Introduction
Cassiae semen, also known as ‘Juemingzi’ in
Chinese, is the dry and mature seed of Cassia obtusifolia L.
or C. tora L., which belong to the Cassia genus of
Leguminosae (1). It is
cultivated in Korea, Japan and China, and is commonly consumed as a
roasted tea (2,3). In traditional Chinese medicine, it
has been used in treatments for hyperlipemia, diabetes mellitus,
Alzheimer's disease, acute liver injury, inflammation, photophobia,
headache, dizziness and hypertension (4–6).
Phytochemical investigations have isolated and
identified >70 compounds, including anthraquinones,
naphthopyrones, volatile oils and sterols (7,8).
Among these, anthraquinones are the primary functional components
and possess a wide spectrum of pharmacological properties (9–11),
including antihyperlipidemic, neuroprotective, hepatoprotective,
antibacterial and antimutagenic activities (12–14).
Naphthopyrones, other primary components, exhibit antidiabetic
(15,16), antimicrobial (17), antiestrogenic (18), antiallergic (19) and anthelmintic effects (20). At present, the Pharmacopoeia of the
People's Republic of China recommends the use of chrysophanol and
aurantio-obtusin as the indicator components, and the quality of
Cassiae semen is evaluated primarily by assessing the
content of these two compounds (1).
The purpose of the present review is to provide
comprehensive information on the ethnobotany, phytochemistry and
pharmacology of Cassiae semen collated from previous
studies, in order to facilitate the further study and application
of Cassiae semen, as well as generate a novel basis for the
associations between structure and activity, and their molecular
mechanisms of action.
Ethnobotany
C. obtusifolia is similar to C. tora
in terms of botanical morphology. The two are an annual, erect,
stout herb, ~1–2 m in length, and their leaves are paripinnate,
typically pubescent and are 4–8 cm in length with a conical gland
between each of the two lowest pairs of leaflets. Leaflets are
formed of 3 leaf pairs and are glaucous, membranous, glabrous or
pubescent, and have obcordate or obovate oblong morphology (2–6 cm
long × 1.5–2.5 cm wide); the base is somewhat oblique, usually
rounded and there are 8–10 pairs of main nerves. The petiolules are
1.5–2 mm in length and their stipules are linear, pilose and
caducous. It blossoms from July to September and produces fruit
from September to October. Flowers are usually in subsessile pairs
in leaf axils, the pedicels are filiform and are 1–1.5 cm in
length. Calyces are ovate, glabrous, membranous and comprised of
five-parts; there are five petals, which are pale yellow, oblong,
obtuse and the upper petal (standard) is two-lobed. The flowers
have 10 stamens, while the upper three are reduced to minute
staminodes. The pods are slender, puberulous, subtetragonous,
obliquely septate and, are ~15 cm in length and 3–4 mm in
width.
However, the seeds of C. obtusifolia are a
dark brown or green-brown, rhombohedral or short cylindrical, and
are 3–7 mm in length and 2–4 mm in width. While C. tora
seeds are a light brown, shiny, short cylindrical, and are 3–5 mm
in length and 2–3 mm in width (1,21).
C. obtusifolia is cultivated in multiple
provinces of China, including Henan, Hubei, Shanxi, Sichuan,
Zhejiang and Anhui, and also other countries, including Korea,
India and Japan. It is primarily distributed in moist and sunny
places, in hillside shrubs and in the sandy soil of river banks
(21).
As a widely used traditional Chinese medicine, there
are some adulterants of this plant, including the seeds of C.
occidentalis (Leguminosae), C. sophera
(Leguminosae) and Sesbania aculeata Pers
(Papilionaceae) (22–24).
To date, a number of methods have been developed to identify and
distinguish these, including experiential identification,
morphological identification, ultraviolet spectrophotometry, the
thin layer chromatography method, high performance liquid
chromatography (HPLC), HPLC-coupled with time-of-flight and ion
trap mass spectrometry, and SDS-PAGE (25–28).
Among these methods, the HPLC method is regarded as the most
popular method for evaluating the quality and authenticity of
Cassiae semen. Chrysophanol and aurantio-obtusin are used as
the indicator compounds to characterize the quality of this plant
and the minimum contents are defined as 0.20 and 0.080%,
respectively, in the Pharmacopoeia of the People's Republic of
China (1).
Phytochemistry
A number of compounds, including anthraquinones,
naphthopyrones, volatile oils and sterols, have been isolated from
Cassiae semen. Anthraquinones and naphthopyrones, which
exhibit multiple pharmacological activities, are considered the
primary active ingredients of Cassiae semen. All compounds
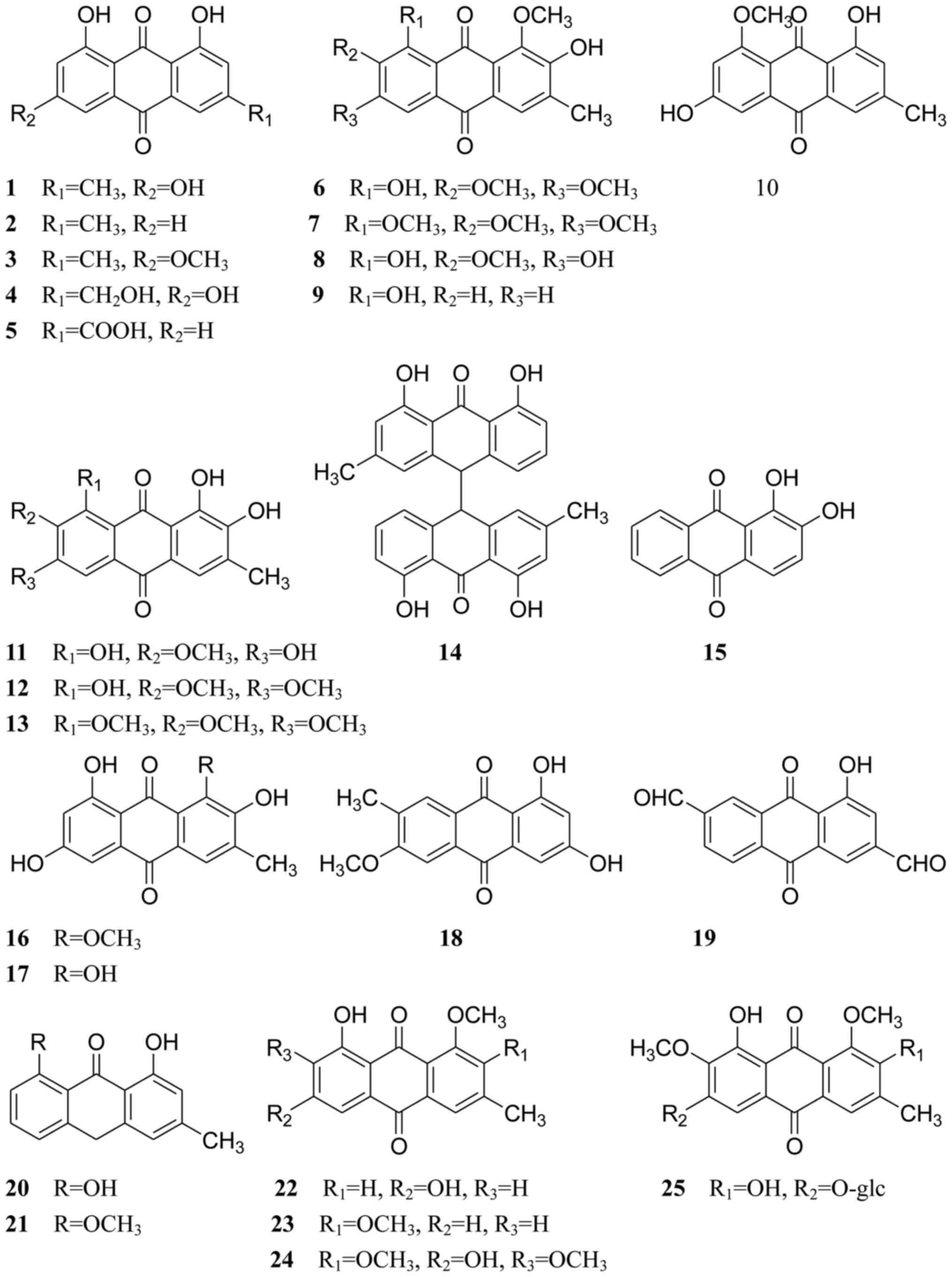
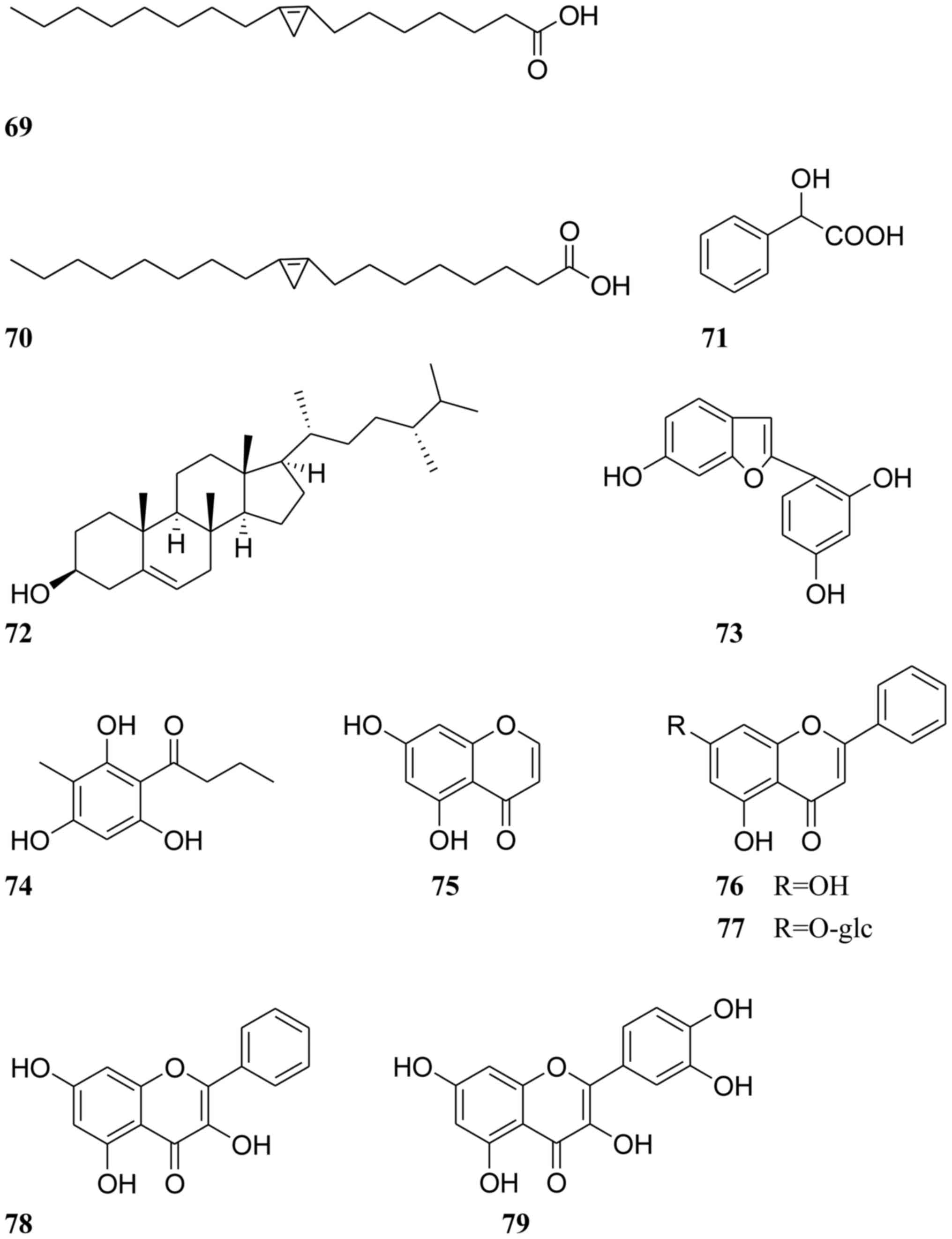
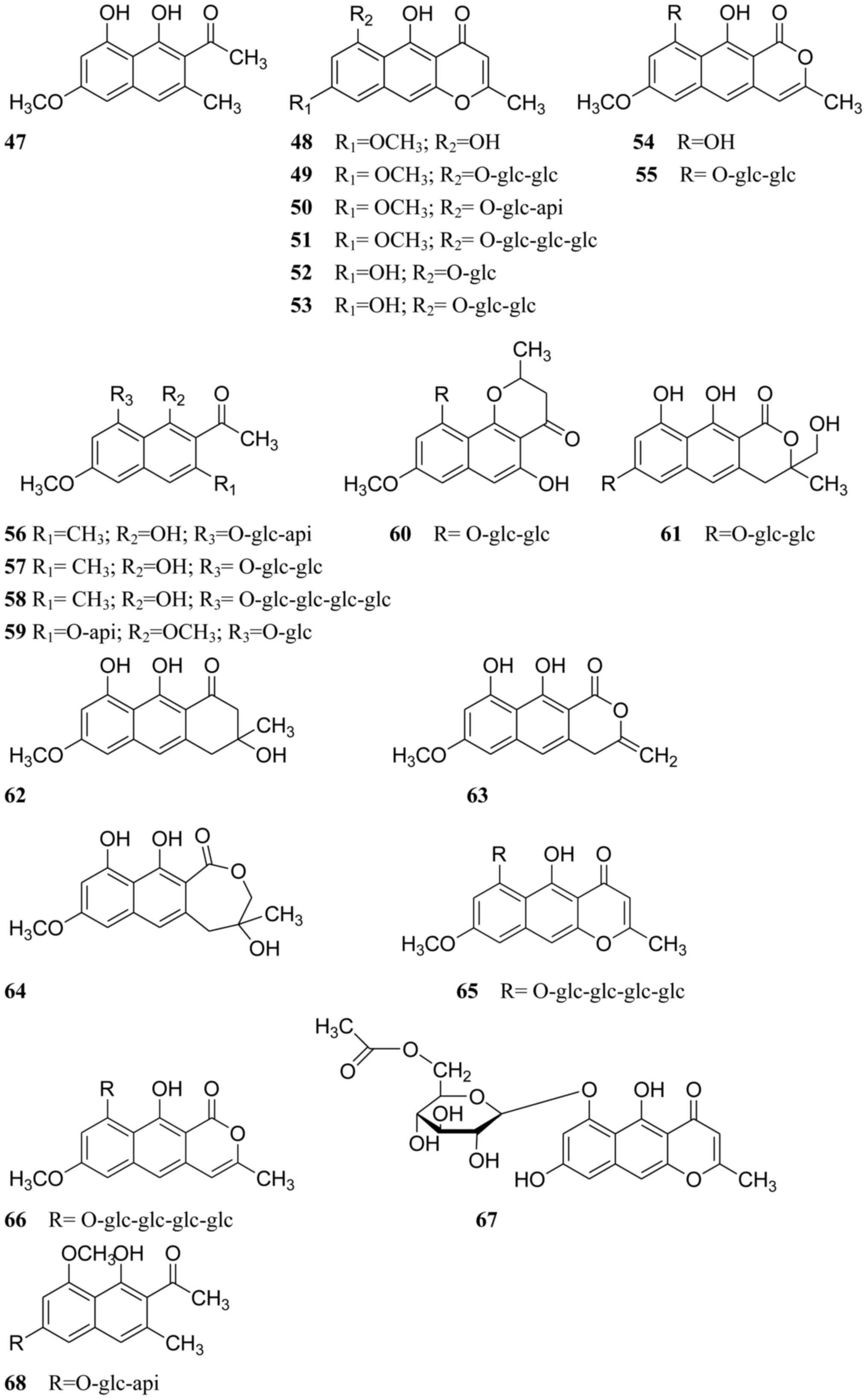
isolated from Cassiae semen are listed in Table I, and their chemical structures are
displayed in Figs. 1–3.
 | Table I.Chemical compounds isolated from
Cassiae semen. |
Table I.
Chemical compounds isolated from
Cassiae semen.
| Classification | No. | Chemical
component | (Refs.) |
|---|
| Anthraquinones | 1 | Emodin | (37,38) |
|
| 2 | Chrysophanol | (34) |
|
