Introduction
Liver cirrhosis is a common disease clinically and
is life-threatening in humans. It features an imbalance in the
synthesis and degradation of extracellular matrix (ECM), primarily
collagen, leading to metabolic disorders of the whole liver and a
series of severe clinical complications. Hepatic fibrosis
constitutes an inevitable pathological stage in the transformation
from multiple chronic liver diseases to cirrhosis, in which the
activation and proliferation of hepatic stellate cells (HSCs) is
pivotal (1). HSC activation and
the following abnormal expression of ECM are essential to the onset
of liver cirrhosis. Transforming growth factor β1 (TGFβ1) functions
as a crucial factor in activating HSCs and promoting its expression
of ECM. The TGFβ1 signal transduction pathway is important in
regulating the expression of ECM in HSCs (2). Therefore, TGFβ1 is one of the
significant targets in the prevention and treatment of hepatic
fibrosis.
In the present study, the optimal small interfering
RNA (siRNA) sequence targeting the TGFβ1 gene was first confirmed,
and the corresponding pGreenPuro/TGFβ1 short hairpin (sh)RNA
lentiviral vector was constructed and transfected into HSC-T6 cells
in vitro to observe the lentiviral vector-mediated effect of
shRNA on TGFβ1 gene silencing and on the expression of collagen
type 1 α1 (Col1a1) of the ECM, which was anticipated to provide an
experimental basis for the prevention and treatment of hepatic
fibrosis.
Materials and methods
Materials
The HSC-T6 cell line was obtained from Shanghai
Aiyan Biotech (Shanghai, China); synthesis of the oligonucleotide
sequences of the three RNA interference (RNAi) sites and the
corresponding controls was performed by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China); the gel extraction kit and plasmid extraction
kit were from Roche Diagnostics (Basel Switzerland); restriction
enzymes, T4 DNA ligase, Taq polymerase and the reverse
transcription-polymerase chain reaction (RT-PCR) kit were from
Takara Bio, Inc. (Otsu, Japan); the pGreenPuro lentiviral vector
was from Shanghai Innovation Biotechnology Co., Ltd. (Shanghai,
China); Lipofectamine® 2000 was from Invitrogen; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); anti-TGFβ1 antibody and
anti-Col1a1 antibody were from Merck Millipore (Darmstadt,
Germany); DNA oligosynthesis of the optimal siRNA sequence, and the
primer synthesis and sequencing were performed by Shanghai Generay
Biotech Co., Ltd. (Shanghai, China).
Designing and screening of the optimal
siRNA site of TGFβ1
A total of three RNAi sites of the TGFβ1 gene of
rats were selected, according to its CDs sequence (site 1,
GTCAACTGTGGAGCAACAC; site 2, GCACCATCCATGACATGAA; site 3,
CCGCAACAACGCAATCTAT). The corresponding siRNAs of the three sites
were synthesized respectively: Site 1 sense,
5′-GUCAACUGUGGAGCAACACdTdT-3′ and antisense,
5′-dTdTCAGUUGACACCUCGUUGUG-3′; site 2 sense,
5′-GCACCAUCCAUGACAUGAAdTdT-3′ and antisense,
5′-dTdTCGUGGUAGGUACUGUACUU-3′; site 3 sense,
5′-CCGCAACAACGCAAUCUAUdTdT-3′ and antisense,
5′-dTdTGGCGUUGUUGCGUUAGAUA-3′. The three pairs of siRNAs were
transfected into HSC-T6 cells, respectively, according to the
manufacturer's protocol.
The detection primers of TGFβ1 (NM_021578.2) and
GAPDH (AF106860.2) of rats were designed based on their respective
gene sequences. The upstream and downstream primers of TGFβ1 were
5′-ACTACGCCAAAGAAGTCACCC-3′ and 5′-TGAGCACTGAAGCGAAAGC-3′,
respectively, and the upstream and downstream primers of GAPDH were
5′-TCTACTGGCGTCTTCA-3′ and 5′-TGAGCCCTTCCACGAT-3′, respectively.
Total RNA of HSC-T6 cells transfected with each siRNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol. With the total RNA of HSC-T6
cells transfected with each siRNA, respectively, and with template
and non-transfected HSC-T6 cells as controls, RT-PCR analysis was
performed to confirm the optimal siRNA site among the three siRNA
sites.
