes humans. Stocks of this strain
were maintained in 10% skimmed milk (Difco; BD BioSciences,
Franklin Lakes, NJ, USA) at −70°C until use. Prior to experiments,
S. mutans was cultured in Bacto™ Todd Hewitt broth (THB; BD
BioSciences) under anaerobic conditions (95% N2 and 5%
CO2) at 37°C for 18 h. Purity of the culture was checked
on Mitis Salivarius agar (Difco; BD BioSciences) and Columbia agar
with 7% sheep blood (E&O Laboratories, Bonnybridge,
Scotland).
Biofilm formation and treatments
To evaluate the effect of treatments, S.
mutans biofilm formation was performed on polystyrene and glass
surfaces in separate experiments. Prior to each experiment, the
optical density (OD) of the bacterial culture was adjusted to 0.2
at 630 nm, using a microplate-reader spectrophotometer. For biofilm
formation on the polysterene surface, 24-well, flat-bottomed,
polystyrene cell culture plates (Sarstedt, Nümbrecht, Germany) were
filled with THB containing 1% sucrose, and then solutions of
methanolic sumac extract (MSE), prepared in sterile distilled water
(Milli-Q water), were added to appropriate wells in the plates at
final concentrations of 4, 5 and 6 mg/ml. Solutions of MG, also
prepared in Milli-Q water, were added to appropriate wells in the
plates at final concentrations of 0.55, 0.7, 0.85 and 1 mg/ml.
Afterwards, S. mutans bacteria was added to the wells at a
final dilution of 1:100, and all of the plates were incubated
anaerobically at 37°C for 24 h. Quantification of the formed
biofilm (biomass) was performed using a colorimetric assay. The
same experimental procedures were used for biofilm formation on the
glass surface, except that sterile glass slides of 1-mm thickness,
cut from standard microscope slides (76×26 mm; Thermo Fisher
Scientific, Inc.), were inserted vertically into wells containing
only MG at the above indicated concentrations prior to inoculation
of bacteria. Quantitative assessment of the developed biofilm was
further performed applying an optical profilometry assay. In these
experiments, plate wells without bacterial cells were used as blank
controls, and the untreated bacteria served as experimental
controls.
Colorimetric assay
After 24 h of incubation, THB was discarded from
plates, wells were rinsed with distilled water to remove loosely
bound bacterial cells, and then adherent bacteria were fixed with
95% ethanol. For quantification of biofilm biomass, the fixed and
air-dried S. mutans biofilm in plate wells was stained with
1 ml/well of 0.01% crystal violet solution (Merck KGaA) for 15 min,
and then the bound dye was extracted using 1 ml/well of 33% acetic
acid solution (Merck KGaA) for 30 min. Afterwards, 200 µl extracted
dye solution from each well was transferred to appropriate wells in
an optically clear, flat-bottomed 96-well microplate. The OD of the
samples was measured at a wavelength of 595 nm with a
microplate-reader spectrophotometer. Background staining was
corrected for by subtracting amount of the staining in blank
wells.
Optical profilometry assay
Following 24 h of incubation, glass slides with
adherent S. mutans biofilm were removed from plate wells,
air-dried and further analyzed using a non-contact optical imaging
profilometer Sensofar PLµ 2300 system (Terrassa, Spain), using a
×50 confocal objective with a view field of 253×190 µm. Primarily,
six regions of the glass slide were scanned seeking to evaluate
surface roughness per slide halfway from bottom to top of visible
biofilm. Afterwards, a vertical scratch was artificially made down
the glass surface by a scalpel in the middle of every slide covered
with biofilm, and then five regions of the glass slide were scanned
to assess the biofilm thickness per slide halfway from the bottom
to the top of the visible biofilm. The bottom of the scratch served
as a reference point for accurate measurement of the biofilm
thickness. All images were captured in a vertical scanning mode,
and data of the collected images were further processed using
Gwyddion software (version 2.40; http://gwyddion.net) to calculate the surface
roughness and biofilm thickness parameters. A median filter (10
pixels or 3 µm) was selected to remove the errors of form and
waviness. Root mean square roughness (Rq), as the
most critical parameter, was calculated to evaluate quantitatively
the slide surface roughness, which indicated adherence of bacteria.
The Rq parameter is an average of the measured
height deviations taken within the evaluation length and measured
from the mean line. Indeed, Rq represents
standard deviation of the surface profile heights, and it is
calculated according to ISO 4287/1-1997 standard by the following
formula:
Rq=1N∑j=1Nrj2
Where N is the number of points within a
sampling length, and rj is the height value at point
j. To measure the biofilm thickness, which indicated the
maturity of biofilm, the height of the artificially produced
vertical scratch on each slide with adherent bacteria was used.
Calculation of the biofilm thickness involved generation of the
height distribution graph curve from the entire area of the scanned
region containing the scratch, followed by Gaussian function
fitting as defined here:
f(x)=y0+aexp[–(x–x0)2/b2]
Where y0 is the peak height,
a is the amplitude (height) distribution,
x0 is the peak position, and b is the
standard deviation. Background for the parameters of surface
roughness and biofilm thickness was corrected for by subtracting
the Rq and thickness values of a blank glass
slide.
Biofilm acidogenicity
S. mutans biofilm formation and treatments
were performed using the same procedures as described above. After
24 h of incubation, the biofilm growth medium (THB) was collected
from the wells of all plates and transferred to 1.5 ml
microcentrifuge tubes. The pH of S. mutans biofilm growth
medium collected in 1.5 ml microcentrifuge tubes was measured with
a microelectrode connected to a benchtop pH meter (Knick 766
Calimatic) at room temperature. The microelectrode was calibrated
using standard pH buffers (pH 4.01 and 7.00) prior to and following
each measurement.
