Introduction
Neuropeptide Y (NPY) is a 36-amino acid peptide
neurotransmitter that is widely distributed throughout the central
and peripheral nervous systems (1,2). In
the central nervous system, NPY has been implicated in controlling
appetite and energy homeostasis (3). In the peripheral nervous system, NPY
functions as part of the sympathetic nervous system, where it is
co-stored and co-released with noradrenaline during nerve
stimulation (3). In addition,
several cell types that produce NPY have been identified, such as
osteoblasts and adipocytes (4–6). In
addition, NPY regulates bone homeostasis via direct and indirect
mechanisms (7,8).
NPY receptors are members of the G-protein-coupled
receptor superfamily. The following five subtypes have been
identified thus far: Y1, Y2, Y4, Y5, and Y6 (9), of which, Y1 and Y2 receptors modulate
bone mass in mice (10). Germline
deletion of either the Y1 or Y2 receptor affects similar anabolic
effects in bones, which leads to increased bone mass due to
activated osteoblasts and an augmented rate of bone formation
(5,11). However, the mechanisms underlying
the function of the Y1 receptor compared with that of the Y2
receptor in bone tissues appear to differ. Germline and conditional
hypothalamic Y2 receptor deletion mice share the same
high-bone-mass phenotype, indicating that the central hypothalamic
Y2 receptor is crucial for this phenotype (11,12).
By contrast, the bone tissue is unaltered by conditional knockout
of the hypothalamic Y1 receptor, indicating a non-hypothalamic
control of bone mass by this receptor subtype (5).
A number of previous studies have demonstrated that
NPY and Y1 receptors are directly involved in regulating
osteoblasts. Y1 receptor expression has been detected in
osteoblastic cells lining endocortical and trabecular bone surfaces
(13), as well as in cultured
primary calvarial osteoblasts (14). In a previous report, Kurebayashi
et al (15) demonstrated
that bone morphogenetic protein (BMP) 2 induced Y1 receptor
expression in myoblastic C2C12 cells. This induction was
additionally observed following co-transfection with Smad1 and
Smad4, which are intracellular signaling molecules of the BMP2
signaling pathway, indicating that Y1 receptor expression depends
upon osteoblast differentiation (15). Mice lacking the Y1 receptor in
specific osteoblasts have a high bone mass phenotype similar to
that of germline Y1 receptor-null mice (16). This suggests that the Y1 receptor
mediates functions in the bone via direct actions on osteoblasts,
and that the osteoblastic Y1 receptor serves a role in the
regulation of bone homeostasis (16). NPY inhibits the cyclic adenosine
monophosphate response to parathyroid hormone and norepinephrine in
cultured osteoblastic cell lines (7,17).
In addition, treatment of cultured osteoblasts or bone marrow
stromal cells with NPY reduces markers of osteoblast
differentiation (7). However,
although the Y1 receptor is expressed in osteoblasts, it is unclear
whether the osteoblastic Y1 receptor regulates osteoblast
differentiation.
The aim of the present study was to investigate the
role of the Y1 receptor in mediating osteoblast differentiation. To
achieve this, RNA-interference was employed to silence the
expression of mouse MC3T3-E1 cells.
Materials and methods
Cell cultures
The mouse MC3T3-E1 osteoblast cell line was obtained
from RIKEN BioResource Center (Tsukuba, Japan) and cultured in
α-minimal essential medium (MEM; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
SAFC Biosciences, Inc., Lenexa, KS, USA) at 37°C in a humidified
atmosphere comprising 5% CO2 in air (18). MC3T3-E1 cells were cultured in
α-MEM containing 10% FBS plus 10 mM β-glycerophosphate
(Sigma-Aldrich; Merck KGaA) and 50 µg/ml ascorbic acid
(Sigma-Aldrich; Merck KGaA) as the differentiation medium. Cell
culture medium was refreshed every 3 days.
Transfection of siRNA
MC3T3-E1 cells were plated 24 h prior to
transfection at a density of 1.7×104
cells/cm2/well (24-well plate) and cultured in α-MEM
(Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS (SAFC
Biosciences, Inc.). Then, the cells were transfected with a
pre-designed Silencer Select siRNA targeting the Y1 receptor (cat.
no. s70765; Ambion; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) or Silencer Negative Control siRNA No. 1 (siCont; Ambion;
Thermo Fisher Scientific, Inc.) at a concentration of 5 nM using
Lipofectamine RNAiMAX Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) for 3 or 6 days, as previously described
(19).
