Introduction
Congenital heart defects occur in ~1% of newborn
children and can be divided into two main groups: Cyanotic
congenital heart defects (CCHD) and acyanotic congenital heart
defects (ACHD) (1). Patients with
CCHD often presents with cyanosis that results from systemic
hypoxia due to deoxygenated blood bypassing the pulmonary
circulation, which is caused by heart structural defects including
tetralogy of Fallot (2). The heart
of a patient with CCHD is also chronically perfused with hypoxic
blood. However, cardiac failure rarely occurs in infants with CCHD,
who can survive for a period of time and resist the
hypoxic-ischemic challenge caused by cardiac surgery (3). An exploration of the underlying
mechanism by which the hearts of children with CCHD adapt to
long-term hypoxia may improve cardioprotection in infants with
CCHD, but remains to be fully elucidated.
The endoplasmic reticulum (ER), a vital organelle
responsible for the correct three-dimensional folding of newly
translated proteins into their native conformation, is vulnerable
to exogenous toxicants (4) and
suboptimal cellular microenvironments, including hypoxia (5). The abnormity of energy metabolism
caused by inadequate oxygen can adversely affect ER protein folding
and initiate ER stress, and sustained and strong ER stress leads to
cell apoptosis. However, cells can initiate the unfolded protein
response (UPR) to defuse the crisis of ER stress by reducing the
generation of new unfolded proteins, facilitating the refolding of
denatured proteins and promoting the degradation of misfolded
proteins (6).
A previous study (7) noted the enhanced UPR process in
myocardial samples from patients with chronic hypoxic CCHD. The
levels of the 78 kDa glucose-regulated protein (GRP78), a major
indicator and molecular chaperone of UPR, were higher in cyanotic
patients. In addition, among the classic three ER stress sensors,
the activating transcription factor 6 (ATF6) pathway was markedly
activated in patients with CCHD. In unstressed cells, GRP78 binds
to ATF6, which is maintained in an inactive 90 kDa form (ATF6-p90).
Upon initial ER stress, the insufficient GRP78 releases ATF6-p90,
which is translocated from the ER to the Golgi apparatus where it
is cleaved into a 50 kDa ATF6-p50 fragment that enters to the
nucleus to mediate the transcriptional activation of several genes
that ameliorate ER stress, including GRP78 (8). Similarly, another study also reported
that enhanced GRP78 and ATF6 protected the heart from ischemic
damage (9). However, little is
known about the endogenous signals that control GRP78 and ATF6
genes expression, particularly in the hypoxic/ischemic heart.
A number of studies (10,11)
suggest the importance of microRNAs (miRNAs) for the regulation of
gene expression in cardiac tissues under physiological and
pathological conditions, and during the heart development process.
A previous study also implicated miRNAs in the regulation of UPR
(12). Therefore, the present
study sought to identify the miRNAs involved in the regulation of
UPR-associated gene expression, including GRP78 and ATF6, within
the hypoxic myocardium. miRNA-199a-5p (miR-199a-5p) has been
reported to be an abundant miRNA species expressed in myocardium
(13,14). The downregulation of miR-199a-5p
has been reported to serve an important role in preventing cardiac
hypertrophy and subsequent heart failure (15). miR-199a-5p is also involved in
pathogenesis of selenium deficiency cardiomyopathy (Keshan disease)
(16). Several lines of evidence
suggest that miR-199a-5p is sensitive to oxygen tension and that
its levels decrease rapidly following hypoxia. Hypoxia-inducible
factor-1α (HIF-1α) is a validated target of miR-199a-5p in various
cells (17). Furthermore,
miR-199a-5p downregulation also results in the upregulation of
sirtuin-1, which in turn contributes to HIF-1α stabilization
(18).
Using bioinformatics prediction programs based on
the human genome, it was previously identified that oxysensitive
miR-199a-5p can simultaneously interact with the 3′-untranslated
region (3′-UTR) of GRP78 and ATF6, which has been identified in
cell types including prostate cancer cells and monocytes from
patients with α-1-antitrypsin deficiency (19,20).
It is well established that miRNAs are not only expressed
differentially, but also act differentially in a tissue- and
disease-specific manner (21,22).
To the best of our knowledge, there have been no reports on
miR-199a-5p-mediated influences on UPR-associated molecules in
cardiomyocytes during either normoxia or hypoxia.
The aim of the present study was to determine
whether changes in miR-199a-5p serve an important role in
regulating UPR in the hearts of patients with CCHD. Initially, the
levels of miR-199a-5p and the effect on ATF6 and GRP78 expression
in cardiomyocytes from patients with CCHD were evaluated.
Subsequently, using an in vitro human cardiomyocyte culture
model under normoxic and hypoxic conditions, the biological effects
of miR-199a-5p in promoting UPR and apoptosis inhibition by
effecting ATF6 and GRP78 expression were demonstrated. The results
may demonstrate a novel mechanism for cardioprotection in CCHD.