| 3 | Physcion | (34) |
|
| 4 | Aloe-emodin | (38) |
|
| 5 | Rhein | (38) |
|
| 6 | Obtusin | (35) |
|
| 7 | Chryso-obtusin | (35) |
|
| 8 |
Aurantio-obtusin | (35) |
|
| 9 | Obtusifolin | (35) |
|
| 10 | Questin | (36) |
|
| 11 |
1-desmethylaurantio-obtusin | (36) |
|
| 12 |
1-desmethylobtusin | (36) |
|
| 13 |
1-desmethylchryso-obtusin | (36) |
|
| 14 |
Chrysophanol-10,10′-bianthrone | (36) |
|
| 15 |
1,2-dihydroxyanthraquinone | (7) |
|
| 16 |
2-hydroxyemodin-1-methylether | (7) |
|
| 17 | Alaternin | (29) |
|
| 18 |
1,3-dihydroxy-6-methoxy-7-methyl
anthraquinone | (30) |
|
| 19 |
1-hydroxy-3,7-diformyl anthraquinone | (30) |
|
| 20 | Chrysarobin | (30,31) |
|
| 21 |
8-O-methylchrysophanol | (32) |
|
| 22 |
1-O-methylemodin | (32) |
|
| 23 |
1,2-dimethoxy-8-hydroxy-3-methyl-9,10-anthraquinone | (32) |
|
| 24 |
l,2,7-trimethoxyl-6,8-dihydroxy-3-methylanthraquinone | (33) |
|
| 25 |
Gluco-aurantioobtusin | (39) |
|
| 26 |
Emodin-6-glucoside | (30) |
|
| 27 |
Physcion-8-O-β-D-glucopyranoside | (7,40) |
|
| 28 |
Physcion-8-O-β-gentiobioside | (41) |
|
| 29 |
Emodin-1-O-β-gentiobioside | (41) |
|
| 30 |
Obtusifolin-2-O-β-D-glucoside | (42) |
|
| 31 |
Chysophanol-1-O-β-gentiobioside | (41) |
|
| 32 |
Alaternin-1-O-β-D-glucopyranoside | (7) |
|
| 33 |
Alaternin-2-O-β-D-glucopyranoside | (29) |
|
| 34 |
Aurantio-obtusin-6-O-β-D-glucopyranoside | (43) |
|
| 35 |
Chryso-obtusin-2-O-β-D-glucopyranoside | (7) |
|
| 36 |
Obtusifolin-2-O-β-D-(6′-O-acetyl)
glucopyranoside | (44) |
|
| 37 |
Emodin-8-O-β-D-glucopyranoside | (45) |
|
| 38 |
2-methoxyl-chrysophanol-8-O-β-D-glucopyranoside | (46) |
|
| 39 |
1-demethylaurantio-obtusin-2-O-β-D-glucopyranoside | (43) |
|
| 40 |
1,7,8-trimethoxyl-2-hydroxyl-3-methylanthraquinone-2-O-β-D-glucopyranoside | (33) |
|
| 41 |
l,7-diinethoxyl-2,8-dihydroxyl-3-methylanthraquinone-2-O-β-D-glucopyranoside | (33) |
|
| 42 |
l,2,7-trimethoxyl-6,8-dihydroxy-3-methylanthraquinone-6-O-β-D-glucopyranoside | (33) |
|
| 43 |
2,8-dimethoxyl-l,6-dihydroxy-3-methylanthraquinone-6-O-β-D-glucopyranoside | (33) |
|
| 44 |
1-[(β-D-glucopyranosyl-(1→3)-O-β-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl)oxy]-8-hydroxy-3-methyl-9,10-anthraquinone | (42) |
|
| 45 |
1-[(β-D-glucopyranosyl-(1→6)-O-β-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl)oxy]-8-hydroxy-3-methyl-9,10-anthraquinone | (42) |
|
| 46 |
4,6,7-trimethoxyl-aloe-emodin-8-O-β-D-glucopyranoside | (46) |
| Naphthopyrones | 47 | Torachrysone | (48) |
|
| 48 | Rubrofusarin | (47) |
|
| 49 |
Rubrofusarin-6-O-β-D-gentiobioside | (47) |
|
| 50 | Toralactone | (49) |
|
| 51 | Rubrofusarin
triglucoside | (17) |
|
| 52 | Cassiaside | (50) |
|
| 53 | Nor-rubrofusarin
gentiobioside | (17) |
|
| 54 | Cassiaside B | (50) |
|
| 55 | Cassiaside C | (50) |
|
| 56 | Torachrysone
apiglucoside | (17) |
|
| 57 | Torachrysone
gentiobioside | (17) |
|
| 58 | Torachrysone
tetraglucoside | (17) |
|
| 59 | Cassiatoroside | (51) |
|
| 60 |
Demethylflavasperone gentiobioside | (17) |
|
| 61 | Cassialactone
gentiobioside | (28) |
|
| 62 | Torosachrysone | (53) |
|
| 63 | Isotoralactone | (53) |
|
| 64 | Cassialactone | (53) |
|
| 65 | Cassiaside
B2 | (19) |
|
| 66 | Cassiaside
C2 | (19) |
|
| 67 |
Nor-rubrofusarin-6-O-β-D(6′-o-acetyl)
glucopyranoside | (54) |
|
| 68 |
l-hydroxyl-2-acetyl-3,8-dimethoxy-6-O-[β-D-apiofuranosyl-(l→2)-β-D-glucopyranosyl]-naphthalene | (55) |
| Other
compounds | 69 | Malvalic acid | (5,57) |
|
| 70 | Sterculic acid | (5) |
|
| 71 | Mandelic acid | (5) |
|
| 72 | Campesterol | (5) |
|
| 73 | Aspidinol | (58) |
|
| 74 |
5,7-dihydroxychromone | (58) |
|
| 75 | Chrysin | (58) |
|
| 76 |
Chrysin-7-O-β-D-glucoside | (58) |
|
| 77 | Galangin | (58) |
|
| 78 | Cyanidenon | (58) |
Anthraquinones
Cassiae semen contains structurally diverse
and biologically active anthraquinones. Thus far, ~53
anthraquinones have been isolated and identified. The predominant
anthraquinones are emodin-type anthraquinones, which include
emodin, chrysophanol, physcion, aloe-emodin, rhein, obtusin,
chryso-obtusin, aurantio-obtusin, obtusifolin, questin,
1-desmethylaurantio-obtusin, 1-desmethylobtusin,
1-desmethylchryso-obtusin, chrysophanol-10,10′-bianthrone,
1,2-dihydroxyanthraquinone, 2-hydroxyemodin-1-methylether,
alaternin, 1,3-dihydroxy-6-methoxy-7-methyl anthraquinone,
1-hydroxy-3,7-diformyl anthraquinone, chrysarobin,
8-O-methylchrysophanol, 1-O-methylemodin,
1,2-dimethoxy-8-hydroxy-3-methyl-9,10-anthraquinone and
l,2,7-trimethoxyl-6,8-dihydroxy-3-methylanthraquinone (compounds
1–24, respectively; Fig. 1); these
have all been isolated from Cassiae semen (29–38).
There are also many combined anthraquinones (compounds 25–46;
Fig. 1), which have been isolated
from the seeds of C. obtusifolia or C. tora (3,7,30,36,39–46).
Naphthopyrones
Naphthopyrones are the other characteristic
components in Cassiae semen. In 1969, torachrysone,
rubrofusarin and rubrofusarin-6-O-β-D-gentiobioside were isolated
from C. tora seeds (compounds 47–49, respectively; Fig. 2) (47,48).
Subsequently, toralactone, rubrofusarin triglucoside, cassiaside,
nor-rubrofusarin gentiobioside, cassiaside B, cassiaside C,
torachrysone apiglucoside, torachrysone gentiobioside, torachrysone