Statistical analysis
Data were analyzed using SPSS version 23.0 (IBM
Corp., Armonk, NY, USA). Differences between the control
(untreated) and treatment groups were evaluated applying one-way
analysis of variance followed by a post hoc least significant
difference test for multiple comparisons. Data are presented as the
mean ± standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of the MSE on S. mutans biofilm
formation on polystyrene surface
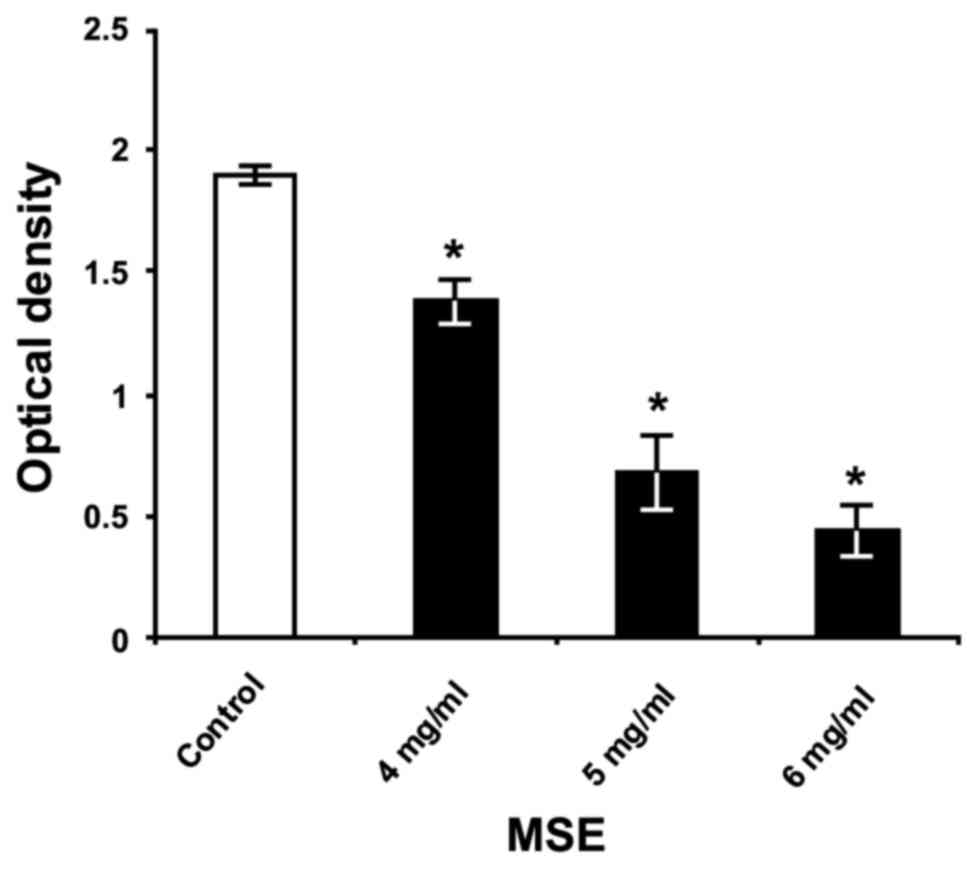
The primary assessment of crude MSE efficiency for
the inhibition of S. mutans biofilm formation showed its
capacity to reduce significantly the biofilm biomass on the
polystyrene surface in a dose-dependent manner (Fig. 1). The greatest inhibitory effect of
crude extract was achieved using a concentration of 6 mg/ml, which
decreased S. mutans biofilm formation by 77%, compared to
the formation of untreated bacteria (P<0.05) cultured in THB
with 1% sucrose.
Analysis of methanol extract of sumac
and isolation of the bioactive phytochemical
