Introduction
Colorectal cancer, including cancerous growths in
the colon, appendix and rectum, is the third most commonly
diagnosed cancer in men and the second most common in women, with
an estimated 1,400,000 cases and a mortality rate of 693,900
worldwide in 2012 (1). Current
treatment for colon cancer includes surgery, radiation, physical
rehabilitation, nutritional therapy and chemotherapy using
cytotoxic drugs (2). However,
these treatments are not curative. Therefore, effective anticancer
drugs for colon cancer are required.
Gossypol is a polyphenolic, yellowish compound
derived from cottonseed extract. It is extensively used as a
contraceptive agent in men (3,4).
Gossypol has been identified as a promising anticancer agent. Moon
et al (3) demonstrated that
gossypol effectively inhibited tumor necrosis factor-α-induced
expression of intercellular adhesion molecule 1 by activating the
suppression of nuclear factor-κB, and in vitro invasion and
adhesion in human breast cancer cells. Another study demonstrated
that gossypol reduced the viability of three prostate cancer cell
lines with a half-maximal inhibitory concentration
(IC50) of between 3 and 5 µmol/l. In addition, gossypol
effectively inhibited prostate tumor-initiating cell-driven tumor
growth in a NOD/SCID xenograft model, in addition to inducing DNA
damage and activating p53 (5).
Knowledge of the molecular mechanisms underlying the anticancer
effects of gossypol against HT-29 human colonic cancer cells is
limited. Autophagy and apoptosis are two evolutionarily conserved
programmed cell death mechanisms, which occur in several
physiological conditions (6).
Autophagy and apoptosis are dysregulated in cancer cells (6); therefore, the present study examined
the effects of gossypol on the apoptosis and autophagy of HT-29
cells.
Materials and methods
Compounds and reagents
Gossypol (>98% pure) was purchased from LKT
Laboratories, Inc. (Hanzhou, China) and reconstituted in DMSO
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). RPMI-1640
medium and fetal bovine serum (FBS) were obtained from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The Cell
counting Kit-8 (CCK-8),
5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetra-ethylbenzimidalyl-carbocyanineiodide
(JC-1) and Annexin V/PI apoptosis detection kits were purchased
from Beyotime Institute of Biotechnology (Nanjing, China).
Antibodies against Caspase-3, B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax), microtubule-associated protein
light chain 3 (LC3), Beclin-1, Cytochrome c (Cyt-c) and β-actin
were commercially available from MBL International Co. (Boston, MA,
USA).
Cell culture
The HT-29 human colon cancer cells were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere
in a 5% CO2 incubator at 37°C.
CCK-8 assay
The growth-inhibitory effect of gossypol was
determined using a CCK-8 assay. Briefly, the HT-29 human colon
cancer cells were seeded at a density of 1×104 cells/100 µl/well in
96-well plates and were cultured overnight for attachment.
Following 24 h of incubation, the HT-29 cells were incubated with
gossypol (5, 10, 20, 40 or 80 µM/l) in 96-well plates for 24 h at
37°C. Following incubation, 10 µl CCK-8 solution was added to each
well and incubated at 37°C for 4 h, and the absorbance was measured
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) at 450 nm.
Analysis of cell apoptosis
Apoptosis kits were used to detect the effect of
gossypol on the HT-29 cells. The cells (5×104 cells/well) were
treated with gossypol (20 and 40 µM/l) for 24 h at 37°C. The cells
were harvested and washed with ice-cold PBS, resuspended with
binding buffer, and simultaneously stained with Annexin-V-FITC and
PI for 5–15 min. The cells were then analyzed immediately using
flow cytometry.
Mitochondrial membrane potential (ΔΨm)
assay
JC-1 staining was used to detect changes in ΔΨm. The
cells (2×105 cells/well) were seeded in 6-well plates and treated
with or without gossypol (20 and 40 µM/l) for 24 h. Following
incubation; the collected cells were incubated with 5 µg/ml JC-1 in
culture medium for 15 min at 37°C. The staining solution was
removed and the cells were washed twice with JC-1 staining buffer.
The cell-associated fluorescence was analyzed using a FACSCalibur
flow cytometer.