Materials and methods
Patients
Subjects with congenital heart disease were
recruited from the Department of Cardiovascular Surgery, Xinqiao
Hospital of Third Military Medical University (Chongqing, China)
between 2013 and 2014. The two separate cohorts comprised 17
patients with a diagnosis of CCHD and 15 ACHD individuals. Clinical
characteristics of the patients are summarized in Table I. The parents of all subjects
provided written informed consent and the study protocol was
approved by the Ethics Committee of the Third Military Medical
University. During cardiac surgery, ventricular specimens were
collected from the right ventricular outflow tract immediately
following cardiopulmonary bypass. Each specimen was immediately
placed in liquid nitrogen for protein and RNA extraction.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | Acyanotic
(n=15) | Cyanotic
(n=17) |
|---|
| Age at surgery
(year)a | 6.7 (2.6–17) | 7.5 (2.9–16) |
| Weight at surgery
(kg)a | 17.6
(12.8–52.8) | 15.7
(11.4–49.2) |
| Artery oxygen
saturationb | 97.3±1.3 |
84.4±5.5d |
| Hemoglobin
(g/dl)b | 12.6±1.1 |
16.9±1.8d |
|
Diagnosesc |
|
Ventricular septal defect | 6 | 0 |
| Atrial
septal defect | 4 | 0 |
|
Tetralogy of Fallot | 0 | 17 |
|
Pulmonary atresia with
ventricular septal defect | 2 | 0 |
|
Pulmonary arteriovenous
malformation | 3 | 0 |
Cell culture
Human cardiac myocytes (HCMs; cat. no. C12810) from
normal ventricle tissue of the adult heart along with the Myocytes
Growth Medium kit (cat. no. C22070) were purchased from PromoCell
GmbH (Heidelberg, Germany). The HCMs express markers of cardiac
differentiation and possess proliferation capacity. The culture
system contained final concentrations of 0.5 ng/ml epidermal growth
factor, 2 ng/ml basic fibroblast growth factor, 5 µg/ml insulin, 5%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 0.5% penicillin/streptomycin. Cells were maintained at
37°C under normoxia (21% O2, 5% CO2 and 74%
N2), moderate hypoxia (3% O2, 5% CO2 and 92%
N2) or severe hypoxia (1% O2, 5% CO2 and 94%
N2). The medium was changed 24 h after seeding and then every other
day. Accutase (Innovative Cell Technologies, Inc., San Diego, CA,
USA) was used to detach HCMs from culture plates for subsequent
analysis. HEK293T cells (American Type Culture Collection, Manassas
VA, USA) used for transfection were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% fetal bovine serum under 5% CO2 and 37°C.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA and small RNA-enriched RNA fractions were
extracted using RNAiso (Takara Biotechnology Co., Ltd., Dalian,
China) and the PureLink miRNA Isolating kit (Invitrogen; Thermo
Fisher Scientific, Inc.), respectively. RNA samples were reverse
transcribed using the PrimeScript® RT reagent kit
(Takara Biotechnology Co., Ltd.). RT-qPCR was performed to analyze
ATF6 and GRP78 mRNA expression using the Power SYBR Green PCR
Master Mix kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following PCR primers were used in the experiments: i)
GRP78, forward 5′-GATAATCAACCAACTGTTAC-3′ and reverse
5′-GTATCCTCTTCACCAGTTGG-3′; ii) ATF6, forward
5′-TGAACTTCGAGGATGGGTTC-3′ and reverse 5′-TCACTCCCTGAGTTCCTGCT-3′
and iii) GAPDH, forward 5′-CGGATTTGGTCGTATTGGG-3′ and reverse
5′-TCTCGCTCCTGGAAGATGG-3′. Mature miR-199a-5p levels were measured
using the GeneCopoeia All-in-One miRNA qRT-PCR Detection kit
(GeneCopoeia, Inc., Rockville, MD, USA). GAPDH mRNA and U6 snRNA
were used as loading controls. Relative expression analysis was
performed using the 2−ΔΔCq method (23).
Transfection of miR-199a-5p mimic and
inhibitor
The miR-199a-5p mimic, miR-199a-5p inhibitor and
negative controls were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequences were as follows: Has-miR-199a-5p
mimic, 5′-CCCAGUGUUCAGACUACCUGUUC-3′ (Sense),
5′-ACAGGUAGUCUGAACACUGGGUU-3′ (Anti-sense); mimic negative control,
5′-UUCUCCGAACGUGUCACGUTT-3′ (Sense), 5′-ACGUGACACGUUCGGAGAATT-3′
(Anti-sense); Has-miR-199a-5p inhibitor,
5′-GAACAGGUAGUCUGAACACUGGG-3′; and inhibitor negative control,
5′-CAGUACUUUUGUGUAGUACAA-3′. HCM cells and HEK293 cells were
transfected 24 h after seeding into 6-well plates at a density of
1.2×105 cells/well. Transfection of miR-199a-5p mimic
(100 nM) and inhibitor (100 nM) with a negative control were
performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 6 h, the transfection solution was removed
and replaced with 2 ml fresh medium. Transfection efficiency
(>90%) was validated by RT-qPCR prior to proceeding to further
experiments (data not shown).
Western blot analysis
Rabbit polyclonal antibodies against GRP78 and GAPDH
(cat. nos. ab108613 and ab9485; Abcam, Cambridge, MA, USA), C/EBP
homologous protein (CHOP), ATF6-p90 and ATF6-p50 (cat. nos. sc793
and sc14253; Santa Cruz Biotechnology, Dallas, TX, USA) were used
as primary antibodies. Total protein was extracted using a
radioimmunoprecipitation assay lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology, Haimen, China), and a
bicinchoninic acid concentration measurement kit (cat. no. P0012;
Beyotime Institute of Biotechnology) was used for the protein
determination. A 5% gel for concentration and an 8% gel for
separation were selected for SDS-PAGE, and 50–70 µg total protein
was loaded per lane. For blotting, the proteins were transferred to
polyvinylidene difluoride membranes by wet transfer. The free sites
were blocked with 5% non-fat milk powder for 1 h at room
temperature, and the membrane was incubated in primary antibody
diluted (1:1,000) in Primary Antibody Dilution Buffer (cat. no.
P0023A; Beyotime Institute of Biotechnology) at 4°C overnight.
Following washing 3 times for 10 min in TBS with Tween, the
membranes were incubated with a horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin G secondary antibody (cat. no.
A0208; diluted 1:1,000; Beyotime Institute of Biotechnology) for 1
h at 37°C. Protein bands were visualized using a Pierce Fast
Western Blot kit, ECL Substrate (cat. no. 35055; Thermo Fisher
Scientific, Inc.). The intensity of the individual bands was
measured with a ChemiDoc XRS system using the Quantity One (version
4.6.3; Bio-Rad Laboratories, Inc., Hercules, CA, USA) or ImageJ
(version 1.45s; National Institutes of Health, Bethesda, MD, USA)
software.
Apoptosis assay
An Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (BD Biosciences Pharmingen, San Diego, CA, USA) was
used according to the manufacturer's protocol. Immunofluorescent
staining of cells was analyzed by flow cytometry (Beckman Coulter,
Brea, CA, USA).
Luciferase reporter plasmid
transfection
The 3′-UTR of ATF6 or GRP78 containing a
miR-199a-5p-binding site amplified from HCM cell cDNA or the mutant
UTRs with 6-bp deletions in the binding site (positions 2–7 of the
seed region) was cloned into the Xba1 site of pGL3-promoter
vector (Promega Corporation, Madison, WI, USA). The primers were
used for the cloning were reported previously by Dai et al
(24): GRP78 3′UTR, forward
5′-TCTAGACTTTTCATTAGCAGTTGCTCACA-3′ and reverse
5′-TCTAGACCCAACATACCAAATACTCCCTC-3′; ATF6 3′UTR, forward
5′-TCTAGAGATCAATGGGCAGGACTACGA-3′ and reverse
5′-TCTAGACCAAATAGATGGGTAGATGATGAAA-3′. The deletion mutations of
the 3′-UTR were introduced by the design of suitable PCR primers.
