Introduction
Lung cancer is the leading cause of
cancer-associated mortality with a consistently poor 5-year
survival rate (1). Despite
improvements in monitoring and clinical treatment strategies, the
5-year overall survival rate in the United States remains ~16%
(2). In 2012, lung cancer occurred
in 1.8 million people and resulted in 1.6 million mortalities
worldwide (3). Unfortunately, its
total incidence is increasing dramatically worldwide (4,5).
Most clinical cases of lung cancer are categorized into two
subtypes: Small-cell lung carcinoma (SCLC) and non-small-cell lung
carcinoma (NSCLC). Approximately 70% of patients newly diagnosed
with either subtype of lung cancer present with locally recurrent
or metastatic lesions following resection (6). Undoubtedly, elucidating the
pathogenesis of lung cancer is important for the effective
treatment of this disease.
MicroRNAs (miRNAs) are a class of short, endogenous,
noncoding RNAs that regulate gene expression. Although miRNAs
account for only a minor fraction of the expressed genome, they
have been demonstrated to have important roles in various cellular
processes, including proliferation, apoptosis, differentiation and
malignant transformation (7–9). It
is estimated that 60% genes are regulated by miRNAs, and changes in
miRNA expression affect physiological and pathological processes
(10). Increasing evidence
indicates that miRNAs have critical functions in the initiation and
progression of various malignancies, including lung cancer
(11,12). For instance, miRNA-145 (miR-145),
miR-328, miR-378 and miR-21 have been reported to have important
roles in lung cancer (13–15). Recently, miR-1284 was reported to
be downregulated in gastric cancer, and miR-1284 overexpression
promoted cell cycle arrested at the G0/G1 phase, accelerated
drug-induced apoptosis, and decreased migration and invasiveness
(16). To the best of our
knowledge, the role of miR-1284 in lung cancer has not been
previously investigated.
The present study aimed to investigate the role of
miR-1284 in lung cancer. Therefore, A549 lung carcinoma cells were
transfected with miR-1284 mimic or miR-1284 inhibitor. Cell
viability, growth and apoptosis were then investigated under the
two conditions. In order to further investigate the underlying
molecular mechanisms, the protein expressions of cell cycle
regulators (p27 and p21), apoptosis-associated proteins, including
Bcl-2 associated X, apoptosis regulator (Bax), pro-caspase-3 and
activated caspase-3, and Myc, were determined by western blot
analysis. Results of the present study may lead to new treatment
options for lung cancer.
Materials and methods
Cell culture
A549 human lung cancer cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
and 5% CO2. The miR-1284 mimic (sense, UCU AUA CAG ACC
CUG GCU UUU C; antisense, AAA GCC AGG GUC UGU AUA GAU U), miR-1284
inhibitor (GAA AAG CCA GGG UCU GUA UAG A) were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The scramble miRNA
control used was a 22 nucleotide random sequence with a
non-targeting RNA duplex (GE Dharmacon; GE Healthcare Life
Sciences, Little Chalfont, UK). The cells were transfected with 50
nM miR-1284 mimic, miR-1284 inhibitor or scramble control by using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's manual. Cells were incubated at
37°C in a CO2 incubator for 24–48 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
At 48 h after transfection was complete, total RNA
(5×106 cells) was extracted by TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and quantified by
using agarose gel (1%) electrophoresis and spectrophotometric
analysis (A260/A280 ratio). For cDNA synthesis, 2 µg total RNA was
reverse-transcribed by using a Transcriptor First-Strand cDNA
Synthesis kit (Roche Molecular Systems, Inc., Pleasanton, CA, USA),
according to the manufacturers protocol. The qPCR was performed by
using FastStart Universal SYBR-Green Master (ROX; Roche Molecular
Systems, Inc.) on the Applied Biosystems 7500 Fast Real-Time PCR
system (Applied Biosystems, Thermo Fisher Scientific, Inc.). The
thermocycling parameters were 95°C for 3 min, followed by 40 cycles
of 95°C for 15 sec and by 60°C for 30 sec. Each sample was run in
triplicate and was normalized to U6 small nuclear RNA levels. The
following primers were used for qPCR: miR-1284,
5′-GGTCTATACAGACCCTGGCTTTTC-3′ (forward) and
5′-CTCAACTGGTGTCGTGGA-3′ (reverse); and U6,
5′-TTATGGGTCCTAGCCTGAC-3′ (forward) and 5′-CACTATTGAGGGTATGC-3′
(reverse). All primers were synthesized by Shanghai GenePharma Co.,
Ltd. Melting curve analysis was performed to confirm the
specificity of the PCR products. The replicates were subsequently
averaged, and fold induction was determined by a ΔΔCq-based fold
change calculation (17).