Western blot analysis of Caspase-3,
Bax, Bcl-2, LC3, Beclin-1, Cyt-c and β-actin
The HT-29 cells were seeded at a density of 1×105
cells/well in 6-well plates and, following incubation for 24 h, the
cells were treated with gossypol (20 and 40 µM/l) for 24 h and then
harvested. The expression levels of Bcl-2, Caspase-3, Bax, LC3 and
Beclin-1, and the release of mitochondrial Cyt-c were then assessed
using western blot analysis. Proteins were extracted from HT-29
cells using a total protein extraction kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the instructions of
the manufacturer. Protein concentrations were determined using a
BCA protein assay kit (Beyotime Institute of Biotechnology).
Aliquots of cell lysates containing 20 µl of protein were separated
on a 12% SDS-PAGE gel, and transferred onto PVDF membranes. The
membranes were blocked with TBST buffer containing 10 mM Tris-HCl
(pH 7.5), 150 mM NaCl and 0.05% Tween-20, containing 5% skimmed
milk, and were then incubated with polyclonal antibodies against
Bcl-2 (cat. no. 2876; 1:500), Bax (cat. no. sc-493; 1:500),
caspase-3 (sc-16647; 1:1,000), Cyt-c (cat. no. 250621; 1:1,000),
LC3 (cat. no. 4108; 1:1,000), beclin-1 (cat. no. 4122; 1:500) and
β-actin (cat. no. 4967; 1:1,000) overnight at 4°C, respectively.
The membrane was then incubated with either HRP-conjugated goat
anti-rabbit IgG (cat. no. sc-2005; 1:10,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or goat anti-mouse IgG (cat.
no. sc-2004; 1:10,000; Santa Cruz Biotechnology, Inc.) secondary
antibodies for 1.5 h at room temperature. Peroxidase activity was
detected via ECL visualization of the bands. The images were
analyzed and quantified using a Quantity One software version 4.6.2
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS
version 10.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. Statistical comparisons were
performed using Student's t-test. P<0.05 was considered
significant a statistically significant difference between
groups.
Results
Inhibitory effects of gossypol on
HT-29 cells
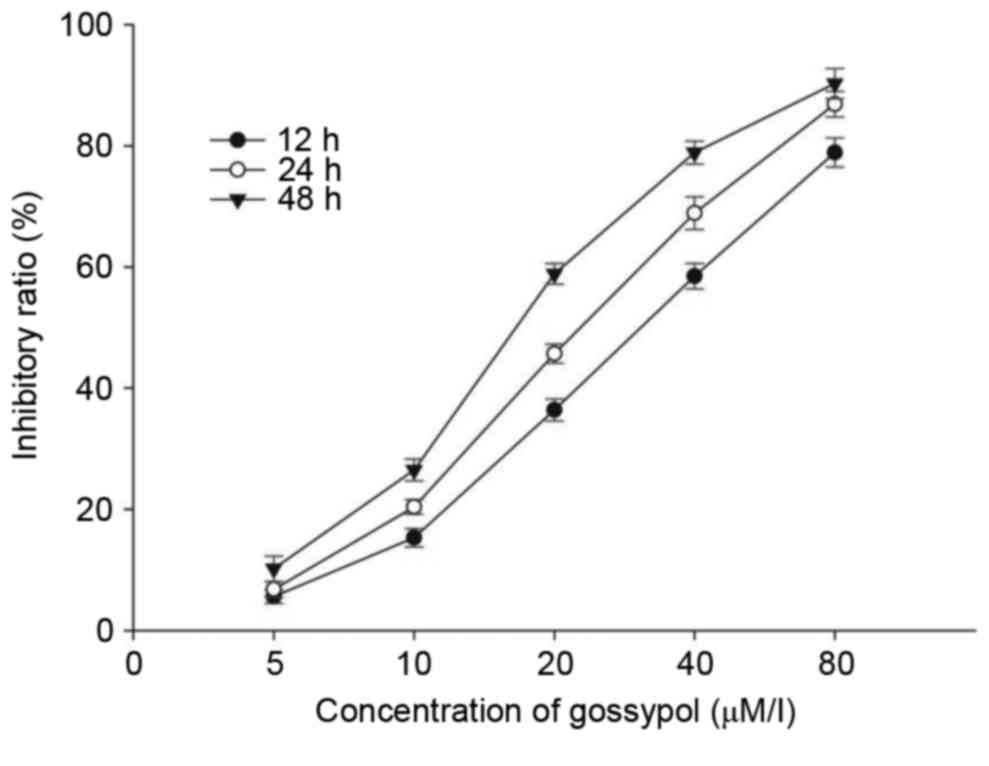
A CCK-8 assay was performed to assess the inhibitory
effects of gossypol on the HT-29 cells. The cells were cultured
with 5–80 µM/l gossypol for 12, 24 and 48 h. As shown in Fig. 1, gossypol inhibited the growth of
HT-29 cells in a time- and dose-dependent manner, with a 12 h
IC50 of 31.20 µM/l, 24 h IC50 of 23.60 µM/l
and 48 h IC50 of 17.97 µM/l. The two concentrations of
20 and 40 µM/l were selected for the following experiments.