Assay of alkaline phosphatase (ALP)
activity
MC3T3-E1 cells were plated onto 24-well plates 24 h
prior to transfection at a density of 1.7×104
cells/cm2/well. They were then transfected with siY1 or
siCont and cultured in α-MEM containing 10% FBS with or without
exposure to differentiation medium containing β-glycerophosphate
and ascorbic acid for 3 or 6 days. Following incubation, the cells
were washed twice with ice cold PBS, and 200 µl of lysis buffer (10
mM of Tris-HCl, pH 8.2, containing 2 mM of MgCl2 and
0.05% Triton X-100) was added to the cells, which were then kept on
ice for 5 min. The cell lysate was sonicated for 1 min and
centrifuged at 1,000 × g for 10 min at 4°C. ALP activity was
assayed using a LabAssay ALP kit (Wako Pure Chemical Industries
Ltd., Osaka, Japan) according to the manufacturer's instructions.
Briefly, 100 µl p-nitrophenyl phosphate substrate was added
to 20 µl of each sample, and the mixture was incubated for 25 min
at 37°C. The reaction was terminated by the addition of 80 µl 0.2 M
NaOH. The optical density at 405 nm was measured using an iMark
Microplate Absorbance Reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Total cell protein was measured using the Bio-Rad Protein
assay kit (Bio-Rad Laboratories, Inc.), and the results were
expressed as the concentration of p-nitrophenol (nmol)
produced/min/mg protein.
MC3T3-E1 cells were plated onto 24-well plates 24 h
prior to transfection at a density of 1.7×104
cells/cm2/well. They were then transfected with siY1 or
siCont and cultured in α-MEM for 6 days. ALP staining was performed
as previously described (18).
Briefly, cells in 24-well plates were rinsed in PBS, fixed in 10%
formalin at room temperature for 1 h, rinsed again with PBS, and
then overlaid with 300 µl 5-bromo-4-chloro-3-indolylphosphate (0.15
mg/ml) and 0.3 mg/ml nitro-blue tetrazolium (Wako Pure Chemical
Industries, Ltd.) in 0.1 M Tris-HCl (pH 9.0), 0.01 N NaOH, and 0.05
mM MgCl2 prior to incubation at room temperature for 1
h. Images were captured using a digital iPhone4 camera (Apple Inc.,
Cupertino, CA, USA), with the same settings applied to all
experimental samples.
Von Kossa staining
Matrix mineralization was analyzed by von Kossa
staining. MC3T3-E1 cells were plated onto 24-well plates 24 h prior
to transfection at a density of 1.7×104
cells/cm2/well. They were then transfected with siY1 or
siCont and cultured in α-MEM for 6 days. Cells in 24-well plates
were washed twice with PBS and then fixed in 10% neutral buffered
formalin at room temperature for 30 min. Following three washes
with deionized water, mineralized matrix was detected by treating
fixed cells with 5% silver nitrate for 12 h under ultraviolet light
at room temperature. The formation of calcium phosphate deposits
was then visualized. Images were acquired using a digital iPhone4
camera (Apple Inc.) with the same settings applied to all
experimental samples.
Quantification of gene expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
MC3T3-E1 cells were plated onto 24-well plates 24 h
prior to transfection at a density of 1.7×104
cells/cm2/well. They were then transfected with siY1 or
siCont and cultured in α-MEM for 1, 2 or 6 days. Total RNA was
extracted from cells in 24-well plate using ISOGEN (Nippon Gene
Co., Ltd., Tokyo, Japan) as previously described (18). RNA was quantified by
spectrophotometric OD260 measurements and quality was assessed by
the OD260/OD280 and OD230/OD280 ratios using a NanoDrop
spectrophotometer (ND-1000; Thermo Fisher Scientific, Inc.). RT-PCR
was performed using a high-capacity cDNA reverse transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The resultant cDNA was then used
as the template for a PCR. qPCR was performed using assay-on-demand
TaqMan Gene Expression assays (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and the following primers: Y1 receptor (cat. no.
Mm00650798_g1), ALP (cat. no. Mm00475834_m1), osteocalcin (cat. no.
Mm03413826_mH), collagen (I) α1 (col1α; cat. no. Mm00801666_g1),
bone sialoprotein (BSP; cat. no. Mm00492555_m1), Runx2 (cat. no.
