Introduction
Neurodegenerative diseases are caused by chronic
progressive degenerative damage of the central nervous system
(1). The number of individuals
suffering from neurodegenerative diseases is growing (2). Although the apoptotic cells in brain
area have been observed in patients with Parkinson's and/or
Alzheimer's disease, the pathogenic mechanism is not clearly
established yet (3). Recent
studies demonstrate that the regulation of apoptosis has become a
target for prevention and treatment in neurodegenerative diseases
(4,5).
Various signals are involved in the apoptotic
process. As a second messenger, intracellular calcium is required
at low level to maintain homeostasis; however, calcium overload
produces large amounts of reactive oxygen species (ROS), which
interferes with the electron transport chain and reduces the
formation of ATP (6). High levels
of calcium and ROS promote the opening of mitochondrial
permeability transition pores, which leads to mitochondrial
membrane potential dissipation (7). Mitochondrial energy dysfunction will
lead to apoptosis (8).
Anti-apoptotic proteins B-cell lymphoma-2 (Bcl-2) and B-cell
lymphoma-extra large (Bcl-xL) are highly concentrated in the outer
membrane of mitochondria and are responsible for
mitochondria-mediated apoptosis (9).
SH-SY5Y human neuroblastoma cells and PC12 rat
pheochromocytoma cells are common cell models for in vitro
investigation of neurodegenerative diseases. PC12 cells have
obvious synapses formation, and are capable of producing
nerve-related proteins following stimulation by nerve growth factor
(NGF) (10). Certain small
synthetic molecules have been reported to exhibit neuroprotective
activities against neurotoxin-induced toxicity in SH-SY5Y and PC12
cells via mitochondrial pathways (11,12).
The present study examined the neuroprotective
effect of a series of 2,2-disubstituted derivatives in
differentiated PC12 (DPC12) cells (Table I). Following morphological
screening, one synthetic small molecule (5zou) exhibited
significant protection against 6-hydroxydopamine (6-OHDA) and
L-glutamic acid (L-Glu)-induced cell damage. The molecular
mechanisms associated with mitochondria were investigated
further.
 | Table I.Neuroprotection screening of zou
compounds. |
Table I.
Neuroprotection screening of zou
compounds.
| Compound | R group | Differentiation
status |
|---|
| 1 |
CON(iPr)2 | N |
| 2 |
CONEt2 | N |
| 3 | CON(OMe)Me | N |
| 4 |
CON(CH2)5 | N |
| 5 |
CON(CH2)4O | Y |
| 6 | CO2Me | N |
| 7 | CN | N |
Materials and methods
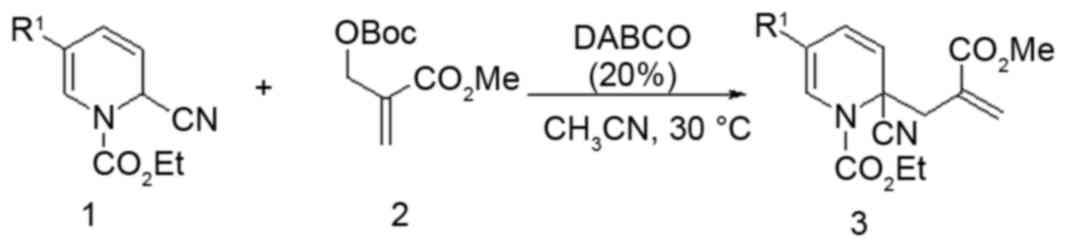
Synthesis of compound 5zou
Under an N2 atmosphere at 30°C were added
1,4-diazabicyclo [2.2.2] octane (20 mol%), compound 1 (as presented
in Fig. 1; 1 mmol, 291 mg), MBH
carbonate (1.2 mmol, 276 mg) and CH3CN (10 ml) to a
dried 10 ml reaction tube. The reaction was monitored by thin layer
chromatography (TLC). Upon completion, the reaction mixture was
concentrated in vacuo. The crude mixture was purified by column
chromatography [silica gel, EtOAc/petroleum ether (60–90°C)] to
provide compound 3 (299 mg, 77% yield). mp: 78.5–79.2°C; 1H NMR
(300 MHz, CDCl3) δ 7.23 (d, J=0.9 Hz, 1H), 6.36 (d, J=0.9 Hz, 1H),
6.13 (dd, J=9.8, 0.9 Hz, 1H), 5.72 (s, 1H), 5.43 (dd, J=9.8, 0.8
Hz, 1H), 4.41 (q, J=7.1 Hz, 2H), 3.72 (s, 3H), 3.70–3.65 (m, 4H),
3.61 (d, J=13.9 Hz, 1H), 3.55–3.50 (m, 4H), 2.83 (d, J=13.8 Hz,
1H), 1.41 (t, J=7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 167.08,
166.92, 152.20, 132.95, 131.96, 130.26, 122.82, 118.35, 117.42,
109.65, 66.90, 64.55, 57.37, 52.42, 45.61, 40.33, 14.33. HRMS
(ESI): calcd. for C19H24N3O6 ([M+H]+): 390.1660, found 390.1650
(13).
