Introduction
Diabetes mellitus is among the most common chronic
metabolism diseases, and its incidence continues to increase.
Epidemiologically, 250 million patients are diagnosed with diabetes
annually, and this number is projected to increase to about 380
million by 2025 (1). Diabetes
mellitus is divided into two subgroups: type 1 diabetes mellitus
(T1DM) and types 2 diabetes mellitus (T2DM). T1DM is an autoimmune
disease characterized by destruction of islet beta cells in the
pancreas, which results in the complete cessation of insulin
production (2). By contrast, T2DM
is a heterogeneous disorder caused by a progressive decline in
insulin resistance in the liver and peripheral tissues, accompanied
by the inability of beta cells to compensate for insulin resistance
(3). Abnormality of glucose
metabolism is the central characteristic of diabetes mellitus.
Accordingly, the pathogenesis of glucose metabolism is a major
topic of research in this field.
The key enzymes, glucose-6-phosphatase (G-6-P) and
glycogen synthase kinase-3 (GSK-3) regulate glycogen and
gluconeogenesis. G-6-P is the rate-limiting enzyme for the
transformation of glucose-6-phosphate to glucose, affecting
glycogen output and blood glucose level (4). GSK-3 is the rate-limiting enzyme
responsible for gluconeogenesis (5). Two types of GSK-3-GSK-3α and
GSK-3β-have been reported to regulate G-6-P expression (6). GSK-3β can phosphorylate glycogen
synthase and inhibit the synthesis of glycogen. In turn, this
process leads to the phosphorylation of insulin receptor
substrate-1 (IRS-1), interrupting insulin signaling and producing
insulin resistance (7). Thus, the
regulation of key enzymes involved in the inhibition of glycogen
synthesis is a critical step in the search for an effective
treatment for diabetes mellitus.
T2DM is a multifactorial disease, with genetic and
environmental factors contributing to its development (8). Although western medicine has achieved
much improvement in T2DM medications, side effects and high costs
continue to pose challenges (9).
As a chronic disease, T2DM requires long-term treatment, and side
effects such as weight gain, bone loss, and increased
cardiovascular risk are unavoidable. Moreover, continuous health
care utilization poses huge economic burdens on societies and
families. Hence, alternative agents with fewer side effects and
lower costs are urgently needed. Herbal medications can be good
alternatives, replacing or at least supplementing western
medications (10,11). Because most of these medications
are extracted from plants, they are characterized by low cost and
few side effects. In Chinese medicine, several thousand years of
history and experience in the use of herbal medications to prevent
and treat T2DM have been documented.
Centella asiatica (C. asiatica) has
been used widely in Chinese medicine to treat varicose veins and
chronic venous insufficiency, and in ointments to treat psoriasis
and help heal minor wounds (12).
Asiatic acid (AA) is an active component of C. asiatica with
many biological activities, including anti-oxidant (13), liver-protecting (14), lipid-lowering (15), anticancer (16), and anti-diabetic (17) effects. Mechanical studies have
suggested that AA lowers glucose levels through anti-inflammatory
action, regulation of glucose metabolism enzymes, and anti-fibrotic
action in the pancreatic islets (15,18).
However, the signaling pathway involved in the effects of AA on
glucose level in diabetes mellitus remains unknown.
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT) pathway is important in the regulation of the
insulin signaling cascade (19).
The activation of PI3K/AKT negatively mediates GSK-3β and affects
glycogen synthesis. In this study, a T2DM (db/db) mouse model was
used to evaluate the PI3K/AKT/GSK-3β signaling pathway involved in
the protective effects of AA on diabetes symptoms.
Materials and methods
Chemicals and reagents
AA (lyophilized powder) was purchased from Chengdu
PuRuiFa Technology Development Co. Ltd (Chengdu, China).
Pioglitazone hydrochloride (PH) (batch no. Zhunzi H20040631) was
purchased from Jiangsu Hengrui Medicine Co., Ltd. (Jiangsu, China).
Assay kits for glucose (glucose oxidase), total cholesterol
(COD-PAP), triglycerides (TG; GOP-PAP), high-density lipoprotein
(HDL) cholesterol (direct hydrogen peroxide scavenging method), and
low-density lipoprotein (LDL) cholesterol (direct surfactant
removal method), and hematoxylin and eosin (H&E) and glycogen
staining kits were obtained from Beijing LEYBOLD Cable Technology
Co., Ltd. (Beijing, China). An immunohistochemical kit and insulin
receptor (InsR) antibody were obtained from Boosen Biological
Technology Co., Ltd. (Beijing, China).
Animal modeling and treatments
Twelve-week-old male db/db mice and age-matched male
C57BL/6J mice were provided by the Model Animal Research Center of
Nanjing University. This study was approved by the ethics committee
of Beijing University of Chinese Medicine. All animals were housed
at the Beijing Animal Experimental Center at a temperature of
23±2°C and humidity of 55±10% with a 12/12-h light/dark cycle. The
animals were provided with food and water ad libitum. Db/db mice
were fed a full-formula high-fat diet (composition: basal diet,
cholesterol, egg-yolk powder, lard, bile salt). C57BL/6J mice were
fed a common basal diet. After 1 week of feeding, tail blood was
collected. A non-random blood glucose concentration >11.1 mmol/l
was considered to indicate successful diabetic modeling.
