Introduction
Tubulointerstitial fibrosis is a common end point of
diabetic nephropathy, allograft rejection and various
glomerulonephritides. Therefore, preventing tubulointerstitial
fibrosis is an important strategy in the treatment of chronic
kidney diseases.
Members of the lysyl oxidase (LOX) family [e.g., LOX
and LOX-like (LOXL)1-4] are responsible for the cross-linking of
collagen and elastin in the extracellular matrix through their
copper-dependent amine oxidase activity. In addition, LOX family
members exhibit various functions in cell proliferation, tumor
invasion and metastasis, and organ development (1). LOXL2 is the most thoroughly studied
of the LOX family members. Its expression is associated with tumor
cell differentiation in colon and esophageal carcinoma (2), and proliferation, migration and
invasion of human hepatocellular carcinoma cells (3). Increased LOXL2 expression is also
associated with poor survival in squamous cell carcinoma of the
larynx and lung (4), and appears
to serve a role in the metastatic potential of breast (5) and gastric carcinoma (6). Potential mechanisms underlying the
effects of LOXL2 include fibroblast activation in the tumor
microenvironment (7), induction of
epithelial-mesenchymal transition in tumor cells (5), and matrix remodeling via regulation
of tissue inhibitor of metalloproteinase-1 and matrix
metalloproteinase-9 (8).
The contribution of LOXL2 to benign fibrosing
diseases has been studied in several organs. Previous studies have
indicated that LOXL2 expression is associated with hepatic fibrosis
in Wilson's disease, primary biliary cirrhosis (9) and hepatocellular carcinoma (10). Elevated serum LOXL2 levels are also
associated with disease progression in idiopathic pulmonary
fibrosis (11), and LOXL2
upregulation is associated with scar formation following glaucoma
surgery (12).
An inhibitory monoclonal antibody to LOXL2 has been
developed; AB0023 binds to human and mouse LOXL2, and AB0024
(simtuzumab) is its humanized form. The antifibrotic effects of
AB0023, AB0024 and other inhibitory antibodies have been determined
in several organs. For example, in a rabbit model of glaucoma
surgery, AB0023 attenuated postoperative fibrosis (12). In BALB/c mice, AB0023 attenuated
tetrachloride-induced hepatic fibrosis and decreased
phosphorylated-Smad3 signaling. In C57BL/6 mice, AB0023 attenuated
high-dose bleomycin-induced pulmonary fibrosis, and this effect was
mediated by inhibiting fibroblast recruitment and activation
(13). Based on the results of
previous animal experiments, clinical trials of simtuzumab for
human fibrosing diseases have been performed. The target diseases
include advanced liver fibrosis due to human immunodeficiency virus
and hepatitis C virus infection (14), and idiopathic pulmonary fibrosis
(clinicaltrials.gov/ct2/show/NCT01769196).
Although fibrosis is a clinically important
pathological process in kidney disease, little is currently known
regarding the expression of LOXL2 in renal tissue and its
contribution to the development of renal tubulointerstitial
fibrosis. In the present study, the expression of LOXL2 in normal
kidney was evaluated in tissues and cell lines. In addition, to
evaluate its possible profibrotic role, LOXL2 expression was
evaluated in the kidneys of mice following the induction of
tubulointerstitial fibrosis.
Materials and methods
Renal cell culture
For the in vitro study, human podocytes and
the most widely used proximal and distal tubular epithelial cell
lines were selected. Immortalized human proximal tubular cells
(HK-2 cells) were purchased from the American Type Culture
Collection (Manassas, VA, USA) and canine tubular cells (MDCK
cells; cat. no. 10034) were purchased from the Korean Cell Line
Bank (Seoul, South Korea). HK-2 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM)/Nutrient Mixture F-12 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). MDCK cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.).
Conditionally immortalized human podocytes were provided by Dr Moin
A. Saleem (University of Bristol, Bristol, UK) and Dr Jun Oh
(University Medical Center Hamburg-Eppendorf, Hamburg, Germany).