Construction and identification of the
TGFβ1 shRNA lentiviral vector
The shRNA targeting the sequence of the optimal
siRNA was designed and synthesized as follows: Sense,
5′-GATCCGTCAACTGTGGAGCAACACCTTCCTGTCAGAGTGTTGCTCCACAGTTGACTTTTTG-3′
and antisense,
5′-AATTCAAAAAGTCAACTGTGGAGCAACACTCTGACAGGAAGGTGTTGCTCCACAGTTGACG-3′;
BamH and EcoR digestion sites were introduced into
the positive-sense and antisense strands, respectively, and
annealed to form a double-stranded structure. The annealed
oligonucleotide fragment was cloned into the pGreenPuro plasmid to
establish the pGreenPuro/TGFβ1 shRNA lentiviral vector, which was
then transfected into competent cells. The positive recombinant
plasmid DNA was identified by restriction enzyme digestion and
sequencing.
Packaging and titer of TGFβ1 shRNA
lentiviral recombinants
Trypsin was used to digest the healthy 293T cells
(Type Culture Collection of Chinese Academy of Sciences, Shanghai,
China), which were in the logarithmic phase, 24 h prior to
transfection, and the cells were then diluted with DMEM
high-glucose medium (DMEM; HyClone; GE Healthcare, Chicago, IL,
USA) containing 10% FBS (catalog no. F2442; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). and, inoculated into a 6-well plate at
5×105 cells/well for incubation at 37°C, 5%
CO2 and saturated humidity. When the culture reached a
70–80% density, Lipofectamine® 2000 gene transfection
reagent was used to mediate transfection of the pGreenPuro/TGFβ1
shRNA lentiviral plasmid DNA with the right sequence into 293T
cells. The medium was renewed at 8 h and cultivation was continued
until 48 h under the same conditions. An inverted fluorescence
microscope was used to observe the expression of GFP and capture
images.
The incubation was continued until 72 h, when the
supernatant of each culture was collected for centrifugation at
4,000 × g and 4°C for 10 min to remove cell debris. A filter (0.45
µm) was used for further filtration. Following concentration, the
supernatant was stored in aliquots at −70°C.
Titers were detected and calculated using the double
dilution method with the following formula: Viral titer = (P ×
N/100 × V) ×1/DF; in which P represents the GFP-positive cell
count, N represents 105, V represents the volume of
viral diluent and DF represents the dilution ratio.
Transfection of HSC-T6 cells with the
pGreenPuro/TGFβ1 shRNA lentiviral plasmid
Trypsin was used to digest the healthy 293T cells in
the logarithmic phase 24 h prior to transfection, following which
the cells were then diluted with DMEM high-glucose medium
containing 10% FBS and inoculated into a 6-well plate at
5×105 cells/well for incubation at 37°C, 5%
CO2 and saturated humidity. When the culture reached a
70–80% density, the pGreenPuro/TGFβ1 shRNA lentiviral solution was
added to the medium for transfection at a ratio of 1:50, with
HSC-T6 cells transfected with empty vector or non-transfected cells
as controls. An inverted fluorescence microscope was used to
observe the expression of GFP and capture images 24–48 h later.
Effects of the pGreenPuro/TGFβ1 shRNA
lentiviral vector on the protein expression of TGFβ1 and Col1a1 in
HSC-T6 cells
At 48 h post-transfection with the pGreenPuro/TGFβ1
shRNA lentiviral vector, the total protein was extracted by western
and IP cell lysis solution (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol from the
HSC-T6 cells. The protein concentration was measured using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology),
and 20 µg protein was separated by 10% SDS-PAGE and transferred
onto nitrocellulose membranes. Membranes were soaked in blocking
buffer containing a TGFβ1 rabbit polyclonal antibody (1:600;
catalog no. sc-146) or a Col1a1 mouse monoclonal antibody (1:500;
catalog no. sc-59772) (both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), respectively, overnight at 4°C. Subsequently,
HRP-labeled mouse anti-rabbit secondary antibody (1:400; catalog
no. A0208) and rabbit anti-mouse secondary antibody (1:400; catalog
no. A0216) (both from Beyotime Institute of Biotechnology, Inc.)
were added, respectively, and incubated at room temperature for 1.5
h. Chemiluminescence substrate was added for co-culture for ~7 min
at 25°C. Images of the results were then captured using GADPH (MW,
3.7×104) as the internal reference.
Effects of TGFβ1 shRNA on the gene
expression of TGFβ1 and COL1 in HSC-T6 cells
The specific detection primers for rat Col1a1
(NM_053304.1) were designed based on its gene sequence: Upstream
5′-TGATGTATGCTTGATCTGTAT-3′ and downstream
5′-CGGGGACCCATTGGACCTGAA-3′. The specific detection primers of rat
TGFβ1 and GAPDH were as described above. Total RNA of HSC-T6 cells
transfected with TGFβ1 shRNA was used as the template, and the gene
expression of TGFβ1 and COL1 were detected using RT-PCR analysis,
with empty vector-transfected cells treated as controls and GADPH
gene as the internal reference. A total of 1.2 µg RNA from each
sample was transcribed into cDNA using the PrimeScript™ RT kit
(Takara Bio, Inc.) according to the manufacturer's protocol.
