Introduction
The first reported case of aluminum toxicity in
humans was from the early 1970s and following this, aluminum
overexpression has been demonstrated to result in severe brain
damage and neurodegeneration (1).
Aluminum-induced neuronal injury is associated with the
neuroinflammatory response (2,3).
Previous studies have indicated that the consumption of
anti-inflammatory medication attenuates aluminum-induced functional
neurotoxicity in rats (4).
Intestinal endotoxemia (IETM) is involved in liver
failure, is associated with lipopolysaccharides (LPS) and has
previously been demonstrated to exhibit a pathogenetic mechanism of
initiation (5). Typically, mild
cases of IETM often result in chronic hepatic failure (6). Inflammation induced by LPS under
conditions of IETM is important in the development of numerous
diseases (7,8), however, if IETM-induced inflammation
occurs during the development of aluminum neurotoxicity, remains to
be elucidated.
The aim of the present study was to investigate the
effect of inflammation induced by IETM in a rat model of aluminum
neurotoxicity established using D-galactose and aluminum
trichloride (AlCl3). D-galactose was used due to its
known neurotoxic effect (9).
Following successful creation of the rat model of aluminum
neurotoxicity, the learning and memory abilities of the rats were
observed. The serum levels of LPS, tumor necrosis factor (TNF)-α,
interleukin (IL)-1, diamine oxidase (DAO), glutamine (Gln) and
glutaminase were detected to evaluate the degree of IETM. The
expression levels of amyloid β-protein (Aβ) precursor (APP),
presenilin 1 (PS1), β-site APP-cleaving enzyme (BACE), zona
occludens protein (ZO)-1 and Aβ1-40 in the brain were observed, in
addition to the total quantity of lysozyme (LYZ) in the liver and
S-100β in the serum of the rats. The results of the present study
reveal the underlying role of LPS in the development of aluminum
toxicity and thus, may provide novel strategies for the prevention
and treatment of associated clinical diseases such as Alzheimer's
disease and amyotrophic lateral sclerosis.
Materials and methods
Experimental protocol and ethics
statement
Rats were provided by Vital River Experimental
Technology Company Ltd. (Beijing, China). A total of 48 adult (age,
9–11 weeks) male Wistar rats, weighing 200–250 g, were housed 6 per
cage at 20–25°C with a relative humidity of 50–60%, a 12 h
light-dark cycle and free access to food and water. Rats were
randomized to either receive vehicle (0.9% NaCl; 85 mg.kg-1.day-1)
or D-galactose intraperitoneal injection (60 mg.kg-1.day-1) and
AlCl3 (25 mg.kg-1.day-1) for 90 days, to establish the
aluminum neurotoxicity model. Following this, the learning and
memory abilities of the rats were observed using a Morris water
maze. The serum levels of LPS, TNF-α, and IL-1 were determined by
the chromogenic Limulus amoebocyte lysate (CLAL) and radioimmunity
assays. The level of DAO was measured using a Diamine oxidase
colorimetric assay kit (Shanghai Bairui Biotech Co., Ltd.,
Shanghai, China) according to the manufacturer's instructions. The
levels of Gln were measured using a Glutamine Colorimetric Assay
kit (Biovision, Inc., Milpitas, CA, USA), according to the
manufacturer's instructions, and glutaminase was measured using the
Glutaminase ELISA assay kit (cat. no. 201233721; Cusabio Biotech
Co., Ltd., Wuhan, Hubei, China) according to the manufacturer's
instructions. All experimental animal procedures were approved by
the Laboratory Animal Use and Care Committee of Shanxi Medical
University (Taiyuan, China; permit no. SXMU-2012-18;) and the
Ethics Committee of Animal Experiments of Shanxi Medical University
(permit no. 20120623-1). All surgery was performed under sodium
pentobarbital anesthesia and all possible efforts were made to
minimize the suffering of the experimental rats. The rats were
sacrificed via anesthetic overdose.
Morris water maze
The Morris water maze was used to test the learning
and memory of the rats for 5 days (n=10/group) and was performed 8
weeks following administration (10). For this experiment, a circular
stainless steel tank (155 cm in diameter and 60 cm deep) containing
water (24–26°C) to a depth of 40 cm was used; the extramaze cues,
which consisted of four uniquely colored and textured inserts
(coarse plastic mesh, metal screen, ridged plastic and sandpaper)
placed on the arms of the plus-maze, were kept constant. The rats
learned to escape from the water by searching for a submerged, and
therefore hidden, Plexiglas platform (10 cm in diameter and 38.5 cm
high), which was kept in a fixed position. To familiarize them with
the experimental situation, prior to the experiment, the rats swam
for 90 sec with the platform removed. During each trial, the animal
was placed in the water facing the wall at one of five designated
starting points. Animals that failed to find the platform within 60
sec were placed onto the platform and kept there for 30 sec, then
the rats were removed from the maze until the next repeat of the
experiment. The time taken to find the platform, termed the escape
latency, was measured. This test was performed once for each of the
5 starting points per day for 5 days.
Clal
The CLAL test kit was obtained from Chromogenix
(cat. no. 20120056; Molndal, Sweden). The analysis was conducted
according to the manufacturer's protocol. The coefficient of
variation was 8% (n=10/group).
Immunohistochemistry
The expression of LYZ in the liver and Aβ1-40 in the
brain (hippocampus) were detected via immunohistochemistry
(11–13). Rabbit anti-rat/−rabbit LYZ and
Aβ1-40 IgG antibodies (Abcam, Shanghai, China) were used. Following
the completion of the Morris water maze experiments, 12 rats (6
rats/group) were sacrificed and the whole brain and liver were
removed and dissected. Prior to sectioning, liver tissue and
hippocampus samples were fixed in 10% formalin for 24 h at room
temperature, then embedded in paraffin. The paraffin blocks were
cooled to −10°C to ease sectioning. Using the Microm HM340 E
microtome (Thermo Fisher Scientific Inc., Waltham, MA, USA), 4-µm
sections were generated. The EnVision™ staining kit was
used according to the manufacturer's instructions, for
immunohistochemical analyses [G|2 Doublestain System, Rabbit/Mouse
(DAB+/Permanent RED); cat. no. K5361; Dako DK; Agilent
Technologies, Inc., Santa Clara, CA, USA]. The optimal antigen
retrieval method and concentration to use were determined per
antibody. Following deparaffinization in dimethylbenzene twice at
room temperature for 5 min each time, the slides were washed in PBS
containing 0.05% Tween-20 prior to antigen retrieval in the
microwave (for liver tissues; 2 min 780 W, 10 min 150 W and 10 min
80 W) or in 70% formic acid (for hippocampus samples). The slides
were placed in cuvettes, as a part of the capillary gap staining
method (Thermo Fisher Scientific, Inc.), which was performed as
described previously (14).