Mm00501584_m1), osterix (cat. no. Mm00504574_m1) and GAPDH
(Pre-Developed TaqMan Assay Reagents mouse GAPDH no. 4352661; all
from Applied Biosystems; Thermo Fisher Scientific, Inc.). A StepOne
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used with the following cycling conditions: 95°C for 10
min, then 40 cycles of 95°C for 15 sec and 60°C for 1 min, as
previously described (19). The
relative level of target gene expression was quantified using the
comparative quantification cycle method (20), with GAPDH as the endogenous
control. The relative expression level of target gene mRNA in the
siCont-transfected group on day 2 was defined as the standard.
Analysis of cell viability
MC3T3-E1 cells were plated at 0.9×104
cells/cm2 and following 24 h of culture, cells were
transfected with siY1 or siCont (both at 5 nM) and incubated again
for 1 day. To quantify the number of viable cells, the
tetrazolium-based colorimetric Cell Counting kit-8 assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was employed. A 30
µl aliquot of the WST-8 substrate [5 mM;
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
monosodium salt] was added to each well. Following incubation for 1
h at 37°C, the optical density was measured at a wavelength of 450
nm using the iMark Microplate Absorbance Reader (Bio-Rad
Laboratories Inc.).
Measurement of caspase-3/7
activity
MC3T3-E1 cells were plated at 1.7×104
cells/cm2 and following culture for 24 h, cells were
transfected with siY1 or siCont (both at 5 nM) and incubated again
for 1 day. The cellular enzymatic activities of caspase-3/7 were
determined using Caspase-Glo 3/7 assay Systems (Promega