Cell culture
PC12 rat adrenal cells (obtained from the American
Type Culture Collection, Manassas, VA, USA; CRL-1721; passages
<10) were cultured in in Dulbecco's modified Eagle medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) which
was supplemented with 5% horse serum (Invitrogen; Thermo Fisher
Scientific, Inc.), 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.), penicillin (100 U/ml), and streptomycin
(100 µg/ml) (Invitrogen; Thermo Fisher Scientific, Inc.), under a
humidified atmosphere containing 5% CO2 at 37°C. The
culture medium was changed every three days. PC12 cells were
differentiated for 48 h using 50 ng/ml of NGF (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) dissolved in DMEM medium containing 1%
FBS, 1% horse serum and 100 U/ml penicillin/streptomycin.
Cellular morphology analysis
PC12 cells were seeded in 6-well plates at 2×104
cells/well. After replacing the medium with serum-free basic
medium, cells were treated with 40 µM of the synthetic compounds
detailed in Table I, and 10, 20
and 40 µM 5zou for 24 h and then imaged using an inverted
microscope (x10; Nikon Corporation, Tokyo, Japan). Cells with
projections and a longer neurite (neurite length range, 5–37 µm),
compared with untreated undifferentiated cells (neurite length
range, 2–15 µm), were considered as differentiated.
Cell viability analysis
DPC12 cells were seeded in 96-well plates at 2×104
cells/well, and pretreated with 5zou (10–40 µM) for 3 h, and then
exposed to 100 µM 6-OHDA and 25 mM L-Glu for another 24 h. After
incubation with MTT solution (0.5 mg/ml) for 4 h at 37°C in
darkness, 100 µl dimethyl sulfoxide was added to dissolve crystals.
A microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to measure the absorbance at 540 nm. Viability values were
expressed as a percentage of that of corresponding control
cells.
Hoechst staining analysis
Nuclear morphological alterations were analyzed by
Hoechst 33342 staining. DPC12 cells were pre-treated with 20 and 40
mM 5zou for 3 h, followed with 24 h co-incubation with 100 µM
6-OHDA and 25 mM L-Glu. Then cells were incubated with Hoechst
33342 (5 µg/ml; Sigma-Aldrich; Merck KGaA) for 15 min at 37°C in
darkness. After washing with PBS, the fluorescence intensity in the
nucleus was captured using a fluorescent microscope (x20; Axio
Observer Z1; Carl Zeiss AG, Oberkochen, Germany). The percentage of
damaged cells was analyzed by measuring the blue fluorescence
intensity using Image J 1.38x software (National Institutes of
Health, Bethesda, MA USA).
Intracellular calcium concentration
([Ca2+]i) analysis
DPC12 cells were seeded in a 6-well plate (2×105
cells/well). The next day, cells were treated with 20 and 40 µM
5zou for 3 h prior to co-incubation with 100 µM 6-OHDA and 25 mM
L-Glu for 12 h. Then, the supernatant was removed, and cells were
incubated with 5 µM Fluo-4-AM (Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 min at 37°C in darkness. After three
washes, cells were observed using a fluorescence microscope (x20;
Axio Observer Z1). The average of green fluorescence intensity was
detected using Image J software and expressed as a percentage of
that of the corresponding control cells.
Mitochondrial membrane potential (MMP)
analysis
DPC12 cells (1×105 cells/well) were seeded into a
6-well plate. Cells were pre-treated with 20 and 40 µM 5zou for 3
h, followed with 12 h co-incubation with 100 µM 6-OHDA and 25 mM
L-Glu. Treated cells were incubated with 2 µM
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanine
iodide (JC-1) (Sigma-Aldrich; Merck KGaA) at 37°C for 10 min.
Fluorescent microscope (x20; CCD camera, Axio Observer Z1; Carl
Zeiss, Germany) was applied to record the fluorescent color in each
group. The ratio of red to green fluorescence intensity was
detected by Image J software and expressed as a percentage of that
of corresponding control cells.
Measurement of ROS
Intracellular ROS levels were analyzed using a ROS
assay kit obtained from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). DPC12 cells were exposed to 20 and 40 µM 5zou for
3 h, and co-incubated with 6-OHDA (100 µM) and L-Glu (25 mM) for 12
h. Treated cells were incubated with 10 µM dichlorofluorescein
diacetate (DCFH-DA) at 37°C for 30 min. The fluorescent color was
photographed by a fluorescent microscope (x20Axio Observer Z1). The
average green fluorescence intensity was detected by Image J and
expressed as a percentage of that of corresponding control
cells.
