Introduction
The Achilles tendon is the strongest tendon in the
human body. Achilles tendon ruptures frequently occur in sporting
activities (1) and patients with
Achilles tendon rupture have been reported to have a 200-fold risk
of sustaining a contralateral rupture (2). Surgery has the highest success rate,
particularly when dealing with elite athletes; however, it is
associated with a significantly high risk of postoperative
complications (3). Non-surgical
treatment, such as exercise, may be considered a promising approach
for Achilles tendon healing. The favorable effect of early
functional rehabilitation has been addressed in previous studies
(4–7). However, the underlying mechanism
involved in active functional rehabilitation-mediated Achilles
tendon healing requires further clarification.
The pathology of the Achilles tendon following
injury is poorly understood. Collagen fibrils represent the
smallest unit in tendons, which aggregate to form fascicles that
are responsible for tensile strength (8). In the immediate area of the rupture
site, a loss of larger collagen fibrils (9), a more evident gelatinolytic activity
and altered matrix metalloprotease (MMP) expression (10) has previously been identified.
Collapsin response mediator protein-2 (CRMP-2),
which is a mammalian homologue of UNC-33, is critical for
determining the fate of axons and dendrites, and contributes to the
establishment and maintenance of neuronal polarity (11). Our previous study identified
several differentially expressed proteins in injured Achilles
tendon tissue (12). Among these
differentially expressed proteins, CRMP-2 expression was
upregulated in animals receiving active rehabilitation treatment,
suggesting that CRMP-2 may be involved in the process of Achilles
tendon healing (12).
The present study established a rat model of
Achilles tendon injury and determined the efficacy of active
mobilization therapy on Achilles tendon healing and collagen fibril
arrangement. In addition, the potential involvement of CRMP-2 in
mobilization-induced Achilles tendon healing was investigated. The
findings suggested that early active exercise may be a potential
strategy for rehabilitation therapy and CRMP-2 may be involved in
efficient Achilles tendon healing.
Materials and methods
Animals
A total of 45 healthy, male, 4-month-old, adult
Sprague Dawley rats, weighing 230±20 g, were obtained from the
Laboratory Animal Center, Xinjiang Medical University (Urumqi,
China). The rats were housed in specific pathogen-free conditions
and had ad libitum access to food and water prior to the
experiment. Housing conditions were thermostatically maintained at
25°C with a 12 h light/dark cycle. The Animal Ethical Committee of
The First Teaching Hospital of Xinjiang Medical University (Urumqi,
China) approved the animal experiments.
Achilles tendon injury model in
rats
In order to establish the rodent Achilles tendon
injury model, all rats were fasted for 12 h and deprived of water
for 4 h. Surgical procedures were conducted under standard aseptic
technique, using a combination of local anesthesia, utilizing 12 ml
0.25% procaine hydrochloride injection (Sichuan Kelun Bio-Tech
Pharmaceutical Co., Ltd., Chengdu, China) and hypnotic induction
(neck massage 45 times per minute) as previously described
(13). Briefly, following
anesthesia, an S-shaped incision was made on the right Achilles
tendon of each rat and the Achilles tendon was carefully freed from
the underlying tissue under aseptic conditions. The Achilles tendon
injury was then repaired using a previously described surgical
procedure (13). The incision was
covered with sterile gauze and a clean and dry bandage. The animals
were returned to their cages postoperatively.
Experimental setup
Rats with Achilles tendon injury were randomly
divided into two groups, the immobilization group (n=15) and the
active mobilization group (n=15). The rats in the immobilization
group had their operated leg immobilized with a fixation brace. The
ankle was fixed at a 90° angle and the knee joint at 85°. Following
leg immobilization with a plaster cast, the animals were returned
to their cages. The rats in the active mobilization group received
training by offering water at a place 13 cm higher than usual
following surgery, in order to increase the rats' active raising
and squatting movements. Therefore, post-operation, the average
number of raising and squatting movements was increased to ~150±15
times/day, to ensure rats continued exercising. All rats were
returned to their cages and did not receive additional treatments.
Additionally, 15 healthy rats were included for the comparison of
structure of Achilles tendon under light microscope.
Inclusion and exclusion criteria
Animals meeting the following criteria post-surgery
were included: i) Stage I incision healing (endogenous healing at
the site of incision) (14); and
ii) stage I tissue healing of the injured Achilles tendon. Animals
were excluded if any of the following situations occurred: i)
Death; ii) infection at the site of incision; iii) sample
contamination; iv) injured Achilles tendon had a gap >1 mm in
length and v) cast was loose or had fallen off.
Sample collection
All rats were sacrificed 14 days after the operation
and the Achilles tendon tissues were carefully removed. Tissue
samples were washed 3 times with cold normal saline and
longitudinally cut into 2 pieces. One piece was used for
histological examination, and the other piece was prepared for
western blotting. For histological examination, each sample was cut
into 30 slices, and used for hematoxylin and eosin (H&E)
staining (10 slices), immunohistochemical staining (10 slices) and
scanning electronic microscopy (SEM) (10 slices). All tissue
samples were stored in liquid nitrogen until use.