Eighteen modeled mice were divided randomly into
control, PH, and AA groups. C57BL/6J mice were used as normal
controls and were given a normal diet. The other mice were fed a
high-fat diet. PH was used as the positive control. PH (12 mg/kg)
and AA (50 mg/kg) were administered orally once per day for 4
consecutive weeks. Saline served as the negative control. The
animals' general condition (mental state, activity, hair color) was
observed and body weight was recorded each week. After 4 weeks of
treatment, the mice were fasted for 12 h. Tail blood was collected
for the measurement of blood glucose and performance of oral
glucose tolerance test (OGTTs) and insulin tolerance test (ITTs) at
0, 30, 60, and 120 min. Arterial blood was obtained for the
measurement of fasting plasma glucose (FPG), carbohydrate (CHO),
TG, HDL, LDL, insulin, and free fatty acids (FFAs). Insulin
resistance was calculated using the following formula: homeostasis
model assessment-estimated insulin resistance (HOMA-IR)
index=glucose level × serum insulin level/22.5 (20).
H&E and periodic acid-Schiff
staining
Liver tissue fixed in paraformaldehyde was used for
H&E and periodic acid-Schiff (PAS) staining. After fixation in
10% neutral formalin, the tissues were embedded in paraffin,
sectioned (5-µm slice thickness) and stained with H&E or
PAS.
Immunohistochemistry
Fixed liver tissue was cryoprotected in 30% sucrose
for 1 h at 4°C and sectioned at 20-µm intervals with a freezing
microtome (Leica, Mannheim, Germany). The sections were then
incubated with the primary antibody (1:1,000) at 4°C overnight.
After washout with phosphate-buffered saline, the second antibody
was added and incubated for 2 h at room temperature. After
3,3-diaminobenzidine (DAB) colorization, the slices were covered
and observed under a microscope (Olympus Corp., Tokyo, Japan). The
optical density was analyzed using Image-Pro Plus software.
Quantitative PCR
Total RNA was extracted from liver tissues using
TRIzol reagent. RNA concentrations were determined
spectrophotometrically, and 1 µg total RNA was reverse transcribed
using an avian myeloblastosis virus reverse-transcriptase kit
(Promega Corp., Madison, WI, USA). PCR primers were as follows:
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH):
5′-TGAAGCAGGCATCTGAGGG-3′ (sense), 5′-CGAAGGTGGAAGAGTGGGAG-3′
(antisense); InsR: 5′-TTTGTCATGGATGGAGGCTA-3′ (sense),
5′-CCTCATCTTGGGGTTGAACT-3′ (antisense); IRS-1:
5′-TCCTATCCCGAAGAGGGTCT-3′ (sense), 5′-TGGGCATATAGCCATCATCA-3′
(antisense); PI3K: 5′-GCTCCTGGAAGCCATTGAGAA-3′ (sense),
5′-CGTCGATCATCTCCAAGTCCAC-3′ (antisense); Akt-1:
5′-CCCTTCTACAACCAGGACCA-3′ (sense), 5′-ATACACATCCTGCCACACGA-3′
(antisense); GSK-3β: 5′-TATTTCCTGGGGACAGTGGT-3′ (sense),
5′-ATTTGCTCCCTTGTTGGTGT-3′ (antisense); G-6-P:
5′-AGCTCCGTGCCTATAATAAAGCAG-3′ (sense),
5′-CATACGTTGGCTTTTTCTTTCCTC-3′ (antisense). The amplification
reactions were carried out with a 7500 Real-Time PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA), with
initial hold steps (50°C for 2 min, followed by 95°C for 10 min)
and 40 cycles of a two-step PCR (95°C for 15 sec and 60°C for 1
min). The comparative computed tomography method was used to
determine the amount of target, normalized to an endogenous
reference (GAPDH) and relative to a calibrator
(2−ΔΔCq).
Statistical analyses
Data are presented as means ± standard deviations.
One-way analysis of variance with post hoc Bonferroni tests for
multiple comparisons was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
After 4 weeks of treatment, mice in the normal
control group displayed normal activity and appearance. In
contrast, the activity and appearance of mice in the model control
group were abnormal. AA and PH treatments ameliorated those
abnormalities. Body weight increased significantly after modeling
compared with the normal control (P<0.05), AA and PH treatments
decreased body weight significantly (P<0.05; Fig. 1A). The FPG level was elevated in
the model group, but this condition was reversed significantly by
the AA and PH treatments (Fig.
1B). The increased INS level after modeling was attenuated by
AA, but aggravated by PH (Fig.
1C). HOMA-IR values were elevated significantly in the model
control group compared with the normal control group (P<0.05;
Fig. 1D). PH and AA treatments
significantly attenuated this elevation (P<0.05).