Western blot
DPC12 cells were pre-treated with 20 and 40 µM 5zou
for 3 h, and followed with 24-h incubation of 6-OHDA (100 µM) and
L-Glu (25 mM). Cells were lysed by radioimmunoprecipitation assay
buffer (Sigma-Aldrich; Merck KGaA) containing 2%
phenylmethanesulfonyl fluoride (Sigma-Aldrich; Merck KGaA) and 1%
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Protein
concentrations were determined using a Standard BCA Protein Assay
kit (Merck KGaA). Proteins (30 µg) were separated on a 12% SDS-PAGE
gel and electrophoretically transferred onto nitrocellulose
membranes (Bio Basic, Inc., Markham, ON, Canada). The membranes
were blocked in 5% bovine serum albumin at room temperature for 4
h, and then incubated with the following primary antibodies (all
diluted 1:1,000) overnight at 4°C: Bcl-2 (ab321224), Bcl-xL
(ab7973), and GAPDH (ab8245) (1:1,000; Abcam, Cambridge, UK) at 4°C
overnight, followed by incubation with appropriate horseradish
peroxidase-conjugated secondary antibodies (sc-2005 and sc-358925)
at dilution of 1:2,000 at 4°C for 4 h (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Chemiluminescence was detected by using
enhanced chemiluminescence detection kits (GE Healthcare Life
Sciences, Little Chalfont, UK).
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were evaluated by one-way analysis of variance to
detect statistical significance, followed by post-hoc multiple
comparisons (Dunn's test) using SPSS 16.0 software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
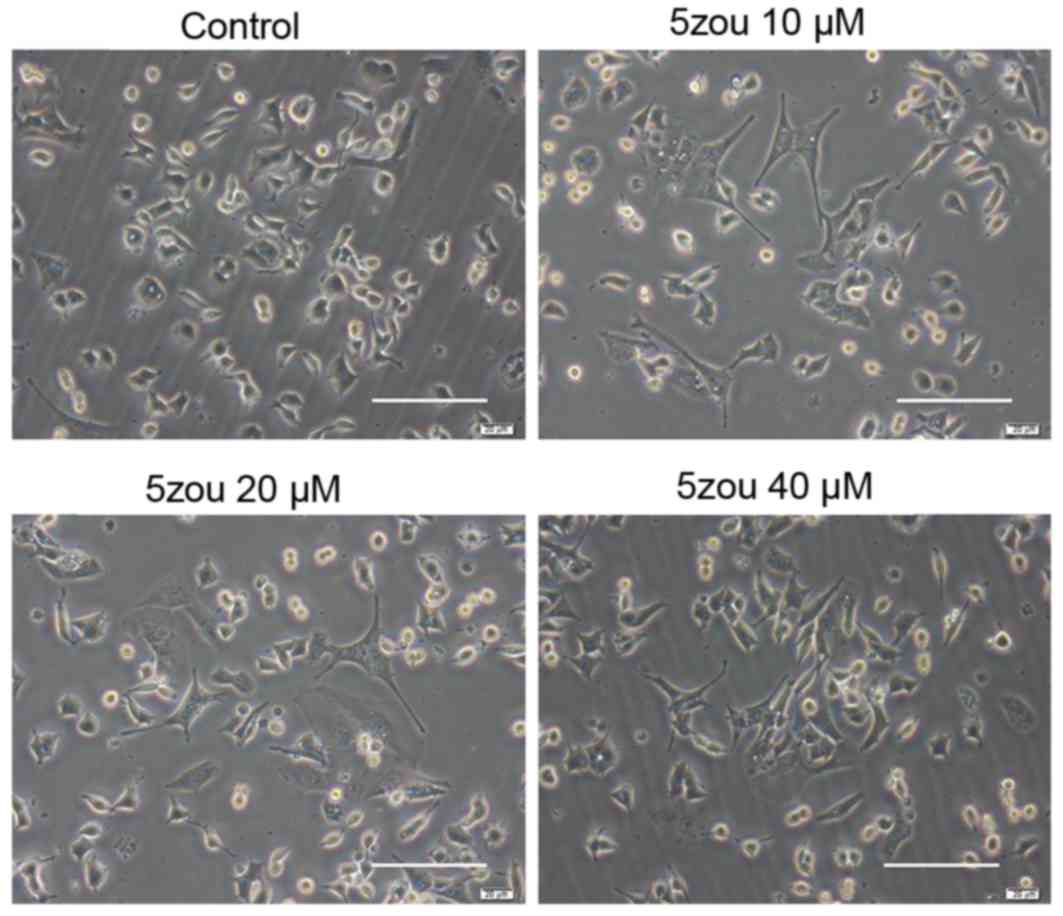
5zou increases DPC12 cells
differentiation
PC12 cells in the control group, treated with basic
DMEM, were round, short spindle and triangular, and their nuclei
were large and round. Cells became a polygonal shaped following
5zou treatment. With the increasing 5zou concentration, cell
differentiation rate was increased (Fig. 2).