Corporation, Madison, WI, USA) as previously described (21). For each reaction, cells were
incubated with a luminogenic substrate containing the
Asp-Glu-Val-Asp sequence, which is cleaved by activated
caspase-3/7, for 1 h at room temperature. Luminescence was
quantified using the MiniLumat LB 9506 Luminometer (Berthold
Technologies GmbH and Co., KG, Bad Wildbad, Germany).
Statistical analysis
All experiments were repeated between three and six
times, and the representative results are presented as the mean ±
standard deviation. The results were analyzed using the F-test
followed by Student's t-test using Microsoft Excel 2010 (Microsoft
Corporation, Redmond, WA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Knockdown of the Y1 receptor induces
ALP activity and mineralization of MC3T3-E1 cells
To evaluate the potential role of Y1 receptor in
osteoblasts, the well-characterized mouse calvaria-derived
pre-osteoblastic cell line, MC3T3-E1, was employed. The effect of
Y1 receptor knockdown using RNA interference was then investigated.
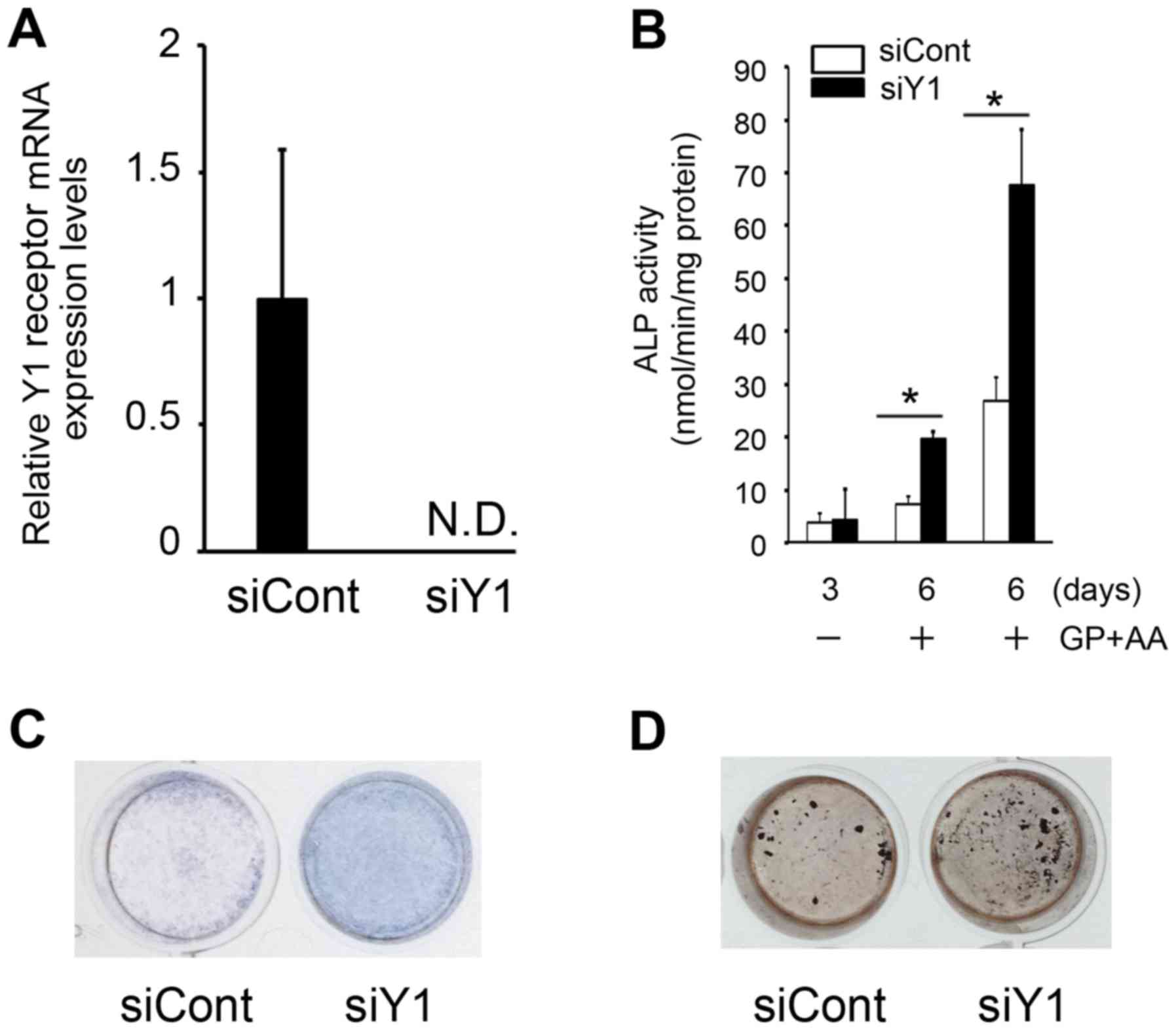
Following transfection of MC3T3-E1 cells with siRNA targeting the
Y1 receptor the mRNA expression levels of the Y1 receptor were
reduced to undetectable levels, which confirmed that the siRNA was
effective in silencing the endogenous Y1 receptor (Fig. 1A). In these cells, ALP activity was
significantly increased at day 6 following Y1 receptor inhibition
when compared with siCont-transfected controls (Fig. 1B and C). In order to determine the
effects of Y1 receptor inhibition, the level of cell matrix
mineralization in vitro was examined. Von Kossa staining
demonstrated that the mineralization of MC3T3-E1 cells was