Effect of gossypol on apoptosis
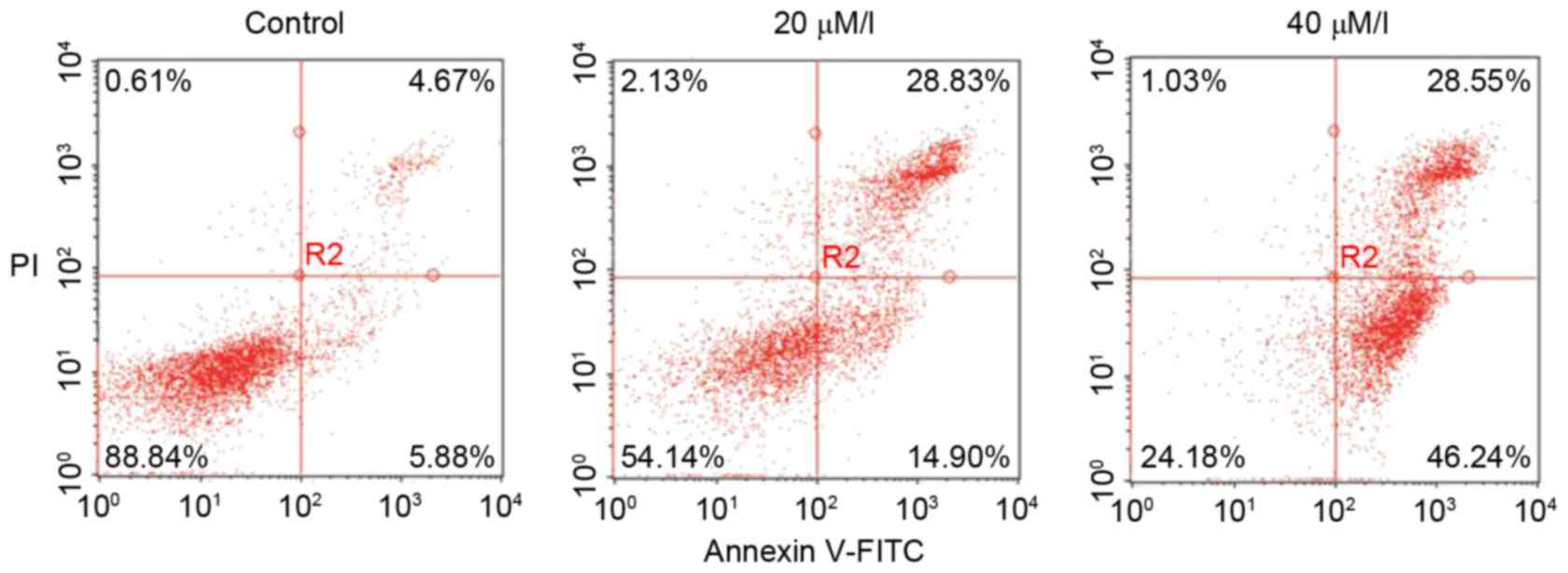
To determine the induction of cell apoptosis by
gossypol, an Annexin V and PI staining assay based on flow
cytometry was used. As shown in Fig.
2, there were significant changes in the proportions of early
and late apoptotic, or necrotic HT-29 cells following exposure to
gossypol for 24 h. Compared with the control group, the proportion
of early apoptotic cells increased from 5.88 to 46.24% and the
proportions of late apoptotic cells increased from 4.67 to
28.55%.