Endogenous peroxidase activity was blocked by the
EnVision™ dual endogenous enzyme block (Agilent
Technologies, Inc.). Following washing with PBS containing 0.05%
Tween-20, a second blocking step was applied using PBS, 0.1% bovine
serum albumin (BSA; Sigma Aldrich; Merck KGaA, Darmstadt, Germany),
1% NHS and 0.2% Triton X-100. Then, the primary antibodies [rabbit
anti-rat/−rabbit LYZ (cat. no. 20120789) and Aβ1-40 (cat. no.
20123349) IgG antibodies; Abcam, Shanghai, China; with a 1:3,000
dilution in 0.1% BSA/PBS] were added for 10 min at room
temperature, followed by washing with PBS containing 0.05% Tween-20
and the addition of the EnVision™ polymer/horseradish
peroxidase (HRP) goat anti-rabbit secondary antibody (cat. no.
201213219; 1:500 dilution; Dako Cytomation; Agilent Technologies,
Inc.) for 10 min at room temperature. Following washing with PBS,
DAB+ (from EnVision™ staining kit; Dako DK; Agilent
Technologies, Inc.) was added to initiate the enzymatic reaction
and allow for visualization of the elements. A total of 10 high
magnification fields were randomly selected from each slice and the
number of positive cells per high magnification field (upright
light microscope; CX31-LV320; Olympus Corporation, Tokyo, Japan)
were counted. Following this, the average number of positive cells
per slice was calculated.
Enzyme-linked immunosorbent assay
(ELISA)
The S-100β level was measured using an S-100β ELISA
kit (cat. no. 20120166; Neogen Europe, Ltd., Lansing, MI, USA)
according to the manufacturer's protocol (n=10/group).
Western blotting
Protein was extracted from fresh brain tissue using
a ProteoExtract Subcellular Proteome Extraction kit (Sigma Aldrich;
Merck KGaA). Protein concentration in the lysates was evaluated
using a bicinchoninic acid protein assay kit according to the
manufacturer's instructions (Thermo Fisher Scientific, Inc.).
Following 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (5 µg protein applied) and transfer onto a
nitrocellulose membrane, the membranes were blocked with 5% BSA
(Sigma Aldrich; Merck KGaA) and 0.05% Tween-20 in Tris-buffered
saline (TBST) for 1 h at room temperature. This was followed by
overnight incubation at room temperature with the following primary
antibodies: Monoclonal rabbit anti-rat ZO-1 antibody (1:200; cat.
no. 201212315; Abcam), monoclonal rabbit anti-rat APP antibody
(1:500; cat. no. 20121232; Abcam), monoclonal rabbit anti-rat PS-1
antibody (1:1,000; cat. no. 20123343; Abcam), monoclonal rabbit
anti-rat BACE1 antibody (1:500; cat. no. 201233561; Abcam) and
monoclonal rabbit anti-rat β-actin antibody (1:1,000, cat. no.
201214231; Abcam). Following three washes with TBST, the membranes
were incubated with a HRP-conjugated anti-rabbit antibody (1:5,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h at room
temperature. The membranes were exposed to film (Kodak, Rochester,
NY, USA) and developed. The films were scanned and analyzed using
the Kodak IS440 Automated Digitizing System and image analytical
software (ImageQuant TL v2005; GE Healthcare Life Sciences,
Uppsala, Sweden) for densitometric analysis. Experiments were
repeated 3 times.
Reverse-transcription polymerase chain
reaction (RT-PCR)
Primers: APP (295 bp), forward
5′-GGATGCGGAGTTCGGACATG-3′ and reverse 5′-GTTCTGACTCTGCTCAAAG-3′;
PS-1 (186 bp), forward 5′-AGATGCCTCCTCTGTCCTCA-3′ and reverse
5′-CCGTCTTTGGGCATACATCT-3′; BACE1 (309 bp), forward
5′-CACCGAGACCGACGAAGAGCC-3′ and reverse
3′-CTAATAGGCTATGGTCATGAGGGT-3′; GAPDH (307 bp), forward
5′-TTGCCAGTTGCTTTAGTGATA-3′ and reverse
5′-CTTTTTCCCCCATTTCATTTC-3′.
Total RNA from fresh brain tissue samples was
extracted using the TRIzol® reagent (Takara Biotechnology Co.,
Ltd., Dalian, China) according to the manufacturer's instructions.
RNA was reverse transcribed to cDNA using the Takara PrimeScript
1st Strand cDNA Synthesis kit, according to the manufacturer's
instructions, and the obtained cDNA was used as a template to
perform PCR amplification. Semi-quantitative RT-PCR was performed
with a mixture of the cDNA product, primers, dNTP and Taq DNA
polymerase (Invitrogen; Thermo Fisher Scientific, Inc.). The
thermocycling conditions used were as follows: 95°C for 30 sec,
55°C for 30 sec and 72°C for 30 sec for 25 cycles, followed by a
final extension at 72°C for 5 min. Results were normalized to the
APP reference gene. Electrophoresis was performed on 1.5%
agarose gel (10 mA; 100 V; 30 min). The images were scanned and
then analyzed with Multi-Analyst software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Data were analyzed using the SPSS software version
22.0 (IBM Corp., Armonk, NY, USA). All values are expressed as the
mean ± standard deviation. Statistical analysis was performed using
an unpaired Student's t-test, multiple-factor repetitive
measurement and one-way repeated measures analysis of variance (for
learning and memory abilities). P<0.05 was considered to
indicate a statistically significant difference.