augmented at day 6 following silencing of the Y1 receptor compared
with siCont-transfected controls (Fig.
1D). These results indicate that ALP expression may be Y1
receptor-dependent, and that the NPY signaling pathway may be
involved in regulating ALP expression and mineralization in
MC3T3-E1 cells.
Knockdown of Y1 receptor upregulates
mRNA expression of specific genes that characterize osteoblastic
differentiation in MC3T3-E1 cells
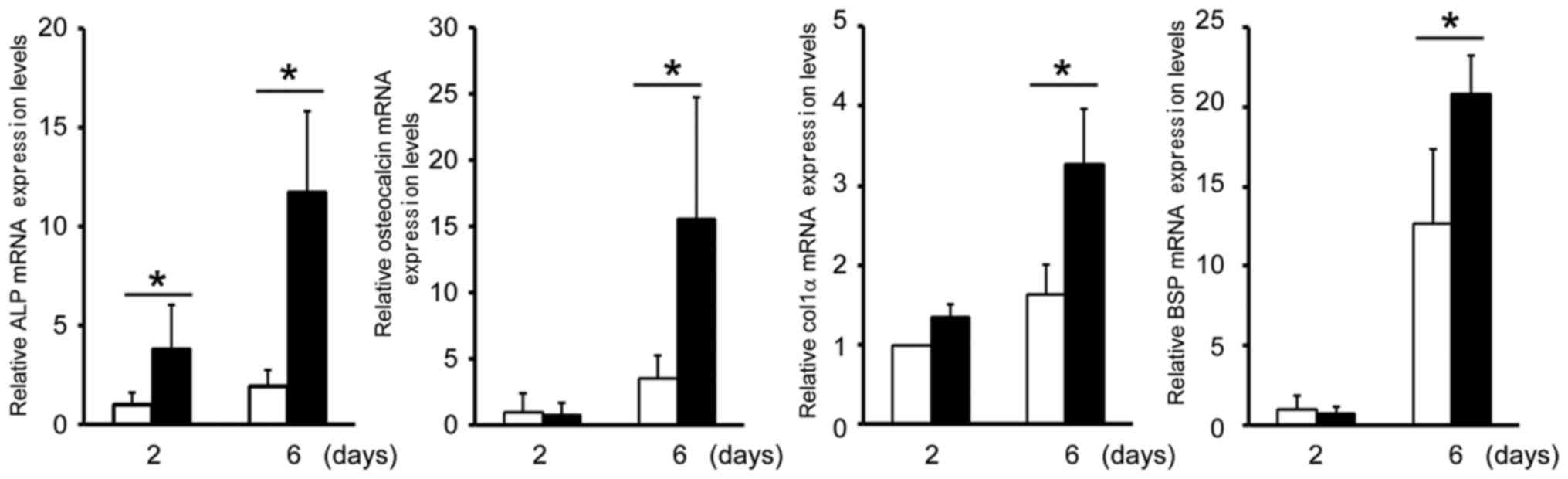
The next aim of the present study was to examine the
effects of Y1 receptor inhibition on the mRNA expression levels of
specific genes in MC3T3-E1 cells that characterize osteoblastic
differentiation. The mRNA expression levels of ALP, osteocalcin,
col1α and BSP were significantly increased in siY1-transfected
cells when compared with controls (Fig. 2), which indicated that osteoblastic
gene expression may be dependent on Y1 receptor expression. These
observations suggest that NPY signaling may be involved in
regulating osteoblastic differentiation.
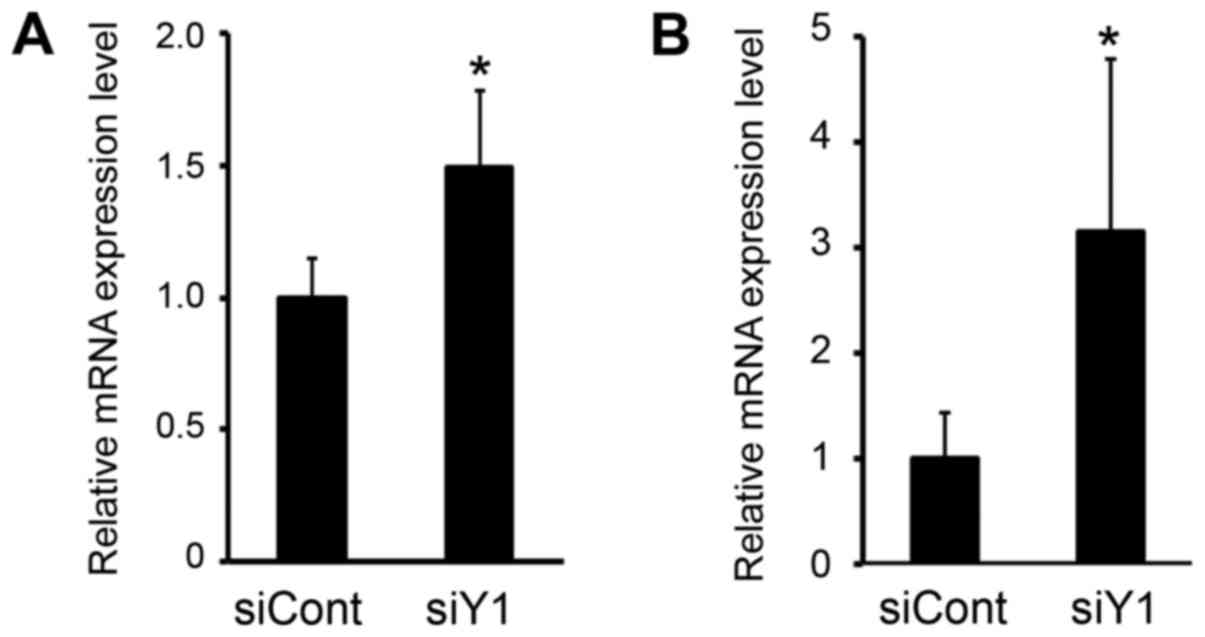
Knockdown of the Y1 receptor
upregulates the mRNA expression levels of runt-related
transcription factor 2 (Runx2) and osterix in MC3T3-E1 cells
The differentiation and function of osteoblasts is
regulated by important transcription factors, such Runx2 and
osterix (22). To investigate the
mechanism by which Y1 receptor inhibition activates osteoblastic
differentiation in MC3T3-E1 cells, the expression level of these
transcription factors was examined. As demonstrated in Fig. 3, Runx2 and osterix mRNA expression
levels were significantly upregulated following siRNA-mediated
knockdown of the Y1 receptor.