Sumac is very rich in phenolic and anthocyanin
compounds. These classes of compounds are known to exhibit a range
of biological activities. In the attempt to isolate the active
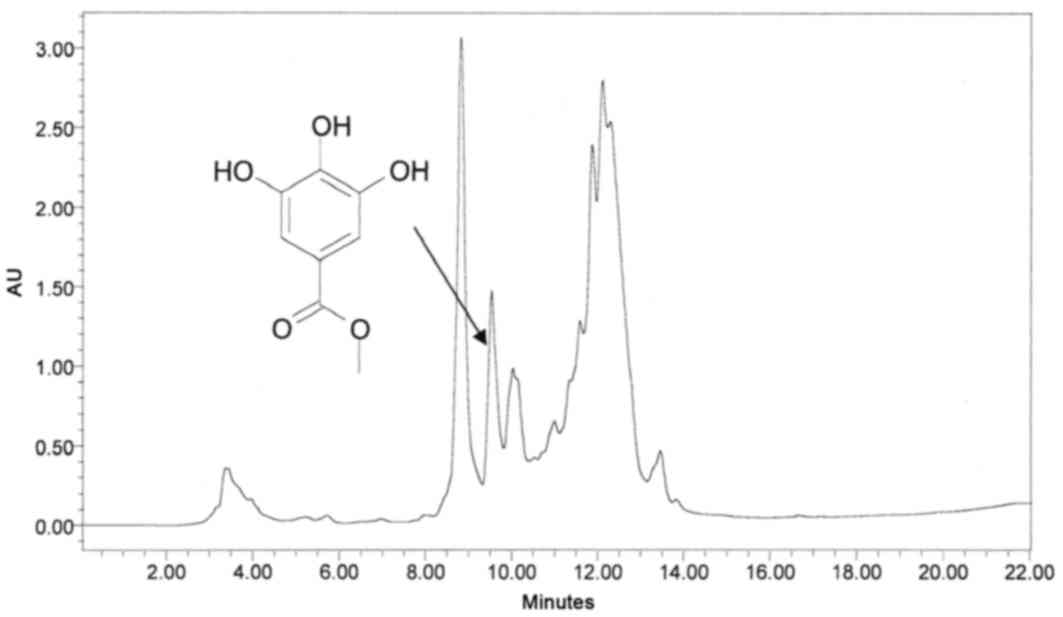
ingredients from sumac, several analytical HPLC runs were performed
using different mobile phases. Upon scaling up using preparative
HPLC, separations were carried out by collecting six fractions,
which were subjected to antibacterial assays. A typical preparative
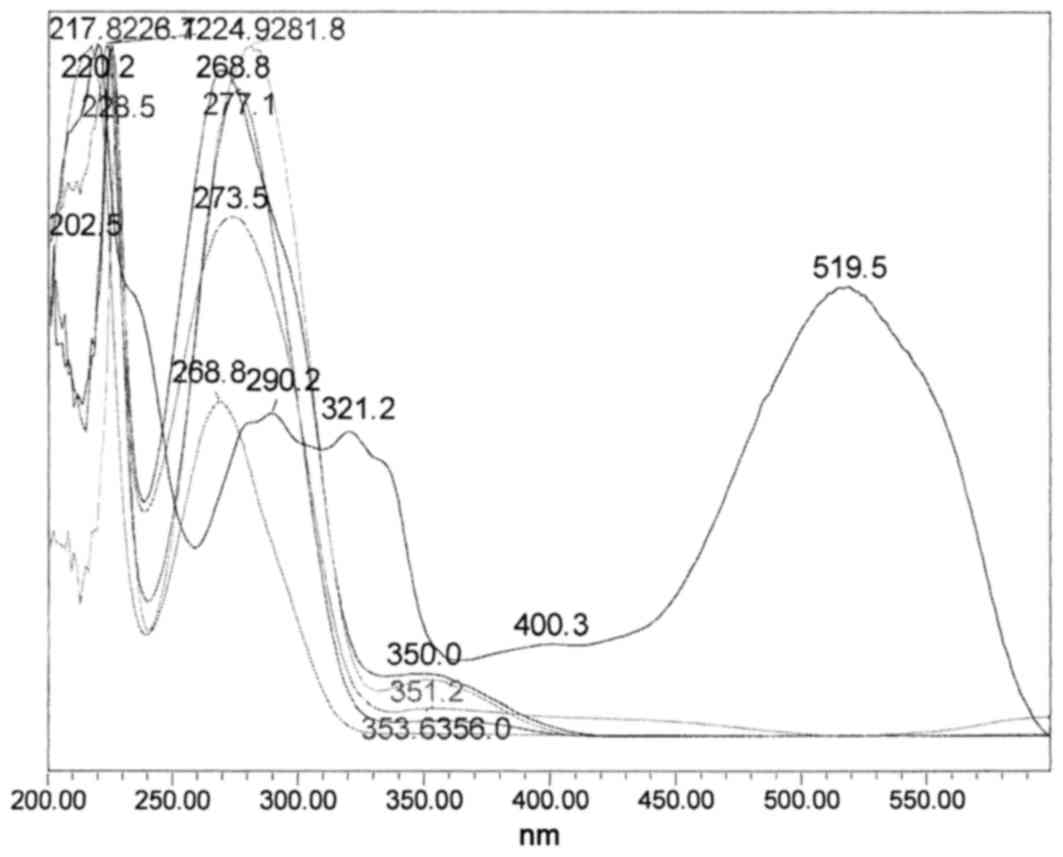
HPLC chromatogram of the crude methanol mixture and its
corresponding overlaid UV-Vis spectra, in the range of 210–500 nm,
are presented in Figs. 2 and
3, respectively.
The ultraviolet-visible spectra demonstrated many
phenolics that possess typical absorption maximums between 268.8
and 277.1 nm. There was also a very strong absorption maximum at
519.5 nm, which is a typical anthocyanin compound that is
responsible for the red pigment in sumac (Fig. 3).
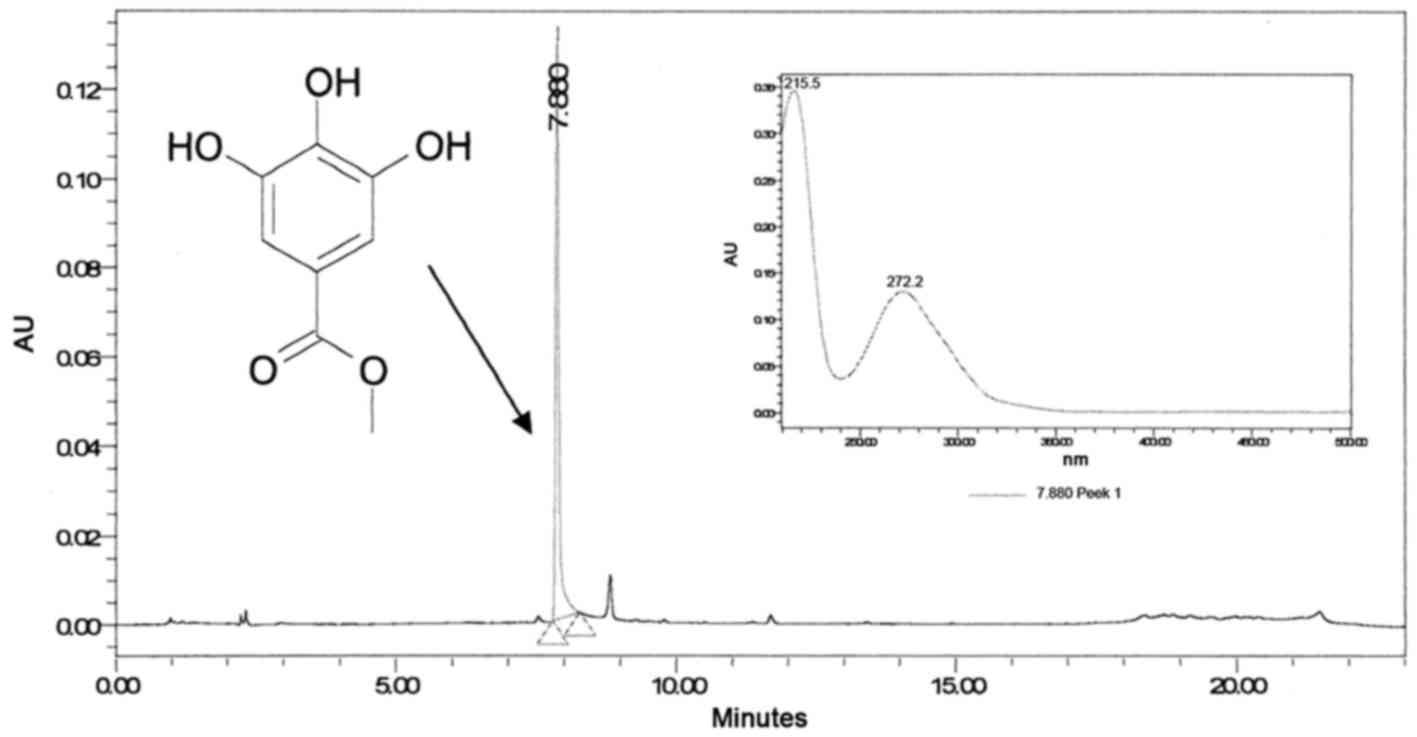
Fraction III (Fig.
