Introduction
Acute kidney injury (AKI) is a common complication
of sepsis, which increases mortality rates to as high as 70%
(1). However, the pathophysiology
of sepsis-induced AKI remains to be fully elucidated (2). Excessive fission and/or insufficient
fusion of mitochondria, which contributes to the progression of
sepsis, may be evoked by oxidative stress, and can lead to the loss
of mitochondrial function and the apoptosis of tubular cells under
stress (3,4).
Human trypsin inhibitor (urinary trypsin inhibitor;
UTI) is a Kunitz-type protease inhibitor, which inhibits the
activity or release of lysosomal enzymes (5,6). The
effects of UTI include the protection of endothelial cells,
suppression of excessive superoxide anion radicals, and reductions
in systemic inflammation and oxidative stress (7). It has been reported that UTI can
protect mitochondria from ischemia functionally and
morphologically, thus benefiting renal function (8,9).
However, the mechanism remains to be elucidated.
In the present study, it was hypothesized that UTI
may have beneficial effects on regulating mitochondrial dynamics.
To confirm this hypothesis, the levels of the mitochondrial fission
protein, death-associated protein kinase 2 (DAPK-2), and two types
of mitochondrial fusion proteins, mitofusin-1 (Mfn1) and
mitofusin-2 (Mfn2), were examined in lipopolysaccharide
(LPS)-induced human kidney-2 (HK-2) cells incubated with or without
UTI. The oxidative activities of inflammatory cytokines, indicated
by maleic dialdehyde (MDA) and superoxide dismutase (SOD), and cell
apoptosis were also measured.
Materials and methods
Drugs, reagents and kits
LPS from Escherichia coli 055:B5 and
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) were obtained from Sigma-Aldrich; Merck Millipore (Darmstadt,
Germany). UTI was purchased from Techpool Bio-pharma Co., Ltd.
(Guangzhou, China). MitoProbe J-aggregate (JC-1;
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanineiodide),
propidium iodide (PI), the Annexin V-FITC Apoptosis Detection kit,
SOD assay kit (cat. no. KGT00150-1) and MDA assay kit (cat. no.
KGT004) were obtained from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). The ATPlite 1 step Luminescence ATP Detection
Assay system was obtained from PerkinElmer, Inc. (Waltham, MA,
USA). The following primary antibodies were used: Caspase-3 (cat.
no. SC-7272; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), caspase-9 (cat. no. 9502S; 1:1,000; CST Biological Reagents
Company Ltd., Shanghai, China), PARP (cat. no. ab96476; 1:1,000;
Abcam, Cambridge, UK), B-cell lymphoma (Bcl)-2 (cat. no. 2870S;
1:1,000; CST Biological Reagents Company Ltd.), Bcl-extra large
(Bcl-xL; cat. no. 2764; 1:1,000; CST Biological Reagents Company
Ltd.), β-actin (cat. no. AP0060; 1:3,000; Bioworld Technology,
Inc., St Louis Park, MN, USA), DAPK-2 (cat. no. 3432–1; 1:1,000;
Epitomics; Abcam), Mfn1 (cat. no. 5870–1; 1:1,000; Epitomics;
Abcam) and Mfn2 (cat. no. 3272–1; 1:1,000; Epitomics; Abcam).
Secondary antibody (horseradish peroxidase-labeled goat anti-mouse
immunoglobulin G) was obtained from Abgent Biotech Co., Ltd. (cat.
no. LP1002a; 1:10,000; Suzhou, China).
Cell culture
The HK-2 cells, an immortalized proximal tubular
epithelial cell line from the normal adult human kidney (10), were obtained from American Type
Culture Collection (Manassas, VA, USA). The HK-2 cells were
cultured in Dulbecco's modified Eagle's medium F-12 (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA; cat. no. SH30023.01)
supplemented with 10% fetal bovine serum (cat. no. 10099-141;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an
atmosphere of 5% CO2 at 37°C.
LPS administration
This procedure was performed to determine the
appropriate concentration of LPS to use in the experiments. The
cell culture medium was replaced with complete medium supplemented
with 0.5% fetal bovine serum and various concentrations of LPS (0,
100, 200, 400, 800, 1,600 and 3,200 ng/ml). Each sample was
incubated for different durations (3, 6, 12 and 24 h) at a density
of 2,500 cells/well at 37°C, which was performed three times.
Cell viability assay
The cell viability was assessed using a commercial
MTT-based assay. This assay detects viable cells based on the
production of the purple compound, formazan, in viable cells.
Following incubation with LPS or LPS+UTI, 10 µl of MTT (5 mg/ml)
was added to each well, and the plates were incubated at 37°C for 4
h. The content of the wells was eluted and the precipitate was
dissolved with 150 µl of MTT solubilization reagent (dimethyl
sulfoxide). The optical density value (OD value) was read at a
wavelength of 490 nm using the Model 680 Microplate Reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell viability was
determined as the ratio of surviving cells in each group divided by
the number in the control ethanol-treated group. The LPS
concentration shown to reduce cell viability the most, but affect
the surrounding environment of the cells the least, was
selected.
UTI administration
This procedure was performed to determine the
appropriate concentrations of UTI to use in the experiments. The
cell culture media were replaced with complete media supplemented
with 10% fetal bovine serum containing LPS at a final concentration
of 800 ng/ml (based on the results of the LPS intervention
experiment) and various concentrations of UTI (100, 150, 200, 250,
300, 350 and 400 U/ml). In the control group, neither LPS nor UTI
was administered. The cell viability assay was then repeated for
selection of the UTI concentrations causing a significant increase
in cell viability.
Groups
Based on the results of the UTI intervention
experiment, the HK-2 cells were randomly divided into a control
group, LPS group and UTI group. The cells were treated with LPS at
a final concentration of 800 ng/ml in the LPS group, and 800 ng/ml
LPS together with 250 U/ml UTI in the UTI group. All cells were
cultured at a density of 2,500 cells/well and maintained at 37°C
for 24 h.
Measurement of inflammatory response
and oxidative activity
Enzyme-linked immunosorbent assay (ELISA) kits were
used in this procedure. Following treatment of cells with LPS and
UTI, as described above, the cell supernatants were collected
(centrifuged at 12,000 × g for 5 min at 37°C) and stored at −80°C.