tetraglucoside, cassiatoroside, demethylflavasperone gentiobioside
and cassialactone gentiobioside were isolated from C. tora
seeds (compounds 50–61, respectively; Fig. 2) (17,28,49–51).
In addition, cassiaside B and cassiaside C were identified in the
seeds of C. obtusifolia (52). Other naphthopyrones, including
torosachrysone, isotoralactone, cassialactone, cassiaside B2,
cassiaside C2, nor-rubrofusarin-6-O-β-D (6′-O-acetyl)
glucopyranoside and
l-hydroxyl-2-acetyl-3,8-dimethoxy-6-O-[β-D-apiofuranosyl-
(l→2)-β-D-glucopyranosyl]-naphthalene were also isolated from C.
obtusifolia seeds (compounds 62–68, respectively; Fig. 2) (19,53–55).
Volatile oils
Li (56) extracted
the volatile oils from Cassiae semen by steam distillation,
and subsequently identified 37 components according to the gas
chromatography/mass spectrometry analysis. Among these peaks, the
major volatile components were 9-octadecenoic acid (E) (22.15%),
n-hexadecanoic acid (12.53%), 9,10-anthracenedione,
1,8-dihydroxy-3-methyl (7.66%), octadecanoic acid (4.56%) and
13-octadecenoic acid methyl ester (Z) (3.84%) (56).
Other compounds
A range of other components have been isolated from
Cassiae semen, including malvalic acid, sterculic acid,
mandelic acid, campesterol, aspidinol and 5,7-dihydroxychromone
(compounds 69–74, respectively; Fig.
3) (5,57,58).
In addition, the four flavonoid compounds, chrysin,
chrysin-7-O-β-D-glucoside, galangin and cyanidenon, were also
obtained and identified from Cassiae semen (compounds 75–78,
respectively; Fig. 3) (58).
Pharmacology
Cassiae semen exerts a great variety
of pharmacological activities due to its complex bioactive
compounds
An overview of the pharmacological studies on
Cassiae semen is presented in detail in the following
sections.
Antihyperlipidemic activity
In traditional Chinese herbal medicine,
Cassiae semen is used for the prevention and treatment of
hyperlipidemia. Several Chinese herbal formulations containing
Cassiae semen is available in the Chinese market for
preventing the formation of atherosclerotic plaques (59). In certain Asian countries,
including China and Korea, it is also commonly drunk as a roasted
tea to reduce body weight (60,61).
Previous studies using mice have evaluated the reductions in blood
lipid contents induced by different Cassiae semen extracts
obtained through different methods, including supercritical fluid
extraction, systematic solvents (petroleum ether, ethyl acetate,
n-butanol, 70% ethanol and water) and ethanol precipitation
following water extraction. The results revealed that the n-butanol
and ethyl acetate extracts were the most effective (62,63).
In addition, the ethanol and aqueous extracts of Cassiae
emen significantly decreased the serum levels of total cholesterol
(TC), triglyceride (TG) and low-density lipoprotein cholesterol
(LDL-C), however, they increased the levels of high-density
lipoprotein cholesterol (HDL-C) (13,64,65).
Similarly, He et al (66)
reported that treatment with the water extract form of C.
obtusifolia seeds decreased the blood-lipid level by inhibiting
cholesterol synthesis. Cho et al (67) demonstrated that soluble fibers from
C. tora seeds markedly decreased liver TC and TG levels in
rats fed with a high-cholesterol diet. The underlying mechanism may
be mediated by increasing fecal bile acid excretion and
downregulating the production of lipogenic enzymes (67). In addition, soluble fibers
decreased the serum levels of TC, TG and LDL-C in patients with
type II diabetes without serious adverse effects (2). Liu et al (68) revealed that the ethanol extract of
Cassiae semen upregulated the expression levels of
peroxisome proliferator-activated receptor (PPAR)-γ, sterol
regulatory element-binding protein-1c, hormone-sensitive lipase and
triacylglycerol hydrolase, however, tumor necrosis factor receptor
superfamily member 6 was downregulated in adipose tissue. The
anti-hyperlipidemia activity of Cassiae semen is primarily
due to its antioxidant components, such as anthraquinones and
polysaccharides. There are a variety of bioactive anthraquinone
components in Cassiae semen, including chrysophanol,
physcion, aurantio-obtusin, obtusifolin and emodin, which have been
observed to decrease the levels of TC and TG (69,70).
Previous studies have demonstrated that anthraquinones isolated
from Cassiae semen were effective substances during
hypolipidemic activities (71,72).
These results were verified by a previous study, which applied an
experimental hyperlipidemic rat model to investigate anthraquinone
treatment (80 and 20 mg/kg, per os, for 20 days). The TC, TG and
LDL-C levels were significantly reduced in a dose-dependent manner,
however, the levels of HDL-C increased. Inhibition of cholesterol
synthesis may be one of the underlying mechanisms involved in
decreasing blood lipid levels (73). Water-soluble polysaccharides (WSPs)
from Cassiae semen markedly inhibited the activities of
α-amylase and pancreatic lipase, however, protease activity
increased. The results demonstrated that WSPs had the ability to
bind to bile acids and reduce the absorption of cholesterol,
indicating that WSPs may have potential as an effective herbal
ingredient in functional food applications (74).