Cell viability assay
Cells in three groups (transfected with miR-1284,
miR-1284 inhibitor and scramble control) were seeded into 96-well
culture plates at 5×103 cells/well and cultured for 1–5
days. Each group had three replicates. Cell viability was then
assayed by adding 20 µl 10 mg/ml
3-[4,5-dimethylthiazol-yl]-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), followed by
incubation for 3 h at 37°C. Then 150 µl dimethyl sulfoxide was
added to each well, and the optical density at 590 nm was recorded
with a Multiskan EX (Thermo Fisher Scientific, Inc.). The results
of three independent experiments are presented as the mean ±
standard deviation.
Bromodeoxyuridine (BrdU) assay
Cells were seeded in 6-well plates at a density of
2×104 cells/well on sterilized coverslips. At 72 h after
transfection, BrdU (Sigma-Aldrich; Merck KGaA) was added to the
medium at a final concentration of 10 µM. After incubation at 37°C
for 5 h in the dark, cells were fixed in cold 70% ethanol for 5
min. DNA was denaturized by adding 1.5 M HCl to the cells for 30
min at room temperature. Cells were washed with 150 µl PBS and 1%
(v/v) bovine serum albumin (Sigma-Aldrich; Merck KGaA) at room
temperature for 20 min for blocking. Immunofluorescence to
visualize incorporated BrdU was performed using a mouse anti-BrdU
antibody (cat no. 560808; 1:2,000; BD Biosciences, San Jose, CA,
USA) according to the manufacturer's instructions, and incubated
for 1 h at room temperature. Then, secondary red-fluorescence
dye-conjugated antibody Alexa Fluor 594 (ab150116; 1:500; Abcam,
Cambridge, UK) was added and incubated for 45 min at room
temperature. VECTASHIELD mounting medium with DAPI (Vector
Laboratories, Burlingame, CA, USA) was used to stain the nuclei.
Images were visualized using a Leica inverted fully automated
microscope (DMI6000B) with digital camera DFC 420 RGB (Leica
Microsystems GmbH, Wetzlar, Germany).
Apoptosis assay
The cells (24 h after transfection) were seeded into
culture dishes at a density of 2×105 cells per culture
dish. Then cells were incubated with Annexin V-fluorescein
isothiocyanate (BD Biosciences) and propidium iodide (PI; BD
Biosciences) for 30 min in the dark at 25°C to analyze the
apoptotic percentage of cells. Cells were analyzed with a
FACSCalibur flow cytometer (BD Biosciences) and the results were
analyzed by using FlowJo 10 software (Tree Star, Inc., Ashland, OR,
USA).
Western blot analysis
Cells (48 h after transfection) were washed once
with PBS and lysed in RIPA buffer (Pierce; Thermo Fisher
Scientific, Inc.). Protein samples were quantified with the Pierce
BCA Protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) and
were then boiled for 10 min in sodium dodecyl sulfate (SDS) sample
buffer. Equal amounts of protein (20 µg/well) were separated by 10%
SDS-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membrane. After blocking with 5% skim milk in 0.05%
TBS-Tween-20 (v/v) for 1 h at room temperature, the membranes were
incubated with the appropriate primary antibodies (at a dilution of
1:1,000) overnight at 4°C. Antibody specific to actin (C-2; cat no.