The levels of inflammatory cytokines interleukin (IL)-6 and tumor
necrosis factor-α (TNF-α) were measured to evaluate the
inflammatory response. SOD and MDA were measured to estimate the
oxidative activities. In all cases, a standard curve was
constructed from standards provided by the manufacturer.
Analysis of mitochondrial
fission/fusion and apoptotic proteins
Western blot analysis was performed to detect the
levels of total proteins. Following treatment with LPS and UTI, as
described above, the cells were lysed using
radioimmunoprecipitation assay buffer [0.6% SDS, 4% glycerine,
12.5% Tris-Hcl (1 M; pH 6.8), H2O], and the
concentrations of total proteins in each sample were measured using
a BCA protein assay kit. The proteins (50 µg/lane) were separated
using SDS-PAGE on a 10% gel and transferred onto PVDF membranes;
the membranes were then blocked in 5% fat-free milk at room
temperature for 2 h. Following incubation with primary antibodies
against caspase-3, caspase-9, PARP, Bcl-2, Bcl-xL and β-actin, and
primary antibodies against DAPK-2, Mfn 1 and Mfn 2 at a dilution of
1:1,000 at 4°C overnight, the membranes were probed with
goat-anti-mouse secondary antibodies at a dilution of 1:10,000 at
37°C for 1 h. The signals were detected and then analyzed using
SensiAnsys software (version JS-680D; Shanghai Peiqing Science
& Technology Co., Ltd., Shanghai, China).
Mitochondrial membrane potential (MMP)
analysis
The fluorescent probe JC-1 was used to detect the
MMP. Following treatment of the cells with LPS and UTI, as
described above, the culture medium was removed and
1–2×106 cells were harvested by trypsinization with
0.25% Trypsin-EDTA. The cells were washed twice with PBS and
centrifuged at 170 × g for 5 min at room temperature. For each
sample, the cells were suspended in 500 µl of JC-1 stock solution
(final concentration, 2 mg/ml; diluted by 1X incubation buffer) and
then incubated for 15–20 min at 37°C. The staining procedure was
performed according to the manufacture's protocol. The stained
cells were then centrifuged at 700 × g for 5 min at room
temperature, washed twice in 1X incubation buffer and re-suspended
in 500 µl 1X incubation buffer. JC-1 aggregates (red fluorescence)
favor high MMP intact cells and, in response to the loss of MMP,
JC-1 monomers are formed showing green fluorescence. The percentage
of cells showing a decrease in red fluorescence was evaluated to
determine the extent of depolarization. The fluorescence intensity
of the red/green ratio was determined semi-quantitatively using
flow cytometry (Beckman Coulter, Inc., Brea, CA, USA). Cells with
collapsed MMPs exhibited a decrease in the red/green fluorescence
intensity ratio.
Measurement of intracellular ATP
Intracellular levels of ATP were measured using a
luminescence ATP detection assay according to the manufacturer's
protocol. Following treatment of the cells with LPS and UTI, as
described above, and equilibrating the microplate at room
temperature for 30 min, 100 µl of the reconstituted lyophilized
substrate solution was added to each well. Each lyophilized
substrate solution vial was reconstituted with 10 ml substrate
buffer and agitated gently until homogenous. The 96-well microplate
was then shaken for 5 min, and the relative light unit (RLU) was
measured using the JANUS® Automated Workstation
(PerkinElmer, Inc.) within 10 min in the dark.
Cell apoptosis assay
The Annexin V-FITC kit was used to detect the
externalization of phosphatidylserine of the cell membrane, one of
the typical markers of early apoptosis. Following treatment of
cells with LPS and UTI as described above, the culture medium was
removed and 1–5×105 cells were harvested by
trypsinization without Trypsin-EDTA, washed twice with PBS and
centrifuged at 700 × g for 10 min at room temperature. Then the
cells were re-suspended in 500 µl of binding buffer. Subsequently,
5 µl Annexin V-FITC and 5 µl of propidium iodide (PI) were added
into each 500 µl of solution according to the manufacturer's
protocol. The cells were then gently vortexed and incubated for
5–15 min at room temperature in the dark. Flow cytometry (Beckman
Coulter, Inc.) was used to analyze the samples within 1 h. The
excitation wavelength was 488 nm and the emission wavelength was
530 nm. The percentage of Annexin V(+)/PI(−) and Annexin V(+)/PI(+)
cells represent the ratios of early and late stage of apoptosis
respectively.
Statistical analysis
All experiments were performed three times. All
numerical data are expressed as the mean ± standard deviation.
Multiple comparison between the groups was performed using one-way
analysis of variance and the least significant difference method.
SPSS 22.0 software (IBM SPSS, Armonk, NY, USA) was used. P<0.05
was considered to indicate a statistically significant difference.
All statistical graphs were produced using OriginPro 9.0 (OriginLab
Corporation, Northampton, MA, USA).
Results
Cell viability
As shown in Fig.
1A, cell viability was reduced as LPS concentration increased.
LPS at concentrations of 1,600 and 3,200 ng/ml caused a rapid
reduction in cell viability in all groups, which may have resulted
from the high osmotic pressure induced by the high drug
concentration on the surrounding environment of the cells. In the 3
and 6 h groups, no significant changes in cell viability were
observed with LPS concentrations of 50–800 ng/ml. In the 12 and 24
h groups, cell viability was significantly decreased with LPS
concentrations of 50–800 ng/ml (P<0.05, vs. control) the lowest
level was observed at 800 ng/ml in the 24 h group. Based on these
results, the HK-2 cells were treated with LPS at 800 ng/ml for 24 h
in the subsequent experiments.