The podocytes were grown in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.), 10% FBS and insulin-transferrin-selenium
supplement (Gibco; Thermo Fisher Scientific, Inc.) at 33°C to
activate the SV40 large T antigen. The cells were then cultured at
37°C for 2 weeks to induce differentiation (15), which was confirmed by western
blotting for synaptopodin (data not presented).
Animal model of tubulointerstitial
fibrosis
Male CD1 mice of 8 weeks of age (Orient Bio, Inc.,
Seongnam, South Korea) were used for the animal experiments. Mice
were housed at 20°C with a 12-h light/dark cycle and free access to
rodent chow and water. Tubulointerstitial fibrosis was induced in 4
mice (mean body weight, 42.5 g) by intraperitoneal injection of
folic acid (240 µg/g body weight), according to previously
described methods (16,17). The folic acid solution was prepared
by dissolving folic acid powder (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in 0.3 M NaHCO3. Control CD1 mice
(n=4; mean body weight, 43.1 g) were intraperitoneally injected
with the same amount of vehicle (NaHCO3). After 4 weeks,
the mice were sacrificed, and the kidneys were harvested. Fresh
frozen tissues were stored at −70°C subsequent to instant freezing
in liquid nitrogen. Additional tissues were fixed in 4%
formaldehyde for 24 h at room temperature and embedded in paraffin
overnight at 55–65°C using an automatic tissue processer (EFTP-FAST
360; Intelsint, Turin, Italy). The present study was approved by
the Institutional Animal Care and Use Committee of Yonsei
University Health System (Seoul, South Korea).
Western blot analysis of LOXL2
expression
HK-2 cells, MDCK cells and differentiated human
podocytes were lysed in radioimmunoprecipitation assay buffer
(Biosesang, Inc., Seongnam, Korea) containing protease inhibitor
cocktail (Roche Diagnostics, Indianapolis, IN, USA). The samples
were centrifuged at 15,871 × g for 30 min at 4°C, and protein
concentration was measured using the bicinchoninic acid protein
assay kit (Thermo Scientific, Inc.) according to the manufacturer's
protocol. Protein samples (50 µg) were separated by 10% SDS-PAGE
for 2 h at 100 V and were then transferred to a polyvinylidene
fluoride membrane. After blocking with 3% skim milk for 1 h at room
temperature, the membrane was incubated with the following primary
antibodies overnight at 4°C: Anti-synaptopodin (cat. no. sc-21537;
1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-LOXL2 (cat. no. ab96233; 1:500; Abcam, Cambridge, MA, USA) and
anti-LOXL2, which has epitope homology to canine species (cat. no.
TA335061; 1:100; OriGene Technologies, Inc., Rockville, MD, USA).
The membrane was then washed with Tris-buffered saline containing
0.1% Tween-20 and was incubated with horseradish peroxidase-labeled
secondary antibodies (cat. no. sc-2020; 1:5,000; Santa Cruz
Biotechnology, Inc.; and cat. no. K4003; 1:5,000; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 1 h at room
temperature. Protein bands were visualized using Pierce Enhanced
Chemiluminescence Western Blotting Substrate (Thermo Fisher
Scientific, Inc.). After stripping the membrane with Restore
Western Blot Stripping Buffer (Thermo Fisher Scientific, Inc.) for
15 min at room temperature, the membrane was incubated with an
anti-β-actin antibody (cat. no. sc-47778; 1:2,000; Santa Cruz
Biotechnology, Inc.), which was used as a loading control. In
addition, fresh frozen kidneys from vehicle or folic acid-injected
mice were homogenized, and western blotting was performed in a
similar manner. Semi-quantification of the bands was performed by
densitometry using Image J software (version 1.50i; National
Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry and
immunofluorescence analysis of LOXL2 in human and mouse
kidneys
LOXL2 expression in human and mouse kidneys was
evaluated by immunohistochemistry and Olympus BX53 light microscope
(Olympus Corporation, Tokyo, Japan). Paraffin-embedded human kidney
tissues from 4 patients were obtained from the surgical pathology
archive of the Department of Pathology, Yonsei University, Gangnam
Severance Hospital (Seoul, South Korea). These tissues were
obtained from a non-neoplastic portion of a nephrectomy specimen of
a renal tumor. The use of archived human tissue was approved by the
institutional review board of Yonsei University, Gangnam Severance
Hospital. The paraffin-embedded kidneys from the aforementioned
vehicle- and folic acid-injected mice were also used for
immunohistochemical analysis of LOXL2 expression.