Results
Learning and memory abilities
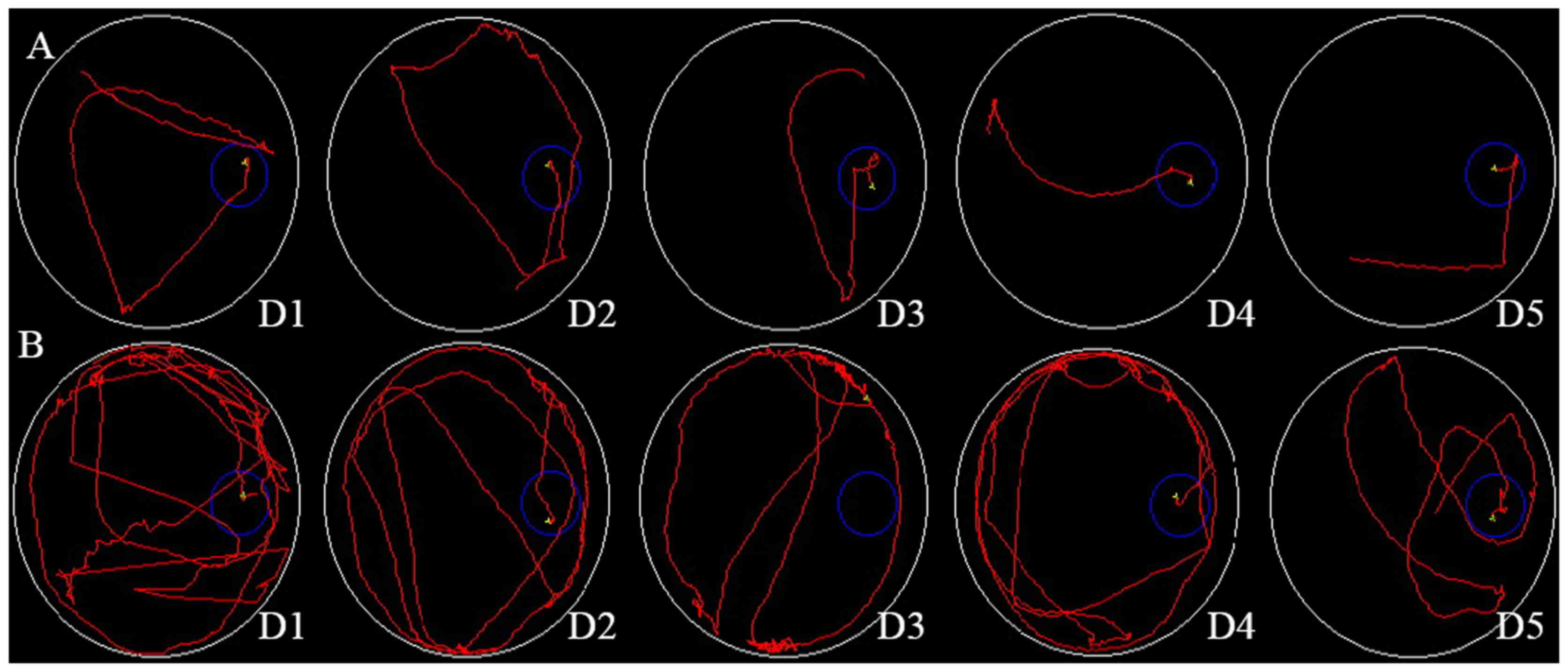
The rat escape latency in the Morris water maze from
days 1–5 was 59±22, 52±18, 38±13, 30±11 and 25±9 sec, respectively,
in the D-galactose + AlCl3 group and 39±12, 30±10, 24±8,
15±7 and 12±5 sec, respectively, in the control group (Fig. 1A and B). These data are
additionally presented in Fig. 1C.
There were no differences in the two groups from days 1–3
(P>0.01). However, from days 4–5, the escape latency in the
D-galactose + AlCl3 group was increased compared with
the control group (30±11 vs. 15±7 sec and 25±9 vs. 12±5 sec,
respectively; P<0.01). From days 4–5, the swim path of the
control group was altered from a random line to a straight line.
However, the swim path of the test group remained random (Fig. 1B). The memory ability was decreased
in the rats treated with D-galactose and AlCl3.