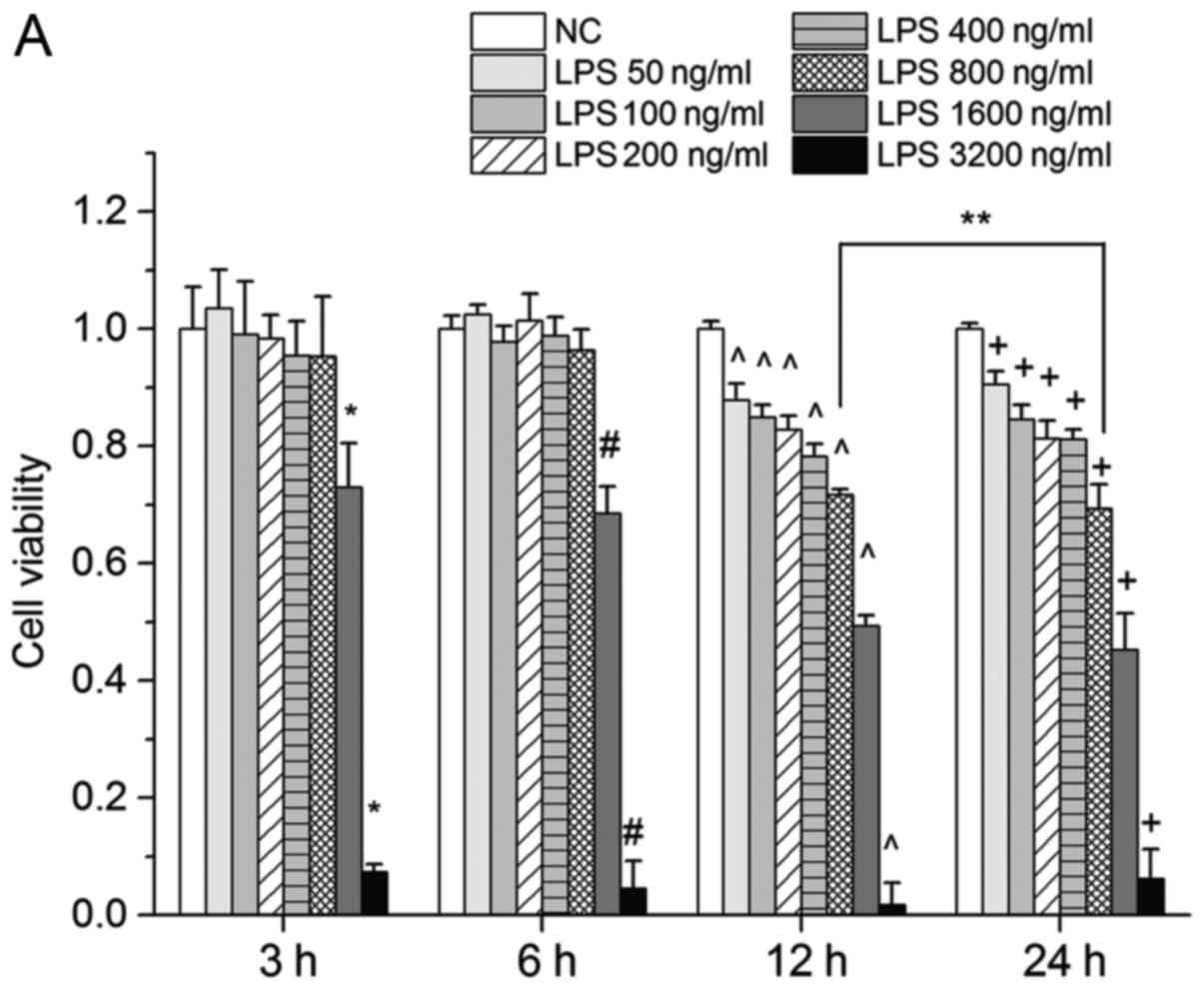 | Figure 1.(A) Effects of LPS on HK-2 cells. Cell
viability is presented as relative MTT activity and determined as
1-ODLPS/ODNC. Cell viability decreased as LPS
concentration increased. When treated with LPS for 3 and 6 h, no
significant change in cell viability was observed. When treated
with LPS at 1,600 and 3,200 mmol/l, cell viability was markedly
reduced. Values are expressed as the mean ± standard deviation of
triplicate experiments. *P<0.05, vs. NC (3 h),
#P<0.05, vs. NC (6 h), ^P<0.05, vs. NC
(12 h), +P<0.05, vs. NC (24 h), **P<0.05 between
groups. (B) Effects of UTI co-treated with LPS (800 ng/ml) in HK-2
cells. Cell viability was presented as relative MTT activity and
determined as 1-ODUTI/ODNC. Cell viability
was positively correlated with UTI concentrations of 100–200 U/ml,
whereas a rapid decrease was observed at concentrations >250
U/ml. The data are presented as the mean ± standard deviation of
triplicate experiments. *P<0.05, vs. NC; **P<0.05, vs. LPS
group. LPS, lipopolysaccharide; UTI, human trypsin inhibitor; NC,
negative control; OD, optical density; MTT, 3-(4,
5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide. |
As shown in Fig.
1B, following co-treatment with different concentrations of UTI
for 24 h, cell viability was positively correlated with drug
concentrations at 100–200 U/ml, but decreased rapidly at
concentrations >250 U/ml, which suggested that concentrations of
UTI >250 U/ml led to an excessive increase in osmotic pressure.
No significant differences in cell viability were observed between
concentrations of UTI at 250 and 200 U/ml. Therefore, 250 U/ml of
UTI was used to evaluate its protective function in subsequent
experiments.
Release of inflammatory cytokines and
oxidative factors induced by LPS is reduced by UTI
As shown in Fig. 2A and
B, following treatment with LPS for 24 h, significant increases
in the release of IL-6 and TNF-α were detected in the HK-2 cells
(P<0.05, vs. control). In the UTI group, the increase in these
two inflammatory cytokines were significantly lower (P<0.05 vs.
LPS group). The oxidation product, MDA, was significantly
increased, whereas the antioxidant enzyme, SOD, was decreased
following treatment with LPS (P<0.05 vs. control). These effects
were less marked in the UTI group (P<0.05 vs. LPS group;
Fig. 2C and D).