Histological examination
Tissue samples from the immobilization (n=13),
active mobilization groups (n=12), and healthy control (n=15) were
fixed in 10% formalin and subsequently dehydrated in an ethanol
series (75, 85, 95 and 100%). The samples were then cleared in
xylene and embedded in paraffin for histological analysis.
Paraffin-embedded tissues were sectioned on a microtome to 4 µm in
an automatic tissue processor (LEICA RM2235; Leica Microsystems,
Inc., Buffalo Grove, IL, USA) and were then stained with H&E. A
total of forty microscopic fields were randomly selected and images
were captured under light microscopy (magnification, ×400; The
automated Leica DM3000 B microscope (Leica Microsystems, Inc.).
SEM analysis
Tissue samples from the immobilization (n=13) and
the active mobilization group (n=12) were fixed in 3%
glutaraldehyde for 2–4 h at 4°C and post-fixed in 1% osmium
tetroxide solution for a further 2 h. Following dehydration in an
ethanol and acetone gradient [50% (v/v) ethanol (10 min), 70% (v/v)
ethanol (10 min), 80% (v/v) ethanol (10 min), 90% (v/v) ethanol (10
min), 90% (v/v) acetone (10 min), 100% acetone (3×15 min)], samples
were impregnated with a mixture of acetone/resin (1:1) with gentle
rotation overnight) and incubated with resin at room temperature
for a further 1 h. Samples were then sectioned with an LKB 2188
ultramicrotome into semi-thin and ultra-thin sections. The
semi-thin sections were stained with toluidine blue and the
ultra-thin sections with super saturated uranyl acetate and lead
citrate. The ultra-thin sections were examined using scanning
electron microscopy (SEM). Images were obtained under a JEOL JEM
1230 SEM (JEOL Ltd., Tokyo, Japan) at magnification ×20,000. The
ultrastructure of 10 randomly selected collagen fibrils was
examined and compared between the experimental groups. The average
circumference of each collagen fibril was quantified and averaged
as previously described (15).
Immunohistochemical analysis
Paraffin-embedded tissue sections prepared at a size
of 25×20×25 mm3 (n=13 for immobilization group; n=12 for
active mobilization group) were exposed to heating in EDTA buffer
(pH 8.0) for antigen retrieval and were then treated with
endogenous peroxidase (3% hydrogen peroxide solution) for 5 min at
room temperature. Following blocking in 5% goat serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30–40 min at
room temperature, sections were immunostained with an anti-CRMP-2
primary antibody (cat. no. sc-30228; 1:1,500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 37°C for 3 h.
Subsequently, sections were washed 3 times in PBS, incubated with a
biotin-labeled goat anti-rabbit secondary antibody (cat. no.
LS-C60867-1000; 1:4,000; LifeSpan BioSciences, Inc., Seattle, WA,
USA) for 20 min at room temperature and incubated with
avidin-conjugated horseradish peroxidase (HRP, Sigma-Aldrich; Merck
KGaA) at a dilution of 1:100 in 0.1 M PBS for 15 min at room
temperature. The sample was washed 3 times in PBS and visualized
using 3, 3-diaminobenzidine tetrahydrochloride. Subsequently,
samples were counterstained with hematoxylin. A total of 5
micrographs were randomly selected from each slice and images were
captured using a LEICA DM3000 microscope and a charge-coupled
device camera at magnification ×400. The mean optical density (MOD)
in each image was analyzed using Image-Pro Plus version 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA), and the
mean CRMP-2 intensity in tissue samples was quantified.
Western blotting
A total of 7 samples were randomly selected from
each experimental group. Total protein was extracted from tissues
using radioimmunoprecipitation assay lysis buffer (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Protein
concentration was quantified using bicinchoninic acid assay kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal amounts
of protein (40 µg) were then separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
polyvinylidene fluoride membrane (0.45 µm; Merck Millipore,
Darmstadt, Germany). Membranes were blocked with Tris-buffered
saline plus 0.1% Tween-20 (TBST) with 5% w/v non-fat dry milk for
50 min at room temperature. Following blocking, the membranes were
incubated with anti-CRMP-2 (cat. no. SC-30228; 1:300; Santa Cruz
Biotechnology, Inc.) or anti-β-actin (cat. no. SC-81178; 1:1,000;
Santa Cruz Biotechnology, Inc.) primary antibodies at 4°C
overnight. Membranes were washed 3 times with TBST and incubated
with HRP-labeled anti-rabbit secondary antibody (cat. no.
LS-C60867-1000; 1:4,000; LifeSpan BioSciences Inc.) for 1 h at room
temperature. Followed by washing with TBST, immunodetected protein
bands were visualized using enhanced chemiluminescence kits
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc.) and images were captured using a Chemi Doc MP gel imaging
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
levels were quantified by analyzing the densitometric values using
ImageJ software version 1.48 for Windows (National Institutes of
Health, Bethesda, MD, USA). The housekeeping protein β-actin was
used as an internal control.
Statistical analysis
Data are presented as the mean and standard
deviation of at least 3 independent experiments and were analyzed
using SPSS version 20.0 software (IBM SPSS, Armonk, NY, USA). Data
with normal distribution was tested for homogeneity of variance.