Reverse transcription was performed under the following conditions:
30°C for 10 min and 42°C for 30 min to synthesize the first chain
of cDNA; 99°C for 5 min to inactivate reverse transcriptase;
cooling at 5°C for 5 min, one circle. The RT product (12 µl) was
diluted in water to a total volume of 50 µl. cDNA was then
amplified by PCR with primers specific to the target TGFβ1 and
Col1a1 sequence. PCR was performed under the following conditions:
Predenaturation at 95°C for 5 min, denaturation at 94°C for 45 sec,
annealing at 57°C for 1 min, extension at 72°C for 1 min (28
circles) and final extension at 72°C for 5 min. The PCR products (5
µl) were subjected to electrophoresis and confirmed using GoldView™
staining.
Results
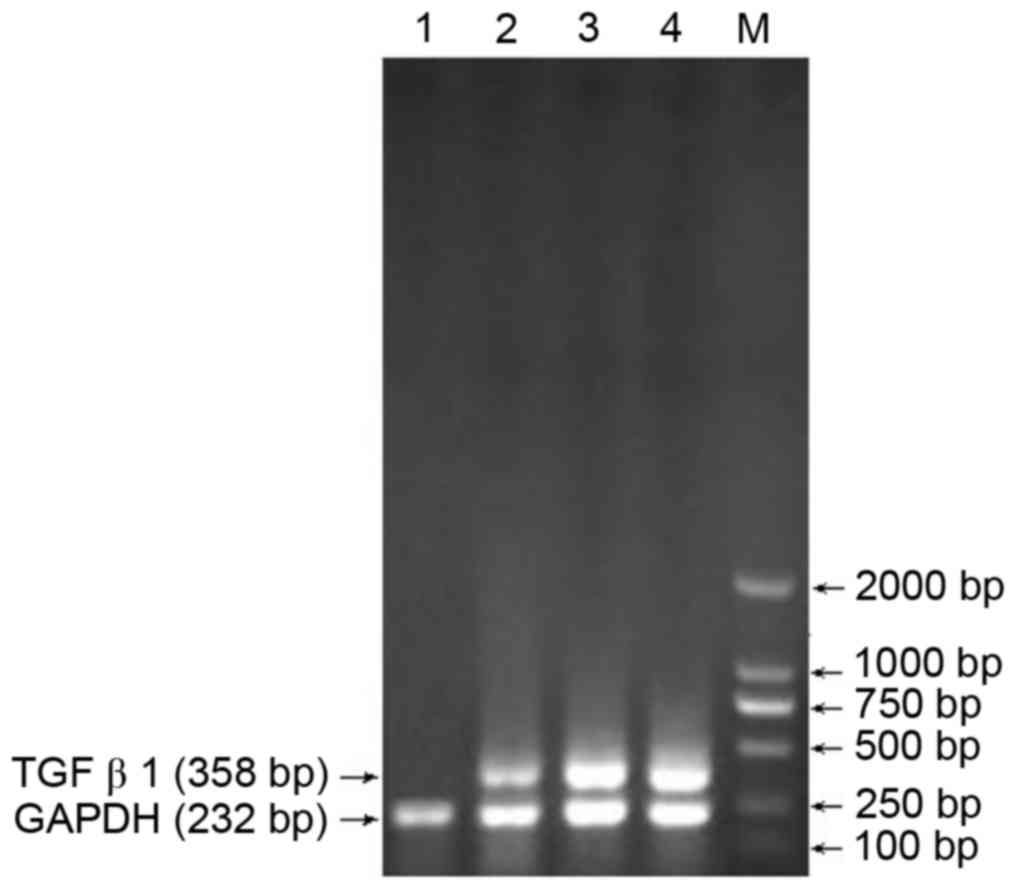
Screening of the optimal siRNA site in
TGFβ1
RT-PCR analysis was used to confirm the optimal
siRNA site among the three sites. Site 1 was identified as optimal,
with the sequence GTCAACTGTGGAGCAACAC (Fig. 1).