4967) was purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA); c-Myc (cat no. ab32072), active caspase-3 (cat no.
ab2302), p27 (cat no. ab54563), p21 (cat no. ab7960) and
pro-caspase-3 (cat no. ab32150) were purchased from Abcam. Bax
antibody (cat no. 2772) was purchased from Cell Signaling
Technology, Inc. Then membranes were washed once with TBS-Tween-20
and incubated with anti-mouse and anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (cat nos. ab131368 and
ab191866, respectively; 1:5,000) for 2 h at room temperature.
Protein bands were visualized by using WEST-ZOL (plus) Western Blot
Detection system (Intron Biotechnology, Inc., Seongnam, Korea).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
statistical software (IBM SPSS, Armonk, NY, USA). All experiments
were repeated three times. The results of multiple experiments were
presented as the mean ± standard deviation. The data were initially
tested for normal distribution using one-sample Kolmogorov-Smirnov
test, and then analyzed by one-way analysis of variance followed by
Dunnett's test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Role of miR-1284 in cell
proliferation
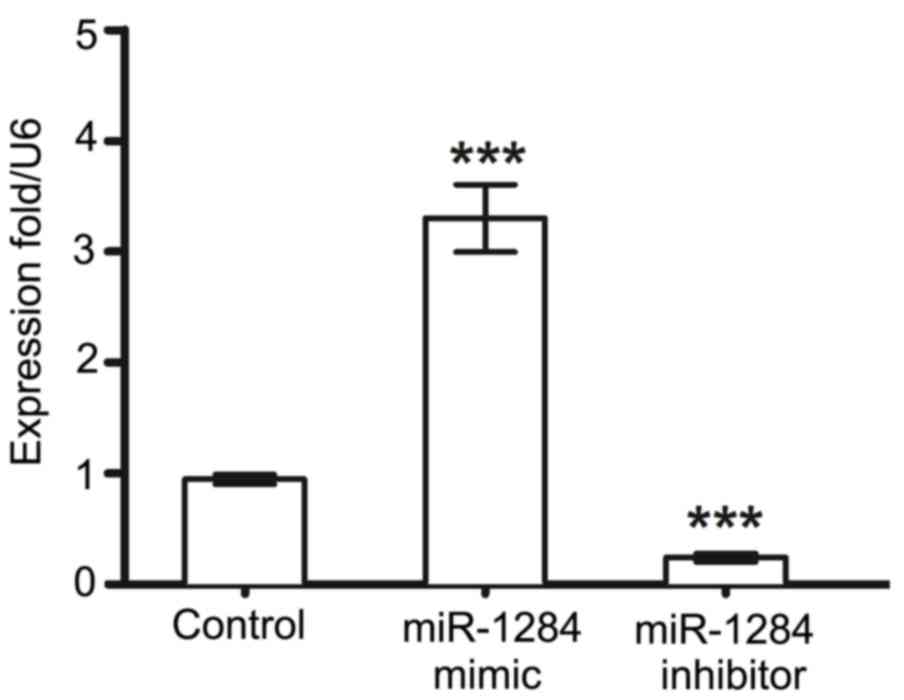