Statistical significance was determined using a Student's t-test
(Independent Samples) for equal variances and Welch's t-test was
used for the unequal variances with a 95% confidence interval.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histological examination
Rats were randomly divided into immobilization
(n=15) and active mobilization groups (n=15). A total of 5 animals
were excluded according to the aforementioned criteria. In the
immobilization group, one animal had an infection at the site of
incision and the cast fell off another animal. In the active
mobilization group, 3 animals were excluded for the following
reasons: Death (n=1), infection at the site of incision (n=1) and
injured Achilles tendon had a gap >1 mm in length (n=1).
Therefore, the immobilization group contained 13 rats and the
active mobilization group contained 12 rats that were used for
subsequent analyses. Histological methods were used to compare the
various pathological manifestations following Achilles tendon
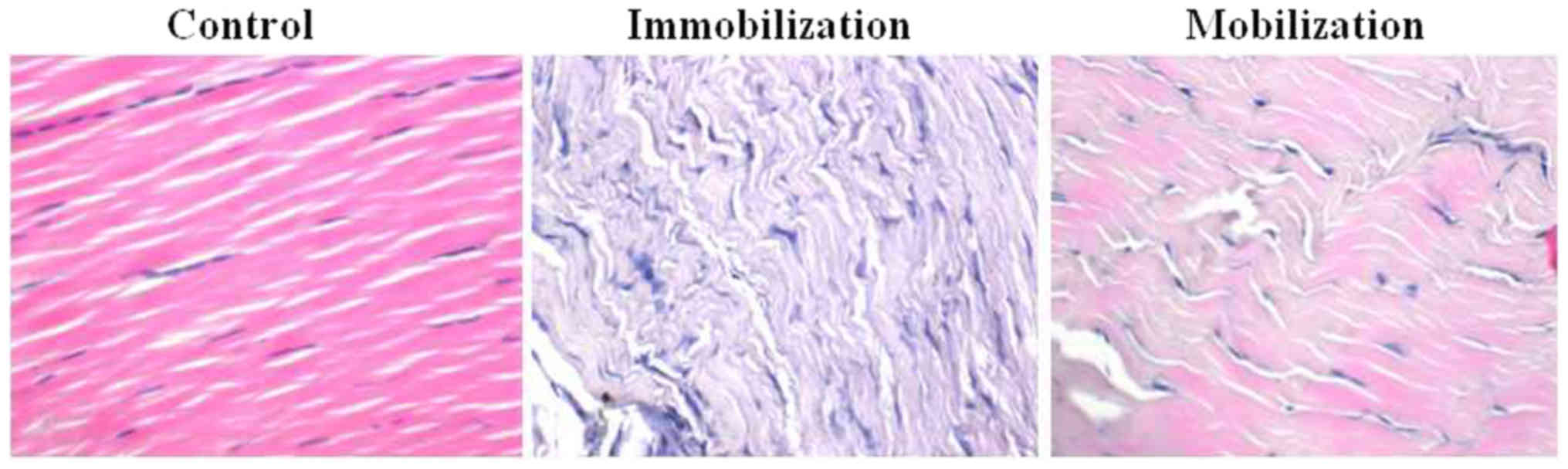
injury. In healthy animals, the structure of the Achilles tendon
was intact, with collagen fibrils, stained in pink, arranged in
regular, tightly lined and evenly arranged parallel arrays with
abundant capillaries (Fig. 1).
Achilles tendon tissue samples derived from the immobilization
group were markedly different in appearance. They exhibited a loss
of collagen fibrils, disturbed collagen fibril arrangement, a
reduced number of fibroblasts, formation of thin-walled
capillaries, formation of granulation tissue at the site of injury
and infiltration of inflammatory cells (Fig. 1). Notably, in the active
mobilization rehabilitation therapy group these pathological
manifestations were reduced. Animals in the mobilization group
exhibited regular collagen fibril arrangement, increased numbers of
collagen fibrils, increased fibroblast number and only moderate
inflammatory cell infiltration (Fig.
1).