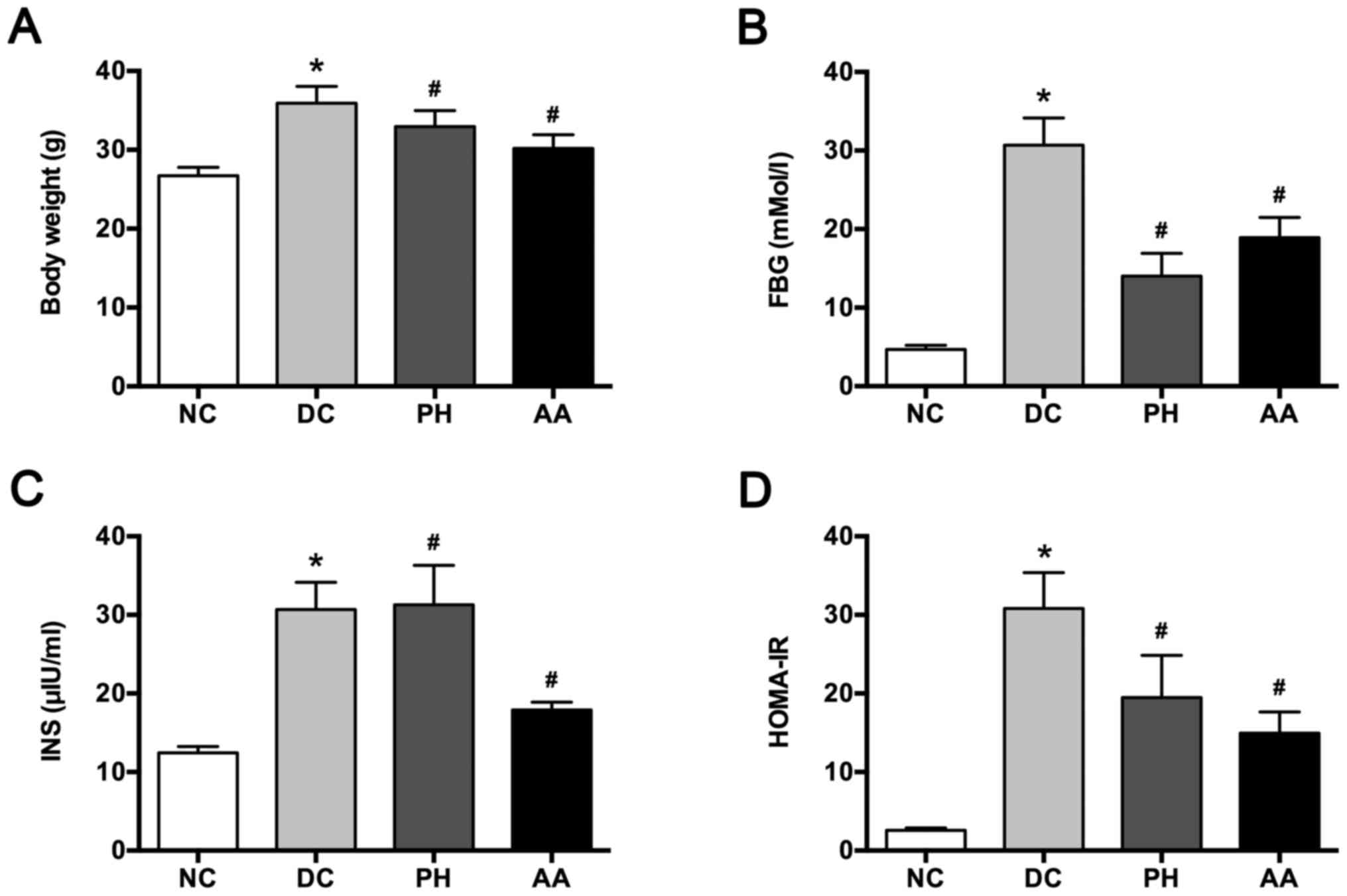 | Figure 1.Effects of AA on (A) body weight, (B)
FPG, (C) INS, and (D) HOMA-IR. Values are mean ± SD for six mice.
*P<0.05 vs. NC, #P<0.05 vs. DC. NC, normal
control; DC, diabetic control; PH, pioglitazone hydrochloride
tablets; AA, asiatic acid; FPG, fasting plasma glucose; INS,
insulin; HOMA-IR, homeostasis model assessment-estimated insulin
resistance. |
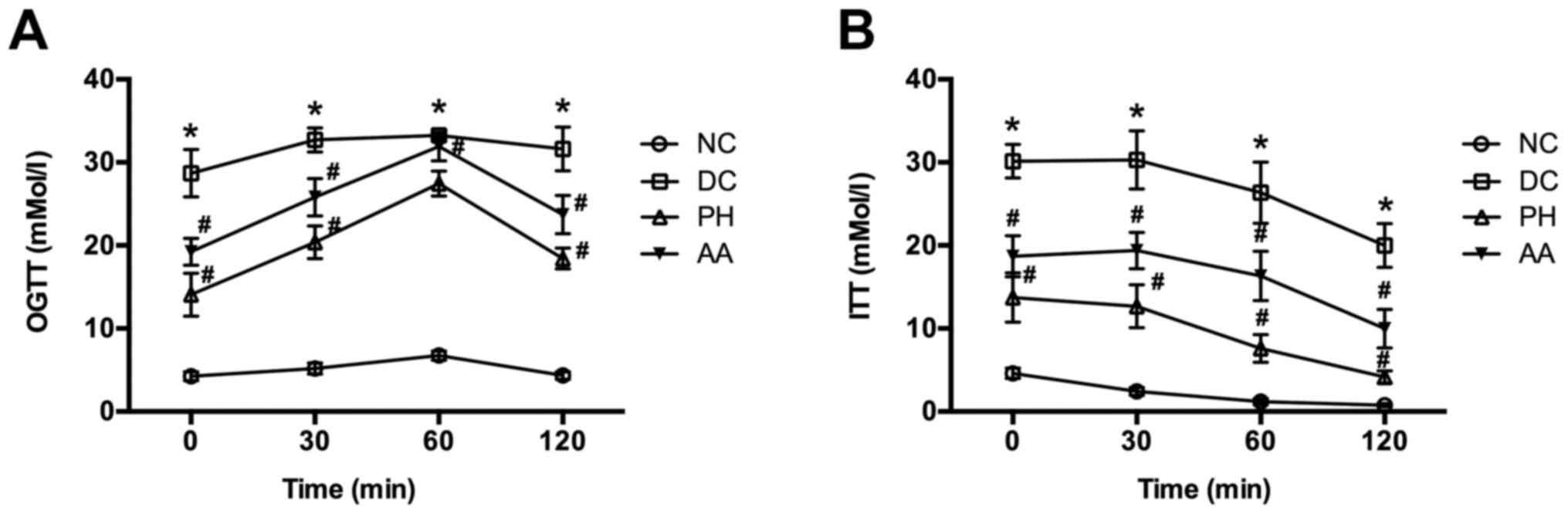
OGTT and ITT values were also higher in the model
control group than in the normal control group at each time point
(P<0.05). AA and PH treatments reversed those abnormalities
(P<0.05 vs. model group; Fig. 2).