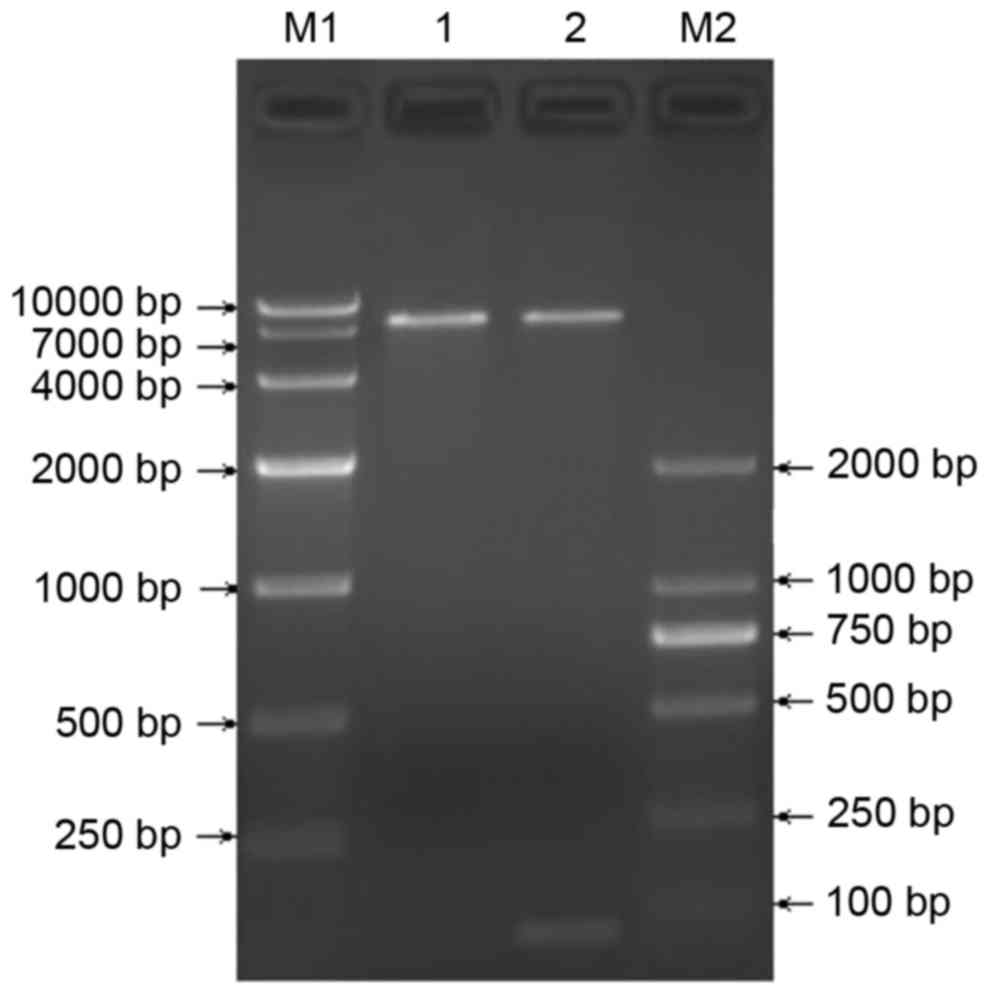
Construction and identification of the
TGFβ1 shRNA lentiviral vector
The recombinant pGreenPuro/TGFβ1 shRNA lentiviral
vector was digested using BamH1 and EcoRI to produce
a 55 bp shRNA fragment, shown by electrophoresis (Fig. 2). Sequencing verified successful
construction of the recombinant pGreenPuro/TGFβ1 shRNA lentiviral
vector carrying TGFβ1 shRNA.
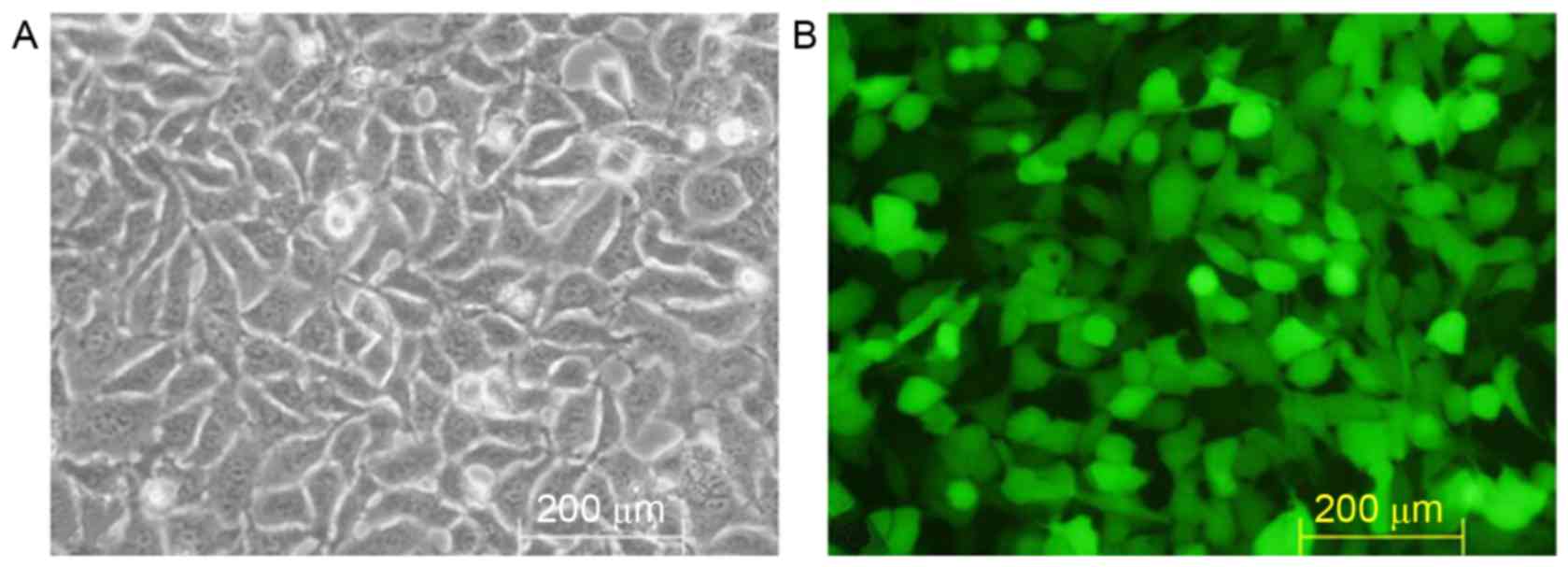
Packaging and titer of TGFβ1 shRNA
lentiviral recombinants
Lipofectamine® 2000 gene transfection