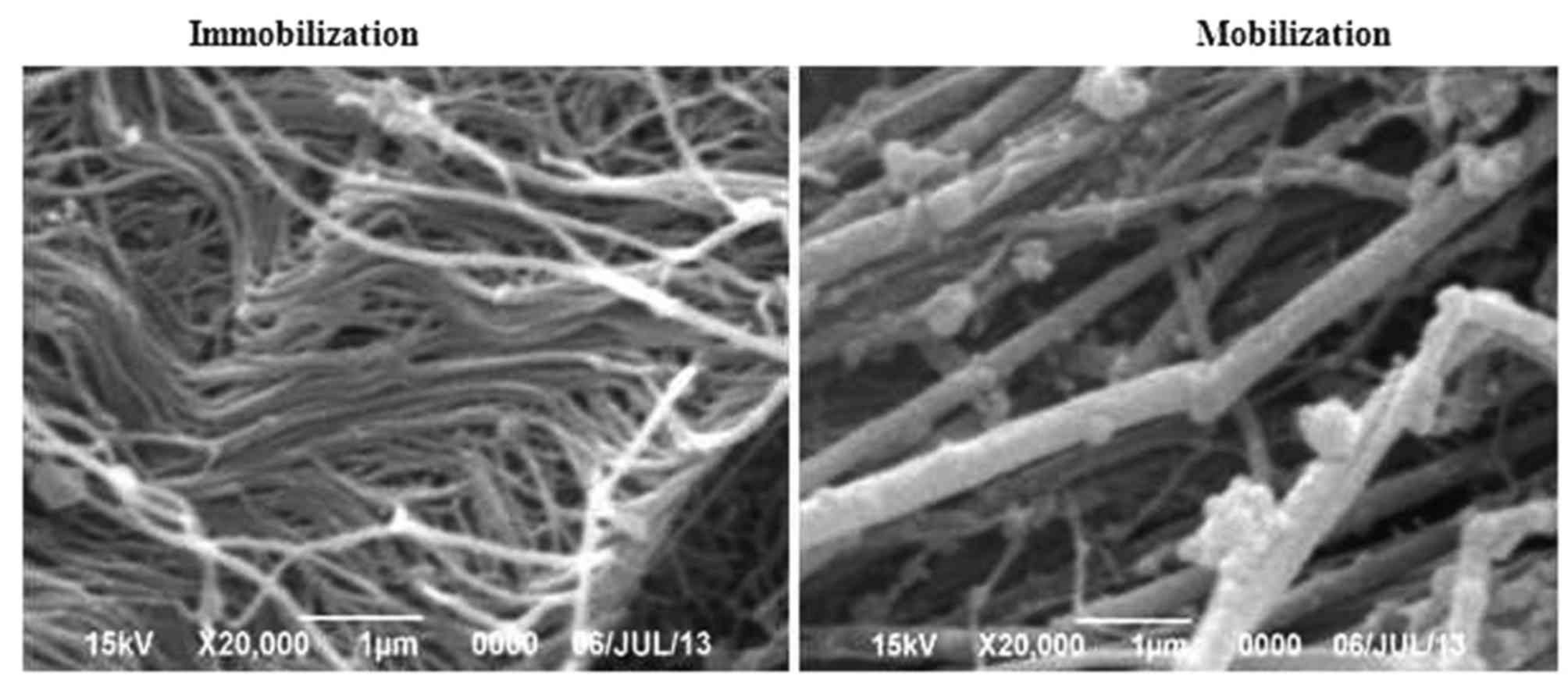
Ultrastructural examination
Differences in the ultrastructure of the Achilles
tendon tissues between the immobilization group and the active
mobilization group were compared using SEM. Similar to the results
of histological staining, the findings revealed that Achilles
tendon injury followed by immobilization resulted in disorganized
collagen fibrils with varied gap size and thickness, with some
collagen fibrils attached to each other, some fibril tangling, and
fragmentation (Fig. 2).
Conversely, the collagen fibrils in the mobilization group were
tightly arranged with even thickness, and uniform intervals were
detected between the collagen fibrils (Fig. 2). These findings suggested that
mobilization therapy may prevent pathological alterations in the