Preliminary experiments to determine whether
transfection with miR-1284 mimic and miR-1284 inhibitor
successfully increased or decreased miR-1284 expression were
performed, and the results indicated that transfection with
miR-1284 mimic successfully increased the expression of miR-1284
and miR-1284 inhibitor transfection reduced levels, compared with
control cells (Fig. 1). To
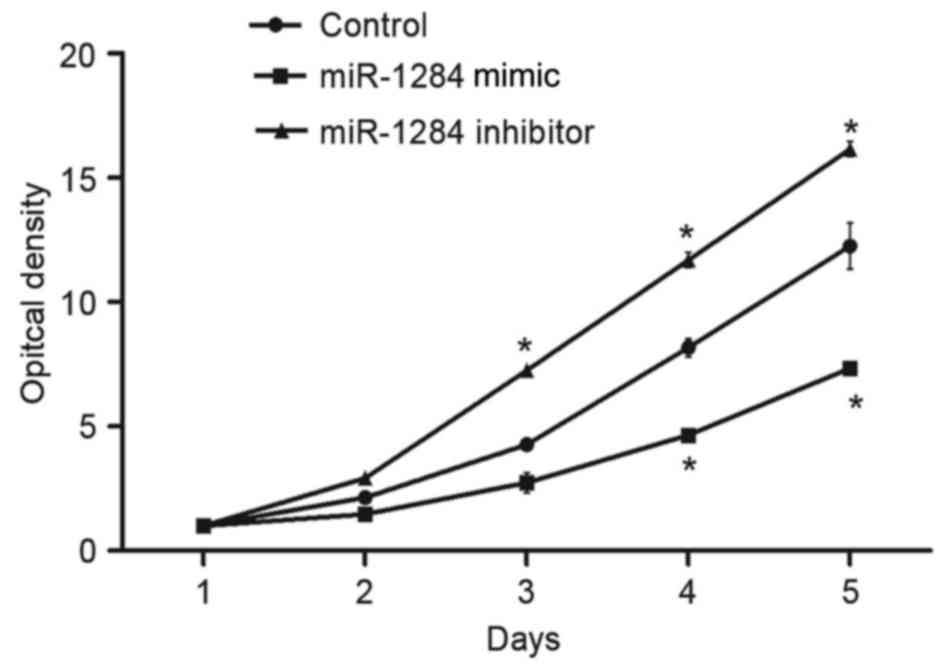
determine the effect of miR-1284 on cell viability, A549 cells were
transfected with miR-1284 or miR-1284 inhibitor and an MTT assay
was performed. As demonstrated in Fig.
2, after transfection with miR-1284 mimic, the cell viability
of A549 cells was decreased significantly compared with control
(P<0.05). When cells were transfected with miR-1284 inhibitor,
the cell viability was significantly increased compared with
control cells (P<0.05).
Role of miR-1284 in cell growth
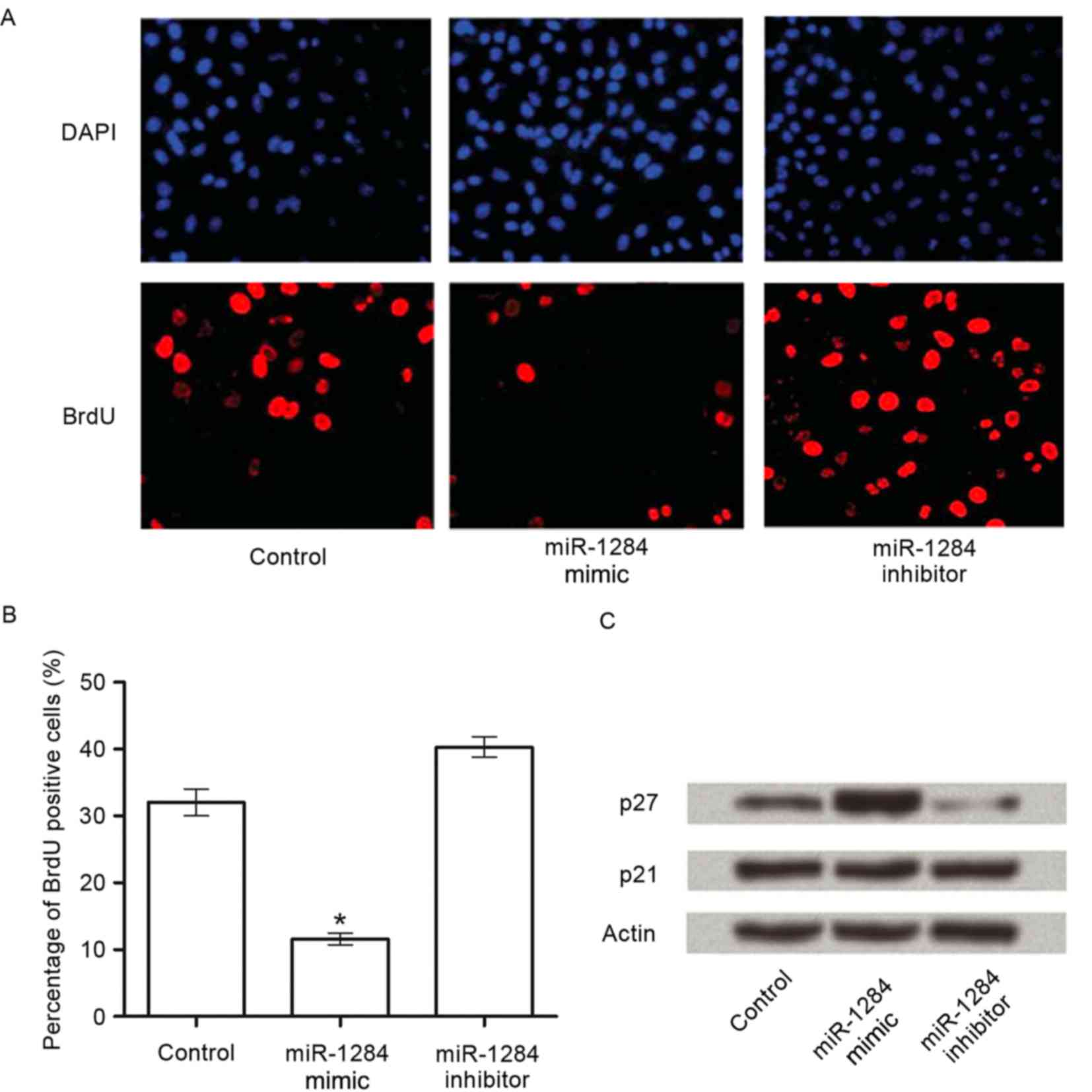
BrdU staining was used to determine the role of
miR-1284 in cell growth. Compared with the control group, a 20.7%
decrease of BrdU-positive cells was detected in response to
miR-1284 overexpression (P<0.05), whereas the miR-1284 inhibitor