Antidiabetic activity
A number of studies have demonstrated that
Cassiae semen exhibits anti-diabetic activity. A total of
three naphthopyrone glucosides (compounds 49, 52 and 55) isolated
from the butanol-soluble extract of Cassia semen have been
evaluated for their inhibitory activity on advanced glycation end
products (AGEs) formation in vitro. The results revealed
that these compounds possessed more potent inhibitory activity
against AGEs compared with the aminoguanidine positive control
(15). In addition,
rubrofusarin-6-O-β-d-gentiobioside (compound 49) and cassiaside
(compound 52) significantly inhibited the expression of
transforming growth factor (TGF)-1 and extracellular matrix protein
in glomerular mesangial cells cultured under diabetic conditions,
suggesting that the active compounds in Cassiae semen may be
effective in the treatment of renal complications associated with
diabetes (16). Similarly, Kim
et al (75) evaluated the
preventive effects of the methanol extract of Cassia semen
(200 mg/kg/day, for 12 weeks) on the development of diabetic
nephropathy in streptozotocin (STZ)-induced diabetic rats. The
results indicated that oral treatment with the Cassia semen
methanol extract inhibited the development of diabetic nephropathy
by inhibiting AGEs accumulation, receptor for advanced
glycosylation end product and cyclooxygenase-2 expression in the
renal cortex of STZ-diabetic rats (75,76).
In addition, Zhu (77) reported
that the water extract of Cassia semen exhibited protective
activity against STZ-induced renal fibrosis in diabetic rats. The
underlying mechanisms may be associated with its ability to
downregulate the expression of TGF-β1, connective tissue growth
factor and mothers against decapentaplegic homolog 3 (smad3), as
well as upregulating the protein expression of smad6 (77).
Neuroprotective activity
The ethanolic extract from the seeds of C.
obtusifolia has been reported to have a neuroprotective effect
in brain disease models. Kim et al (6) suggested that C. obtusifolia
(25, 50 or 100 mg/kg) significantly attenuated scopolamine or
transient bilateral common carotid artery occlusion (2VO)-induced
memory impairment. These effects are mediated by the enhancement of
the cholinergic nervous system via acetylcholinesterase inhibition
in a dose-dependent manner [half maximal inhibitory concentration
(IC50)=81.6 µg/ml] (6).
In addition, C. obtusifolia (10 or 50 mg/kg/day) exhibited a
neuroprotective effect in a mouse transient global ischemia model
due to its anti-inflammatory properties and the induced upregulated
expression of phosphorylated cyclic AMP response element binding
protein and brain-derived neurotrophic factor (78).
Drever et al (79) demonstrated that treatment with
C. obtusifolia (0.1–10 µg/ml) significantly attenuated
secondary calcium dysregulation and cell death induced by
N-methyl-D-aspartate and 3-nitropropionic acid in mouse hippocampal
cultures, and no significant effect on cell death was induced by
incubation with naturally-secreted oligomers of amyloid (A)β. Yi
et al (80) reported for
the first time, that C. obtusifolia (10 µg/ml) ameliorated
the Aβ-induced synaptic dysfunction model through anti-inflammatory
and protein kinase B (Akt)/glycogen synthase kinase-3β pathways.
The results suggested that the neuroprotective effect may be
attributable to obtusifolin (compound 9) and/or alaternin (compound
17) (80). In a further
experiment, C. obtusifolia (0.1–1 µg/ml) inhibited cell
damage against oxidopamine (6-OHDA)-induced dopaminergic (DA)
neural toxicity in PC12 cells through an anti-oxidant and
antimitochondrial-mediated apoptosis mechanism. In a mesencephalic
DA culture, C. obtusifolia (0.1–1 µg/ml) protected the DA
cells against 6-OHDA- and N-methyl-4-phenylpyridinium
iodide-induced toxicities. In addition, C. obtusifolia (50
mg/kg/day for 15 days) significantly protected DA neuronal
degeneration in a
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse
Parkinson's disease (PD) model by inhibiting the movement
impairment and the loss of DA neurons, indicating that C.
obtusifolia may be a useful neuroprotective candidate for PD
(81). In addition, protein and
anthraquinone glucosides from Cassia semen improved learning
and memory capacity, inhibited the malondialdehyde (MDA) and
monoamine oxidase levels, and enhanced the level of superoxide
dismutase (SOD) in the cerebrum of senile mice (82).
Hepatoprotective activity
It was recorded in the Compendium of Materia Medica
that Cassiae semen exhibited the functions of nourishing the
liver and improving vision (83).
In Korea, the aqueous extract of C. tora L. seeds has been
used for the protection of the liver. A weak anti-hepatotoxic
activity in CCl4-induced mice was observed when the drug was
administered orally at a dose of 670 mg/kg (84). The methanol extract of C.
tora L. seeds exhibited significantly protective effects in
primary cultured hepatocytes against CCl4 and toxicity. A total of
two anthraquinone glycosides (compounds 44 and 45) and three
naphthoypyrone glycosides (compounds 49, 51 and 53) from the
methanol extract of C. tora seeds were the primary chemical
constituents (42,50). The preventative effects of
Cassiae semen on acute liver injury in mice induced by CCl4
have also been investigated (85,86).
When compared with the control group, varying concentrations of the
aqueous extract of Cassiae semen significantly increased the
serum levels of SOD, and decreased the serum levels of aspartate
transaminase (AST), alanine transaminase (ALT) and MDA. These
results indicate that Cassiae semen is potentially
beneficial in the treatment or prevention of hepatic damage
(85,86). In addition, the ethanol extract of
Cassiae semen has been observed to increase the serum levels
of SOD and to decrease the serum levels of TG, TC, MDA, AST and ALT
(86). Total anthraquinones from
Cassiae semen exhibited a protective effect on
alcohol-induced acute liver injury in mice by regulating fat
metabolism, improving liver function and increasing the mRNA and
protein expression levels of PPAR-γ (10,87).
Antibacterial activity
Antibacterial activity, an important effect of
Cassiae semen, has been comprehensively investigated.
Naphthalenes (compounds 47 and 50) and anthraquinones (compounds 1,
4 and 5) isolated from C. tora seeds exhibited significant
antibacterial effects on four strains of methicillin-resistant
Staphylococcus aureus [minimal inhibitory concentration
(MIC) was 2–64 µg/ml] and a strain of methicillin-sensitive S.
aureus. In addition, rhein (compound 5) and torachrysone
(compound 47) from the seeds of C. tora exhibited
antibacterial activity against Escherichia coli K12 with MIC
values of 512 and 128 µg/ml, respectively (17). Kim et al (88) were the first to demonstrate that
emodin (compound 1) from C. tora seeds has a median lethal
dose (LC50) value of 0.102, 0.163, 0.385 and 0.046 g/l against
Rhizoctonia solani, Botrytis cinerea, Phytophthora
infestans and Erysiphe graminis, respectively, and
physcion (compound 3) has an LC50 value of 0.248, 0.263, 0.518, and
0.073 g/l against R. solani, B. cinerea, P.
infestans and E. graminis, respectively. In addition,
the LC50 value of rhein (compound 5) is 0.375, 0.478, and 0.047 g/l
against R. solani, B. cinerea and P.
infestans, respectively (88).
It has been reported that ethanol and aqueous
extracts of C. obtusifolia seeds were inhibitory against
Helicobacter pylori strains (MIC were 100 and 60 µg/ml,
respectively) (89). In addition,
1,2-dihydroxyanthraquinone (compound 15) isolated from C.
obtusifolia seeds was revealed to inhibit the growth of
Clostridium perfringens and E. coli., indicating that
this drug exhibited potent growth-inhibiting activities towards
human intestinal bacteria (90).
Li et al (91) demonstrated
that the chloroform extract of the seeds of C. obtusifolia
also exhibited different inhibitory activities against Fusarium
oxysporum and B. cinerea (IC50 values were
0.57 mg/ml and 0.97 mg/ml).
Antioxidant activity
The water extract of C. tora seeds
accelerated the oxidation of deoxyribose induced by
Fe3+-EDTA/H2O2 and exhibited 94%
inhibition of linoleic acid peroxidation at a concentration of 0.2
mg/ml. The underlying mechanisms of this may be mediated by
reducing metal ions, scavenging hydroxyl radical and chelating
ferrousion (92,93). Xv and Hu (94) demonstrated that the water extract
of Cassiae semen exhibited a potent ability to scavenge free
oxygen radicals [IC50 values were 2 mg/ml and 2 µg/ml
for hydroxyl radicals (OH-) and hydrogen peroxide
(H2O2), respectively]. WSP from
Cassiae semen (0.022 mg/ml) effectively inhibited superoxide
radicals (O2-) induced by pyrogallol autoxidation
(95). The inhibitory effects of
WSP on serum levels of MDA were used to evaluate its antioxidation
capabilities. The results demonstrated that WSP decreased MDA serum
levels with an IC50 value of 15.80% (96). In another study, Liu et al
(97) optimized the extraction
conditions for WSP of Cassiae semen (temperature 80°C,
extraction time 3.5 h, solid-liquid ratio 1:30) and observed that
WSP (94.03 µg/ml) had the ability to scavenge hydroxyl and
superoxide radicals with scavenging rates of 43.32 and 64.97%,
respectively.
In addition, ethyl acetate fraction and n-butanol
fraction of Cassiae semen were evaluated by DPPH radical
scavenging activity. The results revealed that the ethyl acetate
fraction had a lower IC50 value of 56.4 g/ml, when
compared with the value of 80.6 g/ml for n-butanol fraction.
1-Desmethylaurantio-obtusin (compound 11) exhibited good scavenging
activity on DPPH with an IC50 value of 4.5±0.7 g/ml,
while aurantio-obtusin-6-O-β-D-glucopyranoside (compound 34) and
questin (compound 10) exhibited moderate antioxidant activity, and
their IC50 values were 103.2±1.5 g/ml and 185.2±1.8
g/ml, respectively. When compared with these results,
chryso-obtusin (compound 7) and aurantio-obtusin (compound 8)
demonstrated weaker antioxidant activity (IC50 >200
µg/ml) (98). The methanolic
extract of C. tora seeds exhibited a high antioxidant
activity on lipid peroxidation (99). Similarly, in another study, Yen
et al (12) demonstrated
that the methanolic extract of C. tora seeds exerted a
greater antioxidant activity than the other organic solvents
(n-hexane and ethyl acetate). Emodin was also revealed to be an
antioxidative component (12). In
addition, alaternin (compound 17), cassiaside (compound 52) and
rubrofusarin-6-O-β-D-gentiobioside (compound 49) isolated from
C. tora seeds exhibited good scavenging activity against
DPPH radicals with IC50 values of 17.59, 32.52 and 18.04
µg/ml, respectively (100).