Human and mouse kidney tissues were cut into 4-µm
sections, deparaffinized, and rehydrated using xylene and ethanol.
Antigen retrieval was conducted by microwaving the tissue sections
in 0.01 M sodium citrate buffer (pH 6.0) for 10 min. Endogenous
peroxidase activity was blocked with 0.3% hydrogen peroxidase for
10 min. The tissue sections were then incubated overnight with a
primary antibody against LOXL2 (cat. no. ab96233; 1:1,000; Abcam)
at 4°C, followed by incubation with a horseradish
peroxidase-labeled secondary antibody (cat. no. K4003; prediluted;
Dako; Agilent Technologies, Inc.) for 1 h at room temperature. The
protein was visualized using the chromogen diaminobenzidine.
Double immunofluorescence staining for LOXL2 along
with synaptopodin was performed in a similar manner. Sections were
incubated with the primary antibody against LOXL2 (1:100) for 4 h
at room temperature, followed by incubation with a Texas
Red-conjugated anti-rabbit immunoglobulin G (cat. no. TI-1000;
1:50; Vector Laboratories, Inc., Burlingame, CA, USA) overnight at
4°C. Subsequently, the tissue sections were incubated with a
primary antibody against synaptopodin (cat. no. 65294; 1:50; Progen
Biotechnik GmbH, Heidelberg, Germany) for 4 h at room temperature,
followed by incubation with fluorescein isothiocyanate-conjugated
anti-mouse secondary antibody (cat. no. FI-2000; 1:50; Vector
Laboratories, Inc.) overnight at 4°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of LOXL2
expression
The mRNA expression levels of LOXL2 in renal cells
and fresh frozen kidneys from the vehicle- or folic acid-injected
mice were analyzed by RT-qPCR. RNA was extracted using the RNeasy
Mini kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol, and the RNA was reverse transcribed using
the QuantiTect Reverse Transcription kit (Qiagen GmbH) according to
the manufacturer's protocol. PCR amplification was performed using
TaqMan Gene Expression Master Mix and an ABI 7900 HT real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
the following thermal cycle: 2 min at 50°C for uracil