caused a 7.3% increase compared with the control group, however,
this was not statistically significant (Fig. 3A and B).
To further understand the mechanisms of miR-1284
induced cell growth inhibition, we analyzed the effect of miR-1284
on cell cycle regulators in A549 cells. A strong increase in p27
was detected in response to the miR-1284 expression compared with
the control. The other major cyclin-dependent kinase inhibitor 1
family member, p21, was unaffected by miR-1284 overexpression or
inhibition (Fig. 3C).
Role of miR-1284 in apoptosis
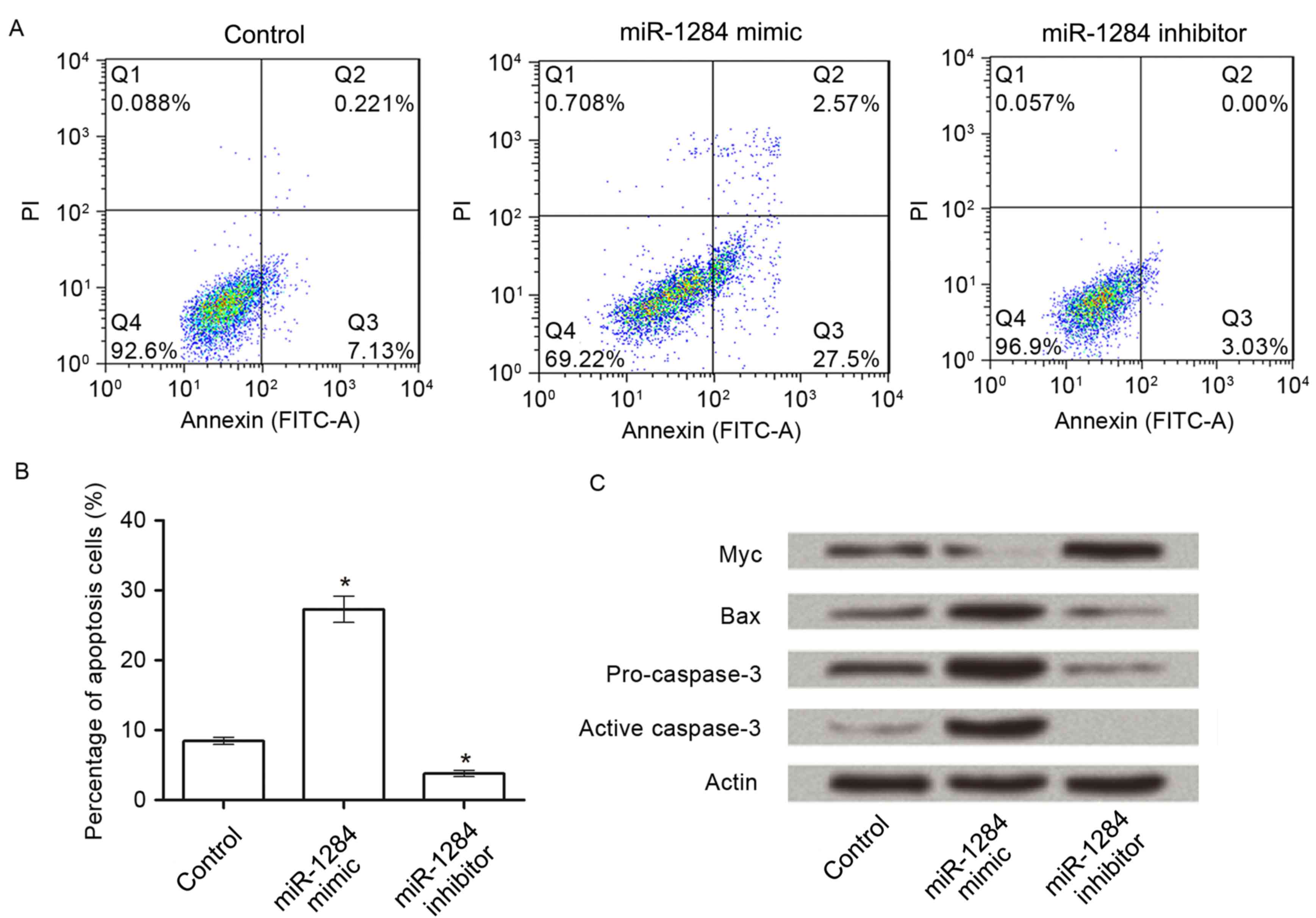
To analyze the effect of miR-1284 on cell apoptosis,
A549 cells were transfected with miR-1284 mimic or inhibitor and an
apoptosis assay was performed. As presented in Fig. 4A and B, following transfection with
miR-1284 mimic in A549 cells, the percentage of apoptotic cells was
significantly increased from 8.0±1.0% (control group) to 27.6±3.2%
(P<0.05). However, when cells were transfected with miR-1284
inhibitor, the percentage (3.09±0.6%) was decreased significantly
compared with the control (P<0.05).
miR-1284 may induce apoptosis by
targeting Myc
To further understand the mechanisms of miR-1284 on
apoptosis, the protein expressions of Myc, Bax, pro-caspase-3 and
activated caspase-3 in the three groups were detected by western
blot. The results in Fig. 4C
demonstrated that, compared with control, the expression of Myc
decreased markedly in the miR-1284 group, and increased markedly in
the miR-1284 inhibitor group. For the other three proteins, their
expressions in the miR-1284 group were higher than in the other two
groups.
Discussion
The results of the present study demonstrated that
miR-1284 has an important role in cell viability, growth and
apoptosis of A549 cells. Following transfection with miR-1284, cell
viability and growth decreased significantly, while apoptosis was
significantly increased. By contrast, miR-1284 inhibition
significantly increased the cell viability and growth, and
decreased apoptosis in A549 cells. Furthermore, the expression of a
cell cycle regulator, p27, was markedly increased and the
expression of Myc was markedly decreased when miR-1284 was
overexpressed in A549 cells. For the other three
apoptosis-associated proteins, their expressions in miR-1284 mimic
group were higher than in the control and miR-1284 inhibitor
groups.