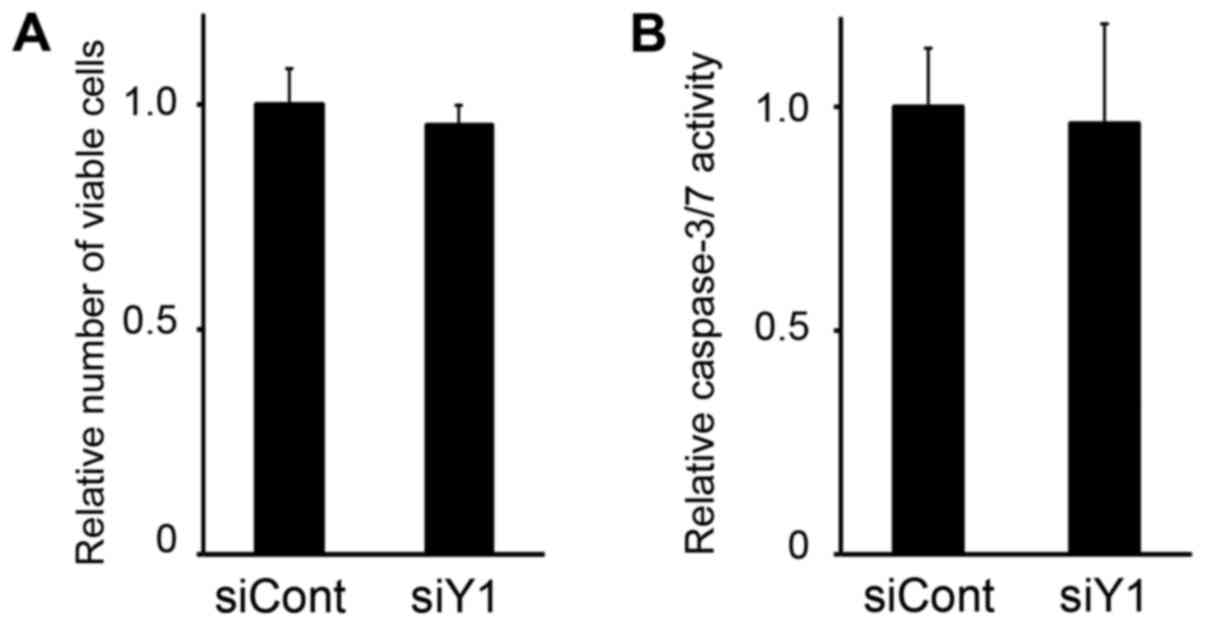
Knockdown of the Y1 receptor does not
affect the viability or apoptosis levels in MC3T3-E1 cells
The effect of Y1 receptor expression downregulation
on the viability of MC3T3-E1 osteoblasts was then examined. As
demonstrated in Fig. 4A,
transfection of MC3T3-E1 with siY1 demonstrated no significant
effect on cell viability when compared with siCont-transfected
controls. Similarly, caspase-3/7 activity, which is activated upon
apoptosis induction, was not observed to increase following
knockdown of the Y1 receptor (Fig.
4B). Therefore, upregulation of osteoblastic gene expression
did not appear to be influenced by cell cycle regulation or
apoptosis induction.
Discussion
The Y1 receptor is a G-protein-coupled receptor
predominantly expressed in the brain and in several types of
peripheral tissues and cells, such as osteoblasts (9,16).
C2C12 cells, a myoblastic cell line with a well-characterized model
system, have been reported to differentiate into myotubes and also
osteoblasts depending upon the specific culture conditions when
incubated with BMP2 (18). A
previous study demonstrated that C2C12 cells do not express Y1
receptor mRNA; however, its expression is induced by BMP2
treatment, which suggests that induction of Y1 receptor expression
may be regulated during the osteoblast differentiation process
(15). The results of the present
study demonstrated that Y1 receptor inhibition in MC3T3-E1 cells
induced osteoblastic differentiation, which may support the notion
that Y1 receptor expression may be a marker of osteoblast
differentiation.