The PCR experiments and construction of the 3′UTR-containing
plasmid based on pmiR-RB-Report™ were completed by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). The constructed vectors were
co-transfected with miR-199a-5p mimics into human embryonic kidney
(HEK) 293T cells or HCM cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Empty vector and pRL-TK (Promega
Corporation) were added as the negative and internal controls.
Cells were lysed after 48 h of transfection and the activities of
the firefly and Renilla luciferases were measured
consecutively using the Dual-Luciferase Reporter Assay System
(Promega Corporation).
Statistical analysis
Results are presented as the mean ± standard
deviation. Differences were evaluated by the unpaired Student's
t-test for comparing the means of the two groups or one-way
analysis of variance followed by a least significance difference
test for multiple comparisons. Statistical analyses were performed
with SPSS software version 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-199a-5p and its
potential targets differ in CCHD and ACHD myocardial tissues
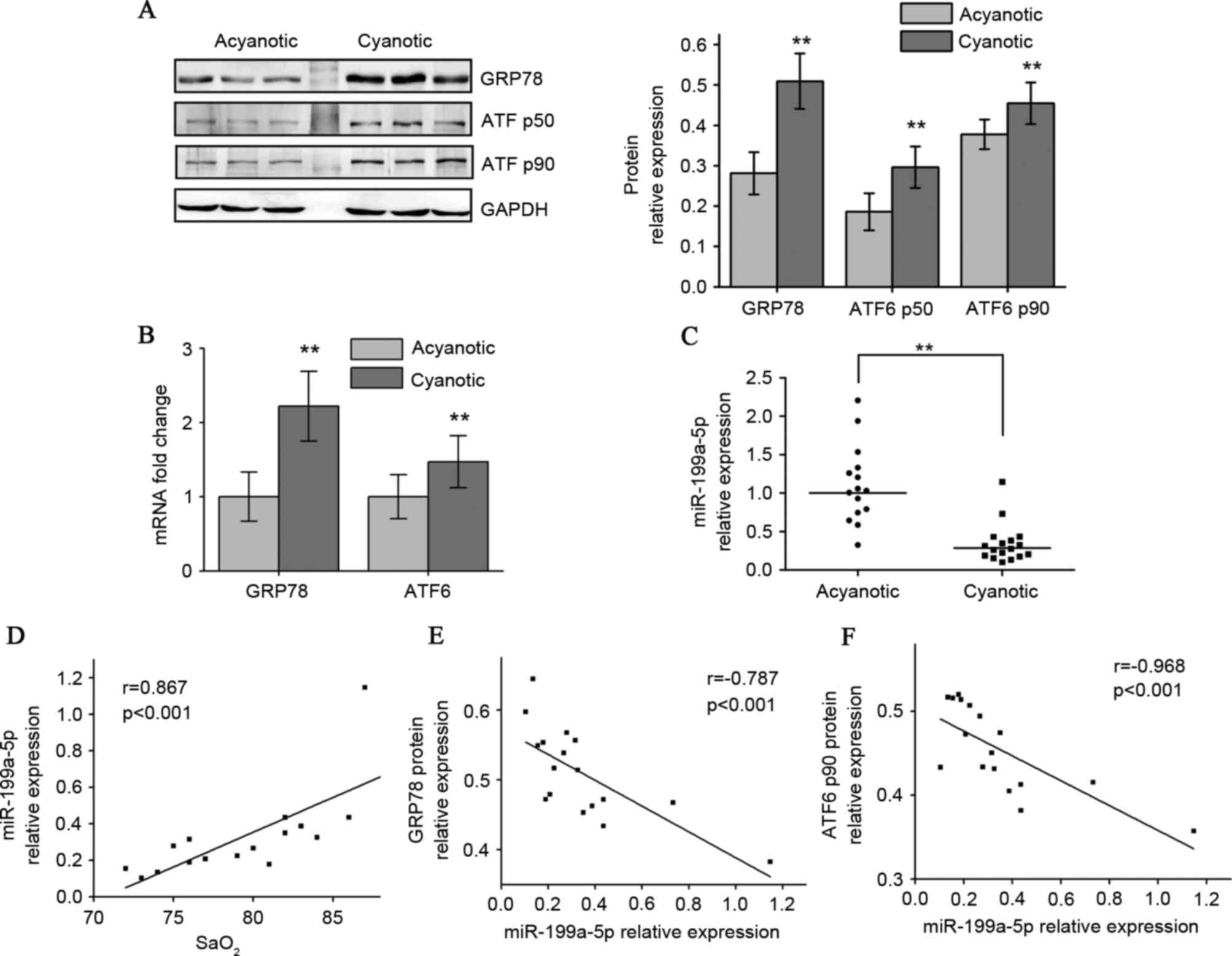
Western blot analysis indicated that the CCHD
cardiac specimens exhibited higher GRP78 protein levels compared
with the ACHD group. Adaptation to chronic hypoxia in CCHD hearts
also resulted in higher p90 full-length ATF6 (native translational
form) and p50 cleaved ATF6 (active form) protein levels (Fig. 1A). RT-qPCR results indicated that
the CCHD group exhibited a significant increase in GRP78 and ATF6
mRNA levels compared with the ACHD group (Fig. 1B). A 5-fold downregulation in
miR-199a-5p levels in CCHD patients was also observed (Fig. 1C). Among the CCHD group, myocardial
miR-199a-5p expression correlated well with arterial oxygen
saturation (SaO2), indicating that the degree of hypoxia
was associated with decreased miR-199a-5p (Fig. 1D). When exploring whether
miR-199a-5p may be clinically relevant to GRP78 and ATF6
expression, an inverse correlation between miR-199a-5p levels and
these two protein levels in CCHD patients was identified (Fig. 1E and F).
Moderate hypoxia enhances
anti-apoptotic UPR and downregulates miR-199a-5p in cultured human
cardiomyocytes
Subsequently, suitable in vitro models were
established to simulate CCHD by culturing HCM cells in different
oxygen environments (21, 3 and 1% O2) for 5 days. The
results demonstrated that Annexin V+ apoptotic rates
markedly increased under 1% O2 hypoxia compared with 21%
O2 normoxia; however, the apoptotic rate did not
significantly increase at 3% O2 compared with 21%
O2 (Fig. 2A).