After 4 weeks of treatment, serum CHO, TG, HDL, LDL,
and FFA levels were markedly elevated in the model group. AA
treatment attenuated CHO, HDL, and FFA levels, and PH treatment
attenuated CHO, TG, and FFA levels (P<0.05; Table I).
 | Table I.Effects of asiatic acid on lipid
levels in db/db mice. |
Table I.
Effects of asiatic acid on lipid
levels in db/db mice.
| Group | n | CHO (mM) | TG (mM) | HDL (mM) | LDL (mM) | FFA (mM) |
|---|
| NC | 6 | 1.80±0.24 | 0.62±0.13 | 0.76±0.11 | 1.03±0.14 | 0.40±0.01 |
| DC | 6 |
2.40±0.61a |
1.24±0.21a |
0.56±0.15a |
1.55±0.55a |
0.49±0.04a |
| PH | 6 |
1.86±0.20b |
0.88±0.26b | 0.63±0.20 | 1.32±0.12 |
0.56±0.06b |
| AA | 6 |
1.67±0.46b | 1.21±0.27 |
0.75±0.14b | 1.53±0.26 |
0.45±0.01b |
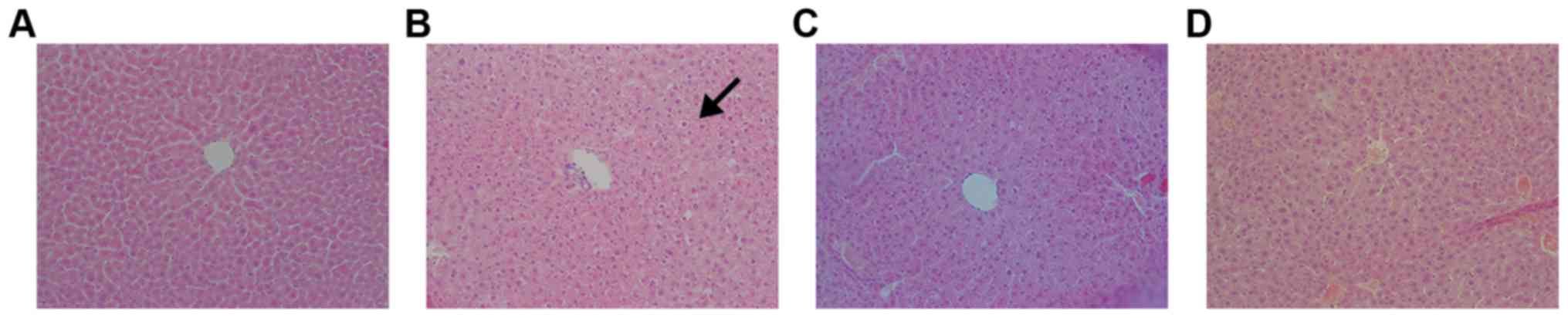
H&E staining showed that hepatic cells were
arranged radially around the central vein in the normal control
group (Fig. 3). Cellular structure
was normal, with centralized nuclei. In the model control group,
H&E staining displayed steatosis in hepatic cells. Moreover,
the cells were arranged irregularly. Portal-area inflammation
infiltration was observed in the model control group. AA and PH
treatments ameliorated these pathological changes, and steatosis
disappeared. Hepatic cells were arranged regularly in these groups,
and minimal inflammation infiltration was observed in the portal
area.