Effects of gossypol on ΔΨm
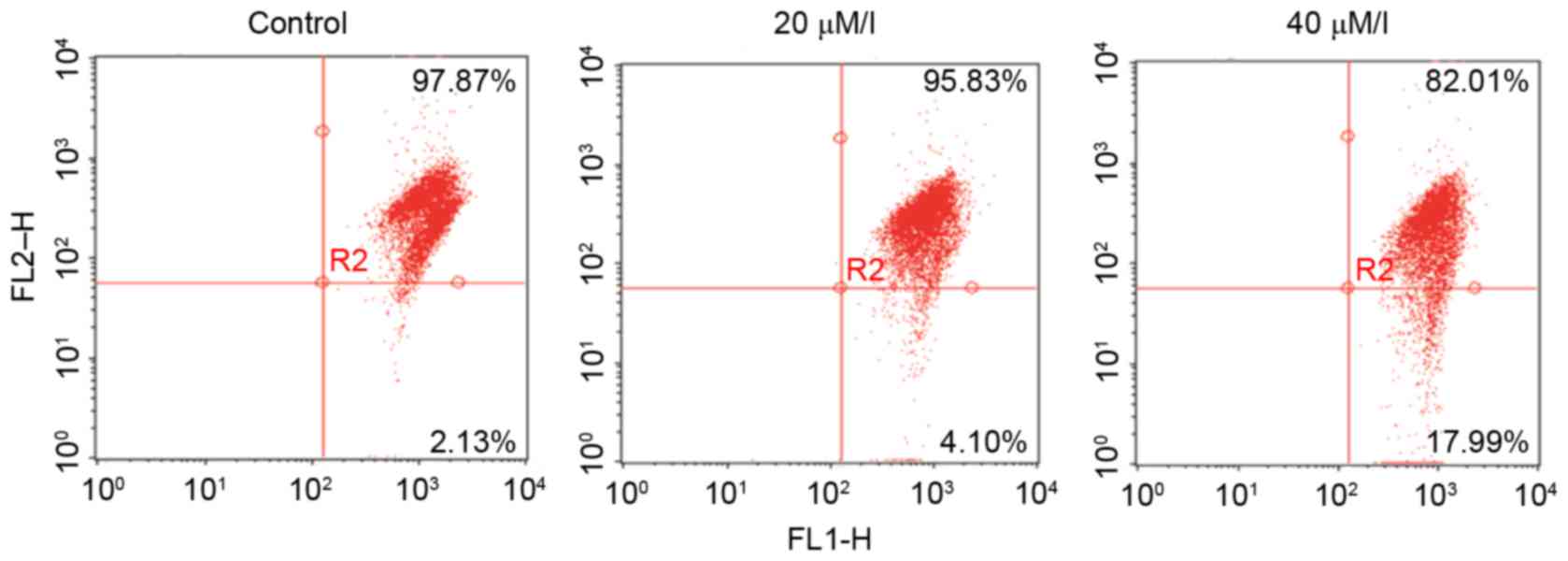
A loss of ΔΨm was detected by the fluorescent dye,
JC-1. As shown in Fig. 3,
treatment with gossypol (20 and 40 µM/l) for 24 h induced a loss in
the ΔΨm of the HT-29 cells, suggesting damage to the
mitochondria.
Expression of proteins of the
apoptotic pathway
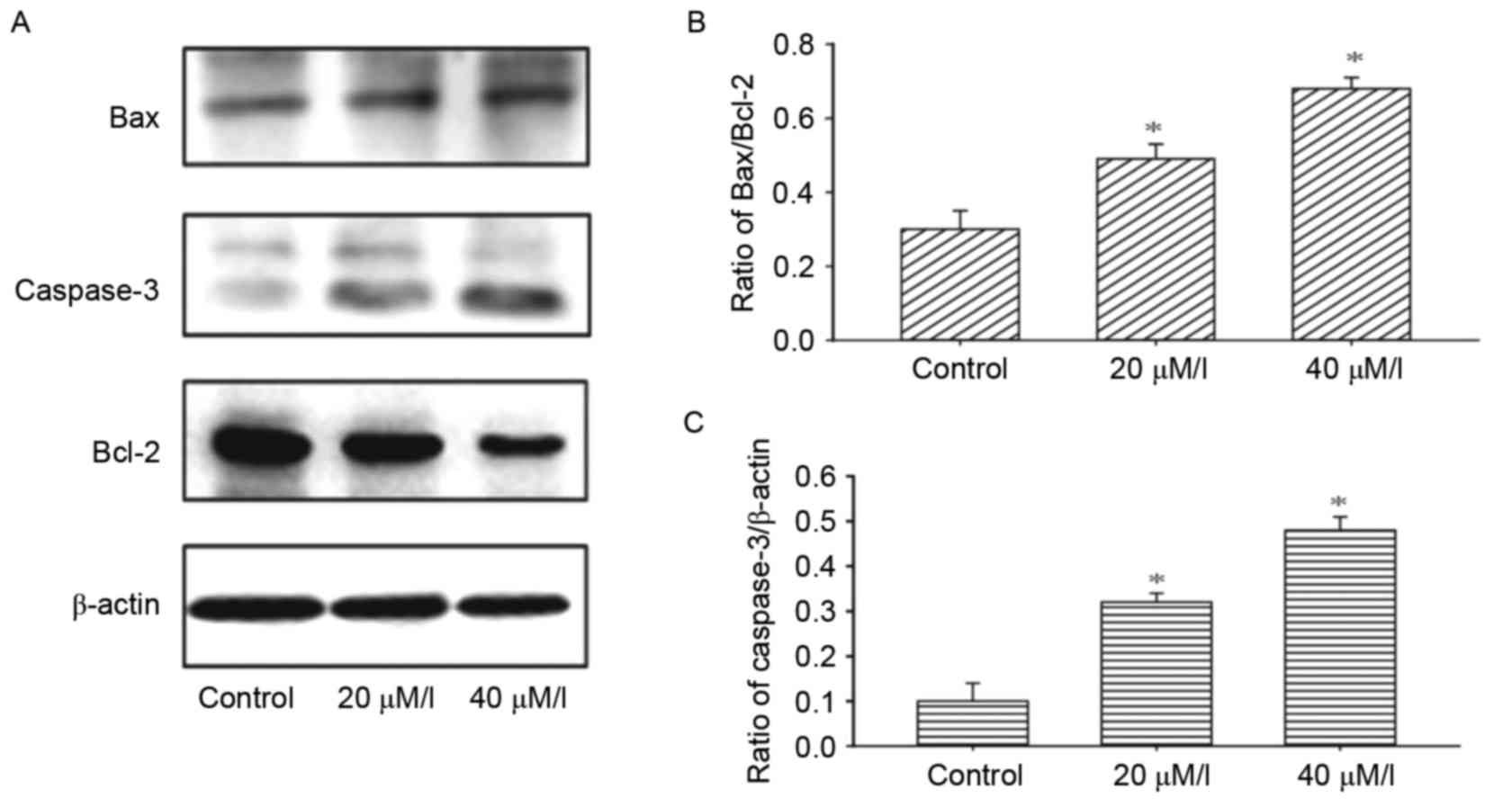
To investigate the mechanism underlying
gossypol-induced apoptosis, the effects of gossypol on the
modification of apoptosis-associated proteins were examined. The
results of the western blot analysis demonstrated that gossypol led
to an increase in the protein levels of Bax, and decrease in the
levels of Bcl-2 in the HT-29 cells following treatment with
gossypol at concentrations of 20 and 40 µM/l (Fig. 4A and B). Gossypol also promoted the
activation of caspase-3 (Fig.
4C).
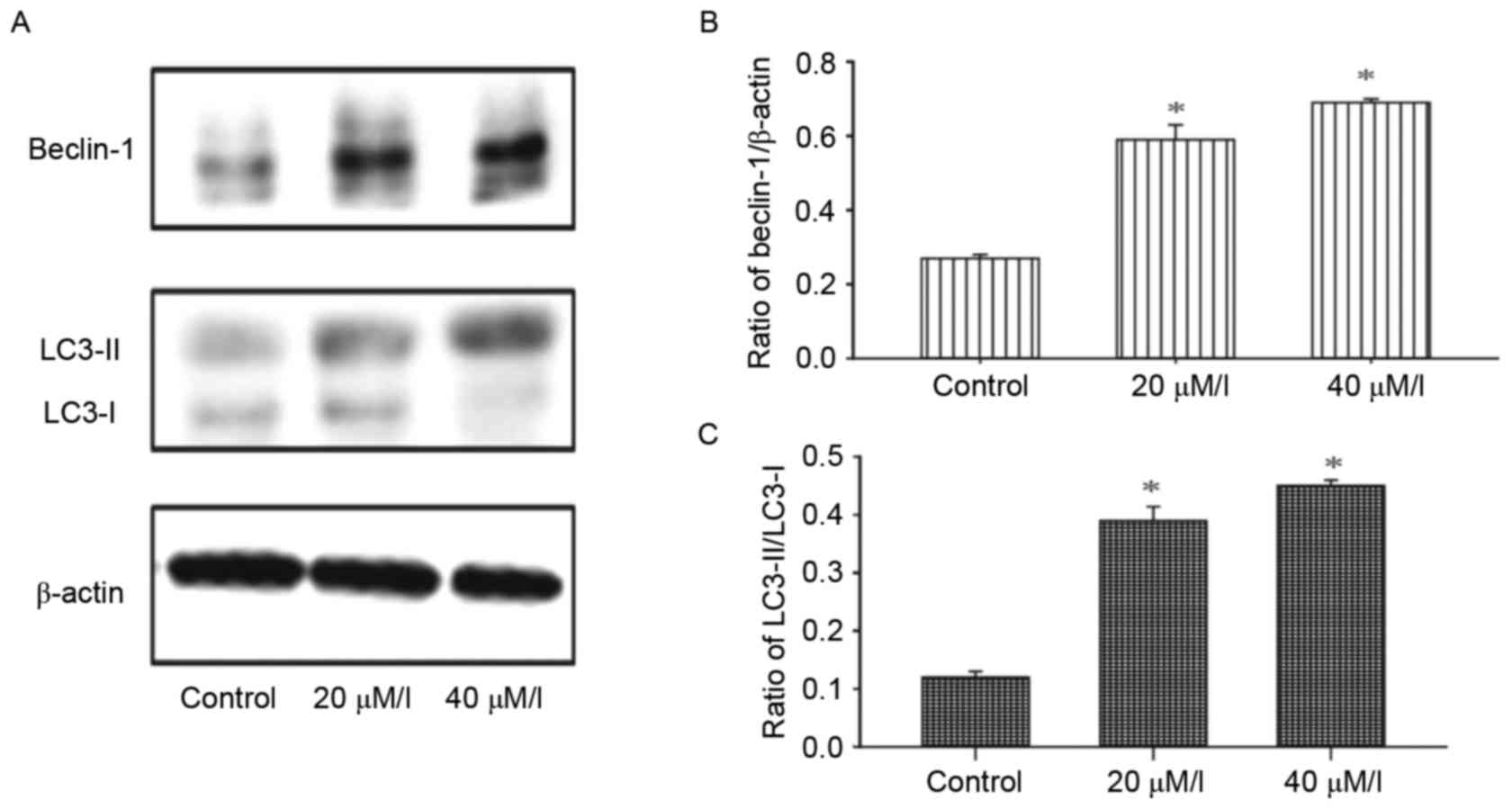
Autophagy is induced by gossypol in