A previous study has reported that altered
expression of miRNAs targeting cyclins, cyclin-dependent kinases
(CDKs) or CDK inhibitors, such as p21 and p27, is involved in the
deregulation of cell cycle progression during tumorigenesis
(18). The results of the present
study indicated that the expression of p27 was increased when
miR-1284 was overexpressed, whereas p21 was unaffected by miR-1284
overexpression or inhibition. p27 belongs to the Cip/Kip family of
CDK inhibitor proteins, which bind to cyclin D alone or its
catalytic subunit CDK4 to control the cell cycle progression at G1
(19). In accordance with this,
the present study demonstrated that cell growth was decreased
significantly by miR-1284 overexpression, indicating that miR-1284
may induce cell growth arrest by upregulating p27 expression in
A549 cells.
Apoptosis is a complex biological process that
enables organisms to kill and remove unwanted cells during their
development, normal homeostasis and disease (20,21).
Evasion of apoptosis is a crucial step in the initiation and
progression of cancer (22). The
caspase family of cysteine proteases have key roles in the
initiation and execution of apoptosis (23,24).
Caspase-3 is a member of the caspase family that exists in the cell
as a low-activity zymogen, pro-caspase-3. Pro-caspase-3 can be
activated by proteolysis, and active caspase-3 is necessary for
initiation of apoptosis (25). In
the current study, miR-1284 overexpression significantly increased
the apoptosis rate of A549 cells. Furthermore, the expression of
pro-caspase-3 and active caspase-3 was also increased by miR-1284,
which indicated that miR-1284 may be involved in accelerating the
apoptosis of A549 cells.
In addition to pro-caspase-3 and active caspase-3,
Bax expression was also increased by miR-1284 overexpression. Bax
is a member of the Bcl-2 apoptosis regulator protein family, which
has been studied intensively owing to their importance in the
regulation of apoptosis and tumorigenesis (26). Bax is a pro-apoptotic protein and
inactive in the cytosol of normal cells but becomes active in
apoptotic cells by changing conformation in a multistep process
(27). In accordance with this,
the results of the present study also demonstrated that Bax was
upregulated in apoptotic cells, further indicating that miR-1284
accelerates apoptosis.
Unlike the aforementioned proteins, the expression
of Myc was decreased in the miR-1284 overexpression group and
increased in the miR-1284 inhibitor group compared with control.
Myc protein is a multifunctional, nuclear phosphoprotein that has a
role in apoptosis. Aberrantly high or deregulated Myc activity is
usually implicated in various types of cancer, and is often
associated with aggressive and poorly differentiated tumors
(28). Cell-autonomous apoptosis
following Myc overexpression has been regarded as a major
tumor-suppression mechanism (29).
Romero et al (30) reported
that amplifications of Myc are one of the most consistent gene
alterations detected in lung cancer. In the current study,
overexpression of Myc was observed in the miR-1284 inhibition
group, which also had the lowest apoptosis rate, suggesting that
Myc may be associated with apoptosis in these cells. Additionally,
the results of the present study also indicated that miR-1284 may
induce apoptosis by targeting Myc in A549 lung cancer cells.
In conclusion, the current study suggests that
miR-1284 affects cell proliferation, growth and apoptosis of lung
cancer cells, indicating that miR-1284 may have a key role in lung
tumorigenesis. Thus, miR-1284 may be a potential therapeutic agent
for the treatment of lung cancer.
References
|
1
|
Peng Y, Dai Y, Hitchcock C, Yang X, Kassis
ES, Liu L, Luo Z, Sun HL, Cui R, Wei H, et al: Insulin growth
factor signaling is regulated by microRNA-486, an underexpressed
microRNA in lung cancer. Proc Natl Acad Sci USA. 110:15043–15058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortettieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Zeng H and Zhang S:
Epidemiology of lung cancer in China. Thoracic Cancer. 6:2092015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janssen-Heijnen ML and Coebergh JW: The
changing epidemiology of lung cancer in Europe. Lung Cancer.