injured Achilles tendon by maintaining the normal structure and
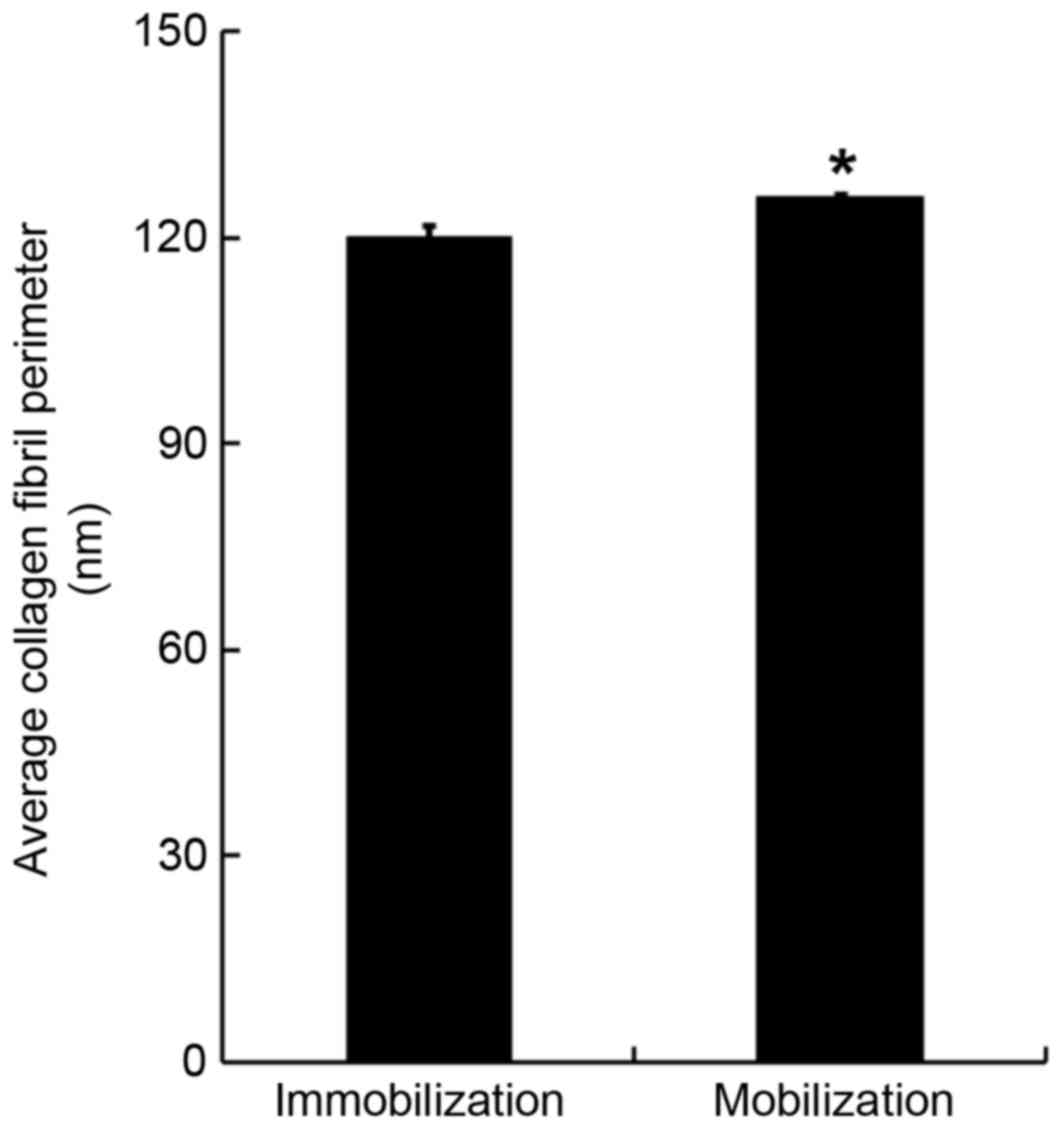
organization of collagen fibrils. The SEM images were also used to
quantify the collagen fibril perimeter. It was demonstrated that
the average collagen fibril perimeter in the mobilization group was
significantly increased compared with in the immobilization group
(125.6±0.8 nm vs. 119.9±1.7 nm; F=6.075, P<0.05; Fig. 3).
CRMP-2 expression
In order to determine the molecular mechanism
underlying mobilization-mediated protection in rats with injured
Achilles tendons, the expression of CRMP-2 was examined.
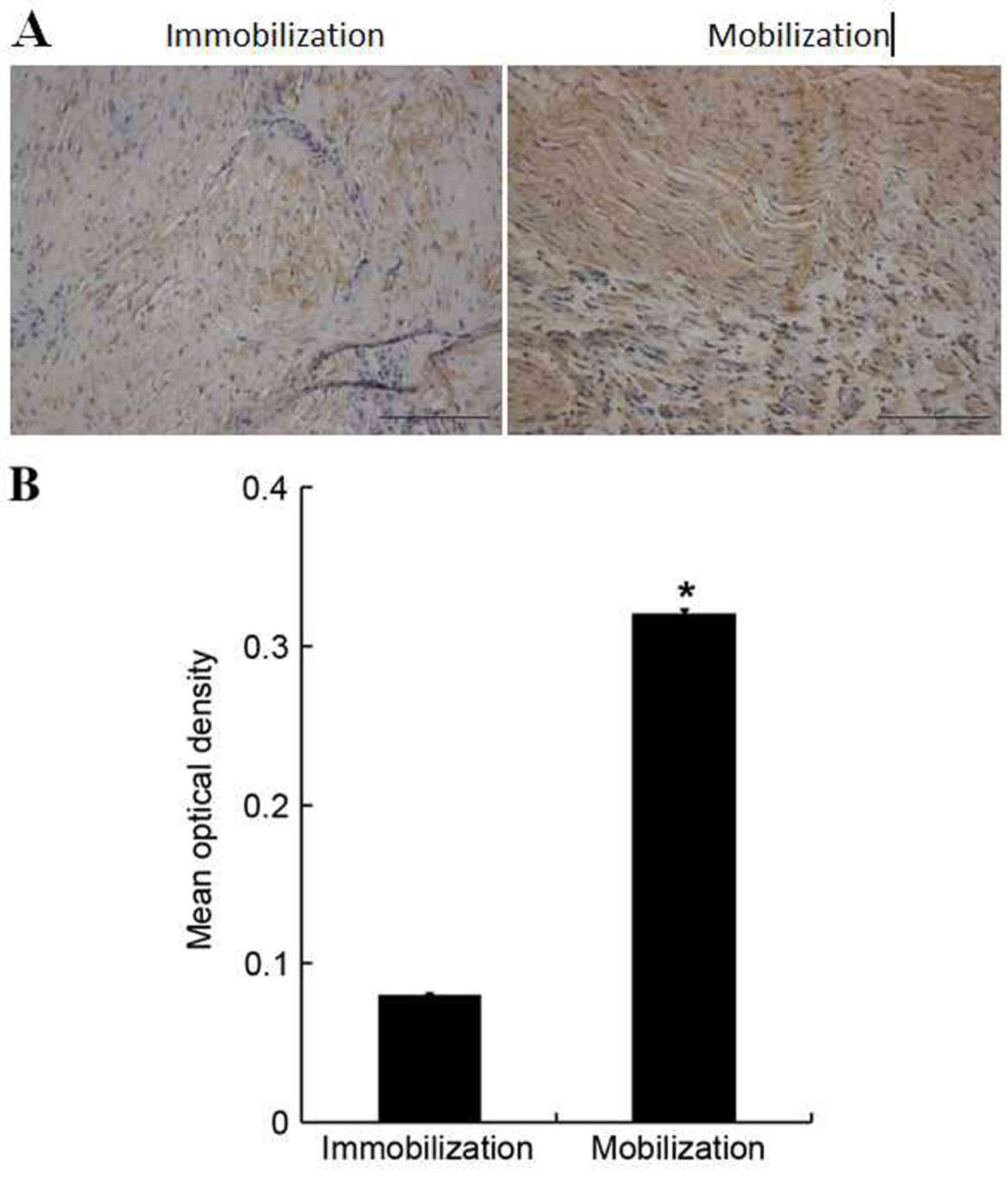
Immunohistological analysis demonstrated a low CRMP-2 expression in