Additionally, the protein expression of CHOP, a pro-apoptotic
transcription factor induced by ER stress, increased significantly
following culture in 1% O2 conditions, but was not
significantly altered by 3% O2 compared with normoxic
conditions (Fig. 2B). Notably,
GRP78 expression at the mRNA and protein levels was significantly
higher under 3% O2 compared with 1% O2
culture. ATF6 expression was increased by hypoxia (3 and 1%)
compared with 21% O2, and not different between the 3
and 1% O2 groups (Fig. 2B
and C). This suggested that 3% O2 may invoke the
protective roles of UPR under hypoxia, while 1% O2
resulted in decompensated ER stress and cell apoptosis. Thus, it
was hypothesized that moderate in vitro hypoxia (3%
O2) represented the pathological process observed in
patients with CCHD.
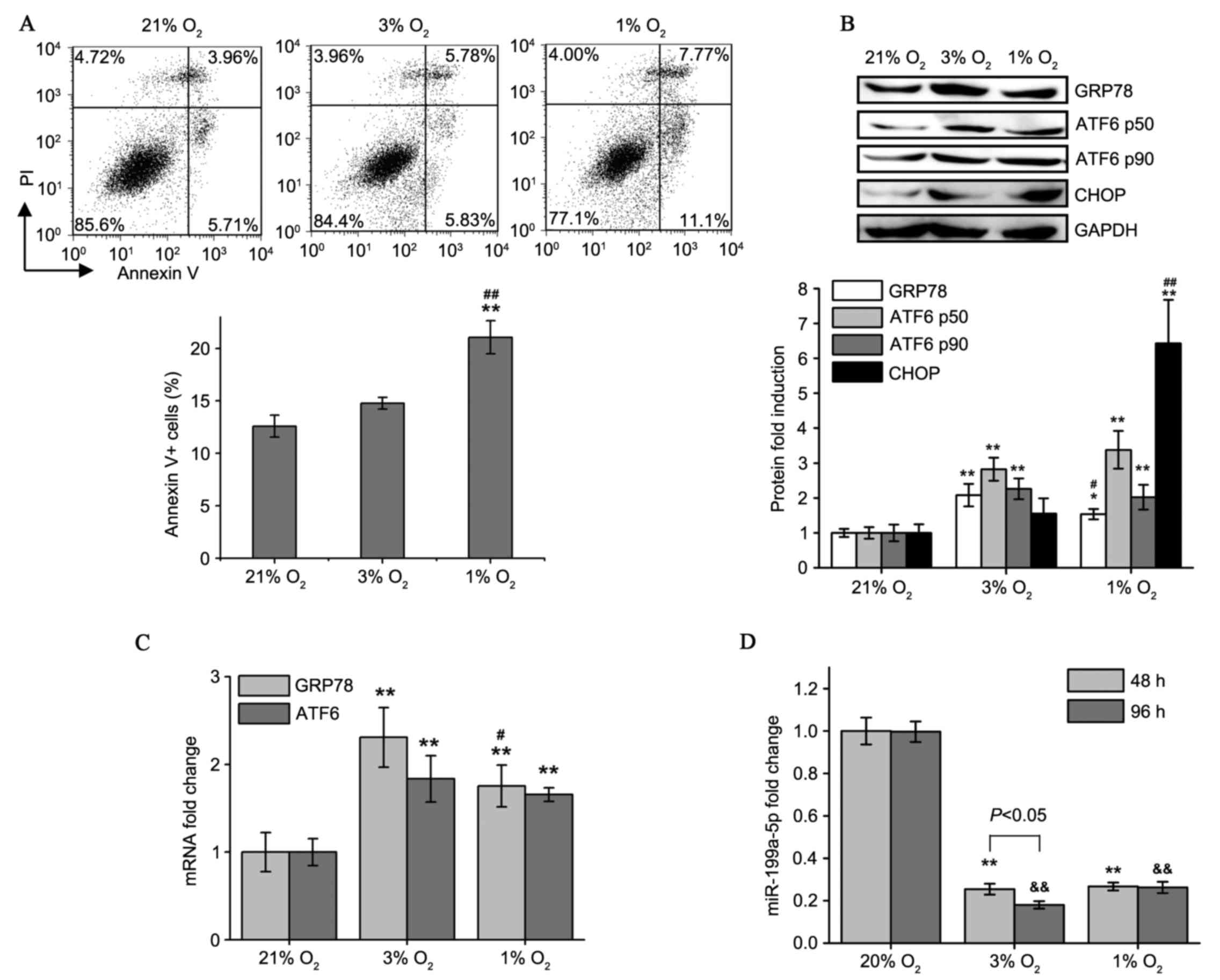 | Figure 2.Moderate and severe hypoxia cause
different apoptosis rates and changes in GRP78, ATF6 and
miR-199a-5p levels. (A) Flow cytometry plots of Annexin V/PI
staining in HCM cells following 21, 3 and 1% O2
treatments for 5 days. Percentages of all Annexin V+
(PI+/−) apoptotic cells were evaluated. (B) Western blot
analysis of GRP78, ATF6α-p50, ATF6α-p90 and CHOP protein expression
in HCM cells cultured under different oxygen tension. (C) Induction
of GRP78 and ATF6 mRNA expression in HCM cells following varying
degrees of hypoxia as detected by reverse
transcription-quantitative polymerase chain reaction. (D)
miR-199a-5p expression levels were determined in 48 and 96 h
hypoxia-cultured HCM cells. Values presented as the mean ± standard
deviation from three independent experiments. *P<0.05,
**P<0.01 and &&P<0.01 compared with the
respective 21% O2-cultured group; #P<0.05
and ##P<0.01 vs. the 3% O2-cultured group.
HCM, human cardiac myocyte; PI, propidium iodide; GRP78, 78 kDa
glucose-regulated protein; ATF6, activating transcription factor 6;
CHOP, C/EBP homologous protein; miR, microRNA. |
As demonstrated in Fig.
2D, exposure to low oxygen levels decreased miR-199a-5p levels.
Culture in 1% O2 did not have a greater effect on
miR-199a-5p levels compared with culture in 3% O2.