2) contains pure compound, which, upon reinjection using
analytical HPLC-PDA, reached a peak at 7.88 min with a maximum
wavelength of 272 nm (Fig. 4).
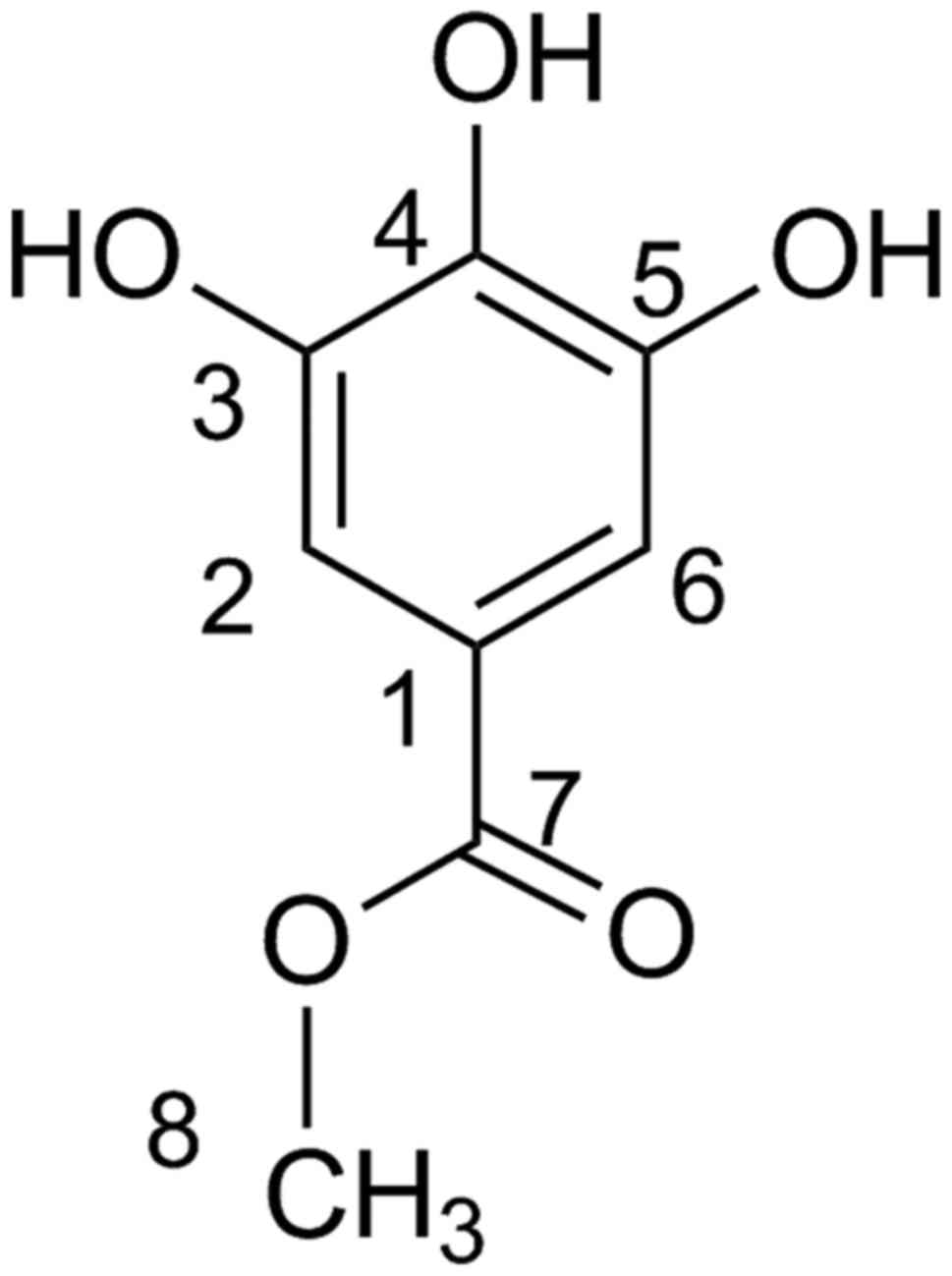
Accurate high-resolution mass spectroscopy (HR-MS)
and 1H-NMR and 13C-NMR disclosed the identity
of the peak at 7.88 min to be MG (Fig.
5). The HR-QTOF-MS using the ESI in the positive mode gave a
protonated molecular ion of [M+H]+ at m/z
185.0452 Da (calculated for
C8H9O5+, m/z
185.0450 Da). 1H-NMR (CD3OD) gave peaks at δ:
3.8 (3H s, -OCH3), 7.05 (2H s, H-2/H-6).
13C-NMR (CD3OD) gave peaks at δ: 52 (C-8),
109 (C-2/C-6), 120.5 (C-1), 139 (C-4), 145.3 (C-3/C-5), 168
(C-7).
Analytical HPLC was used to construct a calibration
curve of MG at 5 levels (10, 25, 50, 100, 250 and 500 ppm) with a
coefficient of determination (R2) of >0.999.