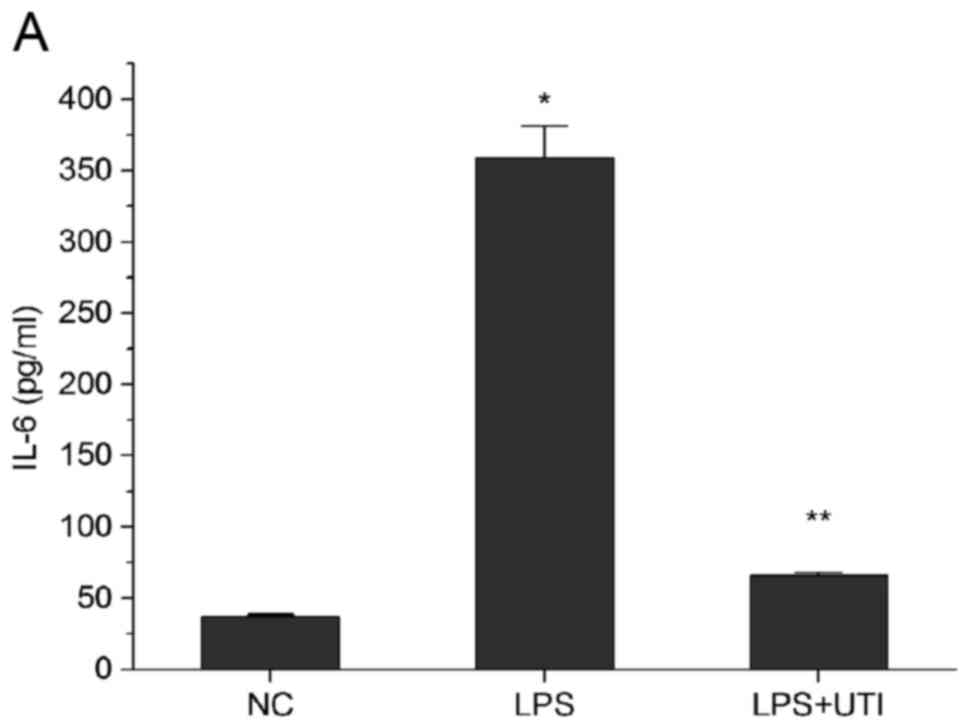 | Figure 2.Release of inflammatory cytokines and
oxidative factors were reduced by UTI. Expression of inflammatory
cytokines (A) IL-6, (B) TNF-α, the (C) oxidation product MDA and
(D) antioxidant SOD were detected using enzyme-linked immunosorbent
assay kits. Expression levels of IL-6 and TNF-α were lowerin the
UTI group, compared with those in the LPS group. Expression of MDA
was higherand that of SOD was lowerin the UTI group. Data are
presented as the mean ± standard deviation of triplicate
experiments. *P<0.05, vs. NC; **P<0.05, vs. LPS group. LPS,
lipopolysaccharide; UTI, human trypsin inhibitor; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α; NC, negative
control; MDA, maleic dialdehyde; SOD, superoxide dismutase. |
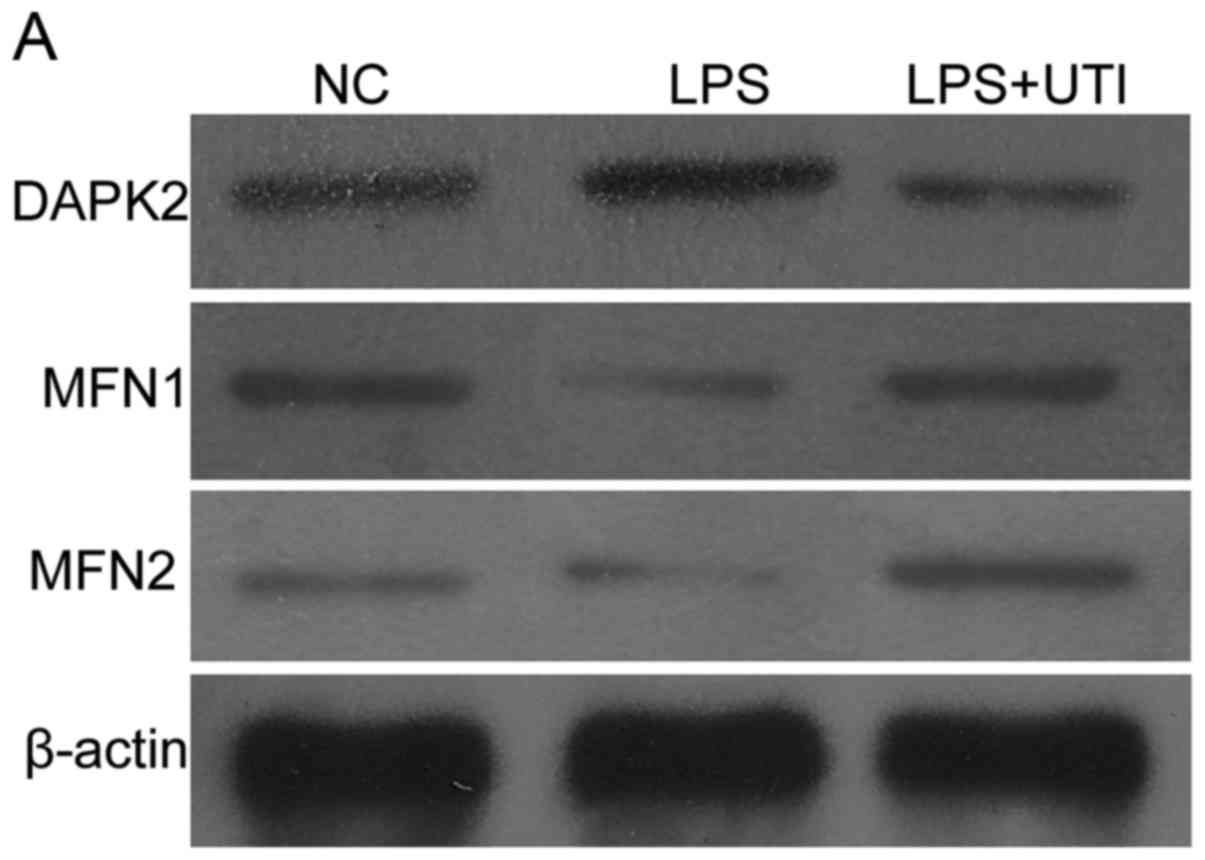
LPS-induced increase in mitochondrial
fission and reduction in mitochondrial fusion are reversed by
UTI
As shown in Fig. 3,
the expression of DAPK2 was increased, and the levels of Mfn1 and
Mfn2 were decreased in the LPS group (P<0.05 vs. control). These
changes suggested that LPS induced increased fission and weakened
fusion of mitochondria. In the cells co-treated with UTI, decreased
expression of DAPK2, and enhanced expression of Mfn1 and Mfn2 were
found, compared with the levels in the UTI group (P<0.05, vs.
LPS group). These changes indicated that UTI increased
mitochondrial fusion and limited mitochondrial fission.