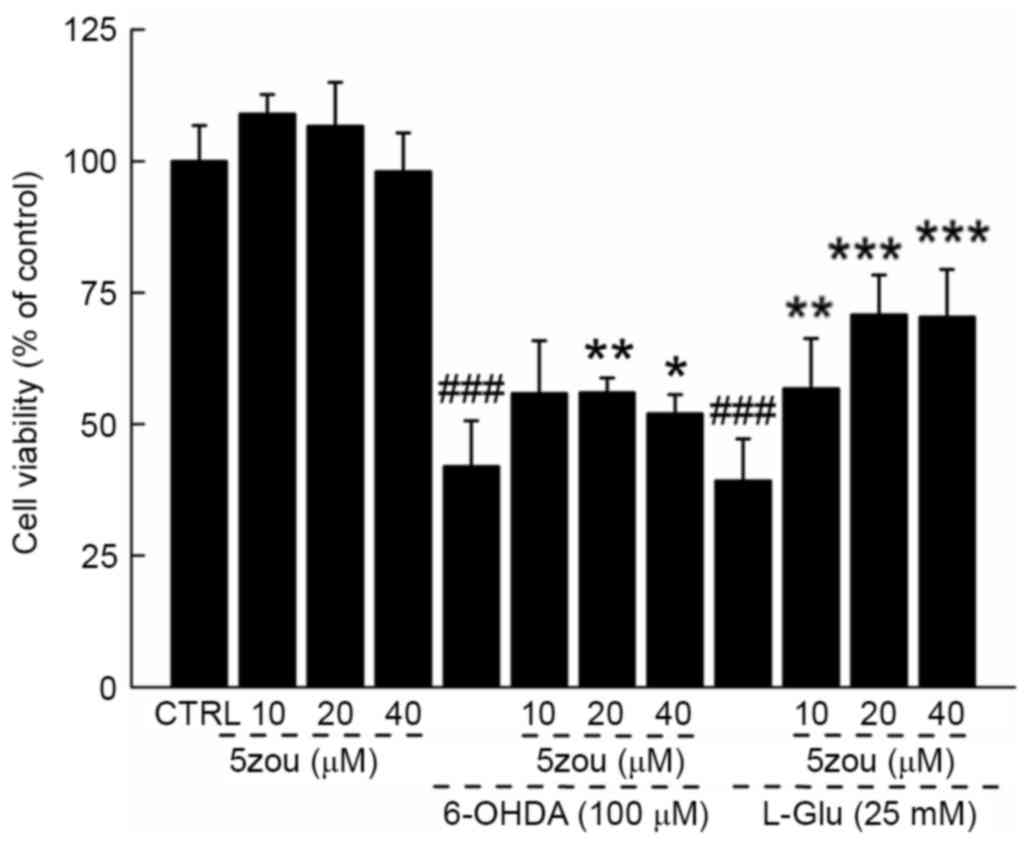
5zou protected neurotoxin-induced
DPC12 cell damage
6-OHDA and L-Glu resulted in a 60.8 and 57.2%
reduction in cell viability, respectively (P<0.001; Fig. 3), which was partially restored
following 5zou pre-treatment (P<0.05; Fig. 3).
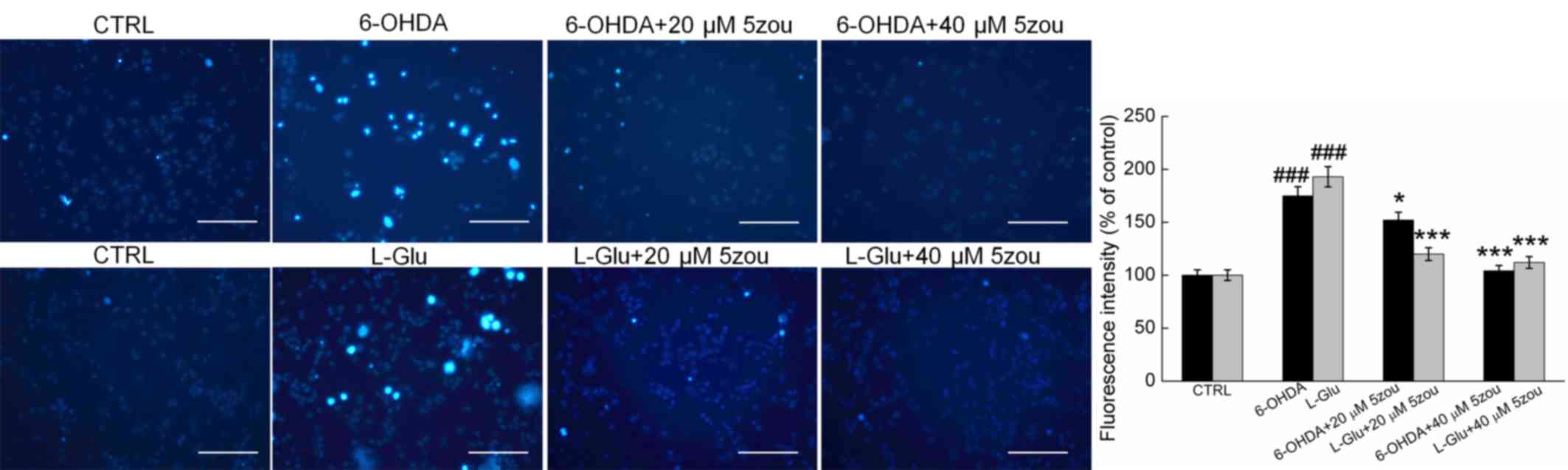
Hoechst 33342 staining demonstrated that the nuclei
in control cells exhibit a uniform weak light blue fluorescence.
However, in 6-OHDA and L-Glu-treated DPC12 cells, most cells were
asymmetrical and appeared chunky in shape with intense blue
fluorescence. Pretreatment with 5zou for 3 h pretreatment strongly
reversed the nuclear damage in DPC12 cells (P<0.05; Fig. 4).