reagent was used to mediate pGreenPuro/TGFβ1 shRNA lentiviral
plasmid DNA transfection of 293T cells (Fig. 3A). Following incubation for 48 h,
marked GFP expression was observed under the fluorescence
microscope (Fig. 3B). The culture
showed a viral titer of 7×107 TU/ml via the double
dilution method.
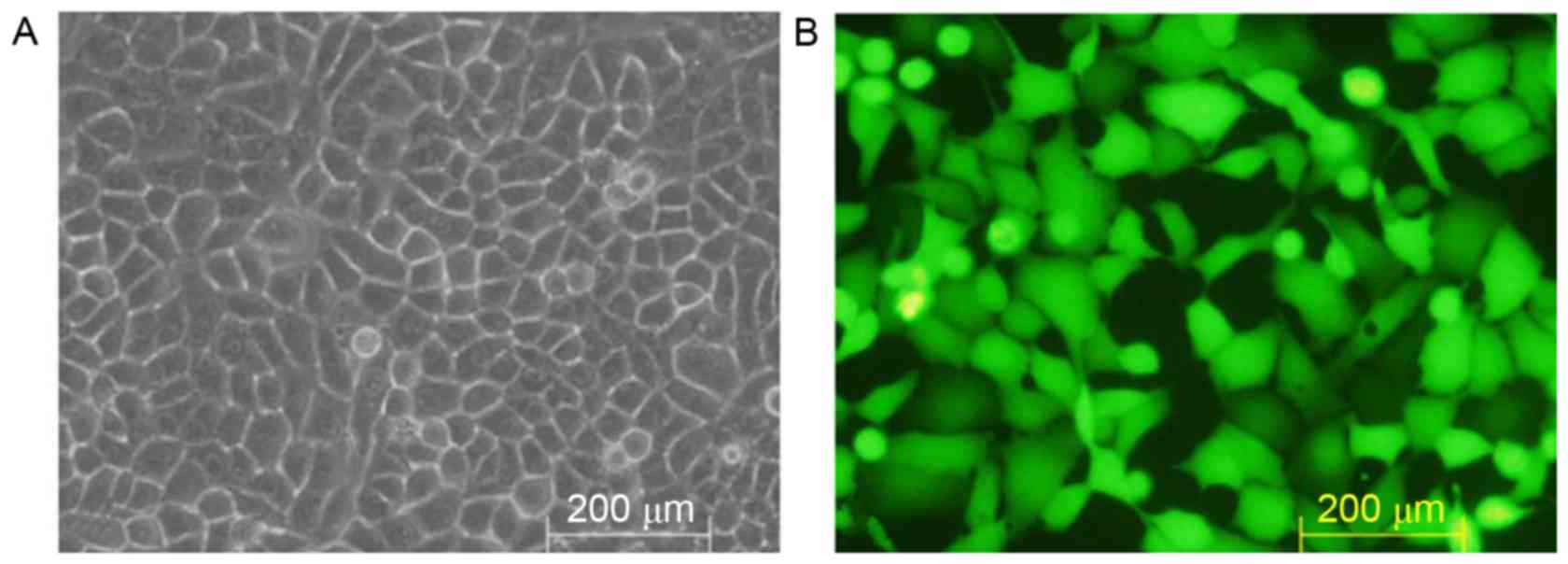
Transfection of HSC-T6 cells with the
pGreenPuro/TGFβ1 shRNA lentiviral plasmid
No expression of GFP was detected in the
non-transfected HSC-T6 cells (Fig.