HT-29 cells
The activity of autophagy in HT-29 cells was
evaluated following treatment with gossypol. Alterations in the
levels of autophagy marker proteins, LC3-I and LC3-II, in HT-29
cells were detected using western blot analysis. As shown in
Fig. 5, the ratio of LC3-II/LC3-I
and Beclin-1 were enhanced in a dose-dependent manner following
exposure to gossypol (20 and 40 µM/l) for 24 h.
Discussion
The aims of the present study were to evaluate the
antitumor activities of gossypol and the possible underlying
molecular mechanism. Gossypol inhibited the proliferation of HT-29
cells in a concentration-dependent manner. The loss of ΔΨm is a
hallmark of the early-stage of apoptosis (7). Mitochondrial involvement in
gossypol-induced apoptosis was assessed using JC-1 staining, and
Annexin V and PI double-staining was used to differentiate early
and late apoptosis. The proportions of early and late apoptotic
cells were higher, compared with those in the control group.
The signal of apoptosis in several human cancer
cells in response to antitumor agents is upregulation of the
mitochondrial apoptotic pathway triggered by an alteration in the
ratio of Bax/Bcl-2 and the activation of caspases (8,9). In
the present study, proteins associated with mitochondria-dependent
apoptosis were measured in the HT-29 cells. The expression of Bcl-2
was significantly decreased following gossypol exposure (20 and 40
µM/l) for 24 h, whereas the expression of Bax was markedly
increased. Therefore, the ratio of Bax/Bcl-2 was increased. A high
Bax/Bcl-2 ratio leads to the release of Cyt-c and activates caspase
3, which is the final step in apoptosis (10,11).
In the present study, caspase-3 was significantly increased
following gossypol exposure (20 and 40 µM/l) for 24 h. These
results suggested that gossypol induced apoptosis via the
mitochondrial apoptotic pathway.