Several in vitro and in vivo studies
have demonstrated that NPY inhibits osteoblast differentiation,
which were summarized by Khor and Baldock (7). In bone marrow stromal cells, NPY
decreases ALP and osteocalcin expression and inhibits
mineralization (23). In a mouse
with osteoblast lineage-specific NPY overexpression, trabecular and
cortical bone volumes were reduced (24). In addition, decreased expression of
osteocalcin was observed in the calvarial osteoblasts of this
mouse. Bone marrow stromal cells derived from mice with a germline
deletion of the Y1 receptor were investigated (16). Consistent with the results of the
present study, Y1 receptor deficiency was observed to enhance
osteoblastic differentiation in mesenchymal progenitor cells
(16). In the current study, the
mRNA expression levels of Runx2 and osterix, which are two
transcription factors that are essential for osteoblastic
differentiation and bone formation, were induced by inhibition of
the Y1 receptor. Runx2 is now known to be the earliest principle
transcription factor involved in the expression of
osteoblast-specific genes and in the differentiation of mesenchymal
stem cells into osteoblasts (22).
In addition, it is thought to function primarily during the
terminal differentiation of osteoblasts (25). Inhibition of the Y1 receptor may
induce Runx2 and osterix activities; consequently, it may
contribute to osteoblastic differentiation and the expression of
ALP, osteocalcin, col1α, and BSP (22). In the present study, inhibition of
the Y1 receptor using siRNA was observed to regulate ALP activity
and mineralization in MC3T3-E1 cells. Together, these results
suggest that the Y1 receptor may additionally be involved in
inhibiting mineral deposition by mature osteoblasts.
It is known that multiple sources of NPY regulate
bone homeostasis. In the peripheral nervous system, NPY is
co-stored and co-released with noradrenaline during nerve
stimulation (3). In addition, NPY
is released into the circulation by sympathetic nerves and the
adrenal medulla (3). Furthermore,
NPY has been detected in bone tissues, including osteoblasts and
osteocytes (14). The results of
the current study demonstrated that inhibition of the Y1 receptor
induces the expression of differentiation-specific genes in
MC3T3-E1 osteoblasts, indicating a role of the local NPY-Y1
receptor axis in regulating osteoblastic differentiation.
A previous study demonstrated that Y1 receptor
antagonism induced by oral administration of BIBO3304
[(R)-N-[[4-(ami-nocarbonylaminomethyl)phenyl]methyl]-N2-(diphenylacetyl)-argininamide
trifluoroacetate], an argininamide derivative originally developed
as a selective Y1 receptor antagonist, enhanced osteoblast activity
in mice (26). This lead to
increased mineral deposition rates in the cortical and cancellous
bones. In addition, oral administration of BIBO3304 was not
observed to produce significant extraskeletal side effects with
regards to body weight, energy metabolism, glucose homeostasis or
food intake (26). It is
hypothesized that a potent Y1 receptor inhibitor or siRNA may be a
novel anabolic agent that may be used as a therapeutic agent to
prevent or reverse bone loss in conditions such as osteoporosis,
through the modulation of osteoblastic activity.
In conclusion, the results of the current study
demonstrated that inhibition of the Y1 receptor might enhance
osteoblastic differentiation. These results provide an explanation
for the mechanism of action of NPY and the Y1 receptor in mediating
osteoblast differentiation, and support a potential role of Y1
receptor inhibition as a novel anabolic strategy for the prevention
of bone loss.
Acknowledgements
The authors would like to thank Dr Kiyomi
Tsuji-Tamura (Hokkaido University, Sapporo, Japan) for the critical
reading of this manuscript and for her valuable suggestions.
References
|
1
|
Baraban SC: Neuropeptide Y and limbic
seizures. Rev Neurosci. 9:117–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cerdá-Reverter JM and Larhammar D:
Neuropeptide Y family of peptides: Structure, anatomical
expression, function, and molecular evolution. Biochem Cell Biol.
78:371–392. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silva AP, Cavadas C and Grouzmann E:
Neuropeptide Y and its receptors as potential therapeutic drug
targets. Clin Chim Acta. 326:3–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo LE, Kitlinska JB, Tilan JU, Li L,
Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R,
et al: Neuropeptide Y acts directly in the periphery on fat tissue
and mediates stress-induced obesity and metabolic syndrome. Nat
Med. 13:803–811. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baldock PA, Allison SJ, Lundberg P, Lee
NJ, Slack K, Lin EJ, Enriquez RF, McDonald MM, Zhang L, During MJ,
et al: Novel role of Y1 receptors in the coordinated regulation of
bone and energy homeostasis. J Biol Chem. 282:19092–19102. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang K, Guan H, Arany E, Hill DJ and Cao
X: Neuropeptide Y is produced in visceral adipose tissue and
promotes proliferation of adipocyte precursor cells via the Y1
receptor. FASEB J. 22:2452–2464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khor EC and Baldock P: The NPY system and
its neural and neuroendocrine regulation of bone. Curr Osteoporos
Rep. 10:160–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horsnell H and Baldock PA: Osteoblastic
actions of the neuropeptide Y system to regulate bone and energy
homeostasis. Curr Osteoporos Rep. 14:26–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin S, Boey D and Herzog H: NPY and Y
receptors: Lessons from transgenic and knockout models.