DNA-glycosylase enzyme incubation and 10 min at 95°C for AmpiTaq
Gold enzyme activation, followed by 40 cycles of 15 sec at 95°C and
1 min at 60°C. TaqMan primer/probes for mouse LOXL2 (cat. no.
Mm00804740_m1) and ribosomal 18S RNA (cat. no. Mm03928990_g1) were
purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.).
Expression was calculated using the 2−ΔΔCq method
(18).
Statistical analysis
Quantitative analysis was performed for the western
blotting and RT-qPCR results. Folic acid-injected and
vehicle-treated control groups were compared (n=4 mice/group). Data
are expressed as the mean ± standard deviation and were compared
using the Mann-Whitney U test. The analyses were performed using
GraphPad Prism 6 for Windows (GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
LOXL2 protein expression in human and
mouse kidneys
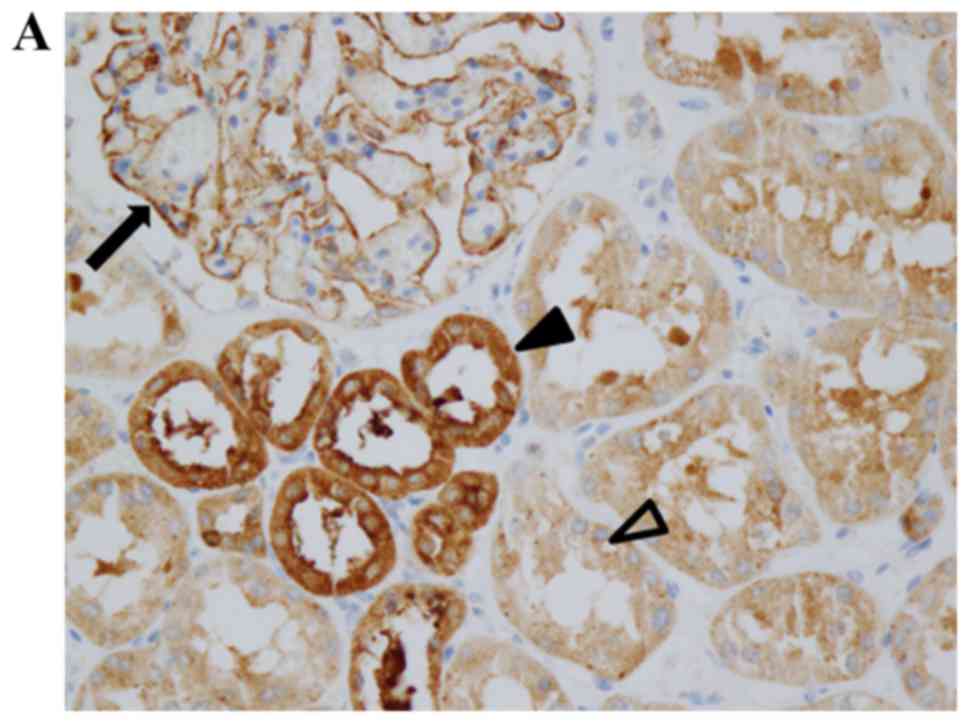
Immunohistochemistry results demonstrated that LOXL2
protein was expressed in the glomeruli and tubular epithelial cells
in human kidneys (Fig. 1A). In the
glomeruli, LOXL2 staining was observed along the outer surface of
capillary loops. In the tubular epithelial cells, LOXL2 staining
was cytoplasmic with no nuclear or membranous staining observed
(Fig. 1A). Proximal and distal
tubules expressed LOXL2; however, more prominent staining was
detected in distal tubular epithelial cells. In the mouse kidney,
LOXL2 staining was also observed in the glomeruli and tubular
epithelial cells (Fig. 1B).
To determine the precise location of LOXL2
expression, double immunofluorescence microscopy, using the
podocyte marker synaptopodin, was performed in the human kidney.
LOXL2 expression was detected in the cytoplasm of podocytes, where
synaptopodin expression was also observed (Fig. 2).
LOXL2 expression in cultured
cells
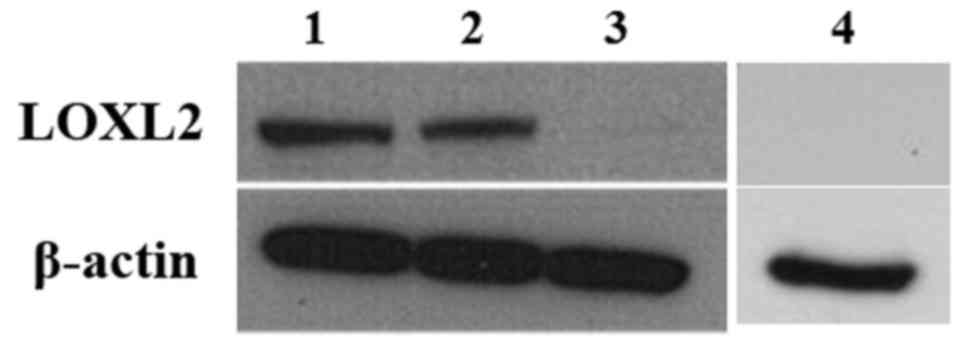
In cultured cell lines, LOXL2 expression was
detected in human podocytes and HK-2 cells, as determined by
western blot analysis, which supported the aforementioned results.
However, MDCK cells, which are tubular cells derived from canine
kidney, did not express LOXL2 (Fig.
3).