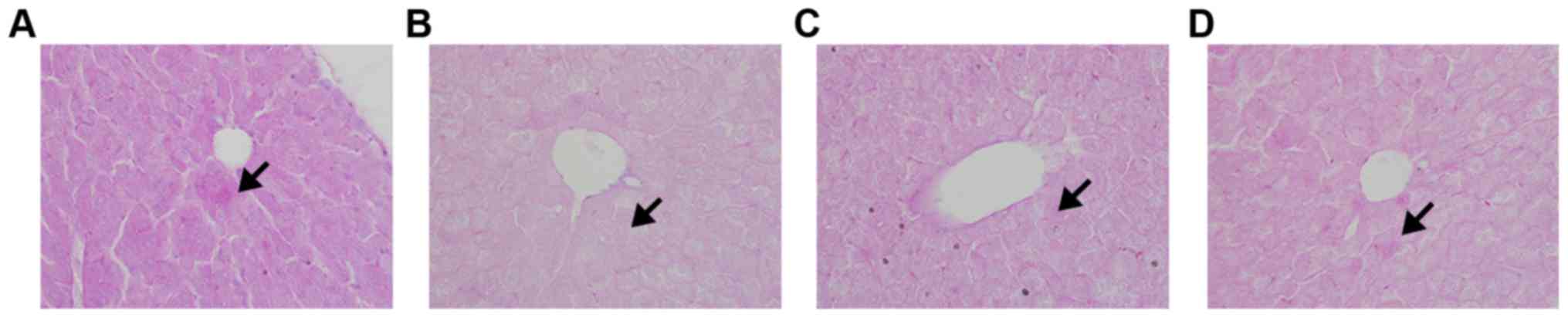
PAS staining revealed a large number of purple
glycogen granules in hepatic cells in the normal control group
(Fig. 4). In contrast, few such
granules were found in the model control group. AA and PH
treatments achieved remarkable recovery of glycogen, although the
numbers of granules remained smaller than in the normal control
(P<0.05).
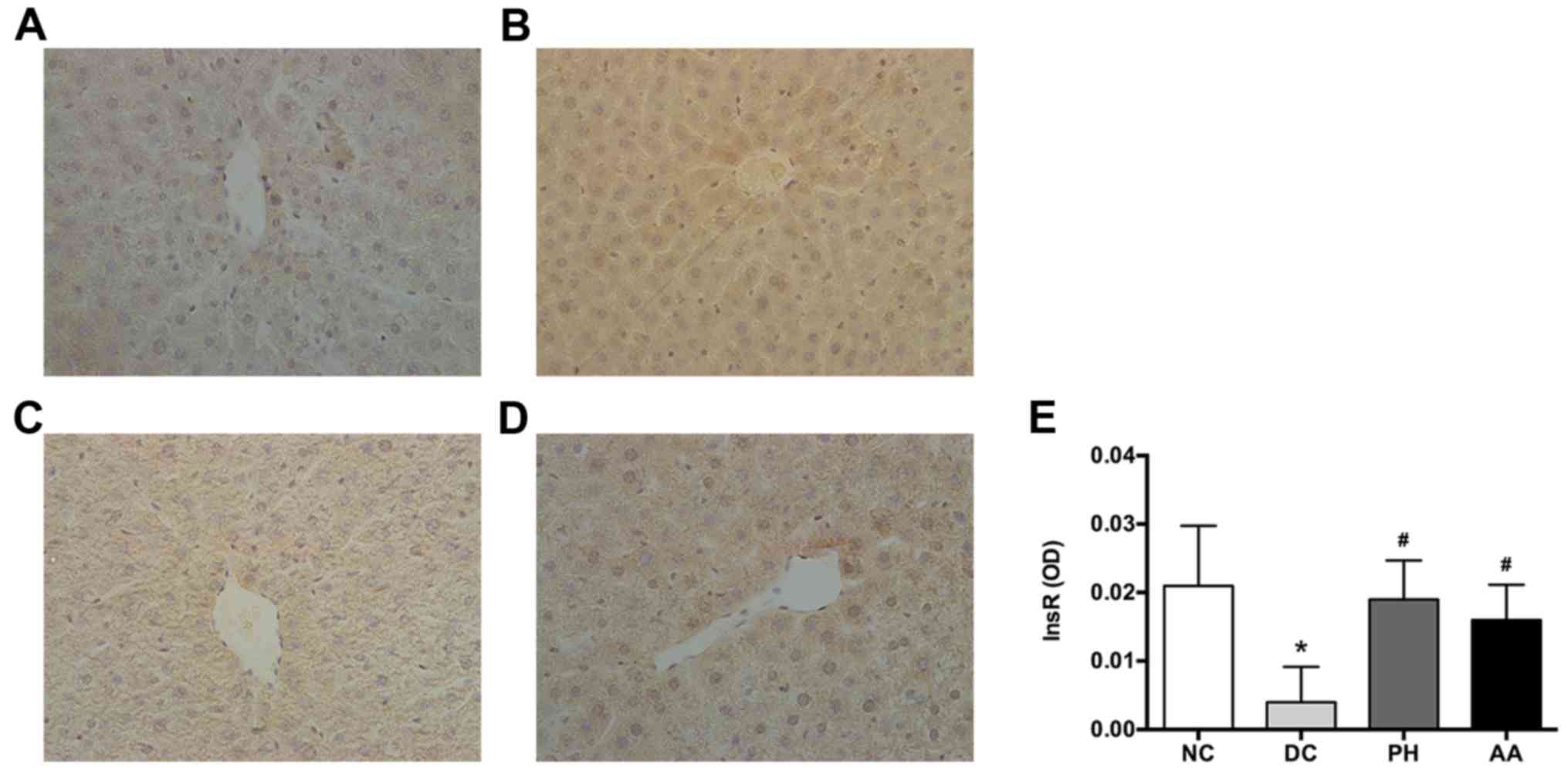
Liver InsR, detected by immunohistochemical
staining, was expressed mainly on cell membranes (Fig. 5). After 4 weeks of treatment, InsR
expression was reduced significantly in the model group compared
with the normal control. By contrast, AA and PH treatments
significantly elevated InsR expression (P<0.05).