Additionally, for the 3% O2 group, prolonged (96 h)
hypoxia was more potent at inhibiting miR-199a-5p than short-term
(48 h) hypoxia. These results indicate that moderate and persistent
hypoxia in patients with CCHD is enough to maintain miR-199a-5p at
a significantly reduced level.
miR-199a-5p overexpression decreased
hypoxia-induced ATF6 and GRP78 expression and increased
apoptosis
To explore whether hypoxia-induced ATF6 and GRP78
expression can be modulated by miR-199a-5p, exogenously synthetic
miRNA mimic and inhibitor were transfected to enhance or knockdown
miR-199a-5p levels in HCM cells cultured under 3% O2 for
96 h. As presented in Fig. 3A and
B, it was identified that transfection with the miR-199a-5p
mimic significantly reduced hypoxic induction of ATF6 and GRP78
protein and mRNA expression compared with the negative mimic
control. By contrast, the miR-199a-5p inhibitor, but not the
inhibitor control, increased ATF6 and GRP78 expression in hypoxic
HCMs. Flow cytometry analysis indicated an increased percentage of
Annexin V+ apoptotic HCMs under hypoxia when
overexpressing miR-199a-5p. By contrast, HCMs transfected with the
miR-199a-5p inhibitor demonstrated a significant reduction in
apoptosis percentages under hypoxia compared with the inhibitor
control (Fig. 3C). Pro-apoptotic
CHOP protein levels also exhibited varying trends similar to the
flow cytometry results following transfection with miR-199a-5p
mimic and inhibitor in hypoxia-cultured HCM cells (Fig. 3D).
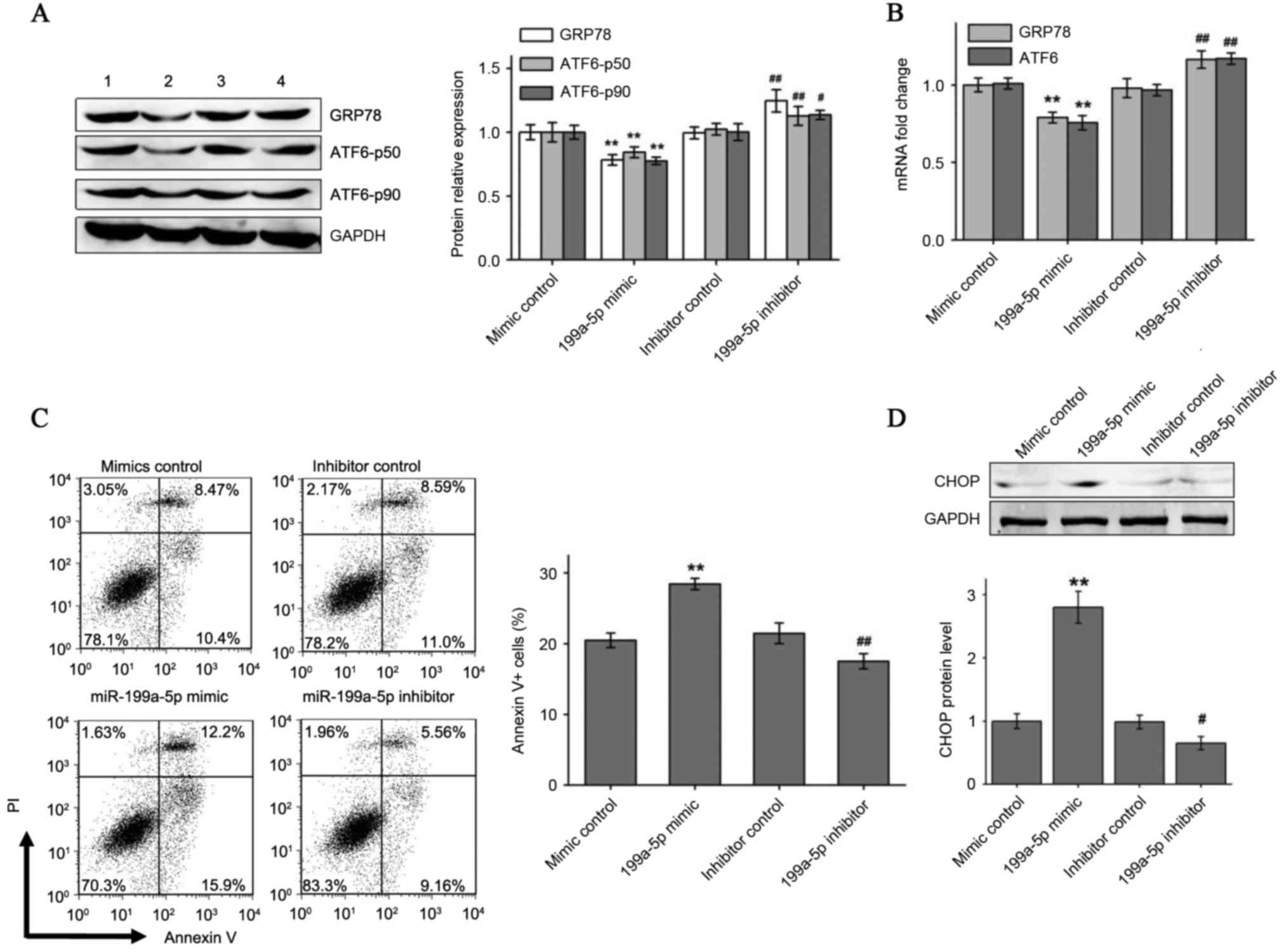 | Figure 3.miR-199a-5p mimic and inhibitor
affect ER stress-associated apoptosis by regulating the expression
of GRP78 and ATF6. (A) GRP78 and full-length and cleaved ATF6
protein levels and (B) GRP78 and ATF6 mRNA levels were measured by
western blotting and reverse transcription-quantitative polymerase
chain reaction, respectively, in 3% O2 cultured HCM
cells transfected with miR-199a-5p mimic or miR-199a-5p inhibitor.