LOXL2 expression in a mouse model of
tubulointerstitial fibrosis
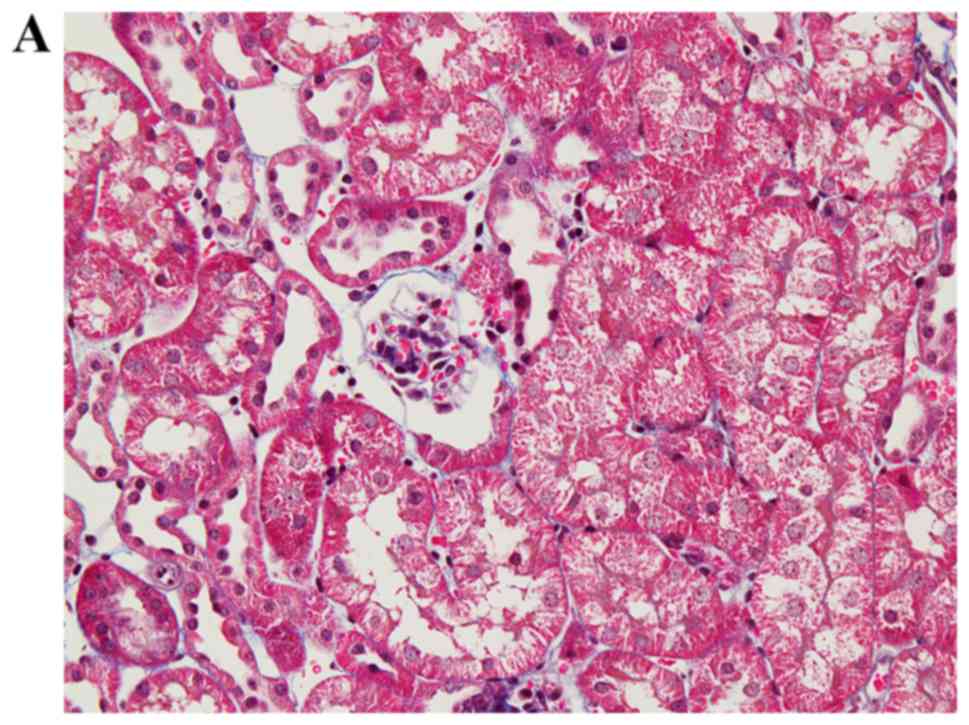
Folic acid injection successfully induced diffuse
renal tubulointerstitial fibrosis in mice (Fig. 4A and B). Immunohistochemistry
analysis of LOXL2 demonstrated strong immunoreactivity in
infiltrating inflammatory cells and the interstitium, in addition
to glomerular and tubular expression (Fig. 4C). RT-qPCR analysis indicated that
the mRNA expression levels of LOXL2 were significantly increased in
folic acid-injected mice compared with vehicle-injected controls
(P=0.029; Fig. 4D). In addition,
as determined by western blotting, the protein expression levels of
LOXL2 were increased in folic acid-injected mice compared with
vehicle-injected mice (P=0.023; Fig.
4E).
Discussion
Tubulointerstitial fibrosis occurs during the
progression of all chronic kidney diseases. Preventing
tubulointerstitial fibrosis, regardless of its etiology, is
important in the preservation of renal function. TGF-β is a key
molecule in the development and progression of tubulointerstitial
fibrosis. Activators of latent TGF-β include integrin αvβ6;
downstream signal transduction molecules of the TGF-β signaling
pathway, such as members of the Smad family and mitogen-activated
protein kinases; and upstream signal transduction molecules.
Contributing signal transduction pathways and mechanisms include
the Wnt/β-catenin pathway, platelet-derived growth factors,
epithelial-mesenchymal transition and autophagy (19). Recently, knowledge regarding the
mechanisms underlying the pathogenesis of tubulointerstitial
fibrosis has increased, and an animal study regarding its
prevention has produced promising results (20). However, few therapeutic agents for
clinical use have been developed. Therefore, a better understanding
of the molecular mechanisms is required for the development of
novel treatment strategies for tubulointerstitial fibrosis.
LOXL2, which serves a role in cancer metastasis, is
also involved in organ fibrosis. Previous studies have demonstrated
the involvement of LOXL2 in hepatic and pulmonary fibrosis, and
clinical trials have evaluated a LOXL2-specific inhibitor (9,11–13).
Although the clinical implications of kidney fibrosis are
substantial, little is currently known regarding LOXL2 expression
in this organ, and the role of LOXL2 in tubulointerstitial fibrosis
remains to be elucidated. Therefore, the present study evaluated
LOXL2 expression in cellular compartments of the kidney and its
possible contribution to tubulointerstitial fibrosis.