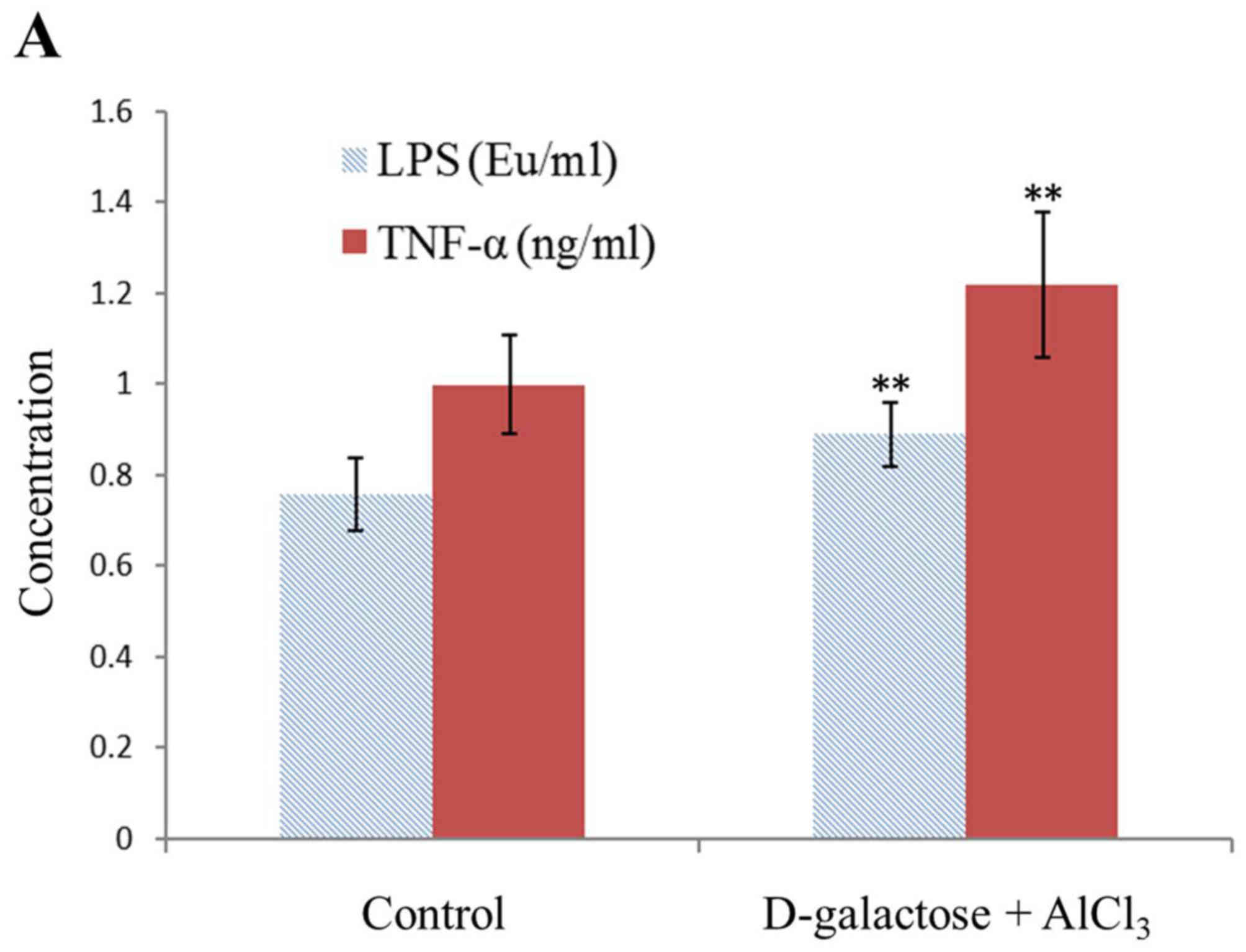
Levels of TNF-α, IL-1, and LPS
The geometric means of the LPS, TNF-α and IL-1
levels in the normal rats were 0.76±0.08 EU/ml, 1.0±0.11 ng/ml and
23.96±3.39 pg/ml, respectively. However, those in the test group
were 0.89±0.07 EU/ml, 1.22±0.16 ng/ml and 38.38±3.48 pg/ml,
respectively, with a significant difference observed (P<0.01;
Fig. 2).
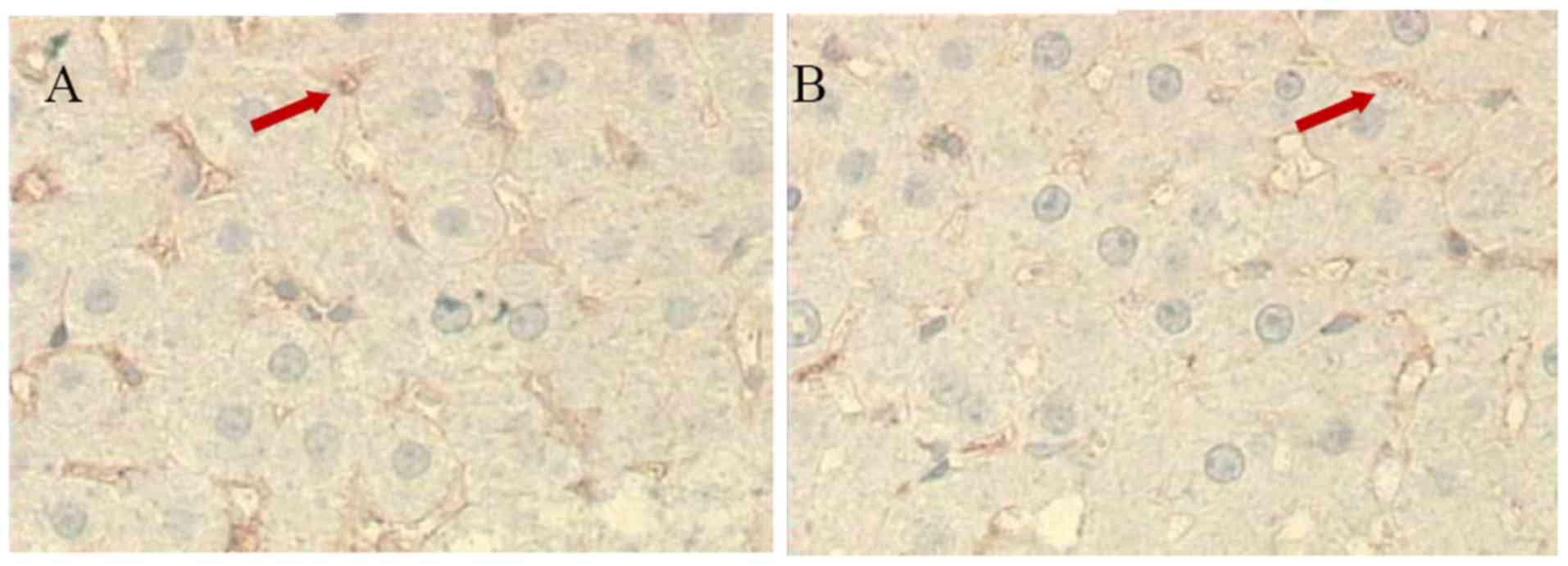
Expression of LYZ in liver
There were numerous brown LYZ cells in the control
rat livers (30.6±8.2; n=10), however fewer LYZ cells were present
in the livers of the aluminum neurotoxicity model rats (18.1±5.1;
n=10; P<0.01 vs. control group; Fig. 3). This observation indicated
decreased Kupffer cell function.
Intestinal mucosal barrier
function
The levels of DAO and Gln in the serum and
intestinal mucosa of the model rats were significantly greater
compared with control rats (P<0.01), whereas the level of
glutaminase was decreased in the model rats compared with control
rats (P<0.01; Table I).
 | Table I.Levels of DAO, glutaminase and Gln in
sera and intestinal mucosa. |
Table I.