4A), whereas marked expression of GFP was observed under the
fluorescence microscope in the HSC-T6 cells transfected with the
pGreenPuro/TGFβ1 shRNA lentiviral plasmid (Fig. 4B).
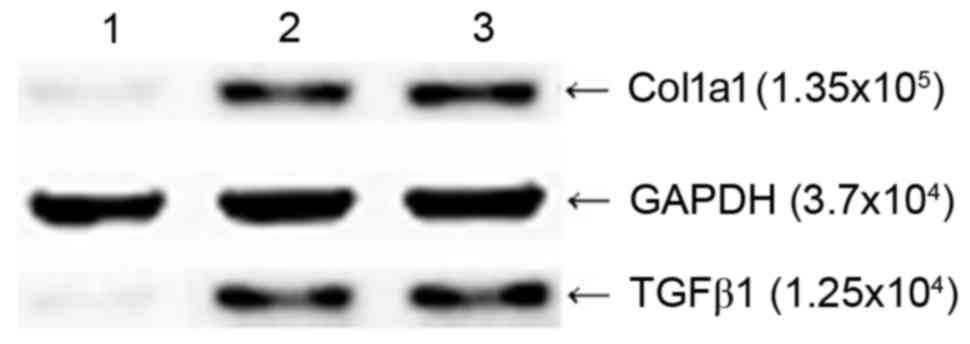
Effects of the pGreenPuro/TGFβ1 shRNA
lentiviral vector on the protein expression of TGFβ1 and Col1a1 in
HSC-T6 cells
The results of the western blot analysis showed that
the HSC-T6 cells transfected with pGreenPuro/TGFβ1 shRNA lentiviral
plasmid produced faintly-stained anti-TGFβ1 and anti-Col1a1 bands
at MW 1.25×104 and 1.35×105, respectively,
whereas the empty vector-transfected HSC-T6 cells and
non-transfected HSC-T6 cells produced intense staining of bands at
the two sites (Fig. 5), which
indicated that silencing of the TGFβ1 gene in HSC-T6 cells at the
RNA level effectively inhibited the expression of Col1a1.
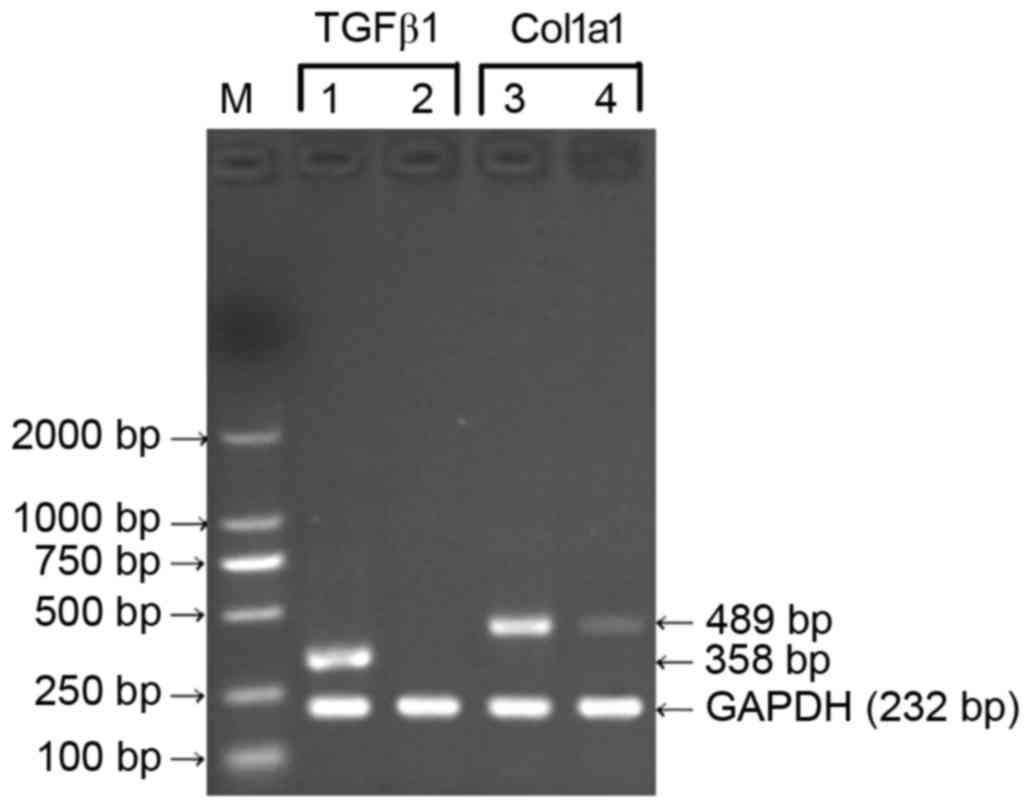
Effects of TGFβ1 shRNA on the gene
expression of TGFβ1 and Col1a1 in HSC-T6 cells
With the total RNA of the HSC-T6 cells 48 h
following transfection with the pGreenPuro/TGFβ1 shRNA lentiviral
plasmid as a template, the RT-PCR assay showed no expression of
TGFβ1 and comparatively mild expression of Col1a1 at the mRNA
level. With total RNA from the HSC-T6 cells at 48 h
post-transfection with the empty plasmid or non-transfected HSC-T6
cells as templates, the results of the RT-PCR assay revealed high
expression levels of TGFβ1 and Col1a1 at the mRNA level (Fig. 6). These results indicated that the
recombinant pGreenPuro/TGFβ1 shRNA lentiviral plasmid effectively
silenced the TGFβ1 gene and downregulated the expression of Col1a1
at the mRNA level.
Discussion
Hepatic fibrosis represents a pathological stage in
which abnormal hyperplasia of intrahepatic connective tissues is
manifested during multiple chronic liver injury repair processes.
It features an imbalance in the generation and degradation of ECM,
which leads to the excessive deposition of ECM, predominantly
Col1a1, and finally results in decreased hepatic function.
HSCs are located in Disse's interspace, close to
liver sinusoidal endothelial cells and hepatocytes. There are only
a small number of HSCs in the normal liver, representing 5–8% of
all hepatocytes. HSCs bind with vitamin A lipid droplets, and
engage in vitamin A metabolism and ECM synthesis (3), which contribute to the excessive
deposition of ECM, particularly under pathological conditions.
Activated HSCs have a leading role in the process of hepatic