Numbers 1–4 at the top of the western blot lanes indicate the mimic
control, miR-199a-5p mimic, the inhibitor control and the
miR-199a-5p inhibitor groups, respectively. (C) miR-199a-5p mimic
significantly increased hypoxia-induced cell apoptosis and
miR-199a-5p inhibitor reduced apoptosis in hypoxic cells. (D)
miR-199a-5p mimic or inhibitor changed CHOP expression in 3%
O2 cultured HCM cells. Data are presented as the mean ±
standard deviation of three independent experiments. **P<0.01
vs. mimic control group; #P<0.05 and
##P<0.01 vs. inhibitor control group. miR, microRNA;
ER, endoplasmic reticulum; HCMs, human cardiac myocyte; GRP78, 78
kDa glucose-regulated protein; ATF6, activating transcription
factor 6; PI, propidium iodide; CHOP, C/EBP homologous protein. |
GRP78 and ATF6 were confirmed to be
target genes for miR-199a-5p
To confirm whether miR-199a-5pcan directly targetthe
ATF6 and GRP78 3′UTRs, a luciferase reporter plasmid containing
wild type ATF6 or GRP78 3′-UTR, and mutants lacking the miR-199a-5p
binding sites were co-transfected with the miR-199a-5p mimic into
HEK293T and HCM cells. It was first observed that the miR-199a-5p
mimic downregulated the relative luciferase activity of wild-type
GRP78 and ATF6 in HEK293T cells cultured in normoxia, a
well-established and widely used protocol for miRNA luciferase
reporter analysis. By contrast, co-transfection of miR-199a-5p
mimics and the vector lacking any 3′-UTR sequences (mutant control)
demonstrated no such repressive effects (Fig. 4A). The results in the human
myocardial cell line also demonstrated that miR-199a-5p
significantly decreased luciferase gene expression in HCM cells
transfected with the reporter vectors containing the ATF6 or GRP78
3′-UTRs compared with the mutant controls and that the inhibition
effect was more pronounced compared with the HEK293T cells
(Fig. 4B). The effects of
endogenous miR-199a-5p on the GRP78 and ATF6 3′-UTR reporters in
HCM cells under normoxia and hypoxia were also examined. In HCM
cells under 3% O2 hypoxia and following reporter
transfection, the wild-type reporters were less repressed compared
with the 21% O2 group (Fig.
4C). These results demonstrated that exogenous miR-199a-5p can
suppress GRP78 and ATF6 expression in normoxic myocardial cells by
targeting their 3′-UTRs, and that hypoxia weakens this suppressive
effect by downregulating intracellular miR-199a-5p levels.
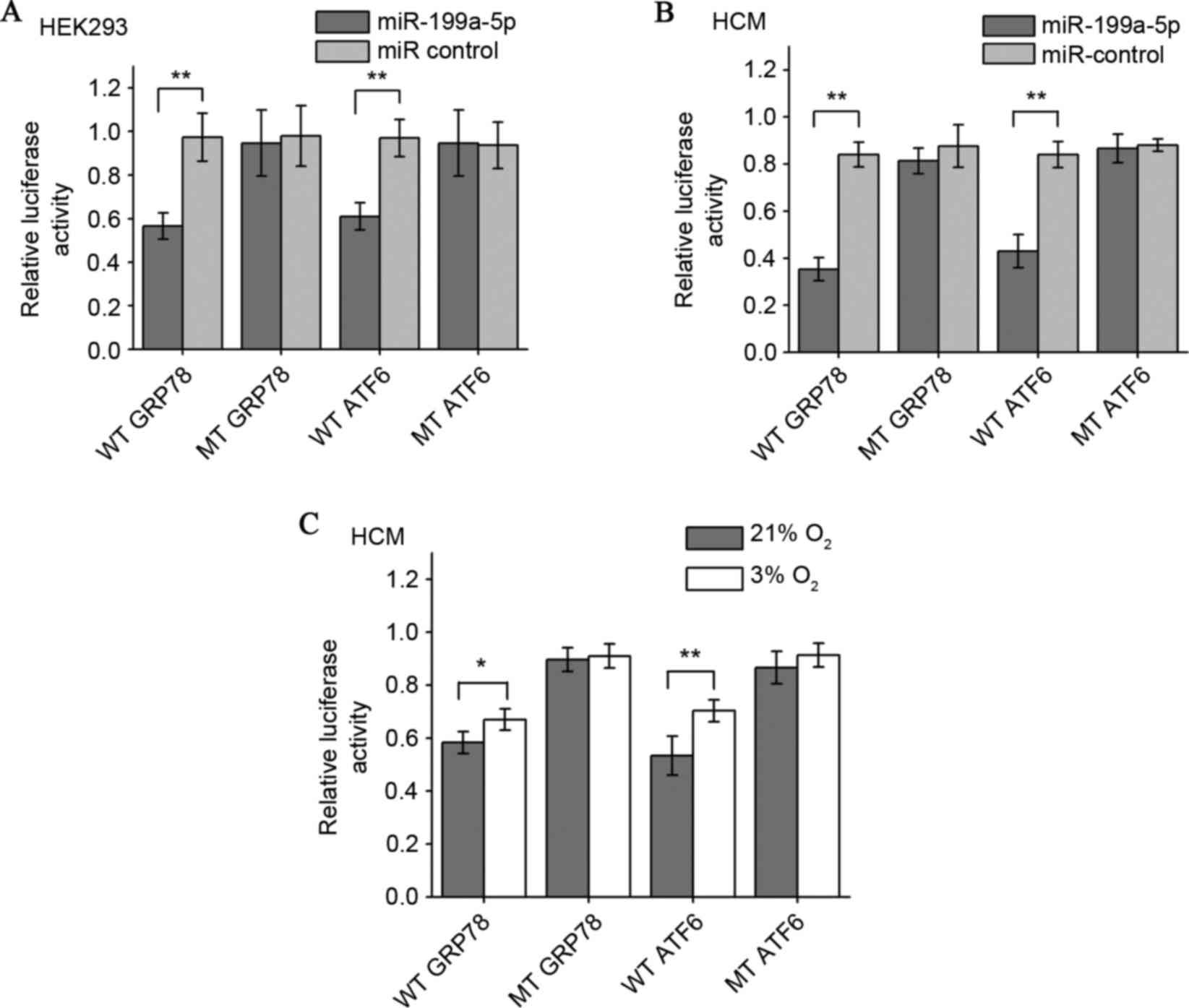 | Figure 4.Establishment of GRP78 and ATF6 as
target genes for miR-199a-5p by luciferase reporter gene assay.
miR-199a-5p mimics decreased the expression of luciferase
activities elicited by vectors containing the 3′-UTR of wild-type
GRP78 and ATF6 mRNAs, but not 3′UTR binding site mutants in (A)
HEK293 cells and (B) HCM cells. Relative luciferase activity was
determined by normalizing firefly to Renilla (control)
luciferase activity. (C) HCM cells transfected with a reporter
containing either wild-type or mutant GRP78 or ATF6 3′-UTRs were
cultured under 21 or 3% O2 for 48 h. The effect of
different endogenous miR-199a-5p levels under normoxia and hypoxia
on luciferase activity was compared. *P<0.05 and **P<0.01.