A previous study reported that LOXL2 expression in
HK-2 cells was increased by hypoxia and hyperglycemia, and this
alteration was associated with hypoxia inducible factor-1α
(21). The present study revealed
that LOXL2 is primarily expressed in tubular cells in the kidney,
particularly in distal tubular cells. There are numerous mechanisms
by which tubular epithelial cells initiate or contribute to the
progression of tubulointerstitial fibrosis. Following hypoxic,
toxic or immunological insult-induced injury, tubular cells secrete
chemoattractants to induce interstitial inflammation (22). TGF-β1 and type III TGF-β1 receptor
have critical roles in the linkage of inflammation and fibrosis via
the TGF-β/Smad3 signaling pathway (23). Epithelial-mesenchymal transition
may also contribute to interstitial fibrosis by providing a source
of fibrogenic myofibroblasts (24). Tubular epithelial cells lose
cell-cell adhesion and acquire myofibroblast properties through the
induction of TGF-β. Other signaling pathways and cellular
components, including autophagy, Wnt/β-catenin signaling and
integrin-linked kinase, are also involved in this process (25,26).
Although the underlying mechanisms remain unclear, it is highly
probable that tubular epithelial cells have a critical role in the
progression of tubulointerstitial fibrosis. The present observation
that LOXL2 is expressed in tubular epithelial cells suggests a role
for LOXL2 in TGF-β-mediated tubulointerstitial fibrosis. This
hypothesis is supported by the increased LOXL2 mRNA and protein
levels detected in the kidneys of mice with folic acid-induced
tubulointerstitial fibrosis.
The association between LOXL2 and TGF-β has been
investigated in numerous studies. Sethi et al demonstrated
that the expression of LOX family genes, including LOXL2, is
induced by TGF-β1, TGF-β2 and TGF-β3, and is mediated by canonical
Smad signaling and noncanonical signaling pathways (27). Voloshenyuk et al reported
that TGF-β1 upregulates LOX expression in cardiac fibroblasts, and
this phenomenon may be prevented by inhibiting Smad3 (28). Conversely, an inhibitory monoclonal
antibody against LOXL2 has been reported to decrease fibroblast
activation and TGF-β signaling, suggesting that LOXL2 serves a role
in activating TGF-β (13). Direct
suppression of TGF-β was not successful in preventing renal
fibrosis due to of the diversity of TGF-β isoforms and their
signaling pathways (29).
Therefore, it may be suggested that LOXL2, and other molecules
involved in TGF-β signaling pathways, should be considered as
therapeutic targets.
The expression of LOXL2 in infiltrating inflammatory
cells detected in the present study is interesting considering that
infiltrating macrophages also express TGF-β (30). This finding suggested that the
potential mechanisms underlying the effects of LOXL2 in
tubulointerstitial fibrosis are complex.
The present study also detected LOXL2 expression in
podocytes, which may have clinical significance. Considerable
evidence supports the role of podocyte injury as a key factor in
the pathogenesis of focal segmental glomerulosclerosis (31–33).
Podocyte detachment is also an important pathogenic mechanism in
the progression of diabetic nephropathy (34), which is characterized by nodular
glomerulosclerosis. Considering the profibrogenic function of LOXL2
in other organs, it is reasonable to hypothesize that podocyte
LOXL2 may contribute to the progression of glomerulosclerosis.
In conclusion, LOXL2, which is a protein involved in
extracellular matrix remodeling and organ fibrosis, is expressed in
renal tubular epithelial cells and podocytes. Improved
understanding regarding the function of LOXL2 in the kidney may
strengthen knowledge of the pathophysiology of tubulointerstitial
fibrosis and glomerulosclerosis, and may lead to the discovery of
novel therapeutic targets.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Science, ICT and Future Planning (grant
no. NRF-2015R1C1A1A02036671).