Hypotensive activity
Aqueous and ethanol extracts of Cassiae
semen have been reported to possess hypotensive effects (101). Koo et al (101) reported that the water extract of
C. tora seeds (3.75, 7.5, 15, 30, 60 and 250 mg/kg)
consistently reduced arterial blood pressure in anesthetized rats.
A potential reflex mechanism of this hypotensive action may involve
a vagal reflex, which reciprocally inhibits the peripheral
vasomotor tone via a reflex reduction in the sympathetic neural
outflow to blood vessels (102).
In addition, the media portion of the medullary reticular formation
has been revealed to be directly involved in the hypotensive effect
of C. tora seeds (103).
Furthermore, the ethanol extract of Cassiae semen
significantly decreased blood pressure in hypertensive rats by
inhibiting receptor-controlled calcium channels on vessels and
regulating the secretion of nitric oxide and inducible nitric oxide
synthase (104).
Other activities
In addition to the pharmacological effects
described above, Cassiae semen and its ingredients have
other pharmacological effects, including estrogenic, anti-allergic,
antigenotoxic, anti-aggregatory, antimutagenic and cardioprotective
effects. Some of these effects are discussed briefly below.
The estrogenic activity of C. obtusifolia
seeds was evaluated by a recombinant yeast screening assay. The
results revealed that 70% EtOH extracts of this drug exhibited
estrogenic relative potency [half maximal effective concentration
(EC50) was 60.2 µg/ml) (105). Cassiaside C2 (compound
66) isolated from C. obtusifolia seeds exhibited a potent
anti-allergic activity by inhibiting the histamine release from
mast cells induced by antigen-antibody reaction (19). Furthermore, gluco-aurantioobtusin
(compound 25) from C. obtusifolia seeds possessed potent
inhibitory activities against arachidonic-acid-, ADP- and
collagen-induced platelet aggregations (39). Wu and Yen (106) demonstrated that the water extract
of C. tora seeds exhibited potential antigenotoxic
activities against the dietary mutagens Glu-P-1 and TrpP-1 in the
Ames test and the Comet assay. The potential mechanisms may be
associated with neutralization of the reactive intermediate of
Trp-P-1 and an antioxidant effect of the tested compounds (106). Anthraquinone aglycones (compounds
2, 7 and 8) and naphthopyrone glycosides (compounds 49 and 52) from
C. tora seeds exhibited significant antimutagenic activity
in vitro. The mechanism associated with these compounds may
be mediated via interactions with a microsomal activating system
(14). Fu et al (107) reported that the water extract of
Cassiae semen (10 mg/kg/day, for one week) effectively
improved myocardial function, and attenuated myocardial ischemia
and reperfusion-induced injury and apoptosis in diabetic animals,
which is potentially attributable to the reduced plasma lipid
levels and the triggered cell survival Akt and extracellular
signal-regulated kinases 1/2 signaling.
Conclusions
In traditional Chinese medicine, Cassiae
semen has long been used to clean the liver, brighten the eye,
loosen the bowel to relieve constipation, and for the treatment of
inflammation, photophobia, headaches, dizziness, hyperlipemia and
Alzheimer's disease. In addition, Cassiae semen is commonly
used in the composition of other herbs. Although modern experiments
have confirmed that this drug alone exhibits multiple
pharmacological activities, it is important to investigate the
molecular mechanisms of Cassiae semen combined with other
herbs based on traditional uses.
A number of studies have investigated the effective
constituents of Cassiae semen from different batches and
geographical areas. HPLC-fingerprint chromatography is a common
method to compare the differences (108–111). Zhang et al (3) developed a sensitive and reliable
ultra-high-performance liquid chromatography-electrospray
ionization-tandem mass spectrometry (UHPLC-ESI-MS/MS) method to
evaluate the quality of Cassiae semen through simultaneous
determination of 13 components, providing a novel basis for the
overall assessment of the quality of this plant. In addition, a
novel nonaqueous capillary electrophoresis method was used for the
analysis of aurantio-obtusin, emodin and rhein in Cassiae
semen with satisfactory results (112). Yang et al (113) was the first to simultaneously
determine 7 anthraquinones in rat plasma by UHPLC-MS/MS following
oral administration of Cassiae semen extract. These results
may support investigations into the bioactivity mechanism and
clinical application of this drug (113). Anthraquinones and naphthopyrones
are considered to be the major constituents. Therefore,
characteristic compounds or a biological index should be
established to evaluate the quality and ensure their clinical
application is suitable. In the Pharmacopoeia of the People's
Republic of China, chrysophanol and aurantio-obtusin are used as
the indicator compounds to characterize the quality of
Cassiae semen with the minimum contents of 0.20 and 0.080%,
respectively (1).
A total of 79 compounds including anthraquinones,
naphthopyrones and volatile oil have been isolated and identified
from Cassiae semen (Table
I; Figs. 1–3). It has also been suggested that
certain efforts should be made to isolate and identify novel
compounds from Cassiae semen, in order to strengthen its
pharmacological profile to develop it further as a candidate for
novel drug developments in the future.
Pharmacological studies have revealed that
Cassiae semen possesses a variety of biological effects,
including anti-hyperlipidemic, anti-diabetic, neuroprotective,
hepatoprotective, antimicrobial, anti-oxidant and hypotensive
activities (5,90,93,114,115). Extracts and compounds responsible
for the pharmacological properties have also been determined, as
presented in Table II. Although
the pharmacological properties of certain traditional uses of
Cassiae semen have been validated, these studies were
primarily conducted in vitro (16,116,117). Therefore, the effects of these
compounds require verification in vivo. In addition, the
association between structure and activity, and the potential
synergistic action exerted by the bioactive compounds requires
further elucidation. It is anticipated that the comprehensive and
current research on the pharmacological activities of extracts, as
well as on active molecules isolated from Cassiae semen,
provided in this review will inspire novel strategies in
therapeutics for curing a number of different ailments.
 | Table II.Pharmacological activities of
Cassiae semen. |
Table II.
Pharmacological activities of
Cassiae semen.
| Pharmacological
activities | Actions |
Extracts/compounds | Application | (Refs.) |
|---|
| Anti-hyperlipidemia
activity | Reduces blood lipid
levels | SFE, systematic
solvents (petroleum ether, ethyl acetate, n.butanol, 70% ethanol
and water) | In vivo | (62,63) |
|
| Decreases the
levels of TC, TG and LDL-C; Increases the level of HDL-C | Ethanol and aqueous
extracts | In vivo | (13,64,65) |
|
| Inhibits the
synthesis of cholesterol | Water extract | In
vitro | (66) |
|
| Increases fecal
bile acid excretion and downregulates the production of lipogenic
enzymes | Soluble fibers | In vivo | (67) |
| Anti-hyperlipidemia
activity | Upregulates the
expression levels of PPARγ, SREBP-1c, HSL and TGH; Downregulates
the levels of FAS | Ethanol
extract | In vivo | (68) |
|
| Decreases TC and TG
levels | Chrysophanol,
physcion, aurantio-obtusin, obtusifolin and emodin | In
vitro | (69,70) |
|
| Decreases TC, TG
and LDL-C levels; Increases HDL-C levels | Anthraquinones | In vivo | (73) |
|
| Binds bile acids
and reduces the absorption of cholesterol | Water-soluble
polysaccharides | In
vitro | (74) |
| Anti-diabetic
activity | Inhibits AGEs
activity | Cassiaside,
cassiaside C, rubrofusarin-6-O-β-D-gentiobioside | In
vitro | (15) |
|
| Inhibits the
expression of TGF-1 and ECM proteins |
Rubrofusarin-6-O-β-D-gentiobioside,
cassiaside | In
vitro | (16) |
|
| Inhibits AGEs
accumulation and, RAGE and COX-2 expression | Methanol
extract | In
vitro/vivo | (75,76) |
|
| Downregulates the
expression of TGF-β1, CTGF and smad3, and upregulates the protein
expression of smad6 | Water extract | In vivo | (77) |
| Neuroprotective
activity | Inhibits AChE
activity | Ethanol
extract | In vivo | (6) |
|
| Upregulates the
expression of pCREB and BDNF | Ethanol
extract | In vivo | (78) |
|
| Attenuates
secondary calcium dysregulation and cell death | Ethanol
extract | In vivo | (79) |
|
| Ameliorates the
Aβ-induced synaptic dysfunction model | Obtusifolin,
alaternin | In vivo | (80) |
|
| Inhibits cell
damage and protects DA neuronal degeneration | Ethanol
extract | In
vitro/vivo | (81) |
|
| Improves learning
and memory capacity; Inhibits MDA and MAO levels; Enhances the
level of SOD | Protein and
anthraquinone glucosides | In vivo | (82) |
| Hepatoprotective
activity | Hepatoprotective
effects | Methanol
extract | In
vitro | (42,50) |
|
| Increases the serum
levels of SOD and decreases the serum levels of AST, ALT and
MDA | Aqueous
extract | In vivo | (85,86) |
|
| Increases the serum
levels of SOD and decreases the serum levels of TG, TC, MDA, AST
and ALT | Ethanol
extract | In vivo | (10,87) |
| Antibacterial
activity |
Anti-Staphylococcus aureus;
Anti-Escherichia coli K12 | Naphthalenes,
anthraquinones | In
vitro | (17) |
|
| Exhibits fungicidal
activity against Botrytis cinerea, Erysiphe graminis,
Phytophthora infestans, Puccinia recondita, Phacelia
grisea, and Rhizoctonia solani. | Anthraquinones | In
vitro | (88) |
|
|
Anti-Helicobacter pylori | Ethanol and aqueous
extracts | In
vitro | (89) |
|
| Anti-Clostridium
perfringens; Anti-E. coli |
1,2-dihydroxyanthraquinone | In
vitro | (90) |
|
| Anti-Fusarium
oxysporum; Anti-B. cinerea | Chloroform
extract | In
vitro | (91) |
| Antioxidant
activity | Accelerates the
oxidation of deoxyribose; inhibits linoleic acid peroxidation | Water extract | In
vitro | (92,93) |
|
| Scavenges free
oxygen radicals | Water extract | In
vitro | (94) |
|
| Inhibits superoxide
radicals | Water-soluble
polysaccharides | In
vitro | (95) |
|
| Decreases MDA serum
levels | Water-soluble
polysaccharides | In
vitro | (96) |
|
| Scavenges hydroxyl
and superoxide radicals | Water-soluble
polysaccharides | In
vitro | (97) |
|
| Scavenges DPPH
radicals | Ethyl acetate
fraction | In
vitro | (98) |
|
| Antioxidant
effect | Methanol
extract | In
vitro | (12) |
|
| Scavenges DPPH
radicals | Alaternin,
cassiaside and rubrofusarin-6-O-β-D-gentiobioside | In
vitro | (100) |
| Hypotensive
activity | Reduces arterial
blood pressure | Water extract | In vivo | (101) |
|
| Hypotensive
activity | Water extract | In vivo | (103) |
|
| Decreases the blood
pressure | Ethanol
extract | In
vitro | (104) |
| Other
activities | Estrogenic
activity | 70% EtOH
extract | In
vitro | (105) |
|
| Inhibits histamine
release from mast cells | Cassiaside
C2 | In
vitro | (19) |
|
| Anti-platelet
aggregation |
Gluco-aurantioobtusin | In
vitro | (39) |
|
| Antigenotoxic
activity | Water extract | In
vitro | (106) |
|
| Antimutagenic
activity | Anthraquinone
aglycones and naphthopyrone glycosides | In
vitro | (14) |
|
| Improves myocardial
function and attenuates MI/R-induced injury | Water extract | In vivo | (107) |
Acknowledgements
The present review was financially supported by the
Collaborative Innovation Construction Plan of Beijing University of
Chinese Medicine (grant no. 2013-XTCX-03).