Neuropeptides. 38:189–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allison SJ, Baldock PA and Herzog H: The
control of bone remodeling by neuropeptide Y receptors. Peptides.
28:320–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baldock PA, Sainsbury A, Couzens M,
Enriquez RF, Thomas GP, Gardiner EM and Herzog H: Hypothalamic Y2
receptors regulate bone formation. J Clin Invest. 109:915–921.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi YC, Lin S, Wong IP, Baldock PA,
Aljanova A, Enriquez RF, Castillo L, Mitchell NF, Ye JM, Zhang L,
et al: NPY neuron-specific Y2 receptors regulate adipose tissue and
trabecular bone but not cortical bone homeostasis in mice. PLoS
One. 5:e113612010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lundberg P, Allison SJ, Lee NJ, Baldock
PA, Brouard N, Rost S, Enriquez RF, Sainsbury A, Lamghari M,
Simmons P, et al: Greater bone formation of Y2 knockout mice is
associated with increased osteoprogenitor numbers and altered Y1
receptor expression. J Biol Chem. 282:19082–19091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Igwe JC, Jiang X, Paic F, Ma L, Adams DJ,
Baldock PA, Pilbeam CC and Kalajzic I: Neuropeptide Y is expressed
by osteocytes and can inhibit osteoblastic activity. J Cell
Biochem. 108:621–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurebayashi N, Sato M, Fujisawa T,
Fukushima K and Tamura M: Regulation of neuropeptide Y Y1 receptor
expression by bone morphogenetic protein 2 in C2C12 myoblasts.
Biochem Biophys Res Commun. 439:506–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee NJ, Nguyen AD, Enriquez RF, Doyle KL,
Sainsbury A, Baldock PA and Herzog H: Osteoblast specific Y1
receptor deletion enhances bone mass. Bone. 48:461–467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bjurholm A, Kreicbergs A, Schultzberg M
and Lerner UH: Parathyroid hormone and noradrenaline-induced
enhancement of cyclic AMP in a cloned osteogenic sarcoma cell line
(UMR 106) is inhibited by neuropeptide Y. Acta Physiol Scand.
134:451–452. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakashima A, Katagiri T and Tamura M:
Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2)
signaling in differentiation pathway of C2C12 myoblasts. J Biol
Chem. 280:37660–37668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uyama M, Sato MM, Kawanami M and Tamura M:
Regulation of osteoblastic differentiation by the proteasome
inhibitor bortezomib. Genes Cells. 17:548–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iizuka S, Oridate N, Nashimoto M, Fukuda S
and Tamura M: Growth inhibition of head and neck squamous cell
carcinoma cells by sgRNA targeting the cyclin D1 mRNA based on TRUE
gene silencing. PLoS One. 9:e1141212014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karsenty G: Transcriptional control of
skeletogenesis. Annu Rev Genomics Hum Genet. 9:183–196. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teixeira L, Sousa DM, Nunes AF, Sousa MM,
Herzog H and Lamghari M: NPY revealed as a critical modulator of
osteoblast function in vitro: New insights into the role of Y1 and
Y2 receptors. J Cell Biochem. 107:908–916. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matic I, Matthews BG, Kizivat T, Igwe JC,
Marijanovic I, Ruohonen ST, Savontaus E, Adams DJ and Kalajzic I:
Bone-specific overexpression of NPY modulates osteogenesis. J
Musculoskelet Neuronal Interact. 12:209–218. 2012.PubMed/NCBI
|
|
25
|
Ryoo HM, Lee MH and Kim YJ: Critical
molecular switches involved in BMP-2-induced osteogenic
differentiation of mesenchymal cells. Gene. 366:51–57. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sousa DM, Baldock PA, Enriquez RF, Zhang
L, Sainsbury A, Lamghari M and Herzog H: Neuropeptide Y Y1 receptor
antagonism increases bone mass in mice. Bone. 51:8–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|