In addition to apoptosis, the present study
investigated the autophagic effects of gossypol on the
proliferation of HT-29 cells. A number of studies have reported
that autophagy eliminates cancer cells, thus autophagy is important
in the fight against cancer. It has been found that autophagy
suppresses the development of carcinogenesis (12,13).
LC3 and Beclin 1 are the most well-known markers of autophagy.
During autophagy, LC3 is transformed from LC3-I to LC3-II for
movement onto isolated membranes and autophagosomes (14,15).
In the present study, the ratio of LC3-II/LC3-I was increased and
the expression of Beclin 1 was upregulated following treatment with
gossypol. Therefore, apoptosis and autophagy were induced in the
HT-29 cells following gossypol treatment. Apoptosis is involved in
eliminating damaged cells and tumor cells.
Although the molecular processes of apoptosis and
autophagy differ, the end result of their actions is to remove
unnecessary cells (16). The
combinatorial use of anticancer agents, which induce autophagy and
apoptosis, can be an effective therapeutic strategy and be used in
treatment against cancer. The data presented in the present study
demonstrated that gossypol induced apoptosis and autophagy, and can
offer potential as a promising anticancer agent for the treatment
of colorectal cancer due to its specific antitumor activity.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2012. View Article : Google Scholar
|
|
2
|
Lee SY, Debnath T, Kim SK and Lim BO:
Anti-cancer effect and apoptosis induction of cordycepin through
DR3 pathway in the human colonic cancer cell HT-29. Food Chem
Toxicol. 60:439–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moon DO, Choi YH, Moon SK, Kim WJ and Kim
GY: Gossypol decreases tumor necrosis factor-α-induced
intercellular adhesion molecule-1 expression via suppression of
NF-κB activity. Food Chem Toxicol. 49:999–1005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balakrishnan K, Wierda WG, Keating MJ and
Gandhi V: Gossypol, a BH3 mimetic, induces apoptosis in chronic
lymphocytic leukemia cells. Blood. 112:1971–1980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Volate SR, Kawasaki BT, Hurt EM, Milner
JA, Kim YS, White J and Farrar WL: Gossypol induces apoptosis by
activating p53 in prostate cancer cells and prostate
tumor-initiating cells. Mol Cancer Ther. 9:461–470. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lisiak N, Paszel-Jaworska A,
Bednarczyk-Cwynar B, Zaprutko L, Kaczmarek M and Rybczyńska M:
Methyl 3-hydroxyimino-11-oxoolean-12-en-28-oate (HIMOXOL), a
synthetic oleanolic acid derivative, induces both apoptosis and
autophagy in MDA-MB-231 breast cancer cells. Chem Biol Interact.
208:47–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu J, Zhou Y, Wang GN, Tai G and Ye XS:
Cell cycle arrest, apoptosis and autophagy induced by iminosugars
on K562 cells. Eur J Pharmacol. 731:65–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Zhou Y, Wang X, Qian W and Han X:
Microcystin-LR induces autophagy and apoptosis in rat Sertoli cells
in vitro. Toxicon. 76:84–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sawada M, Nakashima S, Banno Y, Yamakawa
H, Hayashi K, Takenaka K, Nishimura Y, Sakai N and Nozawa Y:
Ordering of ceramide formation, caspase activation, and Bax/Bcl-2
expression during etoposide-induced apoptosis in C6 glioma cells.
Cell Death Differ. 7:761–772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berardi DE, Campodónico PB, Díaz Bessone
MI, Urtreger AJ and Todaro LB: Autophagy: Friend or foe in breast
cancer development, progression, and treatment. Int J Breast
Cancer. 2011:5950922011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carew JS, Kelly KR and Nawrocki ST:
Autophagy as a target for cancer therapy: New developments. Cancer
Manag Res. 4:357–365. 2012.PubMed/NCBI
|
|
14
|
Backer JM: The regulation and function of
Class III PI3Ks: Novel roles for Vps34. Biochem J. 410:1–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petiot A, Ogier-Denis E, Blommaart EF,
Meijer AJ and Codogno P: Distinct classes of phosphatidylinositol
3′-kinases are involved in signaling pathways that control
macroautophagy in HT-29 cells. J Biol Chem. 275:992–998. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maiuri M, Zalckvar E, Kimchi A and Kroemer
G: Self-eating and self-killing: Crosstalk between autophagy and
apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007. View Article : Google Scholar : PubMed/NCBI
|