Data are presented as the mean ± standard deviation of three
independent experiments. 3′-UTR, 3′-untranslated region; WT,
wild-type 3′-UTR; GRP78, 78 kDa glucose-regulated protein; MT,
mutant 3′-UTR; ATF6, activating transcription factor 6; miR,
microRNA; UTR, untranslated region; HEK, human embryonic kidney
cells; HCM, human cardiac myocyte. |
Discussion
To the best of our knowledge, the present study is
the first to report a mechanism whereby oxygen-sensitive
miR-199a-5p levels change and regulate UPR activation by
controlling GRP78 and ATF6, the two major ER stress sensors and UPR
effectors, thus protecting CCHD patient cardiac myocytes during
chronic hypoxia.
A previous study (25) confirmed that the ATF6 pathway was
activated in the hypoxic myocardium of patients with CCHD. In
addition to functional ATF6-p50, enhanced gene expression of native
ATF6-p90 was also useful in promoting the persistent UPR process by
generating ATF6-p50. It was noted that ATF6 p90 and p50 were
consistently changed in the cardiac tissue of patients with CCHD
and in hypoxic cultured myocardial cells. In addition, the level of
GRP78, a universal ER stress sensor and cytoprotective ER chaperone
closely associated with, but not limited to, the ATF6 pathway, was
also observed to be upregulated in CCHD. It remains to be
elucidated how GRP78 and ATF6 expression are induced by hypoxia,
although HIFs are key modulators for many genes during low oxygen
conditions. It has been reported (26) that there are no HIF binding sites
in the GRP78 promoter and extrinsic HIFs have no effect on the
GRP78 promoter activity. Additionally, until now there have been no
reports that ATF6 expression is directly regulated by HIFs. Thus,
it is possible that GRP78 and ATF6 expression under hypoxic stress
is mainly regulated in a HIF-independent manner.
miRNAs fine-tune the activity of various
protein-coding genes. A recent study (27) reported that miR-199a-5p is
sensitive to hypoxia and decreases quickly to very low levels,
resulting in the release of mRNA targets from its inhibitory
effect, which has already been demonstrated to be an important
mechanism for the promotion of hypoxia-induced gene expression, as
in the case of HIF-1α and vascular endothelial growth factor. The
present study demonstrated that cardiac tissue expression of
miR-199a-5p was markedly decreased in CCHD individuals undergoing
chronic hypoxia. There is a strong linear association between
miR-199a-5p levels and the expression of its potential targets
GRP78 and ATF6, in addition to the oxygen saturation that may
clinically distinguish patients with CCHD from other congenital
heart defects. Notably, to the best of our knowledge, the present
study has obtained the first evidence to demonstrate the causal
association between decreased miR-199a-5p levels, and upregulated
GRP78 and ATF6 expression by conducting experiments in hypoxic
cultured HCMs and by luciferase reporter gene assays. It is
possible that cardiac-enriched miR-199a-5p imposes an important
repression of GRP78 and ATF6 to basal expression under normoxic
conditions. In hypoxia, the downregulation of miR-199a-5p partially
represses this effect, resulting in an increase in expression of
the two genes.
Although it has been reported (22) that miR-199a-5p can affect the
expression of GRP78 and ATF6 in a number of cell types, the present
study confirmed this regulation mechanism for the first time in
cardiomyocytes. The different luciferase reporter results between
HEK293 and HCM cells suggest that these regulatory roles may be
more pronounced in myocardial cells compared with other cells. The
reason may be that the particular cellular contexts in
cardiomyocytes are particularly propitious for miR-199a-5p to
target the GRP78 and ATF6 3′-UTRs. miRNAs post-transcriptionally
downregulate gene expression by either degrading or translationally
repressing target mRNAs. This is dependent upon the
complementarity, number of base pairs and accessibility of the
binding sites. As the mRNA and protein variation of GRP78 and ATF6
(p90) in the present study exhibited a largely synchronous trend
in vivo and in vitro, particularly when exogenously
altering the miR-199a-5p level, it was suggested that translational
repression, in addition to mRNA destabilization, may be involved in
the regulatory mechanism of GRP78 and ATF6 expression by
miR-199a-5p (28).
The results of the present study also demonstrated
that chronic and moderate hypoxia, as observed in clinical patient
with CCHD, can persistently maintain myocardial miR-199a-5p at
lower levels. Low oxygen availability induces changes (up- or
downregulation) in the expression of a specific subset of miRNAs
known as hypoxia miRs (29).
Transcription factors, including HIFs, can directly activate the
transcription of a subset of upregulated hypoxia miRs including
miR-210. However, the mechanisms by which hypoxia selectively
represses other hypoxia miRs have been less well-characterized.
Haghikia et al (30)
demonstrated that signal transducer and activator of transcription
3 (STAT3) protein acts as a potent suppressor of miR-199a-5p
transcription. Coincidentally, the activation of the STAT3 signal
pathway in patients with CCHD has been previously identified
(31). It is speculated that
miR-199a-5p is negatively regulated by an enhanced STAT3 pathway
under hypoxic conditions, although this requires further study for
verification.
In summary, the results of the present study provide
a novel adaptation mechanism of cardiac myocytes to chronic hypoxia
in patients with CCHD. It is proposed that miR-199a-5p may be a new
potential clinical diagnostic and therapeutic target for the
protection of cardiocytes against hypoxia.
Acknowledgements
The present study was supported the National Natural
Science Foundation of China (grant no. 81270228).
Glossary
Abbreviations
Abbreviations:
|
ACHD
|
acyanotic congenital heart defects
|
|
ATF6
|
activating transcription factor 6
|
|
CCHD
|
cyanotic congenital heart defects
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
78 kDa glucose-regulated protein
|
|
HCMs
|
human cardiac myocytes
|
|
HIF
|
hypoxia-inducible factor
|
|
miRNAs
|
microRNAs
|
|
SaO2
|
arterial oxygen saturations
|
|
UPR
|
unfolded protein response
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
UTR
|
untranslated region
|
References
|
1
|
van der Bom T, Zomer AC, Zwinderman AH,
Meijboom FJ, Bouma BJ and Mulder BJ: The changing epidemiology of
congenital heart disease. Nat Rev Cardiol. 8:50–60. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyague NI, Cardoso SM, Meyer F, Ultramari
FT, Araújo FH, Rozkowisk I and Toschi AP: Epidemiological study of
congenital heart defects in children and adolescents. Analysis of
4,538 cases. Arq Bras Cardiol. 80:269–278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oechslin E: Management of adults with
cyanotic congenital heart disease. Heart. 101:485–494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lou Y, Wang Z, Xu Y, Zhou P, Cao J, Li Y,
Chen Y, Sun J and Fu L: Resveratrol prevents doxorubicin-induced
cardiotoxicity in H9c2 cells through the inhibition of endoplasmic
reticulum stress and the activation of the Sirt1 pathway. Int J Mol
Med. 36:873–880. 2015.PubMed/NCBI
|
|
5
|
Schönenberger MJ and Kovacs WJ: Hypoxia
signaling pathways: Modulators of oxygen-related organelles. Front
Cell Dev Biol. 3:422015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu XL and Gao DS: Endoplasmic reticulum
proteins quality control and the unfolded protein response: The
regulative mechanism of organisms against stress injuries.