References
|
1
|
Nishioka T, Eustace A and West C: Lysyl
oxidase: From basic science to future cancer treatment. Cell Struct
Funct. 37:75–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fong SF, Dietzsch E, Fong KS, Hollosi P,
Asuncion L, He Q, Parker MI and Csiszar K: Lysyl oxidase-like 2
expression is increased in colon and esophageal tumors and
associated with less differentiated colon tumors. Genes Chromosomes
Cancer. 46:644–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin ZY, Chuang YH and Chuang WL:
Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and
LOXL2 genes related to promotion of cancer progression in
hepatocellular carcinoma cells. Biomed Pharmacother. 66:525–529.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peinado H, Moreno-Bueno G, Hardisson D,
Pérez-Gómez E, Santos V, Mendiola M, de Diego JI, Nistal M,
Quintanilla M, Portillo F and Cano A: Lysyl oxidase-like 2 as a new
poor prognosis marker of squamous cell carcinomas. Cancer Res.
68:4541–4550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahn SG, Dong SM, Oshima A, Kim WH, Lee HM,
Lee SA, Kwon SH, Lee JH, Lee JM, Jeong J, et al: LOXL2 expression
is associated with invasiveness and negatively influences survival
in breast cancer patients. Breast Cancer Res Treat. 141:89–99.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasashima H, Yashiro M, Kinoshita H,
Fukuoka T, Morisaki T, Masuda G, Sakurai K, Kubo N, Ohira M and
Hirakawa K: Lysyl oxidase-like 2 (LOXL2) from stromal fibroblasts
stimulates the progression of gastric cancer. Cancer Lett.
354:438–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker HE, Bird D, Lang G and Erler JT:
Tumor-secreted LOXL2 activates fibroblasts through FAK signaling.
Mol Cancer Res. 11:1425–1436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barker HE, Chang J, Cox TR, Lang G, Bird
D, Nicolau M, Evans HR, Gartland A and Erler JT: LOXL2-mediated
matrix remodeling in metastasis and mammary gland involution.
Cancer Res. 71:1561–1572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vadasz Z, Kessler O, Akiri G,
Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB and Neufeld G:
Abnormal deposition of collagen around hepatocytes in Wilson's
disease is associated with hepatocyte specific expression of lysyl
oxidase and lysyl oxidase like protein-2. J Hepatol. 43:499–507.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong CC, Tse AP, Huang YP, Zhu YT, Chiu
DK, Lai RK, Au SL, Kai AK, Lee JM, Wei LL, et al: Lysyl
oxidase-like 2 is critical to tumor microenvironment and metastatic
niche formation in hepatocellular carcinoma. Hepatology.
60:1645–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chien JW, Richards TJ, Gibson KF, Zhang Y,
Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N
and O'Riordan T: Serum lysyl oxidase-like 2 levels and idiopathic
pulmonary fibrosis disease progression. Eur Respir J. 43:1430–1438.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Bergen T, Marshall D, Van de Veire S,
Vandewalle E, Moons L, Herman J, Smith V and Stalmans I: The role
of LOX and LOXL2 in scar formation after glaucoma surgery. Invest
Ophthalmol Vis Sci. 54:5788–5796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barry-Hamilton V, Spangler R, Marshall D,
McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien
H, Wai C, et al: Allosteric inhibition of lysyl oxidase-like-2
impedes the development of a pathologic microenvironment. Nat Med.
16:1009–1017. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meissner EG, McLaughlin M, Matthews L,
Gharib AM, Wood BJ, Levy E, Sinkus R, Virtaneva K, Sturdevant D,
Martens C, et al: Simtuzumab treatment of advanced liver fibrosis
in HIV and HCV-infected adults: Results of a 6-month open-label
safety trial. Liver Int. 36:1783–1792. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saleem MA, O'Hare MJ, Reiser J, Coward RJ,
Inward CD, Farren T, Xing CY, Ni L, Mathieson PW and Mundel P: A
conditionally immortalized human podocyte cell line demonstrating
nephrin and podocin expression. J Am Soc Nephrol. 13:630–638.
2002.PubMed/NCBI
|
|
16
|
Long DA, Woolf AS, Suda T and Yuan HT:
Increased renal angiopoietin-1 expression in folic acid-induced
nephrotoxicity in mice. J Am Soc Nephrol. 12:2721–2731.