Levels of DAO, glutaminase and Gln in
sera and intestinal mucosa.
|
|
|
| Gln |
|---|
|
|
|
|
|
|---|
| Group | DAO (U/ml) | Glutaminase
(mmol/h.g) | Sera (mg/dl) | Intestinal mucosa
(mg/g) |
|---|
| Control | 0.52±0.13 | 2.37±0.34 | 50.37±16.85 | 3.28±1.24 |
|
D-galactose+AlCl3 |
1.18±0.36a |
1.24±0.31a |
196.54±4357a |
16.54±6.27a |
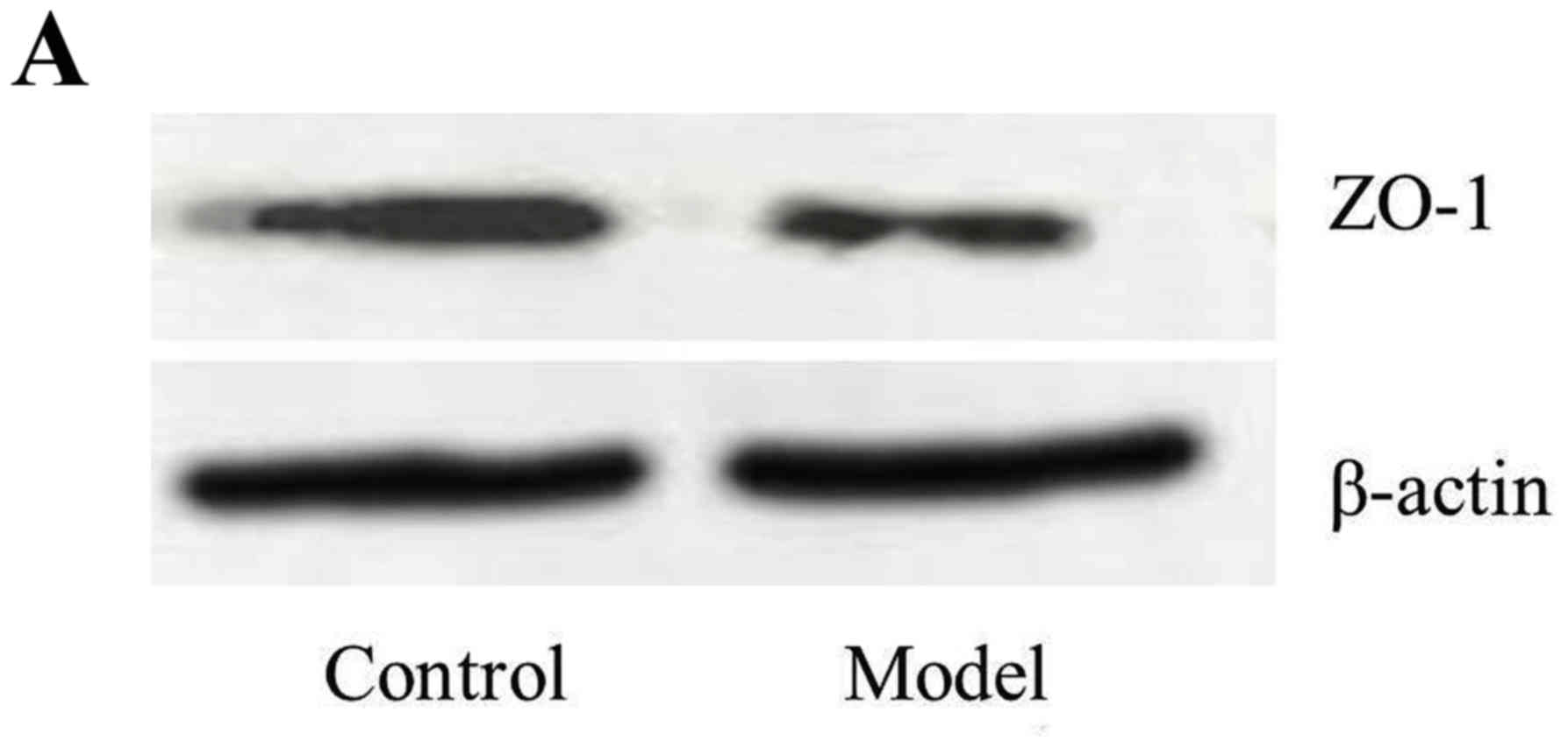
Blood-brain barrier (BBB)
function
The expression of S-100β in the serum (0.33±0.18)
was increased in the model rats compared with control rats
(0.11±0.03; n=10; P<0.01). The expression of ZO-1 in the brain
was increased in the control rats (53.3±8.4; ratio of ZO-1/β-Actin)
compared with the model rats (26.9±5.6; P<0.01; Fig. 4). These data demonstrated that the
BBB function was decreased and permeability was increased in the
model group.
mRNA and protein expression levels of
APP, PS and BACE1
As presented in Fig.
5, the addition of D-galactose and AlCl3 resulted in
a marked increase in the expression of APP, PS1 and BACE1 mRNA
(72±12, 52±10 and 72±15%, respectively) that were ~two-fold
increases compared with control rats (30±6, 32±7 and 36±9%,
respectively). The protein expression levels of APP, PS1 and BACE1
in the model and control groups demonstrated a similar trend to the
aforementioned mRNA results.