Biofactors. 40:569–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jian Z, Li JB, Ma RY, Chen L, Wang XF and
Xiao YB: Pivotal role of activating transcription factor 6α in
myocardial adaptation to chronic hypoxia. Int J Biochem Cell Biol.
44:972–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Glembotski CC: Roles for ATF6 and the
sarco/endoplasmic reticulum protein quality control system in the
heart. J Mol Cell Cardiol. 71:11–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thuerauf DJ, Marcinko M, Gude N, Rubio M,
Sussman MA and Glembotski CC: Activation of the unfolded protein
response in infarcted mouse heart and hypoxic cultured cardiac
myocytes. Circ Res. 99:275–282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Dong X, Zhou Q, Wang H, Qian Y,
Tian W, Ma D and Li X: microRNA expression profiling of heart
tissue during fetal development. Int J Mol Med. 33:1250–1260.
2014.PubMed/NCBI
|
|
11
|
Azzouzi HE, Leptidis S, Doevendans PA and
De Windt LJ: HypoxamiRs: Regulators of cardiac hypoxia and energy
metabolism. Trends Endocrinol Metab. 26:502–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maurel M and Chevet E: Endoplasmic
reticulum stress signaling: The microRNA connection. Am J Physiol
Cell Physiol. 304:C1117–C1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Topkara VK and Mann DL: Role of microRNAs
in cardiac remodeling and heart failure. Cardiovasc Drugs Ther.
25:171–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee DS, Chen JH, Lundy DJ, Liu CH, Hwang
SM, Pabon L, Shieh RC, Chen CC, Wu SN, Yan YT, et al: Defined
MicroRNAs induce aspects of maturation in mouse and human
embryonic-stem-cell-derived cardiomyocytes. Cell Rep. 12:1960–1967.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song XW, Li Q, Lin L, Wang XC, Li DF, Wang
GK, Ren AJ, Wang YR, Qin YW, Yuan WJ and Jing Q: MicroRNAs are
dynamically regulated in hypertrophic hearts, and miR-199a is
essential for the maintenance of cell size in cardiomyocytes. J
Cell Physiol. 225:437–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xing Y, Liu Z, Yang G, Gao D and Niu X:
MicroRNA expression profiles in rats with selenium deficiency and
the possible role of the Wnt/β-catenin signaling pathway in cardiac
dysfunction. Int J Mol Med. 35:143–152. 2015.PubMed/NCBI
|
|
17
|
Ding G, Huang G, Liu HD, Liang HX, Ni YF,
Ding ZH, Ni GY and Hua HW: MiR-199a suppresses the hypoxia-induced
proliferation of non-small cell lung cancer cells through targeting
HIF1α. Mol Cell Biochem. 384:173–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rane S, He M, Sayed D, Vashistha H,
Malhotra A, Sadoshima J, Vatner DE, Vatner SF and Abdellatif M:
Downregulation of miR-199a derepresses hypoxia-inducible
factor-1alpha and Sirtuin 1 and recapitulates hypoxia
preconditioning in cardiac myocytes. Circ Res. 104:879–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su SF, Chang YW, Andreu-Vieyra C, Fang JY,
Yang Z, Han B, Lee AS and Liang G: miR-30d, miR-181a and
miR-199a-5p cooperatively suppress the endoplasmic reticulum
chaperone and signaling regulator GRP78 in cancer. Oncogene.
32:4694–4701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hassan T, Carroll TP, Buckley PG, Cummins
R, O'Neill SJ, McElvaney NG and Greene CM: miR-199a-5p silencing
regulates the unfolded protein response in chronic obstructive
pulmonary disease and α1-antitrypsin deficiency. Am J Respir Crit
Care Med. 189:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang Y, Ridzon D, Wong L and Chen C:
Characterization of microRNA expression profiles in normal human
tissues. BMC Genomics. 8:1662007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nam JW, Rissland OS, Koppstein D,
Abreu-Goodger C, Jan CH, Agarwal V, Yildirim MA, Rodriguez A and
Bartel DP: Global analyses of the effect of different cellular
contexts on microRNA targeting. Mol Cell. 53:1031–1043. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai BH, Geng L, Wang Y, Sui CJ, Xie F,
Shen RX, Shen WF and Yang JM: microRNA-199a-5p protects hepatocytes
from bile acid-induced sustained endoplasmic reticulum stress. Cell
Death Dis. 4:e6042013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Namba T, Ishihara T, Tanaka K, Hoshino T
and Mizushima T: Transcriptional activation of ATF6 by endoplasmic
reticulum stressors. Biochem Biophys Res Commun. 355:543–548. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang SC, Erwin AE and Lee AS:
Glucose-regulated protein (GRP94 and GRP78) genes share common
regulatory domains and are coordinately regulated by common
trans-acting factors. Mol Cell Biol. 9:2153–2162. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai L, Lou W, Zhu J, Zhou X and Di W:
miR-199a inhibits the angiogenic potential of endometrial stromal
cells under hypoxia by targeting HIF-1α/VEGF pathway. Int J Clin
Exp Pathol. 8:4735–4744. 2015.PubMed/NCBI
|
|
28
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nallamshetty S, Chan SY and Loscalzo J:
Hypoxia: A master regulator of microRNA biogenesis and activity.
Free Radic Biol Med. 64:20–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haghikia A, Missol-Kolka E, Tsikas D,
Venturini L, Brundiers S, Castoldi M, Muckenthaler MU, Eder M,
Stapel B, Thum T, et al: Signal transducer and activator of
transcription 3-mediated regulation of miR-199a-5p links
cardiomyocyte and endothelial cell function in the heart: A key
role for ubiquitin-conjugating enzymes. Eur Heart J. 32:1287–1297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu Q, Kong Y, Yu ZB, Bai L and Xiao YB:
Hypoxia-induced SOCS3 is limiting STAT3 phosphorylation and NF-κB
activation in congenital heart disease. Biochimie. 93:909–920.
2011. View Article : Google Scholar : PubMed/NCBI
|