References
|
1
|
Editorial Committee of Chinese
PharmacopoeiaChinese Pharmacopoeia. 2015. Medical Science and
Technology Press; Beijing, China: pp. 1452015
|
|
2
|
Cho SH, Kim TH, Lee NH, Son HS, Cho IJ and
Ha TY: Effects of Cassia tora fiber supplement on serum lipids in
Korean diabetic patients. J Med Food. 8:311–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang W, Wang Y, Wang Q, Yang WJ, Gu Y,
Wang R, Song XM and Wang XJ: Quality evaluation of Semen Cassiae
(Cassia obtusifolia L.) by using ultra-high performance liquid
chromatography coupled with mass spectrometry. J Sep Sci.
35:2054–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen JK and Chen TT: Chinese Medical
Herbology and Pharmacology. CA: Art of Medicine Press; pp.
8032001
|
|
5
|
Hao YJ, Sang YL and Zhao YQ: The
advancement of the studies on the seeds of Cassia obtusifolia.
Chinese Tradit Herb Drugs. 32:858–859. 2001.
|
|
6
|
Kim DH, Yoon BH, Kim YW, Lee S, Shin BY,
Jung JW, Kim HJ, Lee YS, Choi JS, Kim SY, et al: The seed extract
of Cassia obtusifolia ameliorates learning and memory impairments
induced by scopolamine or transient cerebral hypoperfusion in mice.
J Pharmacol Sci. 105:82–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitanaka S, Kimura F and Takido M: Studies
on the Constituents of seeds of Cassia obusifolia LINN. The
structures of two new anthraquinone glycosides. Chem Pharm Bull.
33:1274–1276. 1985. View Article : Google Scholar
|
|
8
|
Zhang C, Li GL, Xiao YQ and Pang LZ: Two
new glycosides from the seeds of Cassia obtusifolia. Chinese Chem
Lett. 20:1097–1099. 2009. View Article : Google Scholar
|
|
9
|
Deng ZY, Zhang JW, Li J, Fan YW, Cao SW,
Huang RL, Yin YL, Zhong HY and Li TJ: Effect of polysaccharides of
cassiae seeds on the intestinal microflora of piglets. Asia Pac J
Clin Nutr. 16:(Suppl 1). 143–147. 2007.PubMed/NCBI
|
|
10
|
Luo X, Xu X, Huang C, Wu X, Liu J, Lan B
and Xu J: Experiment study of total anthraquinone in Cassiae Semen
on lipid peroxidation and PPAR-gamma expression in liver tissues of
rats with alcoholic fatty liver. Zhongguo Zhong Yao Za Zhi.
36:1654–1658. 2011.(In Chinese). PubMed/NCBI
|
|
11
|
Su H, Wang Z and Tang L: Simultaneous
determination of 4 major components in Cassiae Semen obtusifoline
by HPLC. Zhongguo Zhong Yao Za Zhi. 36:1327–1329. 2011.(In
Chinese). PubMed/NCBI
|
|
12
|
Yen GC, Chen HW and Duh PD: Extraction and
identification of an antioxidative component from Jue Ming Zi
(Cassia tora L.). J Agr Food Chem. 46:820–824. 1998. View Article : Google Scholar
|
|
13
|
Patil UK, Saraf S and Dixit VK:
Hypolipidemic activity of seeds of Cassia tora Linn. J
Ethnopharmacol. 90:249–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JS, Lee HJ, Park KY, Ha JO and Kang
SS: In vitro antimutagenic effects of anthraquinone aglycones and
naphthopyrone glycosides from Cassia tora. Planta Med. 63:11–14.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee GY, Jang DS, Lee YM, Kim JM and Kim
JS: Naphthopyrone glucosides from the seeds of Cassia tora with
inhibitory activity on advanced glycation end products (AGEs)
formation. Arch Pharm Res. 29:587–590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung DH, Kim YS, Kim NH, Lee J, Jang DS
and Kim JS: Extract of Cassiae semen and its major compound inhibit
S100b-induced TGF-beta1 and fibronectin expression in mouse
glomerular mesangial cells. Eur J Pharmacol. 641:7–14. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hatano T, Uebayashi H, Ito H, Shiota S,
Tsuchiya T and Yoshida T: Phenolic constituents of Cassia seeds and
antibacterial effect of some naphthalenes and anthraquinones on
methicillin-resistant Staphylococcus aureus. Chem Pharm Bull
(Tokyo). 47:1121–1127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Das C, Dash S, Sahoo DC, Mohanty A and
Rout D: Cassia tora: A phyto-pharmacological overview. Int J Res
Ayurveda Pharm. 2:1162–1174. 2011.
|
|
19
|
Kitanaka S, Nakayama T, Shibano T, Ohkoshi
E and Takido M: Antiallergic agent from natural sources. Structures
and inhibitory effect of histamine release of naphthopyrone
glycosides from seeds of Cassia obtusifolia L. Chem Pharm Bull
(Tokyo). 46:1650–1652. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deore SL, Khadabadi SS, Kamdi KS, Ingle
VP, Kawalkar NG, Sawarkar PS, Patil UA and Vyas AJ: In vitro
Anthelmintic activity of Cassia tora. Int J Chem Tech Res.
1:177–179. 2009.
|
|
21
|
Editorial Board of Flora of China. Science
Publishing House Press; pp. 1261988
|
|
22
|
Du ZX: Comparative identification of
Cassiae Semen and Sesbania aculeata Pers. Chinese Pharm J.
30:204–205. 1995.
|
|
23
|
Zhu SY, Xv HJ and Xv MG: Comparative
identification of Semen Cassiae and Cassia sophera. Chin Tradit
Herb Drugs. 34:379–380. 2003.
|
|
24
|
Liu J, Zou CC and Sun ZF: Comparative
identification of Cassiae Semen and Cassia occidentalis L.
Heilongjiang Med Pharm. 29:63–64. 2006.
|
|
25
|
Hu YJ, Wan L, Zhang JX, et al:
Identification of Cassia obtusifolia L. by TLC. Lishizhen Med Mater
Med Res. 17:21292006.
|
|
26
|
Sun GF: Identification of Cassia
obtusifolia L., Cassia tora. and Cassia occidentalis L. by
SDS-Polyacrylamide gelelectrophoresis. Tianjin Pharm. 8:69–71.
1996.
|
|
27
|
Wang JB, Zhou X and Hu ZF: Quality
evaluation of Cassiae Semen by both indicated component
determination and HPLC fingerprint. Chin Tradit Herb Drugs.
39:917–919. 2008.
|
|
28
|
Luo Y, Zhang L, Wang WH and Li B:
Components identification in Cassiae Semen by HPLC-IT-TOF MS. Chin
J Pharm Anal. 35:1408–1516. 2015.
|
|
29
|
Lee HJ, Choi JS, Jung JH and Kang SS:
Alaternin glucoside isomer from Cassia tora. Phytochemistry.
49:1403–1404. 1998. View Article : Google Scholar
|
|
30
|
Chen QD, Xv R, Xv ZN, et al: Progress in
studies of active coustituents of anthraquinones and their
biological activities from Cassiae Semen. Chins J Mod Appl Pharm.
20:120–123. 2003.
|
|
31
|
DiGiovanni J and Boutwell RK: Tumor
promoting activity of 1, 8-dihydroxy-3-methyl-9-anthrone
(chrysarobin) in female SENCAR mice. Carcinogenesis. 4:281–284.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia ZB, Chen WW, Jiang JX and DING XL:
Study on anthraquinone constituents in the seed of Cassia tora L.
Chem Ind Forest Prod. 29:100–102. 2009.
|
|
33
|
Xv YL: Studies on the chemical
constituents from Semen Cassiae and the influence of processing.