InsR, IRS-1, PI3K, and Akt-1 expressions were
increased significantly in the model group compared with the normal
control group (P<0.05; Fig. 6).
After 4 weeks, AA and PH treatments significantly mitigated the
expressions of InsR, IRS-1, PI3K, and Akt-1 (P<0.05). In
addition, diabetic modeling elevated G-6-P and GSK3β expression,
which was reversed by AA and PH treatments (P<0.05).
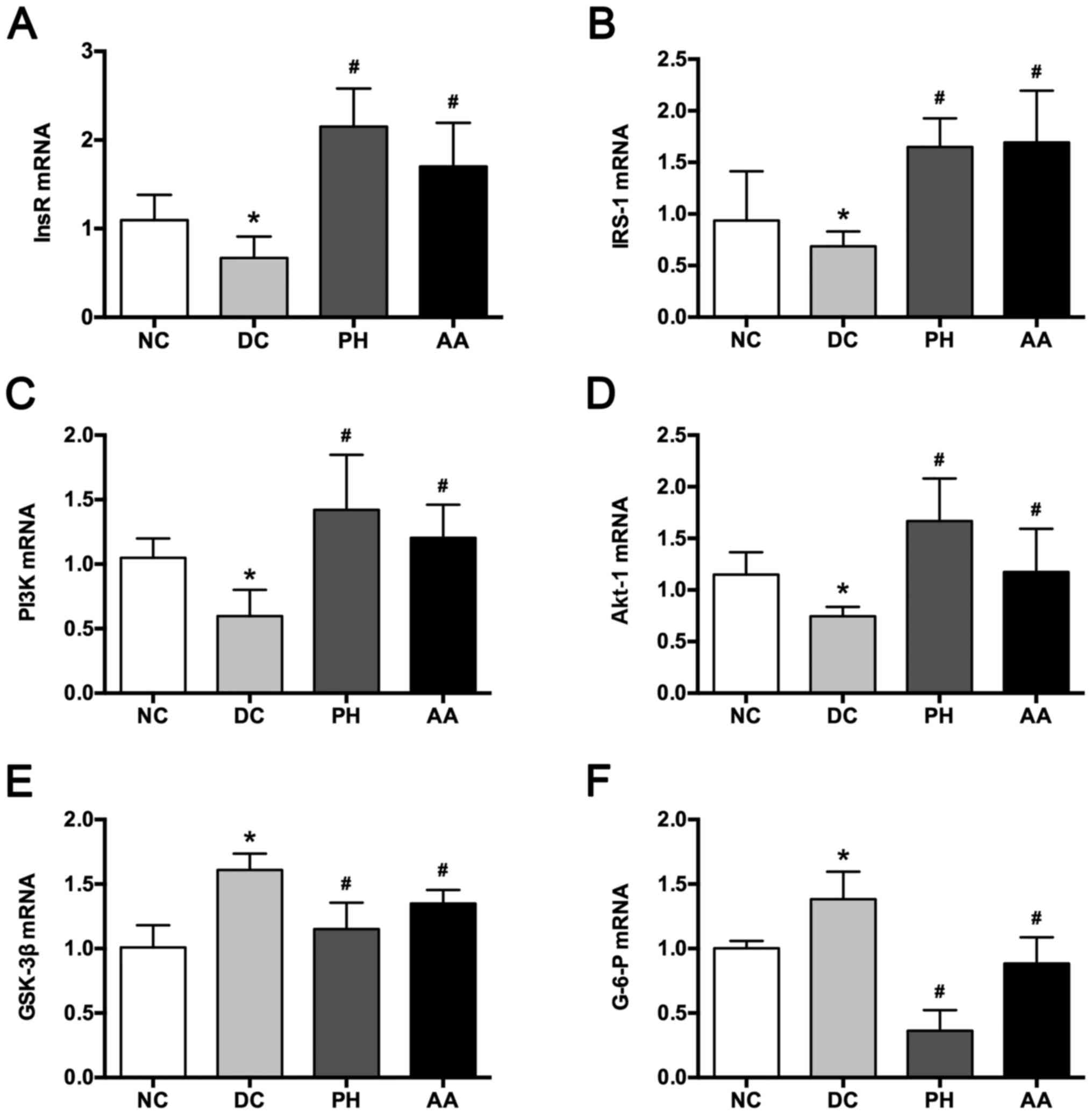 | Figure 6.Effects of AA on (A) InsR, (B) IRS-1,
(C) PI3K, (D) Akt-1, (E) GSK-3β and (F) G-6-P mRNA expression.
Values are mean ± SD for six mice. *P<0.05 vs. NC,
#P<0.05 vs. DC. NC, normal control; DC, diabetic
control; PH, pioglitazone hydrochloride tablets; AA, asiatic acid;
InsR, insulin receptor; IRS-1, insulin receptor substrate-1; PI3K,
phosphatidylinositol 3-kinase; Akt-1, protein kinase B; GSK-3β,
glycogen synthase kinase-3β; G-6-P, glucose-6-phosphatase. |
Discussion
The db/db mouse model is used widely to study
spontaneous T2DM (21).