the Achilles tendon tissues of immobilized animals (Fig. 4A). However, CRMP-2 expression was
upregulated in the mobilization group, as evident by brown-yellow
staining, particularly in the injured site of Achilles tendon
tissues. Following quantification, it was determined that CRMP-2
expression was significantly increased in the Achilles tendon
tissues of the mobilization group compared with the immobilization
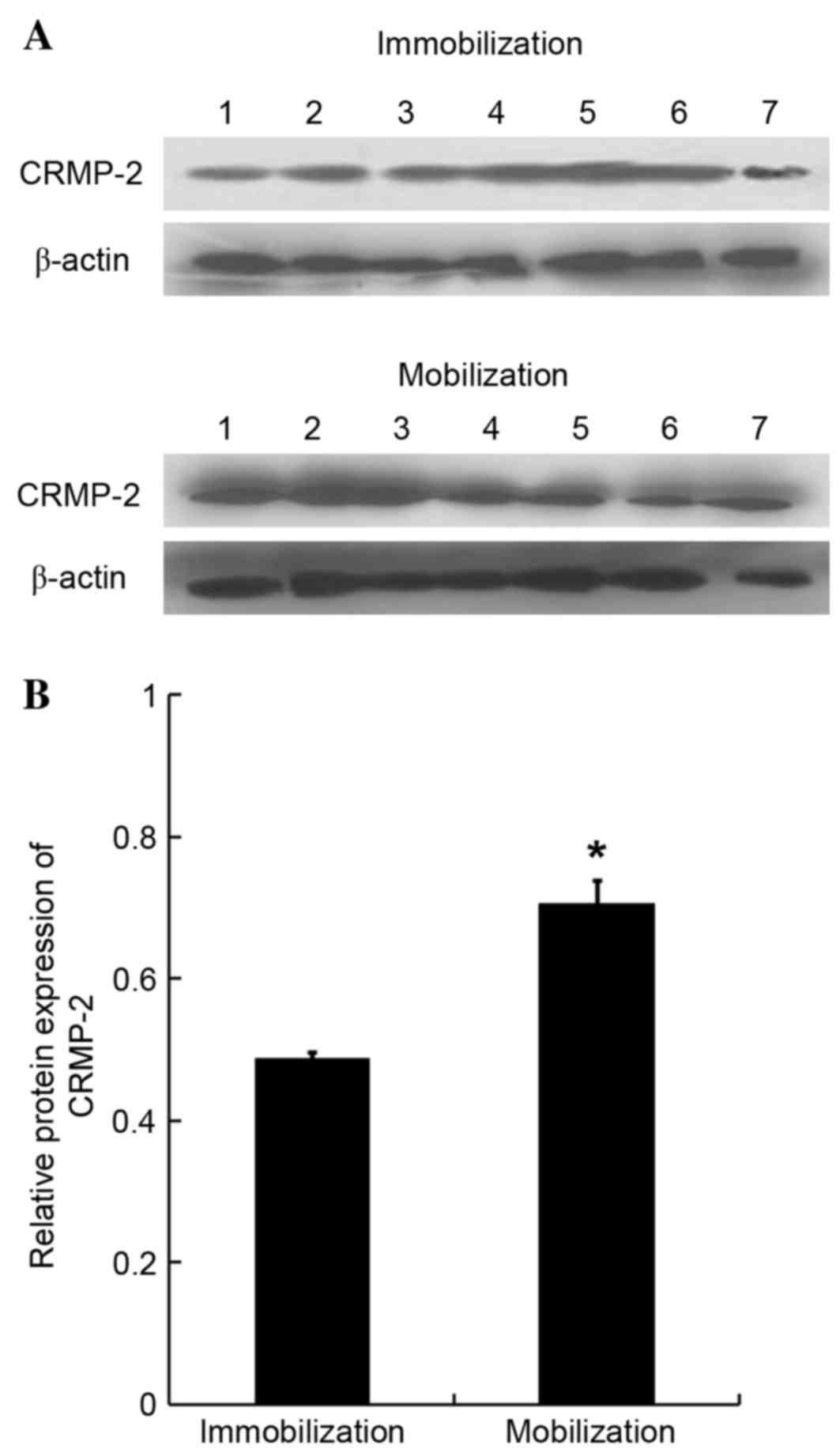
group (0.32±0.00 vs. 0.08±0.00; F=1.332, P<0.05; Fig. 4B). Western blotting
semi-quantification further confirmed that the protein expression
levels of CRMP-2 were significantly upregulated in the Achilles
tendon tissues of the mobilization group compared with the
immobilization group (0.71±0.03 vs. 0.49±0.01 nm; F=3.577,
P<0.05; Fig. 5).