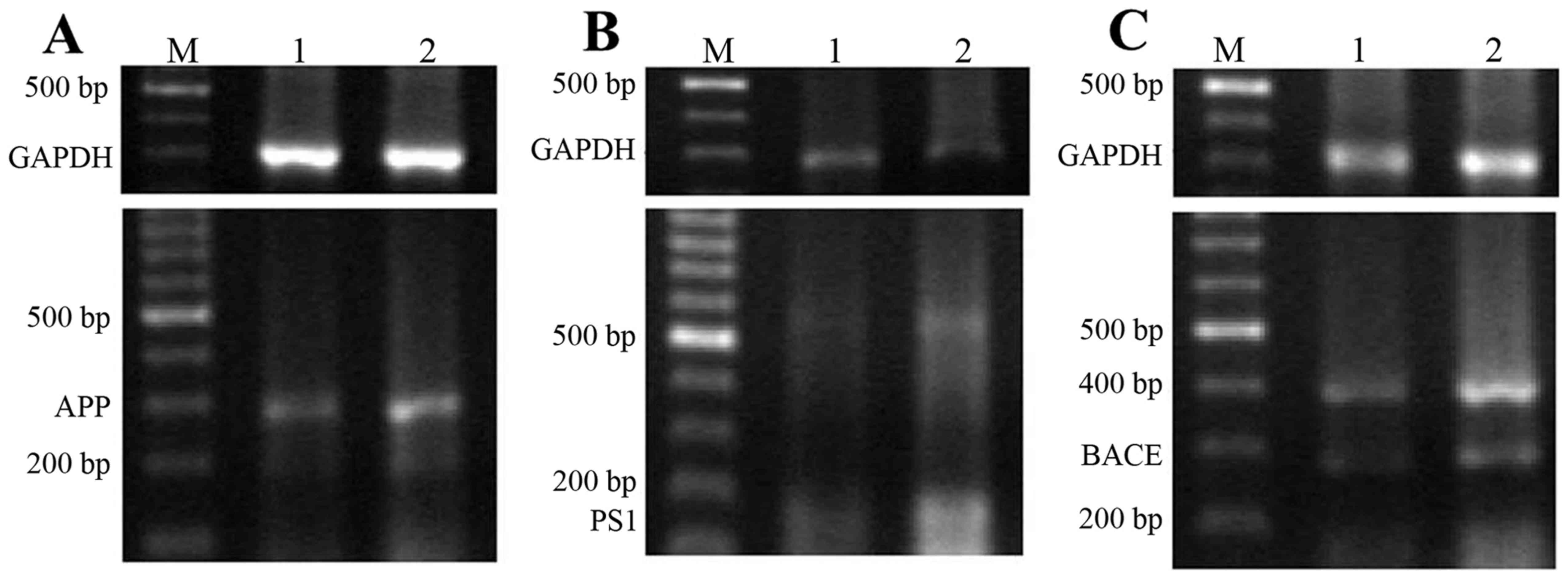 | Figure 5.mRNA and protein expression levels of
APP, PS1 and BACE1 in the rat brain. Expression of (A) APP, (B) PS1
and (C) BACE1 mRNA in the brain detected via reverse
transcription-polymerase chain reaction. (D) Expression of APP, PS1
and BACE1 protein in the brain detected via western blotting. M,
DNA molecule marker; 1, control group; 2, aluminum neurotoxicity
model group; bp, base pairs. APP, amyloid β-protein precursor; PS1,
presenilin 1; BACE, β-site APP-cleaving enzyme. |
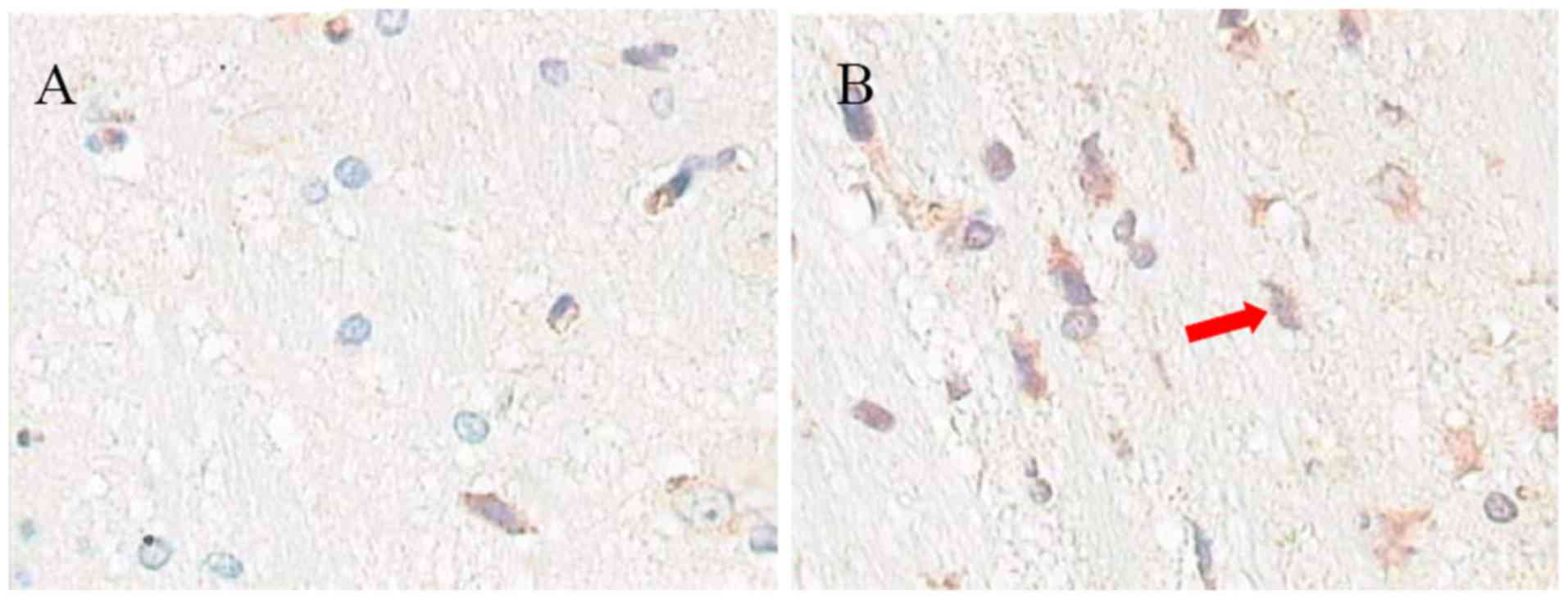
Expression of Aβ1-40
As presented in Fig.