Effects of 5zou on intracellular
Ca2+ concentration, ROS levels and mitochondrial
function
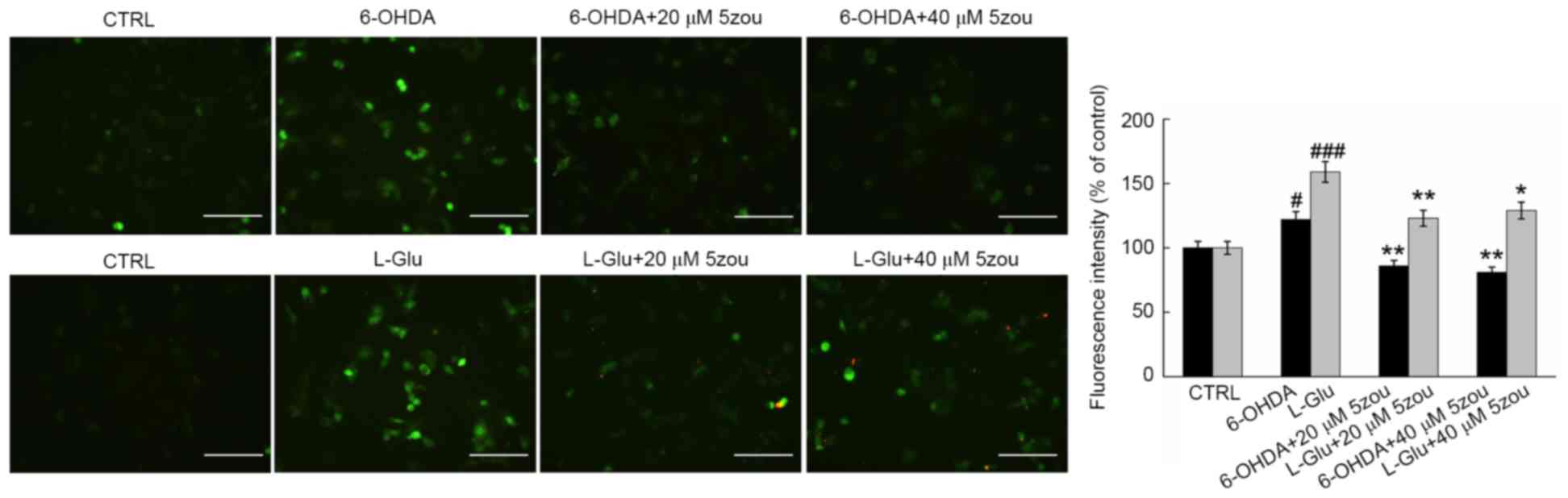
6-OHDA and L-Glu increased the levels of
intracellular Ca2+ in DPC12 cells after 12 h incubation
(Fig. 5). Compared with model
cells, 5zou reduced intracellular Ca2+ concentration,
indicated by reduced green fluorescence (P<0.05; Fig. 5).
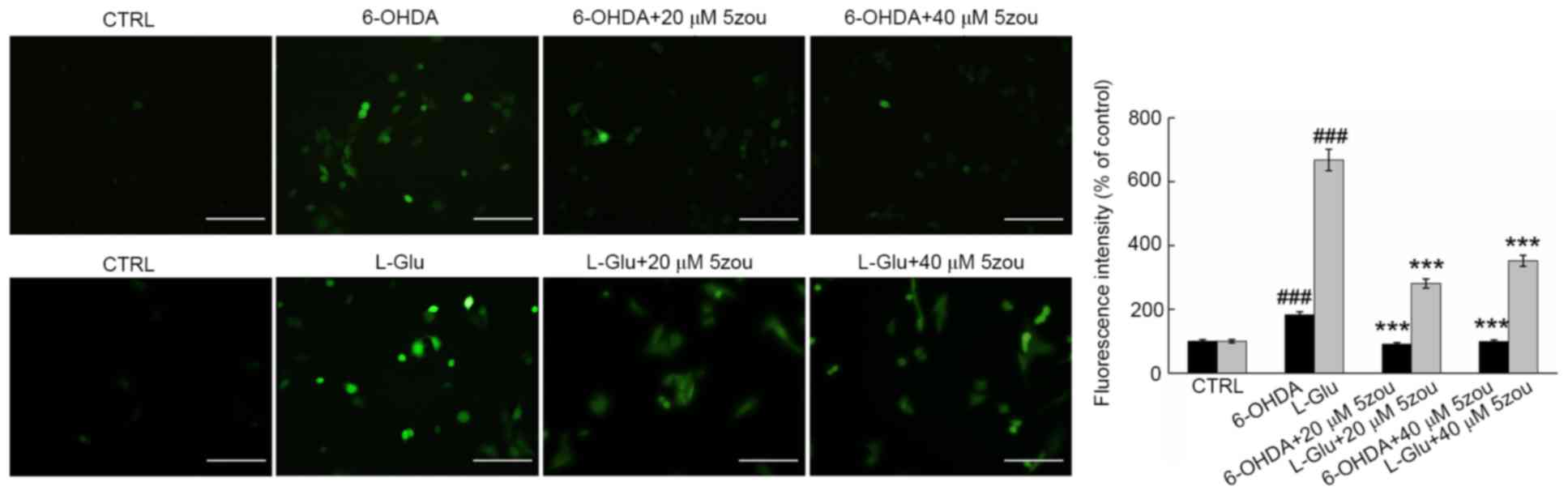
DCFH-DA is oxidized to produce a highly fluorescent
substance when oxidized, and is this used as a probe for measuring
the levels of ROS. High green fluorescence was clearly observed in
DPC12 cells treated with 6-OHDA and L-Glu, which was significantly
reduced in 5zou-treated cells, suggesting 5zou successfully
inhibited ROS accumulation (P<0.05; Fig. 6).