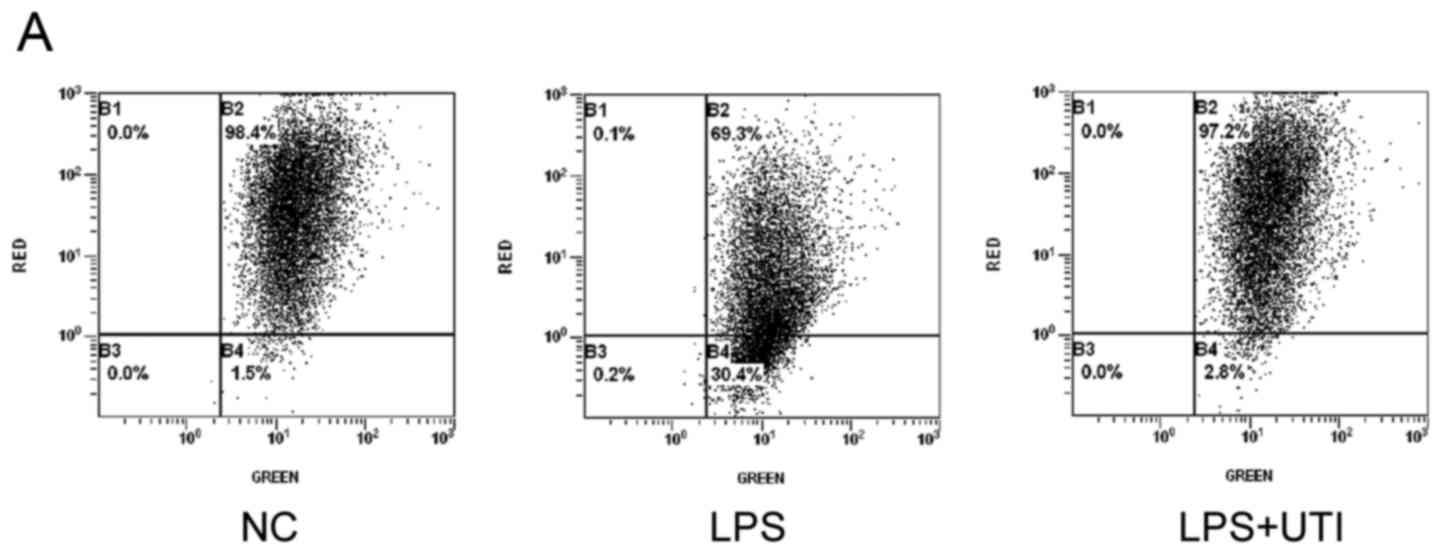
LPS-induced decreases in MMP and
intracellular ATP are reversed by UTI
As shown in Fig. 4A and
B, LPS stimulation led to fewer high red JC-1-positive cells
(69.4±0.75%) compared with the control (98.1±0.3; P<0.05), which
indicated a decrease in MMP. The decrease in MMP was less marked in
the UTI group, compared with that in the LPS group (97.23±0.25 vs.
69.4±0.75%; P<0.05), which indicated amore stabilized
mitochondrial membrane. As presented in Fig. 4C, RLU was significantly decreased
following treatment with LPS for 24 h (0.12±0.05 vs. 0.33±0.03;
P<0.05), compared with that in the negative control, and this
reduction was reversed in the UTI group, compared with that in the
LPS group (0.22±0.04 vs. 0.12±0.05; P<0.05). These results
indicated that co-treatment with UTI prevented the mitochondrial
dysfunction caused by LPS.
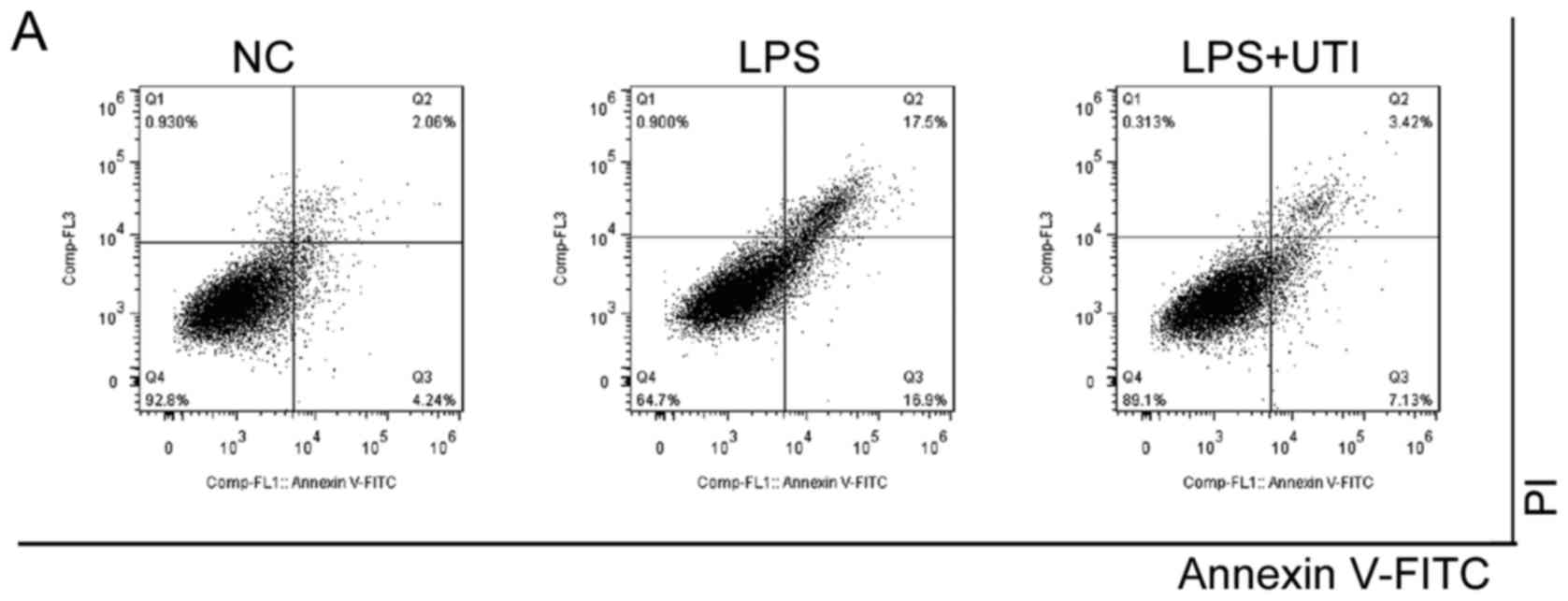
LPS-induced cell apoptosis is
inhibited by UTI
As shown in Fig.