fibrosis, where they multiply in quantity, exhibit phenotype
changes and producing excessive ECM.
Derived from rat primary hepatic stellate cells
infected by the T antigen of SV40, the HSC-T6 cell line is an
active form of HSCs, which can express and secrete collagen matrix,
and is an ideal model for investigating hepatic fibrosis (4).
Myofibroblasts produce abundant collagen fibers
during acute or chronic liver injury, primarily types I and III,
with the level of Col1a1 markedly increased. Type I and III
collagen accumulate excessively in Disse's space and occupies the
interspace of liver sinusoidal endothelial cells, which promotes
the onset and development of hepatic fibrosis. Col1a1 constitutes a
primary stage of ECM in the case of hepatic fibrosis and cirrhosis,
and its expression level can be used as an important indicator to
reflect the degree of hepatic fibrosis. High levels of Col1a1
synthesis is central in the onset and development of hepatic
fibrosis. TGFβ, which is composed of six subtypes: TGFβ1, TGFβ2,
TGFβ3, TGFβ1β2, TGFβ4 and TGFβ5, is essential to the physiological
and pathological status of the liver (5), and TGFβ1 has the highest contribution
to hepatic fibrosis among the multiple coefficient cytokines
(6,7). TGFβ1 regulates ECM deposition
resulting from tissue injury-mediated physiological response and
also pathological reactions. TGFβ1 is predominantly expressed by
liver sinusoidal endothelial cells and Kupffer cells, and mild
expression is observed in HSCs in the normal liver, which is
markedly upregulated in HSCs in the case of liver injury. The
changes in TGFβ1 and Col1a1 showed a consistent trend during
hepatic fibrosis, as TGFβ1 directly or indirectly activated HSCs to
increase the generation of Col1a1-dominating ECM and decrease its
degeneration. The synthesis of ECM, particularly Col1a1, can be
reduced via inhibiting the generation of TGFβ1 and inhibiting its
secretion and amplification processes. Therefore, TGFβ1 functions
as an important initiating factor of hepatic fibrosis. It is clear
that silencing TGFβ1 can be used as an important strategy in the
prevention and treatment of hepatic fibrosis. Inhibiting the
expression or biological activities of TGFβ1 via antisense
oligonucleotides or drugs can reduce the generation of ECM and the
deposition of fibrous protein (8,9),
although this is ineffective. However, the increase of RNAi
techniques provides novel strategies for reducing ECM generation
and fibrous protein deposition via gene silencing.