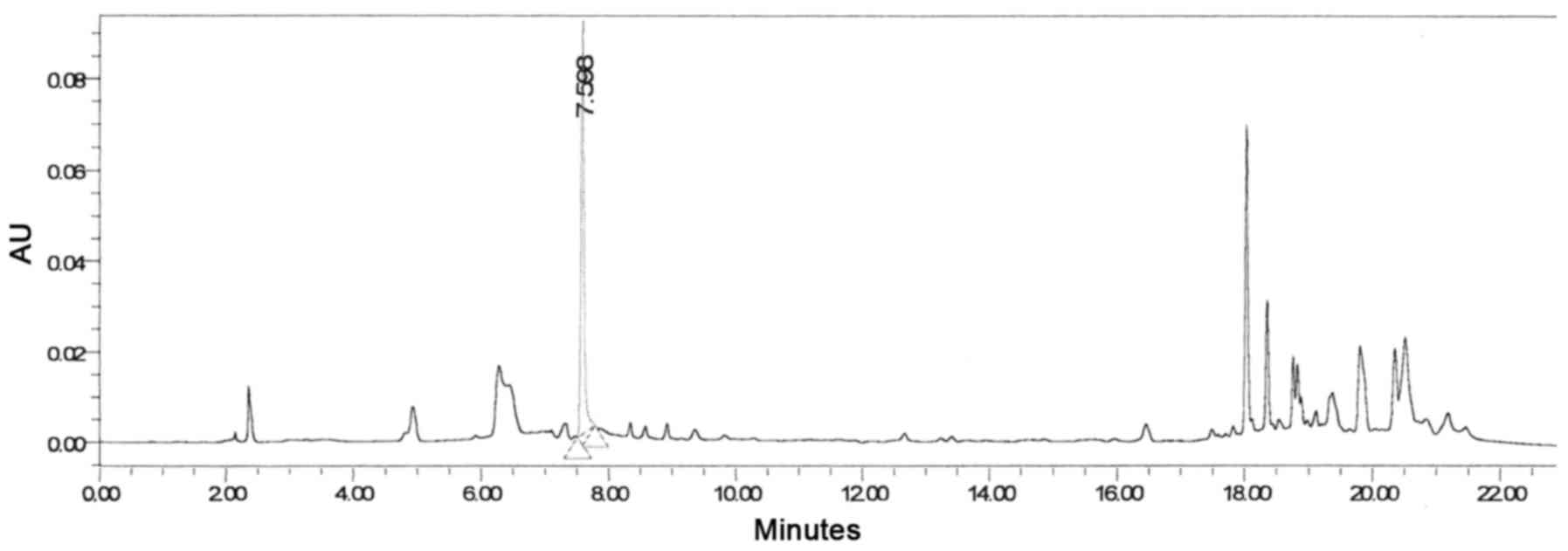
The concentration of MG in the methanol extract was identified to
be 4.85 ppm. Many other peaks that have a more lipophilic character
were eluted between 18 and 22 mins (Fig. 6).
Effect of MG on S. mutans biofilm
formation on polystyrene and glass surfaces
In an effort to evaluate anti-biofilm activity, the
isolated MG (fraction III) was further tested on S. mutans
strain UA159 because of its well-defined features to form biofilms
on various solid surfaces (19,20).
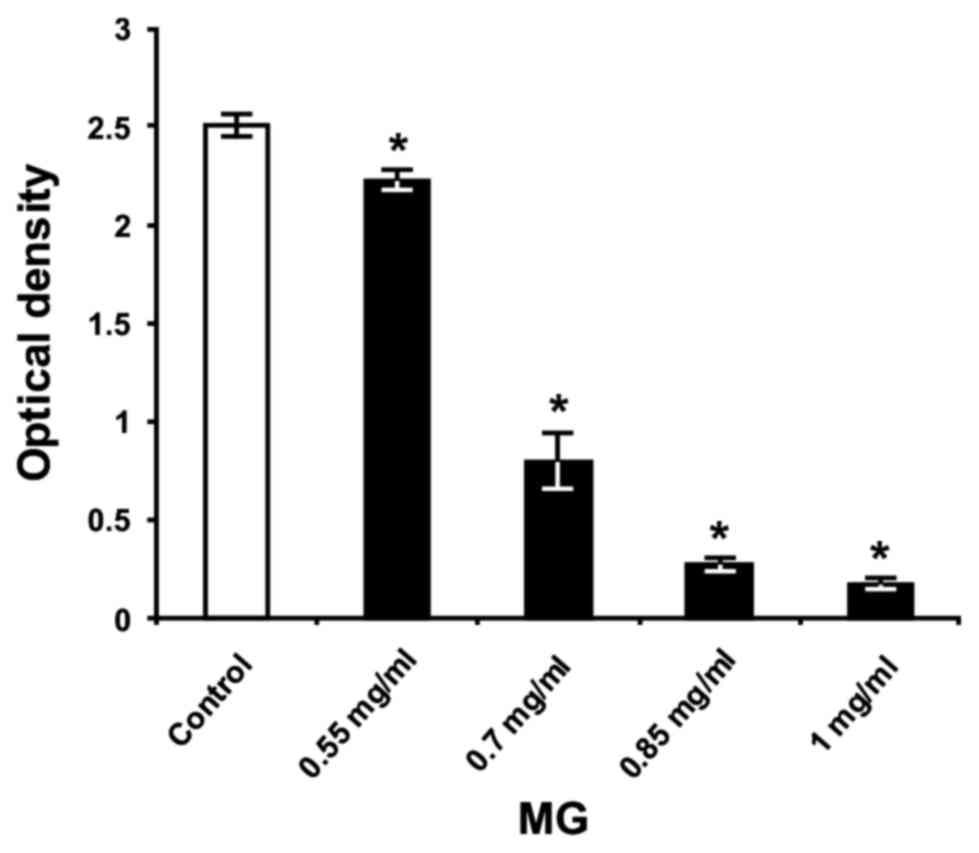
As presented in Fig. 7, all of the
concentrations of MG significantly reduced S. mutans biofilm
biomass on the polystyrene surface, compared with untreated
bacteria (P<0.05) in THB containing 1% sucrose. The anti-biofilm
activity of MG occurred in a dose-dependent manner, exhibiting its
highest effect at concentrations of 0.7, 0.85 and 1 mg/ml, with
biofilm inhibition at 68, 89 and 93%, respectively. These results
confirmed the findings reported by Kang et al (16), where the researchers demonstrated
using beaker-wire tests that 1 mg/ml MG significantly reduced the
wet weight of S. mutans (strain Ingbritt) biofilm biomass in
comparison with untreated bacteria, after 24 h of incubation in
medium containing 5% sucrose.