41:245–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahapatra A: Lung cancer-genomics and
personalized medicine. ACS Chem Biol. 5:529–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dumortier O and Van Obberghen E: MicroRNAs
in pancreas development. Diabetes Obes Metab. 14:(Suppl 3).
S22–S28. 2012. View Article : Google Scholar
|
|
8
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nana-Sinkam SP and Croce CM: Clinical
applications for microRNAs in cancer. Clin Pharmacol Ther.
93:98–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arner P and Kulyté A: MicroRNA regulatory
networks in human adipose tissue and obesity. Nat Rev Endocrinol.
11:276–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patnaik SK, Yendamuri S, Kannisto E,
Kucharczuk JC, Singhal S and Vachani A: MicroRNA expression
profiles of whole blood in lung adenocarcinoma. Plos One.
7:e460452012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen J and Jiang F: Applications of
microRNAs in the diagnosis and prognosis of lung cancer. Expert
Opin Med Diagn. 6:197–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun D, Li X, Ma M, Liu J, Xu Y, Ye L, Hou
H, Wang C, Li X and Jiang Y: The predictive value and potential
mechanisms of miRNA-328 and miRNA-378 for brain metastases in
operable and advanced non-small-cell lung cancer. Jpn J Clin Oncol.
45:464–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng
A and Hu J: miRNA-145 inhibits non-small cell lung cancer cell
proliferation by targeting c-Myc. J Exp Clin Cancer Res.
29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao W, Wei W, Zhan Z, Xie Y and Xiao Q:
miR-1284 modulates multidrug resistance of gastric cancer cells by
targeting EIF4A1. Oncol Rep. 35:2583–2591. 2016.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fernandez S, Risolino M, Mandia N, Talotta
F, Soini Y, Incoronato M, Condorelli G, Banfi S and Verde P:
miR-340 inhibits tumor cell proliferation and induces apoptosis by
targeting multiple negative regulators of p27 in non-small cell
lung cancer. Oncogene. 34:3240–3250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu I, Hengst L and Slingerland J: The Cdk
inhibitor p27 in human cancer: Prognostic potential and relevance
to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mckenzie S and Kyprianou N: Apoptosis
evasion: The role of survival pathways in prostate cancer
progression and therapeutic resistance. J Cell Biochem. 97:18–32.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Green DR: Apoptotic pathways: Paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pop C and Salvesen GS: Human caspases:
Activation, specificity, and regulation. J Biol Chem.
284:21777–217817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maurya SK, Tewari M, Sharma B and Shukla
HS: Expression of procaspase 3 and activated caspase 3 and its
relevance in hormone-responsive gallbladder carcinoma chemotherapy.
Korean J Intern Med. 28:573–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Youle R and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding J, Mooers BH, Zhang Z, Kale J,
Falcone D, McNichol J, Huang B, Zhang XC, Xing C, Andrews DW and
Lin J: After embedding in membranes antiapoptotic Bcl-XL protein
binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax
protein to inhibit apoptotic mitochondrial permeabilization. J Biol
Chem. 289:11873–11896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soucek L, Whitfield JR, Sodir NM,
Massó-Vallés D, Serrano E, Karnezis AN, Swigart LB and Evan GI:
Inhibition of Myc family proteins eradicates KRas-driven lung
cancer in mice. Genes Dev. 27:504–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meyer N, Kim SS and Penn LZ: The
Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol.
16:275–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romero OA, Torres-Diz M, Pros E, Savola S,
Gomez A, Moran S, Saez C, Iwakawa R, Villanueva A, Montuenga LM, et
al: MAX inactivation in small cell lung cancer disrupts MYC-SWI/SNF
programs and is synthetic lethal with BRG1. Cancer Discov.
4:292–303. 2014. View Article : Google Scholar : PubMed/NCBI
|