Genetically, leptin gene mutation leads to abnormality of the
leptin signaling pathway. Major symptoms that appear in this model
include obesity, insulin resistance, high glucose levels, and fatty
liver, which are always found in 6-week-old and typically displayed
in 8-12-week-old mice (22). T2DM
is characterized by absolute or relative deficiency of insulin
secretion. Previously, AA was reported to mitigate the symptoms of
streptozotocin (STZ)-induced diabetes, possibly through
anti-oxidant and anti-inflammatory actions, amelioration of
glycogen metabolism, protection of islets, and insulin resistance
(23). In the present study, we
further demonstrated the protective effects of AA against diabetic
symptoms in a genetic model. Moreover, we showed that the
PI3K/AKT/GSK-3β signaling pathway plays a role in these protective
effects.
Typical symptoms of diabetes were observed in
6-week-old db/db mice fed a high-fat diet, including elevated FPG,
INS, HOMA-IR, and glucose levels and ITT values. The protective
effects of AA observed in this model provide support for the
potential effects of this acid in glucose metabolism. In addition
to its support of glucose control, AA also decreased FFA and CHO
levels and increased HDL levels. In combination with morphological
findings, these results suggest that AA is capable of lowering
lipid levels. Ramachandran also reported that AA prevents lipid
peroxidation in STZ-induced diabetes (24).
Glucose metabolism is regulated by the insulin
signaling pathway. When combined with InsR, insulin initiates the
phosphorylation of its intracellular substrates. PI3K serves as a
docking protein for InsR. Activation of PI3K and its downstream
protein kinase B (Akt) is essential for almost all insulin-induced
glucose or lipid metabolism, such as glucose uptake, glycogen
synthesis, and suppression of triglyceride synthesis (25,26).
GSK-3 differs from other kinases in that it remains active in its
dephosphorylated form. It is inactivated by phosphorylation by
other protein kinases, such as AKT. Insulin activates Akt to
inhibit the activity of GSK-3α or GSK-3β isoforms by
phosphorylating their NH2-terminal serine residues,
terminating their inhibition of glycogen synthase (27). GSK-3β is the predominant regulator
of glycogen synthase in skeletal muscle (28). In diabetes, GSK-3β level and
activity are increased (29). In
the present study, we demonstrated for the first time that AA
decreased PI3K and AKT expression and regulated GSK-3β expression
in diabetes.
G-6-P is a rate-limiting enzyme for gluconeogenesis
and glycogen decomposition, which directly affects the hydrolysis
of G-6-P to glucose. Consistent with previous reports (30), we showed that G-6-P expression was
up-regulated in our diabetic model. Considering the inhibition of
G-6-P by PI3K, GSK-3β might be important in this process. The
effects of AA on G-6-P expression may be exerted via PI-3K,
AKT, and GSK-3β.
Although our data support the effect of AA on PI3
K/AKT/GSK-3β in ameliorating diabetic symptoms, direct evidence of
how AA functions through PI3K/AKT/GSK-3β remains lacking. AA and
its derivatives as potential inhibitors of glycogen phosphorylases
(31). Future studies should
examine the mechanism by which AA regulates the expression of
PI3K/AKT/GSK-3β.
In conclusion, we demonstrated that AA had clear
lipid- and glucose-lowering effects in a db/db mouse model. AA
functions through PI3K/AKT/GSK-3β to facilitate glycogen synthesis.
This experimental evidence provides new clue for the development of
anti-diabetic drugs.
Acknowledgements
This study was supported by grants from the
Innovation Team Project of Beijing University of Chinese Medicine
(no. 2011-CXTD-19) and the International Scientific Collaborative
Project (no. 2012DFG31550).
References
|
1
|
Anna V, van der Ploeg HP, Cheung NW,
Huxley RR and Bauman AE: Sociodemographic correlates of the
increasing trend in prevalence of gestational diabetes mellitus in
a large population of women between 1995 and 2005. Diabetes Care.
31:2288–2293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaul K, Apostolopoulou M and Roden M:
Insulin resistance in type 1 diabetes mellitus. Metabolism.
64:1629–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von W, ilamowitz-Moellendorff A, Hunter
RW, García-Rocha M, Kang L, López-Soldado I, Lantier L, Patel K,
Peggie MW, Martinez-Pons C, Voss M, et al:
Glucose-6-phosphate-mediated activation of liver glycogen synthase
plays a key role in hepatic glycogen synthesis. Diabetes.
62:4070–4082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacAulay K and Woodgett JR: Targeting
glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2
diabetes. Expert Opin Ther Targets. 12:1265–1274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciaraldi TP, Carter L, Mudaliar S and
Henry RR: GSK-3beta and control of glucose metabolism and insulin
action in human skeletal muscle. Mol Cell Endocrinol. 315:153–158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel S, Doble BW, MacAulay K, Sinclair
EM, Drucker DJ and Woodgett JR: Tissue-specific role of glycogen
synthase kinase 3beta in glucose homeostasis and insulin action.