1B, cell viability was preserved in the UTI groups, compared
with that in the LPS group (P<0.05), although UTI concentrations
>250 U/ml led to a descending trend of cell viability. This
suggested that co-treatment with UTI prevented the cell apoptosis
induced by LPS.
The hypothesis of the present study was confirmed
using Annexin V and PI staining. As shown in Fig. 5, the increase in the percentage of
Annexin V-positive cells was marked in the LPS group (34.42±0.64%),
compared with that in the negative control (6.47±0.17; P<0.05),
whereas cell apoptosis was inhibited following co-treatment with
UTI (10.52±0.24), compared with the LPS group (34.42±0.64%;
P<0.05).
As shown in Fig. 6,
significantly higher expression levels of cytochrome c,
caspase-3 and caspase-9 were observed in the HK-2 cells following
incubation with LPS for 24 h (P<0.05, vs. control), whereas
lower expression levels of cytochrome c, caspase-3 and
caspase-9 were observed in the LPS+UTI group. In addition, as shown
in Fig. 7A-C, the protein levels
of Bcl-2 and Bcl-xL were lower in HK-2 cells treated with LPS
(P<0.05 vs. control), where as their expression levels were
increased in the LPS+UTI group (P<0.05). As shown in Fig. 7D, cleavage of PARP was reduced in
the LPS group (Fig. 7D; P<0.05,
vs. control), but levels of PARP were increased in the UTI group
(P<0.05, vs. LPS group).
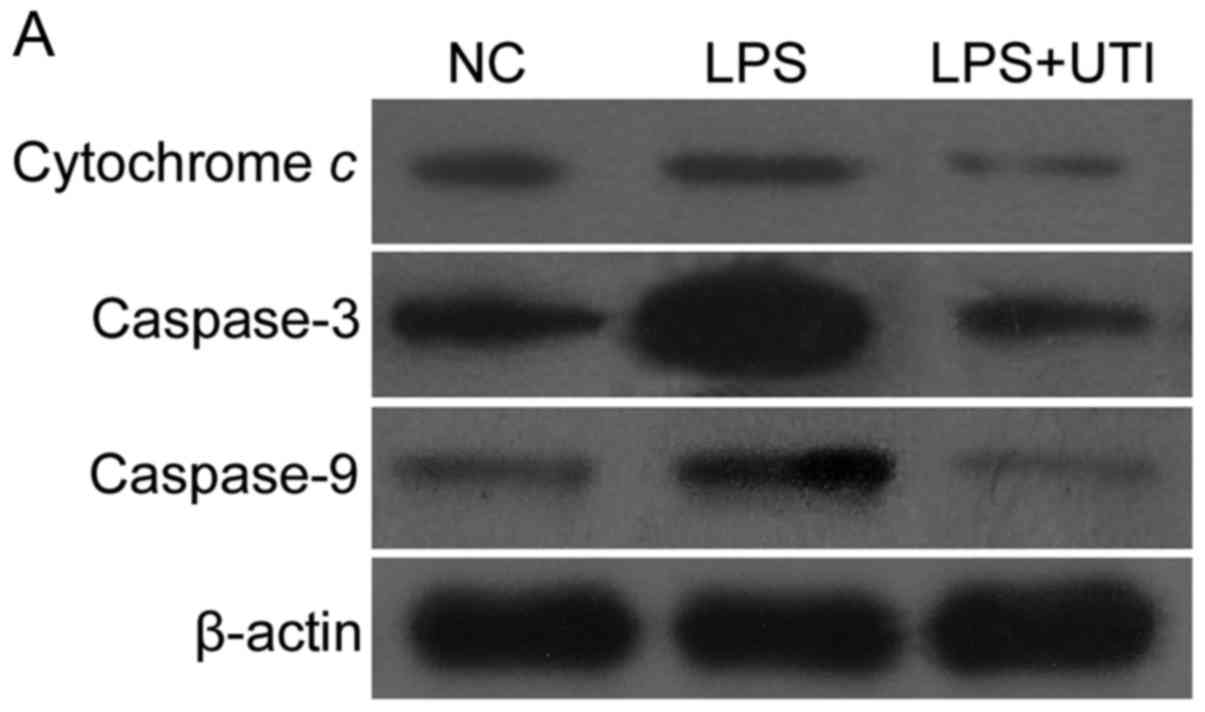 | Figure 6.Expression levels of caspase-3,
caspase-9 and cytochrome c in LPS-induced HK-2 cells are
downregulated by co-treatment with UTI. (A) Expression levels of
caspase-3, caspase-9 and cytochrome c were analyzed using
immunoblotting. The expression ratios of (B) caspase-3, (C)
caspase-9 and (D) cytochrome c were quantitatively
determined using densitometry. The expression of (B) caspase-3, (C)
caspase-9 and (D) cytochrome c increased following treatment
with LPS. In the UTI group, expression levels were decreased. Data
are presented as the mean ± standard deviation of triplicate
experiments. *P<0.05, vs. NC; **P<0.05, vs. LPS group, LPS,
lipopolysaccharide; UTI, human trypsin inhibitor; NC, negative
control. |
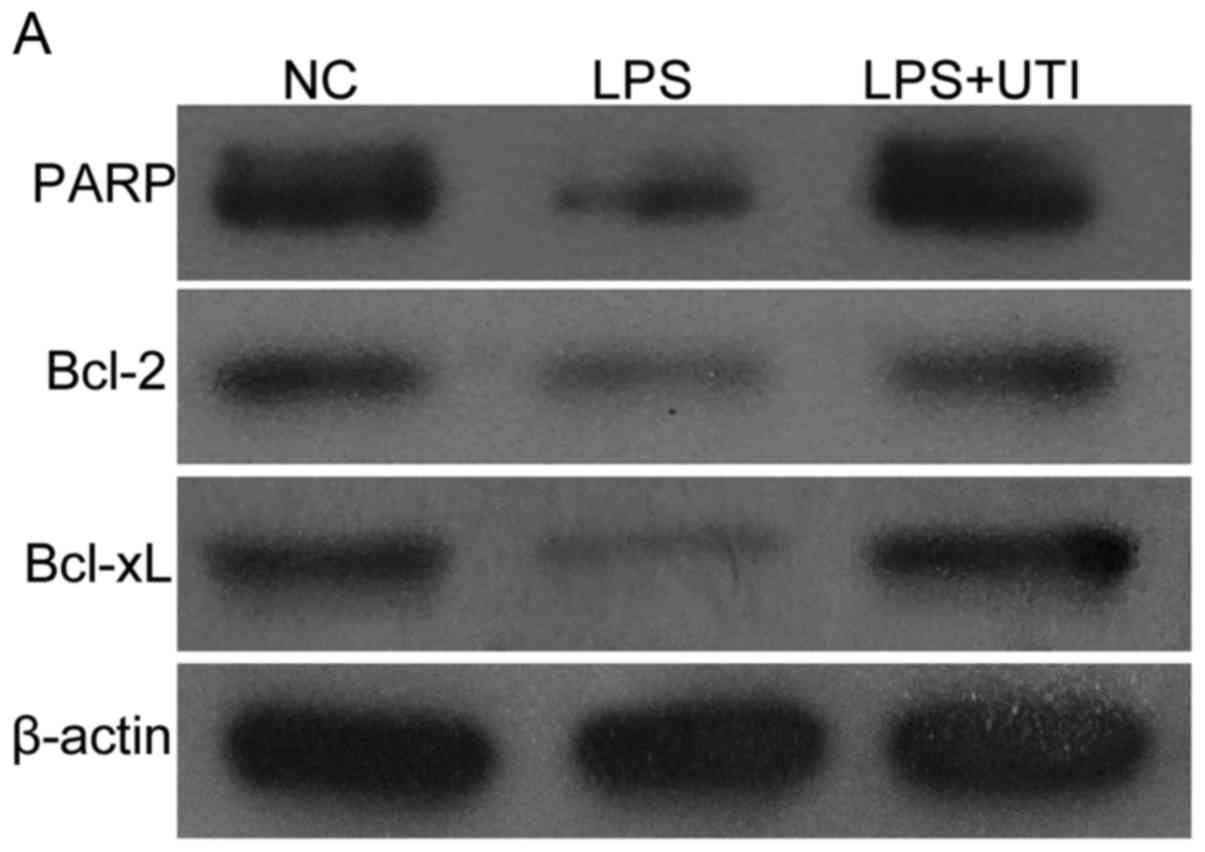 | Figure 7.Expression levels of Bcl-2, Bcl-xL and
PARP in LPS-induced HK-2 cells are upregulated by co-treatment with
UTI. (A) Expression levels of Bcl-2, Bcl-xL and PARP were analyzed
using immunoblotting. The ratios of (B) Bcl-2, (C) Bcl-xL and (D)
PARP were quantitatively determined using densitometry. The
expression levels of (B) Bcl-2, (C) Bcl-xL and (D) PARP were
decreased following treatment with LPS. In the UTI group,
expression levels were increased. Data are presented as the mean ±
standard deviation of triplicate experiments. *P<0.05, vs. NC;
**P<0.05, vs. LPS group. LPS, lipopolysaccharide; UTI, human
trypsin inhibitor; NC, negative control; Bcl-2, B-cell lymphoma-2;
Bcl-xL, Bcl-extra large; PARP, poly ADP-ribose polymerase. |
Discussion
In previous studies, it was found that neither
hemodynamic failure nor ischemia were the primary pathogenic
factors in septic renal damage. Lerolle et al analyzed
postmortem histopathological findings of 19 patients with septic
AKI and reported that tubular cell apoptosis was the major
histological abnormality. Even in patients with severe septic