Beijing: Chinese Academy of Medical Sciences. 1:152014.
|
|
34
|
Takido M: Studies on the constituents of
the seeds of Cassia obtusifolia L. I. The Structure of Obtusifolin.
Chem Pharm Bull. 6:397–400. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takido M: Studies on the constituents of
seeds of Cassia obusifolia L. II. The structure of Obtusin,
Chryso-obutsin and Aurantio-obyusin. Chem Pharm Bull. 8:2461960.
View Article : Google Scholar
|
|
36
|
Kitanaka S and Takido M: Studies on the
constituents of the seeds of Cassia obusifolia: The structures of
three new anthraquinones. Chem Pharm Bull. 32:860–864. 1984.
View Article : Google Scholar
|
|
37
|
Tang LY, Wang ZJ, Fu HM, et al: Study on
anthraquinones constituents from Semen Cassiae. J Chinese Med
Mater. 32:717–719. 2009a.
|
|
38
|
Zhang ZX and Liang YF: Isolation and
identification of chemical constituents from seeds of Cassia
obtusifolia. China Pharm. 23:1782–1783. 2012.
|
|
39
|
Yun-Choi HS, Kim JH and Takido M:
Potential inhibitors of platelet aggregation from plant sources, V.
Anthraquinones from seeds of Cassia obtusifolia and related
compounds. J Nat Prod. 53:630–633. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hao YJ, Sang YL and Zhao YQ: Study on
anthraquinone constituents in Cassiae Semen. Chinese Tradit Herb
Drugs. 34:18–19. 2003.
|
|
41
|
Li CH, Wei XY and Li XE: A new
anthraquinone glycoside from the seed of Cassia obtusifolia.
Chinese Chem Lett. 15:1448–1450. 2004.
|
|
42
|
Wong SM, Wong MM, Seligmann O and Wagner
H: Anthraquinone glycosides from the seeds of Cassia tora.
Phytochemistry. 28:211–214. 1989. View Article : Google Scholar
|
|
43
|
Tang LY, Wang ZJ, Fu MH, He Y, Wu HW and
Huang LQ: A new anthraquinone glycoside from seeds of Cassia
obtusifolia. Chinese Chem Lett. 19:1083–1085. 2008. View Article : Google Scholar
|
|
44
|
Li G, Xiao Y, Li L, Zhang C and Pang Z:
Studies on chemical constituents of roasted seeds of Cassia
obtusifolia. Zhongguo Zhong Yao Za Zhi. 34:54–56. 2009.(In
Chinese). PubMed/NCBI
|
|
45
|
Tang LY, Wang ZJ, He Y, et al: Glycosides
from seeds of Cassia obtusifolia. Chinese J Exp Tradit Med
Formulae. 15:35–37. 2009b.
|
|
46
|
Jia ZB and Ding XL: Anthraquinone
constituents from seeds of Cassia tora L. J Chinese Med Mater.
29:28–29. 2006.
|
|
47
|
Miyuki K, Eisaku M and Shoji S: Chemical
Studies on the Oriental Plant Drugs. XXI. The Constituents of
Cassia tora L. (2). A Glycoside of Rubrofusarin. Chem Pharm Bull.
17:458–461. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shibata S, Morishita E and Kaneda M:
Chemical Studies on the Oriental Plant Drugs. XX. The Constituents
of Cassia tora L. (1). The Structure of Torachrysone. Chem Pharm
Bull. 17:454–457. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takahashi S and Takido M: Studies on the
constituents of the seeds of Cassia tora L. II. (On the purgative
crude drugs. VII). The structure of the new naphthopyrone
derivative, toralactone. Yakugaku Zasshi. 93:2611973. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wong SM, Wong MM, Seligmann O and Wagner
H: New antihepatotoxic naphthopyrone glycosides from the seeds of
Cassia tora. Planta Med. 55:276–280. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi JS, Jung JH, Lee HJ, Lee HJ, Lee JH
and Kang SS: A naphthalene glycoside from Cassia tora.
Phytochemistry. 40:997–999. 1995. View Article : Google Scholar
|
|
52
|
Tianaka S and Takido M: Studies on the
constituents of the seeds of Cassia obtusifolia L. The structures
of two naphthopyrone glycosides. Chem Pharm Bull. 36:3980–3984.
1988. View Article : Google Scholar
|
|
53
|
Kitanaka S and Miehio T: Studies on the
constituents of the seeds of Cassia obtusifolia: The structures of
two new lactones, isotoralactone and cassialactone. Phytochemistry.
20:1951–1953. 1981. View Article : Google Scholar
|
|
54
|
Li L, Zhang C, Xiao YQ, Li W, Yin X, Chen
D, Tian G and Wang Y: Glycosides of roasted seeds of Cassia
obtusifolia. Zhongguo Zhong Yao Za Zhi. 35:1566–1568.
2010.PubMed/NCBI
|
|
55
|
Wang ZJ, Wu QP, Tang LY, Fu MH, He Y, Gong
QF and Hung LQ: Two new glycosides from the genus of Cassia.
Chinese Chem Lett. 18:1218–1220. 2007. View Article : Google Scholar
|
|
56
|
Li YM: Comparison of volatile components
of Cassiae Semen and semen seeds tea. J Med Plants Res.
6:3865–3869. 2012.
|
|
57
|
Jiao SF and Han HD: Studies on chemical
constituents of Cassiae Semen. Chinese J Clin Ration Drug Use.
3:81–82. 2010.
|
|
58
|
Wu XH: Study on the chemical constituents,
quality control and metabolism of Cassia obtusifolia. Wuhan:
Huazhong University of Science and Technology. 1:11–12. 2010.
|
|
59
|
Guan Y and Zhao S: Yishou jiangzhi
(de-blood-lipid) tablets in the treatment of hyperlipidemia. J
Tradit Chin Med. 15:178–179. 1995.PubMed/NCBI
|
|
60
|
Yang Y, Liu J and Lai XH: Observation on
cassia seed tea combined with walking exercise on weight loss in
the elderly. Modern Prev Med. 40:2468–2474. 2013.
|
|
61
|
Lai XH: Double intervention of cassia seed
tea and sports on older women weight loss. Chinese J Gerontol.
31:2402–2404. 2011.
|
|
62
|
Zhang R, Feng ML and Wu YP: Experimental
study on the active situs of fetid cassia seed to reduce blood
lipid and their dose-effect relation. China Remedies Clin.
5:183–185. 2005b.
|
|
63
|
Zhang JX, Wan L, Hu YJ, Qu OL and Shi JY:
Study on the effective part of reducing blood lipid in Semen
Cassiae. Lishizhen Med Mater Med Res. 17:904–905. 2006.
|
|
64
|
Li CH, Li XE and Guo BJ: The effects of
Cassia obtusifolia seeds extracts on reducing blood lipid. J South
China Normal Univ. 98:29–32. 2002.
|
|
65
|
Wang YH, Gao L, Zhou WJ and Ma WJ: Effects
of ethanol extraction from Cassiae Semen on Serum IL-6 and TNF-α in
hyperlipidemia rats. Chinese J Exp Tradit Med Formulae. 20:178–181.
2014.
|
|
66
|
He JY, Liu SQ, Peng YF, et al: Study of
the Mechanism of Cassia Obtusifolia L in decreasing blood -lipid.
China Pharm. 14:202–203. 2003.
|
|
67
|
Cho IJ, Lee C and Ha TY: Hypolipidemic
effect of soluble fiber isolated from seeds of Cassia tora Linn. In
rats fed a high-cholesterol diet. J Agric Food Chem. 55:1592–1596.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu SM, Sun C and Xie WH: Effect of Semen
Cassiae extracts on expression of lipogenesis genes in
hyperlipidemia model mice. Chinese Tradit Herb Drugs. 40:583–587.
2009.
|
|
69
|
Wei N, Lv HR and Liu MF: Study on the
chemical constitutions of reducing blood lipid in Cassiae Semen.
Guangdong Chem Ind. 39:99–100. 2012.
|
|
70
|
Li BL: The active ingredients of reducing
blood lipid in Semen Cassia. China Prac Med. 7:172–173. 2012.
|
|
71
|
Guo CY, Horn W and Pin DD: Extraction and
identification of an antioxidative component from Jue Ming Zi
(Cassia tora L.). J Agric Food Chem. 46:820–824. 1998. View Article : Google Scholar
|
|
72
|
Li XE and Guo BJ: Effects of protein and
anthraquinone glucosides from Cassia seed on serum lipid of
hyperlipidemia rats. Zhongguo Zhong Yao Za Zhi. 27:374–376.
2002.(In Chinese). PubMed/NCBI
|
|
73
|
Li CH, Li XE, Fang KY and Guo BJ: Effects
of anthraquinones from Cassia obtusifolia L. on cholesterol
biosynthesis in cells. J Clin Rehabilit Tissue Eng Res.
12:6593–6596. 2008.
|
|
74
|
Huang YL, Chow CJ and Tsai YH:
Composition, characteristics, and in-vitro physiological effects of
the water-soluble polysaccharides from Cassia seed. Food Chem.
134:1967–1972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim YS, Jung DH, Sohn E, Lee YM, Kim CS
and Kim JS: Extract of Cassiae semen attenuates diabetic
nephropathy via inhibition of advanced glycation end products
accumulation in streptozotocin-induced diabetic rats.
Phytomedicine. 21:734–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jung DH, Kim YS, Sohn EJ, et al: Effects
of CS, a BuOH-Soluble Fraction of Cassiae Semen Methanolic Extract,
on COX-2 Expression in Renal Cortex of STZ-Induced Diabetic Rats
and Cultured Glomerular Mesangial Cells. Diabetes. 56:pA155.