6, the expression of Aβ1-40 in the hippocampal brain of the
aluminum neurotoxicity model rats (24.1±5.1) was increased compared
with control rats (8.6±2.2; n=10; P<0.05; Fig. 6C).
Discussion
The pathological presentation of aluminum
neurotoxicity involves regionalized neuronal dysfunction/apoptosis,
lesions termed neurofibrillary tangles and deposition of Aβ protein
(15,16). In previous studies, hypotheses have
been proposed to link the lesions and cytopathology of aluminum
neurotoxicity with the development of inflammation (2). Inflammation has previously been
suggested to act as a marker and potential inducer of aluminum
neurotoxicity (17).
Han (5), Han
(6) and Zhao and Han (7) first suggested that liver injury may
result from increased levels of LPS. Advanced cases of IETM
typically result in excessive inflammation with severe hepatic
necrosis, hepatitis and acute liver failure. The human endotoxin
model, an in vivo model of systemic inflammation in which
LPS is injected or infused intravenously into healthy volunteers,
may potentially be of use in elucidating the underlying mechanisms
involved (18).
Cerebral deposition of Aβ is a feature of aluminum
neurotoxicity. Induction of Aβ overexpression via AlCl3
or D-galactose in the rat or mouse brain results in animal models
of aluminum neurotoxicity and aging. Aluminum and D-galactose
affect the expression of Aβ metabolism-associated molecules,
suggesting that this mouse model may be useful for studying the
mechanisms and biomarkers of aluminum neurotoxicity (9).
In the present study, the escape latency of rats
treated with D-galactose and AlCl3 was increased
compared with the control group. The model rats exhibited a longer
escape latency to find the platform when compared with the control
rats. D-Galactose and AlCl3 resulted in increased mRNA
and protein expression levels of APP, PS1 and BACE. The expression
of Aβ was additionally increased. These results verify those from
previous reports that suggest high doses of D-galactose and
AlCl3 are associated with neurotoxicity (9). To the best of our knowledge, the
present study demonstrated for the first time that the levels of
LPS, TNF-α and IL-1 in the blood of rats treated with D-galactose
and AlCl3 were significantly increased compared with the
control group.
LPS is a potent inducer of inflammatory cytokines
including TNF-α, IL-6 and IL-1β in macrophages and microglia
(19–21). Bacterial LPS induces numerous host
responses that are beneficial and detrimental (22). Liu et al (23) demonstrated that cluster of
differentiation 14 interacts with Aβ fibrils and therefore
contributes to microglial phagocytosis of Aβ42 fibrils.
Inflammation potentially increases the level of Aβ present in the
brain via three potential mechanisms: Increased influx, decreased
efflux and increased neuronal production (24).
TNF-α is a primary pro-inflammatory cytokine and its
role in the pathogenesis of aluminum neurotoxicity has previously
been investigated (25). TNF-α
influences the expression or metabolism of various molecules that
are involved in the development of aluminum neurotoxicity,
including Aβ (26,27). TNF is upregulated in the mouse
brain following exposure to aluminum (28).
IL-1, which is an immune regulatory cytokine, may
promote the merging and secretion of APP (29) and Aβ (30). Anti-inflammatory drugs have
previously been suggested as a treatment for aluminum
neurotoxicity. Tsunoda et al (28) suggests that IL-1β may be involved
in one of the key disease mechanisms for aluminum neurotoxicity. Of
all the numerous inflammatory cytokines associated with aluminum
neurotoxicity, IL-1β in particular, has been identified to exhibit
an important pathogenic role (27).
Furthermore, the Kupffer cell function in the model
rat liver appeared to be decreased and the barrier function of the
intestinal mucosa and BBB exhibited damage. A previous study
suggested that aluminum may stimulate Fe2+-supported
lipid peroxidation by binding to the membrane, thus affecting the
BBB permeability (31). During BBB
maturation and alteration, the brain endothelia develop a
functional polarity and the membrane glycoprotein containing
α-D-galactose is downregulated (32). Therefore, the LPS from the
intestinal mucosa are able to penetrate the intestinal mucosal
barrier and enter the serum. In addition, the impaired function of
Kupffer cells leads to decreased removal of plasma LPS. The high
level of LPS subsequently activates the Kupffer cells, releasing
TNF-α and IL-1 and finally leads to IETM. The BBB damage allows LPS
to readily enter the brain and induce inflammation.
In conclusion, the present study demonstrated that
IETM was present in the rat model of aluminum neurotoxicity
established by D-galactose and AlCl3 and may be
important in the development of neurotoxicity.
Acknowledgements
The authors would like to thank Mr. Yuanchang Zhao
and Mrs. Jiahui Zhao (Department of Pathophysiology, Shanxi Medical
University, Taiyuan, Shanxi, China) for their technical
assistance.
References
|
1
|
Zhang Q, Li N, Jiao X, Qin X, Kaur R, Lu
X, Song J, Wang L, Wang J and Niu Q: Caspase-3 short hairpin RNAs:
A potential therapeutic agent in neurodegeneration of
aluminum-exposed animal model. Curr Alzheimer Res. 11:961–970.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campbell A: The role of aluminum and
copper on neuroinflammation and Alzheimer's disease. J Alzheimers
Dis. 10:165–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castorina A, Tiralongo A, Giunta S,
Carnazza ML, Scapagnini G and D'Agata V: Early effects of aluminum
chloride on beta-secretase mRNA expression in a neuronal model of
beta-amyloid toxicity. Cell Biol Toxicol. 26:367–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sethi P, Jyoti A, Hussain E and Sharma D:
Curcumin attenuates aluminium-induced functional neurotoxicity in
rats. Pharmacol Biochem Behav. 93:31–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han DW: Intestinal endotoxemia and liver
disease-IETM theory of liver failure. Chin J Hepatol. 3:134–137.
1995.
|
|
6
|
Han DW: Intestinal endotoxemia as a
pathogenetic mechanism in liver failure. World J Gaotroenteral.
8:961–965. 2002. View Article : Google Scholar
|
|
7
|
Zhao LF and Han DW: Clinical significance
of endotoxemia in liver diseases. Shijie Huaren Xiaohua Zazhi.