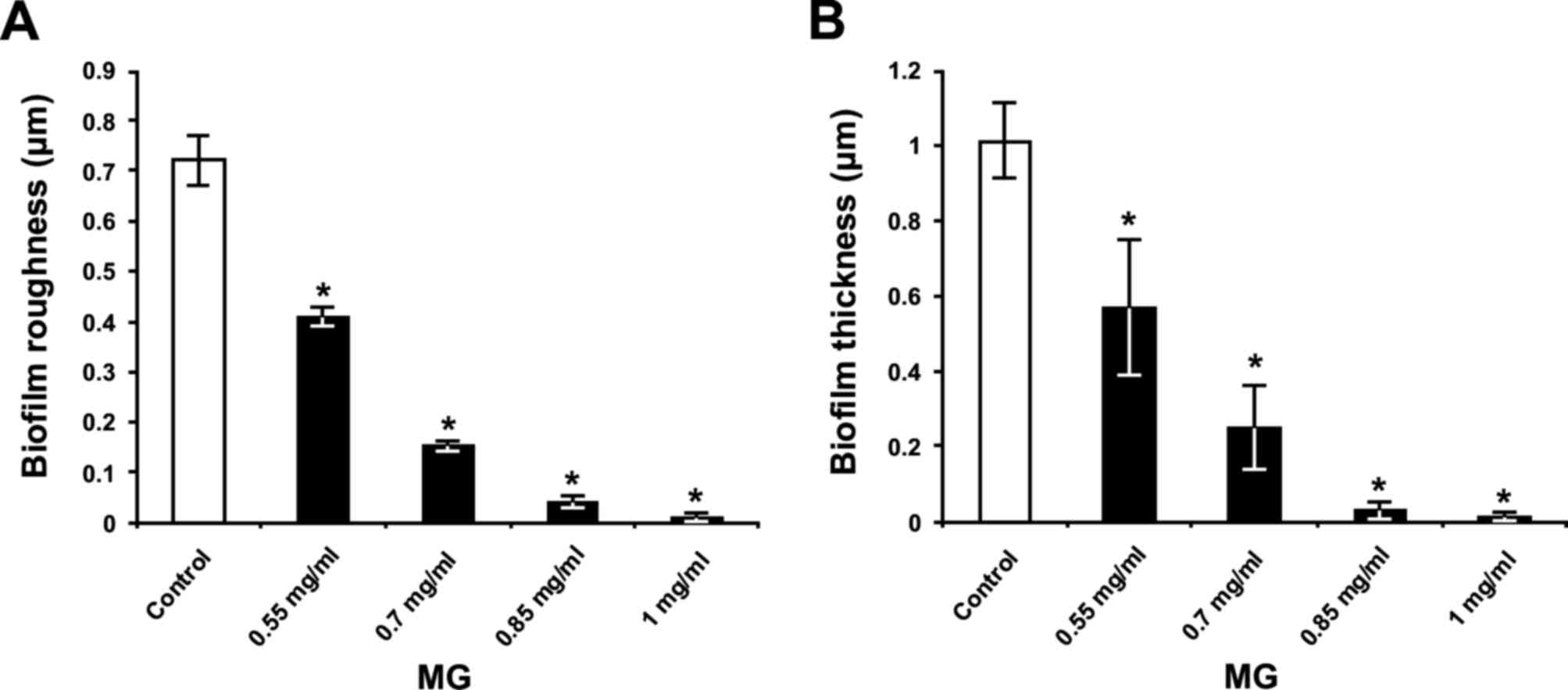
The optical profilometry technique, applied in
analysis of the surfaces of glass slides with S. mutans
biofilm, was used to confirm the results of the colorimetric assay.
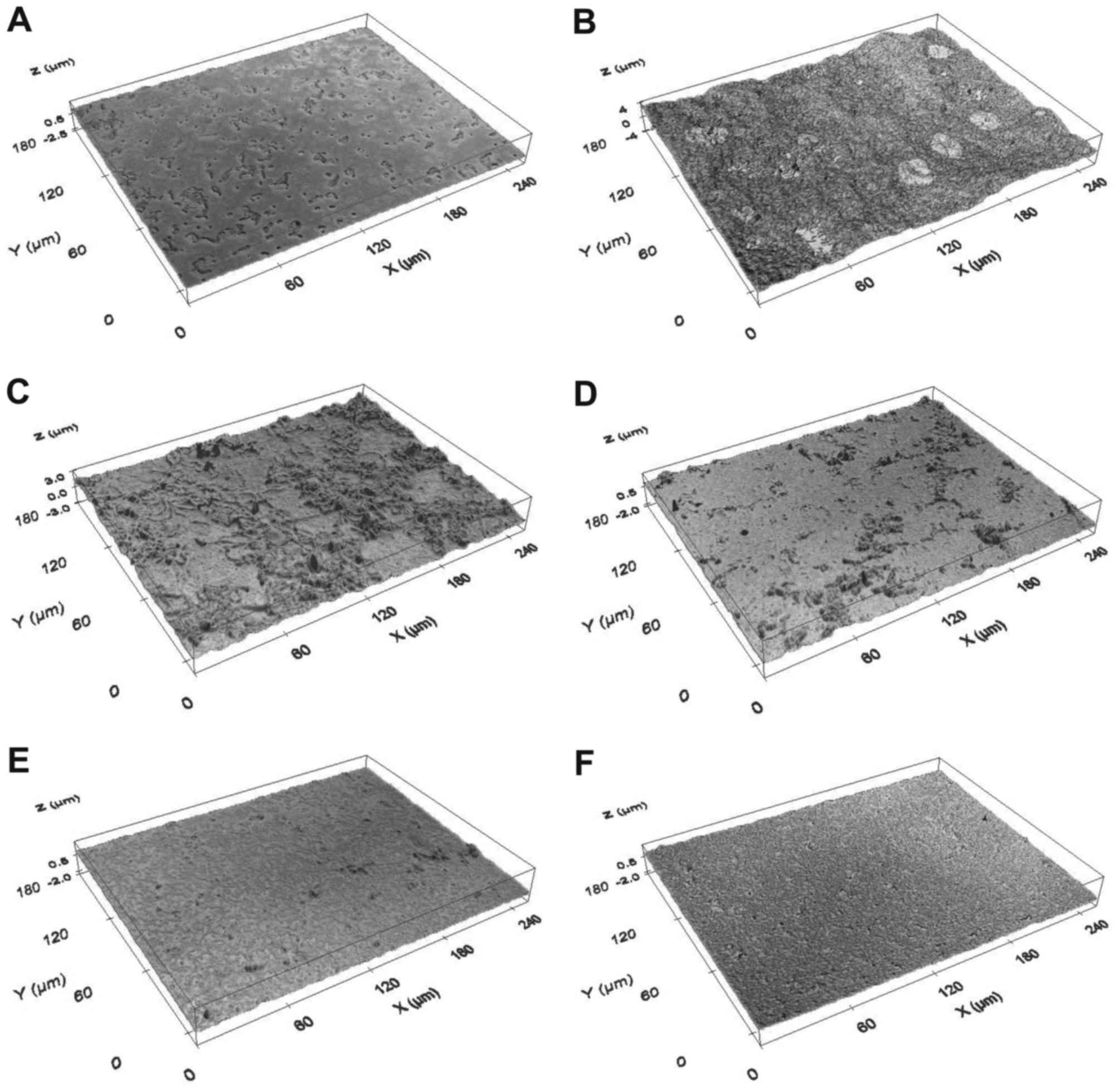
Firstly, compared with bacteria incubated without MG and sucrose
(Fig. 8A), where the
Rq and thickness parameters for the untreated
bacteria grown without sucrose were 0.04±0.01 and 0.02±0.01 µm,
respectively, presence of 1% sucrose in THB induced adherence of
the bacteria to glass slides and subsequent maturation of the
biofilm (Fig. 8B). Secondly, in
the THB with 1% sucrose, exposure of S. mutans to 0.55, 0.7,
0.85 and 1 mg/ml MG (Figs. 8C-F,
respectively) decreased formation of the biofilm on glass surface
in a dose-dependent manner. Quantification revealed that surface
roughness parameter Rq of the biofilm (Fig. 9A) and biofilm thickness (Fig. 9B) were increased in control
bacteria (Fig. 9); however, MG
treatment inhibited this effect in a dose-dependent manner
(P<0.05; Fig 9). In this
respect, MG concentrations of 0.85 and 1 mg/ml exhibited the
greatest effects for reducing the surface roughness parameter
Rq of biofilm, by 94 and 99%, respectively
(Fig. 9A). Furthermore, these
concentrations of MG decreased S. mutans biofilm thickness
by 97 and 99%, respectively (Fig.
9B).
Effect of MG on S. mutans biofilm
acidogenicity
The pH measurements of the collected S.
mutans biofilm growth medium demonstrated that bacteria grown
in THB with 1% sucrose produced organic acids from fermentation of
this carbohydrate, leading to an ~1.8-fold decrease in pH, compared
with the blank group (Tables I and
II). This substantial decrease of
pH indicated increased acidogenicity in the S. mutans
biofilm. However, exposure of the bacteria to crude MSE led to
augmentation of pH in a dose-dependent manner, though not up to the
pH levels of the blank group (Table
I). In contrast, treatment of S. mutans bacteria with
the isolated MG significantly prevented a decrease in the pH level,
compared with untreated bacteria grown in THB with 1% sucrose
(P<0.05), by increasing the pH almost up to the pH levels of the
blank group (Table II). This
preventive effect of MG occurred in a dose-dependent manner, and MG
concentrations from 0.7 to 1 mg/ml increased pH by 96–97%.
Therefore, similarly to reduction of the biofilm biomass, crude MSE
was unable to fully suppress the pH decrease in the S.
mutans biofilm due to reduced concentration of the biologically
active compound within it. However, the isolated MG was able to
almost completely inhibit the acidogenicity of the S. mutans
biofilm.
 | Table I.pH levels of the Streptococcus
mutans biofilm growth medium after 24 h of incubation in the