shock, tubular necrosis was minimal (11). It was reported that inflammation
and renal cellular apoptosis were the essential factors (12,13).
Mitochondria are dynamic organelles, which
constantly undergo fission and fusion, during which they may
exhibit a tubular or fragmented morphotype, or they may be
assembled into networks in response to cellular energy demands and
environmental challenges (14,15).
In mammalian cells, DAPK2 (also known as Drp1) is one of the
primary mitochondrial fission proteins, and the large mitochondrial
GTPases, Mfn1 and Mfn2 are important in mitochondrial fusion
(16). The suppression of Drp-1
has been reported to maintain mitochondrial function and reduce the
apoptosis of tubular cells induced by ischemic injury,
rhabdomyolysis and cisplatin nephrotoxin (17,18).
MFN2 deficiency has been shown to promote Bcl-2-associated X
protein-mediated mitochondrial outer membrane injury and increase
the apoptosis of tubule epithelial cells under stress (19). These findings indicate that the
regulation of mitochondrial dynamics may be one approach in
protecting mitochondrial function and reducing tubule epithelial
cell apoptosis. However, there have been no studies focusing on the
mitochondria dynamics of tubule epithelial cells in septic AKI.
In the present ex vivo study, it was
confirmed that excessive mitochondrial fission and insufficient
mitochondrial fusion were present in LPS-induced HK-2 cells.
Following stimulation with LPS, the HK-2 cells showed significantly
increased expression of DAPK2, and decreases in the expression
levels of Mfn1 and Mfn2, indicating an increase of mitochondrial
fission and decrease of mitochondrial fusion. Decreased
mitochondrial dysfunction and cell apoptosis were also detected, in
addition to increased levels of cytochrome c, caspase-3
andcaspase-9, and decreased levels of Bcl-2, Bcl-xL and PARP.
It has been shown previously that UTI can suppress
the excessive generation of superoxide anion radicals, systemic
inflammation, oxidative stress and endothelial injury caused by
endotoxins (7), particularly
against LPS-induced kidney injury (20). In a previous rat model of ischemia,
UTI was shown to have protective effects on mitochondria in the
kidney (8,9).
In the present study, when co-treated with UTI, the
HK-2 cells showed higher expression levels of Mfn1 and Mfn2, and
lower expression levels of Drp1, suggesting a positive role of UTI
in preventing excessive mitochondrial fission. Mitochondrial
dysfunction and cell apoptosis were also decreased as a subsequent
effect. As previous studies have already shown that oxidative
stress and inflammation can induce disordered mitochondrial fission
(21,22), the present study hypothesized that
the regulatory effect of UTI on mitochondrial dynamics may function
by suppressing the oxidative and inflammatory damage caused by LPS.
The decreased levels of IL-6, TNF-α and MDA, and the increased
expression of SOD support this hypothesis.
Taken together, the results of the present study
suggested that UTI protected human tubular epithelial cells from
apoptosis by preventing the excessive mitochondrial fission induced
by LPS. The effect may result from its antioxidant and
anti-inflammatory effects. Future investigations are required to
evaluate the effect of UTI on mitochondrial dynamics in an animal
model of sepsis.