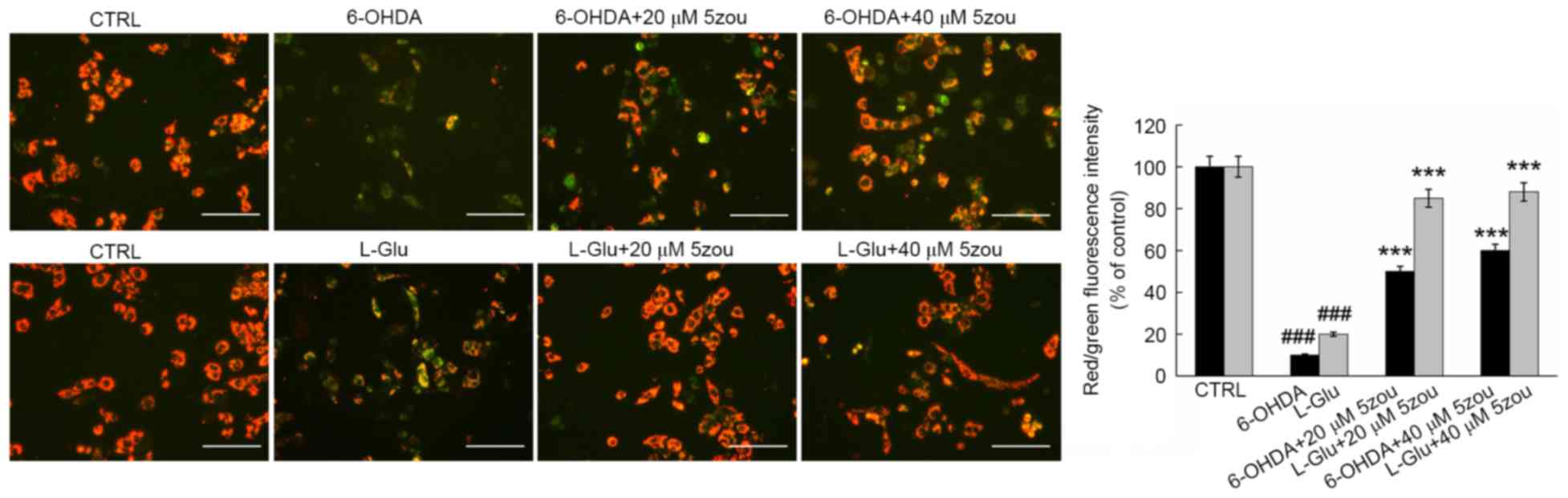
JC-1 staining is widely used as an indicator of the
MMP, producing red florescence in normal cells and green
fluorescence in unhealthy cells. In L-Glu and 6-OHDA treated cells,
weak red fluorescence and strong green fluorescence were observed
(P<0.001; Fig. 7). 5zou
alleviated MMP dissipation in neurotoxin-induced DPC12 cells,
indicated by the enhancement of red fluorescence (P<0.001;
Fig. 7).
Effects of 5zou on Bcl-2 and Bcl-xL
expression in DPC12 cells
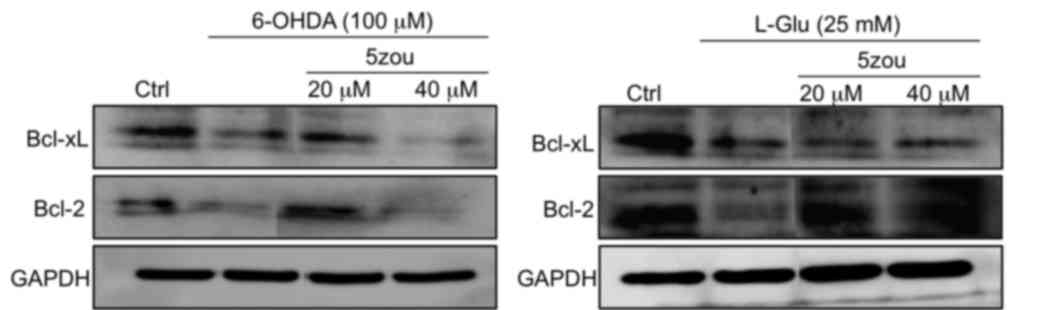
Compared with the control group, the protein
expressions levels of Bcl-xL and Bcl-2 protein were decreased in
PC12 cells treated with 6-OHDA and L-Glu compared with control
cells (Fig. 8). Pretreatment with
5zou, especially at 20 µM, increased the levels of Bcl-2 and Bcl-xL
compared with 6-OHDA and L-Glu treatments. However, 40 µM 5zou
failed to reverse the low expression levels of Bcl-xL and Bcl-2 in
6-OHDA-treated cells (Fig. 8).