Mol Cell Biol. 28:6314–6328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park KS: The search for genetic risk
factors of type 2 diabetes mellitus. Diabetes Metab J. 35:12–22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R and
Matthews DR: Management of hyperglycaemia in type 2 diabetes: A
patient-centered approach. Position statement of the American
Diabetes Association (ADA) and the European Association for the
Study of Diabetes (EASD). Diabetologia. 55:1577–1596. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang LX, Liu TH, Huang ZT, Li JE and Wu
LL: Research progress on the mechanism of single-Chinese medicinal
herbs in treating diabetes mellitus. Chin J Integr Med. 17:235–240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prabhakar PK and Doble M: Mechanism of
action of natural products used in the treatment of diabetes
mellitus. Chin J Integr Med. 17:563–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orhan IE: Centella asiatica (L.) Urban:
From traditional medicine to modern medicine with neuroprotective
potential. Evid Based Complement Alternat Med. 2012:9462592012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pakdeechote P, Bunbupha S, Kukongviriyapan
U, Prachaney P, Khrisanapant W and Kukongviriyapan V: Asiatic acid
alleviates hemodynamic and metabolic alterations via restoring
eNOS/iNOS expression, oxidative stress, and inflammation in
diet-induced metabolic syndrome rats. Nutrients. 6:355–370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Yang F, Yuan M, Jiang L, Yuan L,
Zhang X, Li Y, Dong L, Bao X and Yin S: Synthesis and evaluation of
asiatic acid derivatives as anti-fibrotic agents:
Structure/activity studies. Steroids. 96:44–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramachandran V, Saravanan R and
Senthilraja P: Antidiabetic and antihyperlipidemic activity of
asiatic acid in diabetic rats, role of HMG CoA: In vivo and in
silico approaches. Phytomedicine. 21:225–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Ai L, Lv T, Jiang X and Liu F:
Asiatic acid, a triterpene, inhibits cell proliferation through
regulating the expression of focal adhesion kinase in multiple
myeloma cells. Oncol Lett. 6:1762–1766. 2013.PubMed/NCBI
|
|
17
|
Wang X, Lu Q, Yu DS, Chen YP, Shang J,
Zhang LY, Sun HB and Liu J: Asiatic acid mitigates hyperglycemia
and reduces islet fibrosis in Goto-Kakizaki rat, a spontaneous type
2 diabetic animal model. Chin J Nat Med. 13:529–534.
2015.PubMed/NCBI
|
|
18
|
Ramachandran V and Saravanan R: Efficacy
of asiatic acid, a pentacyclic triterpene on attenuating the key
enzymes activities of carbohydrate metabolism in
streptozotocin-induced diabetic rats. Phytomedicine. 20:230–236.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramachandran V and Saravanan R: Glucose
uptake through translocation and activation of GLUT4 in PI3K/Akt
signaling pathway by asiatic acid in diabetic rats. Hum Exp
Toxicol. 34:884–893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McKeown NM, Meigs JB, Liu S, Saltzman E,
Wilson PW and Jacques PF: Carbohydrate nutrition, insulin
resistance, and the prevalence of the metabolic syndrome in the
Framingham Offspring Cohort. Diabetes Care. 27:538–546. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King AJ: The use of animal models in
diabetes research. Br J Pharmacol. 166:877–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YW, Sun GD, Sun J, Liu SJ, Wang J, Xu
XH and Miao LN: Spontaneous type 2 diabetic rodent models. J
Diabetes Res. 2013:4017232013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, He T, Lu Q, Shang J, Sun H and
Zhang L: Asiatic acid preserves beta cell mass and mitigates
hyperglycemia in streptozocin-induced diabetic rats. Diabetes Metab
Res Rev. 26:448–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramachandran V and Saravanan R: Asiatic
acid prevents lipid peroxidation and improves antioxidant status in
rats with streptozotocin-induced diabetes. J Funct Foods.
5:1077–1087. 2013. View Article : Google Scholar
|
|
25
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo S: Insulin signaling, resistance, and
the metabolic syndrome: Insights from mouse models into disease
mechanisms. J Endocrinol. 220:T1–T23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fornoni A, Pileggi A, Molano RD, Sanabria
NY, Tejada T, Gonzalez-Quintana J, Ichii H, Inverardi L, Ricordi C
and Pastori RL: Inhibition of c-jun N terminal kinase (JNK)
improves functional beta cell mass in human islets and leads to AKT
and glycogen synthase kinase-3 (GSK-3) phosphorylation.
Diabetologia. 51:298–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bertsch S, Lang CH and Vary TC: Inhibition
of glycogen synthase kinase 3[beta] activity with lithium in vitro
attenuates sepsis-induced changes in muscle protein turnover.
Shock. 35:266–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nikoulina SE, Ciaraldi TP, Mudaliar S,
Mohideen P, Carter L and Henry RR: Potential role of glycogen
synthase kinase-3 in skeletal muscle insulin resistance of type 2
diabetes. Diabetes. 49:263–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang SJ, Dong H, Li JB, Xu LJ, Zou X,
Wang KF, Lu FE and Yi P: Berberine inhibits hepatic gluconeogenesis
via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced
diabetic rats. World J Gastroenterol. 21:7777–7785. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Chen J, Gong Y, Liu J, Zhang L,
Hua W and Sun H: Synthesis and biological evaluation of asiatic
acid derivatives as inhibitors of glycogen phosphorylases. Chem
Biodivers. 6:864–874. 2009. View Article : Google Scholar : PubMed/NCBI
|