2001.PubMed/NCBI
|
|
17
|
Stallons LJ, Whitaker RM and Schnellmann
RG: Suppressed mitochondrial biogenesis in folic acid-induced acute
kidney injury and early fibrosis. Toxicol Lett. 224:326–332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-beta: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marek I, Lichtneger T, Cordasic N, Hilgers
KF, Volkert G, Fahlbusch F, Rascher W, Hartner A and
Menendez-Castro C: Alpha8 integrin (Itga8) signalling attenuates
chronic renal interstitial fibrosis by reducing fibroblast
activation, not by interfering with regulation of cell turnover.
PLoS One. 11:e01504712016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sumual S, Saad S, Tang O, Yong R, McGinn
S, Chen XM and Pollock CA: Differential regulation of Snail by
hypoxia and hyperglycemia in human proximal tubule cells. Int J
Biochem Cell Biol. 42:1689–1697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
Inflammatory processes in renal fibrosis. Nat Rev Nephrol.
10:493–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng XM, Huang XR, Xiao J, Chen HY, Zhong
X, Chung AC and Lan HY: Diverse roles of TGF-b receptor II in renal
fibrosis and inflammation in vivo and in vitro. J Pathol.
227:175–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mack M and Yanagita M: Origin of
myofibroblasts and cellular events triggering fibrosis. Kidney Int.
87:297–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao L, Wang M, Yang S, Liu F and Sun L: A
glimpse of the pathogenetic mechanisms of Wnt/b-catenin signaling
in diabetic nephropathy. Biomed Res Int. 2013:9870642013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang M, Wang H, Rao P, Zhao Y, Xie J, Cao
Q, Wang Y, Wang YM, Lee VW, Alexander SI, et al: Autophagy links
beta-catenin and Smad signaling to promote epithelial-mesenchymal
transition via upregulation of integrin linked kinase. Int J
Biochem Cell Biol. 76:123–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sethi A, Mao W, Wordinger RJ and Clark AF:
Transforming growth factor-beta induces extracellular matrix
protein cross-linking lysyl oxidase (LOX) genes in human trabecular
meshwork cells. Invest Ophthalmol Vis Sci. 52:5240–5250. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Voloshenyuk TG, Landesman ES, Khoutorova
E, Hart AD and Gardner JD: Induction of cardiac fibroblast lysyl
oxidase by TGF-b1 requires PI3K/Akt, Smad3, and MAPK signaling.
Cytokine. 55:90–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neelisetty S, Alford C, Reynolds K,
Woodbury L, Nlandu-Khodo S, Yang H, Fogo AB, Hao CM, Harris RC,
Zent R and Gewin L: Renal fibrosis is not reduced by blocking
transforming growth factor-b signaling in matrix-producing
interstitial cells. Kidney Int. 88:503–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MG, Kim SC, Ko YS, Lee HY, Jo SK and
Cho W: The role of M2 macrophages in the progression of chronic
kidney disease following acute kidney injury. PLoS One.
10:e01439612015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang JW, Pardo V, Sageshima J, Chen L,
Tsai HL, Reiser J, Wei C, Ciancio G, Burke GW III and Fornoni A:
Podocyte foot process effacement in postreperfusion allograft
biopsies correlates with early recurrence of proteinuria in focal
segmental glomerulosclerosis. Transplantation. 93:1238–1244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alachkar N, Wei C, Arend LJ, Jackson AM,
Racusen LC, Fornoni A, Burke G, Rabb H, Kakkad K, Reiser J and
Estrella MM: Podocyte effacement closely links to suPAR levels at
time of posttransplantation focal segmental glomerulosclerosis
occurrence and improves with therapy. Transplantation. 96:649–656.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wharram BL, Goyal M, Wiggins JE, Sanden
SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman
LB and Wiggins RC: Podocyte depletion causes glomerulosclerosis:
Diphtheria toxin-induced podocyte depletion in rats expressing
human diphtheria toxin receptor transgene. J Am Soc Nephrol.
16:2941–2952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maezawa Y, Takemoto M and Yokote K: Cell
biology of diabetic nephropathy: Roles of endothelial cells,
tubulointerstitial cells and podocytes. J Diabetes Investig.
6:3–15. 2015. View Article : Google Scholar : PubMed/NCBI
|