Discussion
The prevalence of Achilles tendon rupture in humans
is relatively high, particularly in athletes. Compounding the
problem is the fact that surgical outcomes are frequently poor due
to various postoperative complications. It has previously been
suggested that immobilization of the digit or limb may promote
faster healing; however, this technique may eventually lead to the
formation of adhesions between the tendon and tendon sheath, which
may result in friction and reduced gliding (16). Therefore, the present study
established a rodent model of Achilles tendon injury and
demonstrated that active mobilization following Achilles tendon
injury may promote healing by upregulation of CRMP-2 expression,
particularly at the site of the injured Achilles tendon. In
addition, mobilization through active exercise resulted in reduced
pathological alterations in the injured Achilles tendon; rats in
the mobilization group exhibited regular collagen fibril
arrangement, increased collagen fibrils, elevated number of
fibroblasts and moderate inflammatory cell infiltration. Consistent
with the results of previous studies that identified a strong
positive correlation between collagen fibril diameter and tendon
strength (8,17), the present study indicated that
mobilization treatment significantly increased the mean collagen
fibril perimeter. It is possible that these early adaptive
responses may trigger long-term tendon structure remodeling and
lead to changes in the tendon's mechanical properties (14,18–20).
Various factors and mediators have been proposed to
serve essential roles in the mechanobiological responses of cells,
including collagens, proteoglycans and MMPs (19). In our previous study, protein
expression was compared in rabbits from immobilization and active
mobilization groups, and several differentially expressed proteins
were identified in the rabbits receiving mobilization treatment,
including myosin light chain 1, phosphoglycerate kinase, F-actin
capping protein subunit α 1, gi|45478150-LRRGT00155 protein, prolyl
4-hydroxylase, α I subunit isoform 2 precursor and CRMP-2 (12). The present study detected increased
CRMP-2 protein expression levels in rats with injured Achilles
tendons that received mobilization treatment. The healing process
consists of three phases: i) Inflammatory phase, ii) proliferative
phase and iii) maturation phase. The increased CRMP-2 expression
was particularly evident at the site of injury and it is possible
that the increased CRMP-2 level occurred during the proliferative
phase in injured animals that received mobilization treatment. A
previous study demonstrated that active Achilles tendon
kinesitherapy promoted neurite regeneration of the ruptured
Achilles tendon (12); therefore,
it is possible that active exercises promoted the Achilles tendon
healing process by accelerating the local neurite regeneration and
nerve repair mediated by CRMP-2 at the injury site. In addition, it
has previously been reported that CRMP-2 accelerated axon
regeneration of nerve-injured motor neurons in rats (21). These findings provide promising
insights into tendon healing at the molecular level; however, the
precise role of CRMP-2 in regulating nerve repair and Achilles
tendon healing requires further investigation. These findings are
consistent with those of Ackermann et al (22) and Bring et al (23), and are in accordance with the
findings of our previous studies (1,12,13,20,24,25).
In conclusion, the present study revealed that
active mobilization therapy reduced Achilles tendon damage in a
rodent model. Specifically, collagen fibril structure and
arrangement was similar to that of healthy animals and was improved
when compared with animals that did not undergo mobilization
therapy. Notably, the protection of Achilles tendon injury achieved
by active mobilization therapy may be associated with increased
CRMP-2 protein expression, particularly at the site of injury.
Future studies should focus on the underlying mechanism of
CRMP-2-mediated Achilles tendon repair following acute injury using
conditional knockout mice or cellular gene manipulation. The in
situ expression of CRMP-2 should also be investigated in future
clinical studies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460337).
References
|
1
|
Jielile J, Sabirhazi G, Chen J, Aldyarhan
K, Zheyiken J, Zhao Q and Bai J: Novel surgical technique and early
kinesiotherapy for acute Achilles tendon rupture. Foot Ankle Int.
33:1119–1127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arøen A, Helgø D, Granlund OG and Bahr R:
Contralateral tendon rupture risk is increased in individuals with
a previous Achilles tendon rupture. Scand J Med Sci Sports.
14:30–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan RJ, Fick D, Keogh A, Crawford J,
Brammar T and Parker M: Treatment of acute achilles tendon
ruptures. A meta-analysis of randomized, controlled trials. J Bone
Joint Surg Am. 87:2202–2210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hufner TM, Brandes DB, Thermann H, Richter
M, Knobloch K and Krettek C: Long-term results after functional
nonoperative treatment of achilles tendon rupture. Foot Ankle Int.