7:391–393. 1999.
|
|
8
|
Zhou X, Han D, Xu R, Li S, Wu H, Qu C,
Wang F, Wang X and Zhao Y: A model of metabolic syndrome and
related diseases with intestinal endotoxemia in rats fed a high fat
and high sucrose diet. PLoS One. 9:e1151482014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang W, Shi L, Chen L, Zhang B, Ma K, Liu
Y and Qian Y: Protective effects of perindopril on d-galactose and
aluminum trichloride induced neurotoxicity via the apoptosis of
mitochondria-mediated intrinsic pathway in the hippocampus of mice.
Brain Res Bull. 109:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarrete M, Núñez H, Ruiz S, Soto-Moyano
R, Valladares L, White A and Pérez H: Prenatal undernutrition
decreases the sensitivity of the hypothalamo-pituitary-adrenal axis
in rat, as revealed by subcutaneous and intra-paraventricular
dexamethasone challenges. Neurosci Lett. 419:99–103. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coria F, Castaño EM and Frangione B: Brain
amyloid in normal aging and cerebral amyloid angiopathy is
antigenically related Alzheimer's disease beta-protein. Am J
Pathol. 129:422–428. 1987.PubMed/NCBI
|
|
12
|
Davies L, Wolska B, Hilbich C, Multhaup G,
Martins R, Simms G, Beyreuther K and Masters CL: A4 amyloid protein
deposition and the diagnosis of Alzheimer's disease: Prevalence in
aged brains determined by immunocytochemistry compared with
conventional neuropathologic techniques. Neurology. 38:1688–1693.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitamoto T, Ogomori K, Tateishi J and
Prusiner SB: Formic acid pretreatment enhances immunostaining of
cerebral and systemic amyloids. Lab Invest. 57:230–236.
1987.PubMed/NCBI
|
|
14
|
Philippens IH, Ormel PR, Baarends G,
Johansson M, Remarque EJ and Doverskog M: Acceleration of
amyloidosis by inflammation in the amyloid-beta marmoset monkey
model of Alzheimer's disease. J Alzheimers Dis. 55:101–113. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perl DP and Pendlebury WW: Aluminum
neurotoxicity-potential role in the pathogenesis of neurofibrillary
tangle formation. Can J Neurol Sci. 13 4 Suppl:S441–S445. 1986.
View Article : Google Scholar
|
|
16
|
Kawahara M, Muramoto K, Kobayashi K, Mori
H and Kuroda Y: Aluminum promotes the aggregation of Alzheimer's
amyloid beta-protein in vitro. Biochem Biophys Res Commun.
198:531–535. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prakash D, Gopinath K and Sudhandiran G:
Fisetin enhances behavioral performances and attenuates reactive
gliosis and inflammation during aluminum chloride-induced
neurotoxicity. Neuromolecular Med. 15:192–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreasen AS, Krabbe KS, Krogh-Madsen R,
Taudorf S, Pedersen BK and Møller K: Human endotoxemia as a model
of systemic inflammation. Curr Med Chem. 15:1697–1705. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Cui Z, Wang S and Zhang D: CD93 and
GIPC expression and localization during central nervous system
inflammation. Neural Regen Res. 9:1995–2001. 2014.PubMed/NCBI
|
|
20
|
Mandrekar-Colucci S and Landreth GE:
Microglia and inflammation in Alzheimer's disease. CNS Neurol
Disord Drug Targets. 9:156–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skelly DT, Hennessy E, Dansereau MA and
Cunningham C: A systematic analysis of the peripheral and CNS
effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6
challenges in C57BL/6 mice. PLoS One. 8:e691232013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang YH, Lee CH, Monroy RL, Dwivedi RS,
Odeyale C and Newball HH: Uptake, distribution and fate of
bacterial lipopolysaccharides in monocytes and macrophages: An
ultrastructural and functional correlation. Electron Microsc Rev.
5:381–419. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Walter S, Stagi M, Cherny D,
Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H and
Fassbender K: LPS receptor (CD14): A receptor for phagocytosis of
Alzheimer's amyloid peptide. Brain. 128:1778–1789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaeger LB, Dohgu S, Sultana R, Lynch JL,
Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA,
Butterfield DA and Banks WA: Lipopolysaccharide alters the
blood-brain barrier transport of amyloid beta protein: A mechanism
for inflammation in the progression of Alzheimer's disease. Brain
Behav Immun. 23:507–517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu L, Zhang K, Hu G, Yan H, Xie C and Wu
X: Inflammatory response and neuronal necrosis in rats with
cerebral ischemia. Neural Regen Res. 9:1753–1762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaky A, Mohammad B, Moftah M, Kandeel KM
and Bassiouny AR: Apurinic/apyrimidinic endonuclease 1 is a key
modulator of aluminum-induced neuroinflammation. BMC Neurosci.
14:262013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nedzvetsky VS, Tuzcu M, Yasar A,
Tikhomirov AA and Baydas G: Effects of vitamin E against aluminum
neurotoxicity in rats. Biochemistry (Mosc). 71:239–244. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsunoda M and Sharma RP: Modulation of
tumor necrosis factor alpha expression in mouse brain after
exposure to aluminum in drinking water. Arch Toxicol. 73:419–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mackenzie IR: Anti-inflammatory drugs and
Alzheimer-type pathology in aging. Neurology. 54:732–734. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samy AS and Igwe OJ: Regulation of
IL-1β-induced cyclooxygenase-2 expression by interactions of Aβ
peptide, apolipoprotein E and nitric oxide in human neuroglioma. J
Mol Neurosci. 47:533–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gutteridge JM, Quinlan GJ, Clark I and
Halliwell B: Aluminium salts accelerate peroxidation of membrane
lipids stimulated by iron salts. Biochim Biophys Acta. 835:441–447.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu CH, Wen CY, Shieh JY and Ling EA:
Remodeling of membrane-bound glycoproteins containing
alpha-D-galactose in the cerebral endothelial cells of rats during
blood-brain barrier maturation and alteration. J Hirnforsch.
38:541–552. 1997.PubMed/NCBI
|