Discussion
As the most important excitatory neurotransmitter in
the central nervous system, glutamic acid has important roles in
the formation of synapses. However, markedly increased glutamate
levels, caused by physical or chemical factors, leads to excitatory
neurotoxicity (14). 6-OHDA, a
catecholamine hydroxylated derivative, is commonly used as a
selective catecholaminergic nerve agent (15). In the present study, 6-OHDA and
L-Glu were used to induce PC12 cell damage, and the protective
effect of 5zou on cell apoptosis and the associated molecular
mechanisms were subsequently examined.
In the present study, 5zou was been demonstrated to
have a significant protective effect on 6-OHDA and L-Glu-induced
cell viability reduction, Ca2+ overload, ROS
accumulation and MMP dissipation. Mitochondria are the main
organelle involved in cell energy metabolism. Intracellular
Ca2+ overload is one of the main causes of mitochondrial
dysfunction (16). Mitochondrial
permeability is associated with cell toxicity, oxidative damage and
apoptosis. The leakage from the mitochondrial respiratory chain
produces superoxide radicals, then causes ROS generation, which
will ultimately lead to intracellular oxidative stress (17). ROS accumulation may further induce
mitochondrial damage, which affects the respiratory chain and the
inner and outer mitochondrial membrane proteins, resulting in
cytochrome C release, and triggering of the mitochondrial apoptosis
pathway (18). The imbalance of
intracellular ROS homeostasis is considered to be responsible for
cell oxidative damage (19). The
data of the present study demonstrated that 5zou exhibited strong
protective effects against neurotoxin-induced DPC12 cell damage
associated with ROS-dependent mitochondrial apoptosis.
Previous studies have demonstrated that the balance
of Bcl-2 family members regulates mitochondrial function. 6-OHDA
and L-Glu induce mitochondrial dysfunction by regulating the
Bcl-2/Bax ratio in SH-SY5Y or PC12 cells, thus contributing to cell
apoptosis (20,21). Bcl-2 suppresses apoptosis via two
distinct pathways. The BH1-3 domains of Bcl-2 form a hydrophobic
pocket, binding and inhibiting pro-apoptotic proteins, and
additionally, inositol 1,4,5-trisphosphate receptors prevent
Ca2+ influx-induced apoptosis (22). 5zou was demonstrated to improve the
neurotoxin-induced MMP dissipation, and also inhibit the abnormal
changes to Bcl-2 and Bcl-xL expressions. Thus, 5zou affects the
mitochondrial apoptosis pathway to inhibit 6-OHDA and L-Glu-induced
cell damage.
A high concentration of L-Glu was used in the
present study. For in vitro experiments, 20–25 mM of L-Glu
has been previously used to induce damage of DPC12 cells (23,24).
In previous experiments performed by our group, 25 mM L-Glu was
used to establish neurotoxic DPC12 cell models (25). In the present study, fluorescence
intensity measurements were normalized by cell counting of stained
and unstained cells, which is a potential limitation; future
studies will involve counterstaining, to further improve the
accuracy of the results.
Notably, the results indicated that the same
concentration of 5zou exhibited different effects on 6-OHDA and
L-Glu damaged PC12 cells, which may be related to different
injuries on the neurons, occurring via different signaling
pathways. In conclusion, the present study demonstrated the
neuroprotective effect of a novel compound (5zou). The effect of
5zou may be associated with the mitochondrial apoptotic
pathway.
Acknowledgements
This work was supported by the Natural Science
Foundation of China (grant no. 81402955), China's Post-doctoral
Science Foundation (grant no. 2016M591495) and Post-doctoral
Science Research Project in Jilin Province of China. Authors thank
Professor Weiwei Liao (Department of Organic Chemistry, Jilin
University, Changchun, China) for providing the 2,2-disubstituted
derivatives.
References
|
1
|
Amor S, Puentes F, Baker D and van der
Valk P: Inflammation in neurodegenerative diseases. Immunology.
129:154–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Head E: Combining an antioxidant-fortified
diet with behavioral enrichment leads to cognitive improvement and
reduced brain pathology in aging canines: Strategies for healthy
aging. Ann N Y Acad Sci. 1114:398–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurny A, Michalowska-Wender G and Wender
M: Impact of L-DOPA treatment of patients with Parkinson's disease
on mononuclear subsets and phagocytosis in the peripheral blood.
Folia Neuropathol. 51:127–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balez R, Steiner N, Engel M, Muñoz SS, Lum
JS, Wu Y, Wang D, Vallotton P, Sachdev P, O'Connor M, et al:
Neuroprotective effects of apigenin against inflammation, neuronal
excitability and apoptosis in an induced pluripotent stem cell
model of Alzheimer's disease. Sci Rep. 6:314502016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, An S, Hu W, Teng M, Wang X, Qu Y,
Liu Y, Yuan Y and Wang D: The neuroprotective properties of
hericium erinaceus in glutamate-damaged differentiated PC12 Cells
and an alzheimer's disease mouse model. Int J Mol Sci. 17:pii:
E18102016. View Article : Google Scholar
|
|
6
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akopova OV, Kolchynskayia LY, Nosar VY,
Smyrnov AN, Malisheva MK, Man'kovskaia YN and Sahach VF: The effect
of permeability transition pore opening on reactive oxygen species
production in rat brain mitochondria. Ukr Biokhim Zh (1999).