presence of 1% sucrose and different concentrations of methanolic
sumac extract. |
Table I.
pH levels of the Streptococcus
mutans biofilm growth medium after 24 h of incubation in the
presence of 1% sucrose and different concentrations of methanolic
sumac extract.
| Experimental
group | pH |
|---|
| Blank |
7.20±0.04a |
| Control | 4.07±0.03 |
| MSE (4 mg/ml) |
4.77±0.09a |
| MSE (5 mg/ml) |
5.21±0.11a |
| MSE (6 mg/ml) |
5.30±0.07a |
 | Table II.pH levels of the Streptococcus
mutans biofilm growth medium after 24 h of incubation in the
presence of 1% sucrose and different concentrations of methyl
gallate. |
Table II.
pH levels of the Streptococcus
mutans biofilm growth medium after 24 h of incubation in the
presence of 1% sucrose and different concentrations of methyl
gallate.
| Experimental
group | pH |
|---|
| Blank |
7.47±0.02a |
| Control | 4.28±0.02 |
| MG (0.55
mg/ml) |
6.14±0.17a |
| MG (0.7 mg/ml) |
7.17±0.03a |
| MG (0.85
mg/ml) |
7.27±0.01a |
| MG (1 mg/ml) |
7.28±0.02a |
Discussion
The present study demonstrated that the dominant and
most antibacterial active compound of Rhus coriaria L. is
MG. Using an in vitro system with S. mutans bacteria,
it was demonstrated that MG inhibits growth of bacteria, suppresses
the biofilm development on different solid surfaces (polystyrene,
glass) and prevents the pH decrease in biofilm. Notably, these
actions of MG occurred in a dose-dependent manner. It should be
noted that Vahid-Dastjerdi et al (21) demonstrated the significant effect
of Rhus coriaria L. water extract in reducing the expression
of S. mutans gtfB, gtfC and gtfD genes. Another study
performed by Thimothe et al (22) demonstrated that red wine grape
phenolic extracts, containing high amounts of gallic acid, may
inhibit the activity of GtfB and C enzymes at 70–85% (22). Furthermore, in the same study,
these phenolic extracts also suppressed S. mutans F-ATPase
activity by 30–65%. In addition, the findings reported by Choi
et al (23) indicated that
MG isolated from Galla Rhois may attenuate the action of
proton-driven ATPases in Salmonella spp. bacteria. Finally,
it is noteworthy that, in the presence of sucrose, S. mutans
adhesion to glass surface and subsequent formation of the biofilm
is mainly dependent on the activity of Gtfs, especially those
synthesizing water-insoluble glucans (24). Taking into consideration the
results of the present study and the studies outlined herein, it
may be reasonable to hypothesize that the anti-biofilm effect of MG
occurred because of the downregulation of glucosyltransferases, and
the decrease of acidogenicity was due to inhibition of F-ATPases in
S. mutans bacteria. However, the exact mechanisms for these
biological effects of MG require elucidation through further
studies.