In conclusion, the present study showed that UTI
prevented mitochondrial dysfunction caused by LPS by promoting
mitochondrial fusion and limiting mitochondrial fission, thus
reducing the apoptosis of LPS-induced HK-2 cells. This protective
effect may be accomplished through decreasing the release of
inflammatory cytokines and limiting oxidative activities.
Acknowledgements
This study was part of the Program of The National
Natural Science Foundation of China (grant no. 81201452).
Glossary
Abbreviations
Abbreviations:
|
UTI
|
human trypsin inhibitor
|
|
LPS
|
lipopolysaccharide
|
|
AKI
|
acute kidney injury
|
|
HK-2
|
human kidney 2
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-6
|
interleukin 6
|
|
MDA
|
maleic dialdehyde
|
|
SOD
|
superoxide dismutase
|
|
DAPK-2
|
death-associated protein kinase 2
|
|
Mfn1
|
mitofusin-1
|
|
Mfn2
|
mitofusin-2
|
|
MMP
|
mitochondrial membrane potential
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bcl-xL
|
B-cell lymphoma-extra large
|
|
PARP
|
poly ADP-ribose polymerase
|
|
MTT
|
3-(4, 5-dimethyl-2-thiazolyl)-2,
5-diphenyl-2-H-tetrazolium bromide
|
|
OD
|
optical density
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
NC
|
negative control
|
References
|
1
|
Schrier RW and Wang W: Acute renal failure
and sepsis. N Engl J Med. 351:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Russell JA: Management of sepsis. N Engl J
Med. 355:1699–1713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gonzalez AS, Elguero ME, Finocchietto P,
Holod S, Romorini L, Miriuka SG, Peralta JG, Poderoso JJ and
Carreras MC: Abnormal mitochondrial fusion-fission balance
contributes to the progression of experimental sepsis. Free Radic
Res. 48:769–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jendrach M, Mai S, Pohl S, Vöth M and
Bereiter-Hahn J: Short- and long-term alterations of mitochondrial
morphology, dynamics and mtDNA after transient oxidative stress.
Mitochondrion. 8:293–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Binns OA, DeLima NF, Buchanan SA, Mauney
MC, Cope JT, Thies SD, Shockey KS, Tribble CG and Kron IL:
Neutrophil endopeptidase inhibitor improves pulmonary function
during reperfusion after eighteen-hour preservation. J Thorac
Cardiovasc Surg. 112:607–613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirose J, Ozawa T, Miura T, Isaji M, Nagao
Y, Yamashiro K, Nii A, Kato K and Uemura A: Human neutrophil
elastase degrades inter-alpha-trypsin inhibitor to liberate urinary
trypsin inhibitor related proteins. Biol Pharm Bull. 21:651–656.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka R, Fujita M, Tsuruta R, Fujimoto K,
Aki HS, Kumagai K, Aoki T, Kobayashi A, Izumi T, Kasaoka S, et al:
Urinary trypsin inhibitor suppresses excessive generation of
superoxide anion radical, systemic inflammation, oxidative stress
and endothelial injury in endotoxemic rats. Inflamm Res.
59:597–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueki M, Yokono S and Ogli K: Effects of
ulinastation on rat renal energy metabolism and blood flow in
hemorrhagic shock. J Anesth. 9:65–69. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taie S, Yokono S, Ueki M and Ogli K:
Effects of ulinastatin (urinary trypsin inhibitor) on ATP,
intracellular pH, andintracellular sodium transients during
ischemia and reperfusion in the rat kidney in vivo. J Anesth.
15:33–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryan MJ, Johnson G, Kirk J, Fuerstenberg
SM, Zager RA and Torok-Storb B: HK-2: An immortalized proximal
tubule epithelial cell line from normal adult human kidney. Kidney
Int. 45:48–57. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lerolle N, Nochy D, Guérot E, Bruneval P,
Fagon JY, Diehl JL and Hill G: Histopathology of septic shock
induced acute kidney injury: Apoptosis and leukocytic infiltration.
Intensive Care Med. 36:471–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacobs R, Honore PM, Joannes-Boyau O, Boer
W, De Regt J, De Waele E, Collin V and Spapen HD: Septic acute
kidney injury: The culprit is inflammatory apoptosis rather than
ischemic necrosis. Blood Purif. 32:262–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koçkara A and Kayataş M: Renal cell
apoptosis and new treatment options in sepsis-induced acute kidney
injury. Ren Fail. 35:291–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nunnari J, Wong ED, Meeusen S and Wagner
JA: Studying the behavior of mitochondria. Methods Enzymol.
351:381–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palmer CS, Osellame LD, Stojanovski D and
Ryan MT: The regulation of mitochondrial morphology: Intricate
mechanisms and dynamic machinery. Cell Signal. 23:1534–1545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang WX, Wu WH, Qiu HY, Bo H and Huang SM:
Amelioration of rhabdomyolysis-induced renal mitochondrial injury
and apoptosis through suppression of Drp-1 translocation. J
Nephrol. 26:1073–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Jiang F, Chen Y, Luo J, Liu S,
Zhang B, Ye Z, Wang W, Liang X and Shi W: Necrostatin-1 attenuates
ischemia injury induced cell death in rat tubular cell line NRK-52E
through decreased Drp1 expression. Int J Mol Sci. 14:24742–24754.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gall JM, Wang Z, Liesa M, Molina A, Havasi
A, Schwartz JH, Shirihai O, Borkan SC and Bonegio RG: Role of
mitofusin 2 in the renal stress response. PLoS One. 7:e310742012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ueki M, Taie S, Chujo K, Asaga T, Iwanaga
Y, Ono J and Maekawa N: Urinary trypsin inhibitor reduces
inflammatory response in kidney induced by lipopolysaccharide. J
Biosci Bioeng. 104:315–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu S, Zhou F, Zhang Z and Xing D:
Mitochondrial oxidative stress causes mitochondrial fragmentation
via differential modulation of mitochondrial fission-fusion
proteins. FEBS J. 278:941–954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Motori E, Puyal J, Toni N, Ghanem A,
Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann
KK, Götz M, et al: Inflammation-induced alteration of astrocyte
mitochondrial dynamics requires autophagy for mitochondrial network
maintenance. Cell Metab. 18:844–859. 2013. View Article : Google Scholar : PubMed/NCBI
|