RNAi refers to sequence-specific posttranscriptional
gene silencing in which endogenous or exogenous double-stranded RNA
combines with and degrades intracellular mRNA with the homologous
sequence (10). The RNAi technique
has high specificity, efficiency and stability (11), and is a useful tool with which gene
function and interrelation among upstream and downstream molecules
in signal transduction systems are investigated. However, this
technique is limited by the fact that the transfection of
short-chain RNA can only be sustained for a few days in mammalian
cells, whereas lentiviral vector techniques can realize longer term
gene expression inhibition, which is more appropriate for in
vivo experiments (12).
Therefore, the present study selected three RNAi
sites in the rat TGFβ1 gene according to its CDs sequence, and the
optimal siRNA sequence was confirmed using RT-PCR analysis.
Subsequently, shRNA targeting the sequence of the optimal siRNA was
designed, synthesized and cloned into the human pGreenPuro plasmid
to establish the pGreenPuro/TGFβ1 shRNA lentiviral vector, and
HSC-T6 cells were effectively transfected with this recombinant
lentiviral vector. RT-PCR and western blot analyses confirmed that
the pGreenPuro/TGFβ1 shRNA lentiviral vector effectively mediated
the expression of TGFβ1 in the HSC-T6 cells silenced by TGFβ1
shRNA, and simultaneously downregulated the expression of Col1a1.
These results indicated that TGFβ1 shRNA downregulated the
activating signal of HSCs to reduce the generation of
Col1a1-dominating ECM of HSCs, to alleviate or inhibit hepatic
fibrosis. Therefore, TGFβ1 shRNA is among the novel strategies for
the prevention and treatment of hepatic fibrosis.
The present study was limited by the fact that the
expression of Col1a1 was detected only at the mRNA and protein
levels following TGF-β interference. The secretion of Col1a1 and
changes in other components of the ECM, including collagen type
III, also require consideration in the overall situation, requiring
further investigation.
Acknowledgements
This study was supported by the National Natural
Science Foundation (grant no. 81273918), the Project of Traditional
Chinese Medicine Science and Technology of Chongqing (grant no.
2012-2-63) and the Specific Project of Traditional Chinese Medicine
of Chinese PLA, Chongqing, China (grant no. 2010ZYZ231).
References
|
1
|
Mann DA and Marra F: Fibrogenic signalling
in hepatic stellate cells. J Hepatol. 52:949–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu L, Zheng N, He Q, Li R, Zhang K and
Liang T: Puerarin, isolated from Pueraria lobata (Willd.),
protects against hepatotoxicity via specific inhibition of the
TGF-β1/Smad signaling pathway, thereby leading to anti-fibrotic
effect. Phytomedicine. 20:1172–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atzori L, Poli G and Perra A: Hepatic
stellate cell: A star cell in the live. Int J Biochem Cell Biol.
41:1639–1642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogel S, Piantedosi R, Frank J, Lalazar A,
Rockey DC, Friedman SL and Blaner WS: An immortalized rat liver
stellate cell line (HSC-T6): A new cell model for the study of
retinoid metabolism in vitro. J Lipid Res. 41:882–893.
2000.PubMed/NCBI
|
|
5
|
Baghy K, Iozzo RV and Kovalszky I:
Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem
Cytochem. 60:262–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inagaki Y, Higashiyama R and Higashi K:
Novel anti-fibrotic modalities for liver fibrosis: Molecular
targeting and regenerative medicine in fibrosis therapy. J
Gastroenterol Hepatol. 27:(Suppl 2). S85–S88. 2012. View Article : Google Scholar
|
|
7
|
Gressner OA, Rizk MS, Kovalenko E,
Weiskirchen R and Gressner AM: Changing the pathogenetic roadmap of
liver fibrosis? Where did it start; where will it go? J
Gastroenterol Hepatol. 23:1024–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGFbeta1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ieronimakis N, Hays AL, Janebodin K,
Mahoney WM Jr, Duffield JS, Majesky MW and Reyes M: Coronary
adventitial cells are linked to perivascular cardiac fibrosis via
TGFβ1 signaling in the mdx mouse model of Duchenne muscular
dystrophy. J Mol Cell Cardiol. 63:122–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ashihara E, Kawata E and Maekawa T: Future
prospect of RNA interference for cancer therapies. Curr Drug
Targets. 11:345–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J and Xie J: Progress on RNAi-based
molecular medicines. Int J Nanomedicine. 7:3971–3980. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng Z, Zhang C and Chen J:
Lentivirus-mediated RNA interference of DC-STAMP expression
inhibits the fusion and resorptive activity of human osteoclasts. J
Bone Miner Metab. 31:409–416. 2013. View Article : Google Scholar : PubMed/NCBI
|