In conclusion, the present study demonstrated that
Rhus coriaria L. (sumac) contains the biologically active
antibacterial natural compound MG. Additionally, MG was revealed to
inhibit S. mutans biofilm formation on polystyrene and glass
surfaces, and suppress the acidogenicity of the S. mutans
biofilm. Taken together, these findings are important for the
development of novel pharmaceuticals and formulations of natural
products and extracts that possess anti-biofilm activities with
primary applications for oral health, and in a broader context, for
the treatment of various bacterial infections.
Acknowledgements
The present study was supported by the Al-Qasemi
Research Foundation, the Ministry of Science, Technology and Space
(Israel) and the Faculty of Medicine, Vilnius University
(Lithuania).
Glossary
Abbreviations
Abbreviations:
|
S. mutans
|
Streptococcus mutans
|
|
MSE
|
methanolic sumac extract
|
|
MG
|
methyl gallate
|
|
HPLC
|
high-performance liquid
chromatography
|
References
|
1
|
Klein MI, Hwang G, Santos PH, Campanella
OH and Koo H: Streptococcus mutans-derived extracellular
matrix in cariogenic oral biofilms. Front Cell Infect Microbiol.
5:102015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quivey RG, Koo H, Lemos J and
Kopycka-Kedzierawski DT: Dental caries: General concepts. Oral
microbiology and immunology. 2nd. Lamont RJ, Hajishengallis GN and
Jenkinson HF: ASM Press; pp. 229–250. 2014
|
|
3
|
Kassebaum NJ, Bernabé E, Dahiya M,
Bhandari B, Murray CJ and Marcenes W: Global burden of untreated
caries: A systematic review and metaregression. J Dent Res.
94:650–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krzyściak W, Jurczak A, Kościelniak D,
Bystrowska B and Skalniak A: The virulence of Streptococcus
mutans and the ability to form biofilms. Eur J Clin Microbiol
Infect Dis. 33:499–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koo H, Falsetta ML and Klein MI: The
exopolysaccharide matrix: A virulence determinant of cariogenic
biofilm. J Dent Res. 92:1065–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo L, McLean JS, Lux R, He X and Shi W:
The well-coordinated linkage between acidogenicity and aciduricity
via insoluble glucans on the surface of Streptococcus
mutans. Sci Rep. 5:180152015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venkatesan N, Perumal G and Doble M:
Bacterial resistance in biofilm-associated bacteria. Future
Microbiol. 10:1743–1750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoyle BD and Costerton JW: Bacterial
resistance to antibiotics: The role of biofilms. Prog Drug Res.
37:91–105. 1991.PubMed/NCBI
|
|
9
|
Gademann K: Copy, edit, and paste: Natural
product approaches to biomaterials and neuroengineering. Acc Chem
Res. 48:731–739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cragg GM and Newman DJ: Medicinals for the
millennia: The historical record. Ann N Y Acad Sci. 953:3–25. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaid H, Raiyn J, Nasser A and Rayan A:
Physicochemical properties of natural based products versus
synthetic chemicals. Open Nutraceuticals J. 3:194–202. 2010.
View Article : Google Scholar
|
|
12
|
Awaisheh SS and Ibrahim SA: Screening of
antibacterial activity of lactic acid bacteria against different
pathogens found in vacuum-packaged meat products. Foodborne Pathog
Dis. 6:1125–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kadan S, Rayan M and Rayan A: Anticancer
activity of anise (Pimpinella anisum L.) seed extract. Open
Nutraceuticals J. 6:1–5. 2013. View Article : Google Scholar
|
|
14
|
Abachi S, Lee S and Rupasinghe HP:
Molecular mechanisms of inhibition of streptococcus species by
phytochemicals. Molecules. 21:pii: E215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Reidah IM, Ali-Shtayeh MS, Jamous RM,
Arráez-Román D and Segura-Carretero A: HPLC-DAD-ESI-MS/MS screening
of bioactive components from Rhus coriaria L. (Sumac)
fruits. Food Chem. 166:179–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang MS, Oh JS, Kang IC, Hong SJ and Choi
CH: Inhibitory effect of methyl gallate and gallic acid on oral
bacteria. J Microbiol. 46:744–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh K, Senadheera DB, Lévesque CM and
Cvitkovitch DG: The copYAZ operon functions in copper efflux,
biofilm formation, genetic transformation, and stress tolerance in
Streptococcus mutans. J Bacteriol. 197:2545–2557. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Merritt JH, Kadouri DE and O'Toole GA:
Growing and analyzing static biofilms. Curr Protoc Microbiol
Chapter. 1:Unit 1B.1. 2005. View Article : Google Scholar
|
|
19
|
Shemesh M, Tam A and Steinberg D:
Differential gene expression profiling of Streptococcus
mutans cultured under biofilm and planktonic conditions.
Microbiology. 153:1307–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shemesh M, Tam A, Aharoni R and Steinberg
D: Genetic adaptation of Streptococcus mutans during biofilm
formation on different types of surfaces. BMC Microbiol. 10:512010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vahid-Dastjerdi E, Monadi E, Khalighi HR
and Torshabi M: Down-regulation of glycosyl transferase genes in
Streptococcus mutans by Punica granatum L. flower and
Rhus coriaria L. fruit water extracts. Iran J Pharm Res.
15:513–519. 2016.PubMed/NCBI
|
|
22
|
Thimothe J, Bonsi IA, Padilla-Zakour OI
and Koo H: Chemical characterization of red wine grape (Vitis
vinifera and Vitis interspecific hybrids) and pomace
phenolic extracts and their biological activity against
Streptococcus mutans. J Agric Food Chem. 55:10200–10207.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JG, Mun SH, Chahar HS, Bharaj P, Kang
OH, Kim SG, Shin DW and Kwon DY: Methyl gallate from Galla rhois
successfully controls clinical isolates of Salmonella infection in
both in vitro and in vivo systems. PLoS One. 9:e1026972014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ooshima T, Matsumura M, Hoshino T,
Kawabata S, Sobue S and Fujiwara T: Contributions of three
glycosyltransferases to sucrose-dependent adherence of
Streptococcus mutans. J Dent Res. 80:1672–1677. 2001.
View Article : Google Scholar : PubMed/NCBI
|