83:46–55. 2011.PubMed/NCBI
|
|
8
|
Liu CY, Lee CF and Wei YH: Role of
reactive oxygen species-elicited apoptosis in the pathophysiology
of mitochondrial and neurodegenerative diseases associated with
mitochondrial DNA mutations. J Formos Med Assoc. 108:599–611. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh KJ, Lee SC, Choi HJ, Oh DY, Kim SC, Min
do S, Kim JM, Lee KS and Han JS: Role of phospholipase D2 in
anti-apoptotic signaling through increased expressions of Bcl-2 and
Bcl-xL. J Cell Biochem. 101:1409–1422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su WT and Shih YA: Nanofiber containing
carbon nanotubes enhanced PC12 cell proliferation and
neuritogenesis by electrical stimulation. Biomed Mater Eng. 26
Suppl 1:S189–S195. 2015.PubMed/NCBI
|
|
11
|
Jackson TC, Verrier JD and Kochanek PM:
Anthraquinone-2-sulfonic acid (AQ2S) is a novel neurotherapeutic
agent. Cell Death Dis. 4:e4512013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim IS, Koppula S, Kim BW, Song MD, Jung
JY, Lee G, Lee HS and Choi DK: A novel synthetic compound PHID
(8-Phenyl-6a, 7, 8, 9, 9a, 10-hexahydro-6H-isoindolo [5, 6-g]
quinoxaline-7, 9-dione) protects SH-SY5Y cells against
MPP(+)-induced cytotoxicity through inhibition of reactive oxygen
species generation and JNK signaling. Eur J Pharmacol. 650:48–57.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou GF, Hu ZP, Zhang SQ and Liao WW:
Synthesis of functionalized 1,2-dihydropyridines bearing quaternary
carbon centers via an organocatalytic allylic alkylation.
Tetrahedron Lett. 56:937–940. 2015. View Article : Google Scholar
|
|
14
|
Shimmyo Y, Kihara T, Akaike A, Niidome T
and Sugimoto H: Three distinct neuroprotective functions of
myricetin against glutamate-induced neuronal cell death:
Involvement of direct inhibition of caspase-3. J Neurosci Res.
86:1836–1845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yong Y, Ding H, Fan Z, Luo J and Ke ZJ:
Lithium fails to protect dopaminergic neurons in the 6-OHDA model
of Parkinson's disease. Neurochem Res. 36:367–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garcia-Rivas GJ and Torre-Amione G:
Abnormal mitochondrial function during ischemia reperfusion
provides targets for pharmacological therapy. Methodist Debakey
Cardiovasc J. 5:2–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poyton RO, Ball KA and Castello PR:
Mitochondrial generation of free radicals and hypoxic signaling.
Trends Endocrinol Metab. 20:332–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinnally KW and Antonsson B: A tale of two
mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis.
12:857–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goitre L, Balzac F, Degani S, Degan P,
Marchi S, Pinton P and Retta SF: KRIT1 regulates the homeostasis of
intracellular reactive oxygen species. PLoS One. 5:e117862010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pislar AH, Zidar N, Kikelj D and Kos J:
Cathepsin X promotes 6-hydroxydopamine-induced apoptosis of PC12
and SH-SY5Y cells. Neuropharmacology. 82:121–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun R, Wang K, Wu D, Li X and Ou Y:
Protective effect of paeoniflorin against glutamate-induced
neurotoxicity in PC12 cells via Bcl-2/Bax signal pathway. Folia
Neuropathol. 50:270–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavik AR, Zhong F, Chang MJ, Greenberg E,
Choudhary Y, Smith MR, McColl KS, Pink J, Reu FJ, Matsuyama S and
Distelhorst CW: A synthetic peptide targeting the BH4 domain of
Bcl-2 induces apoptosis in multiple myeloma and follicular lymphoma
cells alone or in combination with agents targeting the BH3-binding
pocket of Bcl-2. Oncotarget. 6:27388–27402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Tan QR and Zhang ZJ:
Neuroprotective effects of paeoniflorin, but not the isomer
albiflorin, are associated with the suppression of intracellular
calcium and calcium/calmodulin protein kinase II in PC12 cells. J
Mol Neurosci. 51:581–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Cheong YK, Wang H, Ren G and Yang Z:
Neuroprotective effects of etidronate and 2,3,3-trisphosphonate
against glutamate-induced toxicity in PC12 cells. Neurochem Res.
41:844–854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu S, Wang D, Zhang J, Du M, Cheng Y, Liu
Y, Zhang N, Wang D and Wu Y: Mitochondria related pathway is
essential for polysaccharides purified from sparassis crispa
mediated neuro-protection against glutamate-induced toxicity in
differentiated PC12 cells. Int J Mol Sci. 17:pii: E1332016.
View Article : Google Scholar
|