2007.
|
|
77
|
Zhu TC: Inhibitory Effects of Cassia Seed
on the Renal Fibrosis in Diabetic Rats. Chin J Exp Tradit Med
Formulae. 18:315–319. 2012.
|
|
78
|
Kim DH, Kim S, Jung WY, Park SJ, Park DH,
Kim JM, Cheong JH and Ryu JH: The neuroprotective effects of the
seeds of Cassia obtusifolia on transient cerebral global ischemia
in mice. Food Chem Toxicol. 47:1473–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Drever BD, Anderson WG, Riedel G, Kim DH,
Ryu JH, Choi DY and Platt B: The seed extract of Cassia obtusifolia
offers neuroprotection to mouse hippocampal cultures. J Pharmacol
Sci. 107:380–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yi JH, Park HJ, Lee S, Jung JW, Kim BC,
Lee YC, Ryu JH and Kim DH: Cassia obtusifolia seed ameliorates
amyloid β-induced synaptic dysfunction through anti-inflammatory
and Akt/GSK-3β pathways. J Ethnopharmacol. 178:50–57. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ju MS, Kim HG, Choi JG, Ryu JH, Hur J, Kim
YJ and Oh MS: Cassiae semen, a seed of Cassia obtusifolia, has
neuroprotective effects in Parkinson's disease models. Food Chem
Toxicol. 48:2037–2044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liu JZ, Lin X, Li XE and Guo BJ: Effect of
protein and anthraquinone glucosides from Semen Cassiae on learning
and memory capacity and related substances of senile mice induced
by D-galactone. Zhongguo Zhong Yao Za Zhi. 32:516–519. 2007.(In
Chinese). PubMed/NCBI
|
|
83
|
Jiangsu New Medical College: Encyclopedia
of Chinese MateriaMedica, I. Shanghai Science and Technology Press;
Shanghai, China: pp. 949841975
|
|
84
|
Yun HS and Chang IM: Plants with liver
protective activities (I). Korean J Pharmacogn. 8:125–129.
1977.
|
|
85
|
Gao Q, Xu H and Chen J: Liver-protective
and bowel-lubricating and defecation-promoting effects of crude and
processed Semen Cassiae. Tradit Chinese Drug Res Clin Pharmacol.
18:194–196. 2007.
|
|
86
|
Lin DJ and Jin Z: Experimental Study on
Protective Effect of Semen Cassiae Extract Against Acute Liver
Injury. Lishizhen Med Mater Med Res. 17:214–215. 2006.
|
|
87
|
Niu YF, Zhao T, Zeng T, et al: Study on
the protective effect of Cassiae Semen extract against
alcohol-induced acute liver injury in mice. J Toxicol. 24:58–61.
2010.
|
|
88
|
Kim YM, Lee CH, Kim HG and Lee HS:
Anthraquinones isolated from Cassia tora (Leguminosae) seed show an
antifungal property against phytopathogenic fungi. J Agr Food Chem.
52:6096–6100. 2004. View Article : Google Scholar
|
|
89
|
Li Y, Xu C, Zhang Q, Liu JY and Tan RX: In
vitro anti-Helicobacter pylori action of 30 Chinese herbal
medicines used to treat ulcer diseases. J Ethnopharmacol.
98:329–333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sung BK, Kim MK, Lee WH, Lee DH and Lee
HS: Growth responses of Cassia obtusifolia toward human intestinal
bacteria. Fitoterapia. 75:505–509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li XH, Gong CR, Cao H, et al: Primary
study on inhabiting of the extracts from Cassia obtusifolia seeds
against Fusarium oxysporum and Botrytis cinerea. J Shanxi Agric
Univ. 26:348–350. 2006.
|
|
92
|
Yen GC and Chung DY: Antioxidant Effects
of Extracts from Cassia tora L. Prepared under different degrees of
roasting on the oxidative damage to biomolecules. J Agr Food Chem.
47:1326–1332. 1999. View Article : Google Scholar
|
|
93
|
Yen GC and Chuang DY: Antioxidant
properties of water extracts from Cassia tora L. In relation to the
degree of roasting. J Agr Food Chem. 48:2760–2765. 2000. View Article : Google Scholar
|
|
94
|
Xv JG and Hu QP: Study on Free Radical
Scavenging Capacity by Cassia Seed Water Extract in vitro. Food
Sci. 27:73–75. 2006.
|
|
95
|
Guo CQ, Yan J, Wu XY, Xu GY, Fan CH and
Gou XJ: Study on purification and antioxidation of water-soluble
polysaccharide isolated from semen cassia. Food Sci. 28:205–208.
2007.
|
|
96
|
Liu J, Deng ZY and Yu HH: Antioxidation
Study on Water-soluble Polysaccharide Isolated from Semen Cassiae.
Food Sci. 27:61–63. 2006.
|
|
97
|
Liu C, Liu Q, Sun J, Jiang Bb and Yan J:
Extraction of water-soluble polysaccharide and the antioxidant
activity from Cassiae Semen. J Food Drug Anal. 22:492–499. 2014.
View Article : Google Scholar
|
|
98
|
Zeng H, Liu Q, Wang M, Jiang S, Zhang L,
He X, Wang J and Chen X: Target-guided separation of antioxidants
from Semen cassia via off-line two-dimensional high-speed
counter-current chromatography combined with complexation and
extrusion elution mode. J Chromatogr B. 1001:58–65. 2015.
View Article : Google Scholar
|
|
99
|
Kim SY, Kim JH, Kim SK, Oh MJ and Jung MY:
Antioxidant activities of selected oriental herb extracts. J Am Oil
Chem Soc. 71:633–640. 1994. View Article : Google Scholar
|
|
100
|
Choi JS, Lee HJ and Kang SS: Alatemin,
cassiaside and rubrofusarin gentiobioside, radical scavenging
principles from the seeds of Cassia tora on 1,
1-diphenyl-2-picrylhydrazyl (DPPH) radical. Arch Pharm Res.
17:462–466. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Koo A, Wang JC and Li KM: Extraction of
hypotensive principles from seeds of Cassia tora. Am J Chin Med
(Gard City N Y). 4:245–248. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Koo A, Chan WS and Li KM: A possible
reflex mechanism of hypotensive action of extract from Cassia tora
seeds. Am J Chin Med (Gard City N Y). 4:249–255. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chan SH, Koo A and Li KM: The involvement
of medullary reticular formation in the hypotensive effect of
extracts from seeds of Cassia tora. Am J Chin Med (Gard City N Y).
4:383–389. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Mao WH, Shang QH, Liu AD, et al: Effects
of Cassiae Semen Extracts on Vasorelaxation and Its Mechanisms in
Rat Aorta. Chin J Hypertens. 18:60–63. 2010.
|
|
105
|
Zhang CZ, Wang SX, Zhang Y, Chen JP and
Liang XM: In vitro estrogenic activities of Chinese medicinal
plants traditionally used for the management of menopausal
symptoms. J Ethnopharmacol. 98:295–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wu CH and Yen GC: Antigenotoxic properties
of Cassia tea (Cassia tora L.): Mechanism of action and the
influence of roasting process. Life Sci. 76:85–101. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fu F, Tian F, Zhou H, Lv W, Tie R, Ji L,
Li R, Shi Z, Yu L, Liang X, et al: Semen cassiae attenuates
myocardial ischemia and reperfusion injury in high-fat diet
streptozotocin-induced type 2 diabetic rats. Am J Chinese Med.
42:95–108. 2014. View Article : Google Scholar
|
|
108
|
Zhang P and Chen JW: HPLC Fingerprint of
Cassiae Semen. Chinese Tradit Herb Drugs. 38:372–375. 2007.
|
|
109
|
Luo W, Liu B, Wang W, et al: HPLC
fingerprint chromatogram of Cassiae Semen. J Beijing Univ Tradit
Chin Med. 32:115–117. 2009.
|
|
110
|
Wang WY, Zhao Q, Zhang TJ, et al: HPLC
Fingerprint and chemical pattern recognition of Cassiae Semen.
Chinese Tradit Herb Drugs. 40:1638–1641. 2009.
|
|
111
|
Tang YL, Liang TZ, Zhang HX and Xu JY:
Study on fingerprint of Cassiae Semen, ultramicro powder and powder
particle. Zhong Yao Cai. 34:1861–1866. 2011.(In Chinese).
PubMed/NCBI
|
|
112
|
Wang N, Wu Y, Wu X, Liang S and Sun H: A
novel nonaqueous capillary electrophoresis method for effective
separation and simultaneous determination of aurantio-obtusin,
emodin and rhein in semen cassiae and cassia seed tea. Anal
Methods. 6:5133–5139. 2014. View Article : Google Scholar
|
|
113
|
Yang C, Wang S, Guo X, Sun J, Liu L and Wu
L: Simultaneous determination of seven anthraquinones in rat plasma
by Ultra High Performance Liquid Chromatography-tandem Mass
Spectrometry and pharmacokinetic study after oral administration of
Semen Cassiae extract. J Ethnopharmacol. 169:305–313. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hyun SK, Lee H, Kang SS, Chung HY and Choi
JS: Inhibitory activities of Cassia tora and its anthraquinone
constituents on angiotensin-converting enzyme. Phytother Res.
23:178–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dhanasekaran M, Ignacimuthu S and Agastian
P: Potential hepatoprotective activity of ononitol monohydrate
isolated from Cassia tora L. on carbon tetrachloride induced
hepatotoxicity in Wistar rats. Phytomedicine. 16:891–895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Jung HA, Ali MY, Jung HJ, Jeong HO, Chung
HY and Choi JS: Inhibitory activities of major anthraquinones and
other constituents from Cassia obtusifolia against β-secretase and
cholinesterases. J Ethnopharmacol. 191:152–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wen CC, Shyur LF, Jan JT, Liang PH, Kuo
CJ, Arulselvan P, Wu JB, Kuo SC and Yang NS: Traditional Chinese
medicine herbal extracts of Cibotium barometz, Gentiana scabra,
Dioscorea batatas, Cassia tora and Taxillus chinensis inhibit
SARS-CoV replication. J Tradit Complement Med. 1:41–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|