27:167–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Metz R, Kerkhoffs GM, Verleisdonk EJ and
van der Heijden GJ: Acute Achilles tendon rupture: Minimally
invasive surgery versus non operative treatment, with immediate
full weight bearing. Design of a randomized controlled trial. BMC
Musculoskelet Disord. 8:1082007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soroceanu A, Sidhwa F, Aarabi S, Kaufman A
and Glazebrook M: Surgical versus nonsurgical treatment of acute
Achilles tendon rupture: A meta-analysis of randomized trials. J
Bone Joint Surg Am. 94:2136–2143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Devries G: Surgical and nonsurgical
treatment of achilles tendon rupture: The favorable effect of early
functional rehabilitation. Clin J Sport Med. 24:159–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Battaglia TC, Clark RT, Chhabra A, Gaschen
V, Hunziker EB and Mikic B: Ultrastructural determinants of murine
achilles tendon strength during healing. Connect Tissue Res.
44:218–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magnusson SP, Qvortrup K, Larsen JO,
Rosager S, Hanson P, Aagaard P, Krogsgaard M and Kjaer M: Collagen
fibril size and crimp morphology in ruptured and intact Achilles
tendons. Matrix Biol. 21:369–377. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karousou E, Ronga M, Vigetti D, Passi A
and Maffulli N: Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in
human achilles tendon rupture. Clin Orthop Relat Res.
466:1577–1582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshimura T, Kawano Y, Arimura N, Kawabata
S, Kikuchi A and Kaibuchi K: GSK-3beta regulates phosphorylation of
CRMP-2 and neuronal polarity. Cell. 120:137–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jielile J, Aibai M, Sabirhazi G, Shawutali
N, Tangkejie W, Badelhan A, Nuerduola Y, Satewalede T, Buranbai D,
Hunapia B, et al: Active Achilles tendon kinesitherapy accelerates
Achilles tendon repair by promoting neurite regeneration. Neural
Regen Res. 7:2801–2810. 2012.PubMed/NCBI
|
|
13
|
Jielile J, Bai JP, Sabirhazi G, Redat D,
Yilihamu T, Xinlin B, Hu G, Tang B, Liang B and Sun Q: Factors
influencing the tensile strength of repaired Achilles tendon: A
biomechanical experiment study. Clin Biomech (Bristol, Avon).
25:789–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang B, Bai JP and Jielile J: The
histological effects of early mobilization in the healing after
Achilles tendon of rabbits rupture repair. J Chin Physicians.
12:19–22. 2010.
|
|
15
|
Jacob KM and Paterson R: Surgical repair
followed by functional rehabilitation for acute and chronic
achilles tendon injuries: Excellent functional results, patient
satisfaction and no reruptures. ANZ J Surg. 77:287–291. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
James R, Kesturu G, Balian G and Chhabra
AB: Tendon: Biology, biomechanics, repair, growth factors, and
evolving treatment options. J Hand Surg Am. 33:102–112. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Derwin KA and Soslowsky LJ: A quantitative
investigation of structure-function relationships in a tendon
fascicle model. J Biomech Eng. 121:598–604. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JH: Mechanobiology of tendon. J
Biomech. 39:1563–1582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JH and Thampatty BP: An introductory
review of cell mechanobiology. Biomech Model Mechanobiol. 5:1–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiasharete J and Bai JP: Mechanobiology
during Achilles tendon healing. Chin J Clin Rehabil Tissue Eng Res.
11:17–20. 2008.
|
|
21
|
Suzuki Y, Nakagomi S, Namikawa K,
Kiryu-Seo S, Inagaki N, Kaibuchi K, Aizawa H, Kikuchi K and Kiyama
H: Collapsin response mediator protein-2 accelerates axon
regeneration of nerve-injured motor neurons of rat. J Neurochem.
86:1042–1050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ackermann PW, Ahmed M and Kreicbergs A:
Early nerve regeneration after achilles tendon rupture-a
prerequisite for healing? A study in the rat. J Orthop Res.
20:849–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bring DK, Kreicbergs A, Renstrom PA and
Ackermann PW: Physical activity modulates nerve plasticity and
stimulates repair after Achilles tendon rupture. J Orthop Res.
25:164–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jielile J, Badalihan A, Qianman B,
Satewalede T, Wuerliebieke J, Kelamu M and Jialihasi A: Clinical
outcome of exercise therapy and early post-operative rehabilitation
for treatment of neglected Achilles tendon rupture: A randomized
study. Knee Surg Sports Traumatol Arthrosc. 24:2148–2155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Badalihan A, Aihemaiti A, Shawutali N,
Jielile J, Jialihasi A, Tangkejie W, Nuerdoula Y, Satewalede T,
Hunapiya B, Niyazebieke H, et al: Outcome of a one-stage tensile
stress surgical technique and early postoperative rehabilitation in
the treatment of neglected achilles tendon rupture. J Foot Ankle
Surg. 54:153–159. 2015. View Article : Google Scholar : PubMed/NCBI
|