Introduction
Oxidative stress is a key factor in the development
of cardiovascular disease (1). A
growing body of evidence indicates the close correlation between
oxidative stress and the abnormalities in endothelial function.
Increased oxidative stress is a major cause of endothelial
dysfunction by prolonging cell proliferation (2), disturbing cell cycle (3), increasing the production of reactive
oxygen species (ROS) (2),
promoting inflammatory responses (4), and activating various intracellular
signal transduction pathways (5).
Furthermore, systemic and vascular ROS generation modulates
inflammatory responses that contribute to microvascular and
macrovascular damage (6). Hydrogen
peroxide (H2O2), one of the ROS, activates
protein tyrosine kinases, resulting in stimulation of downstream
signaling events that regulate gene expression and in subsequent
modification of cardiovascular cells.
Ophiopogon japonicus is a plant, used in
traditional Chinese medicine, and widely distributed in Southeast
Asia (7). Previous studies have
revealed that O. japonicus exhibitsanti-inflammatory
properties and beneficial cardiovascular effects, including
anti-ischemia and anti-arrhythmic effects, inhibiting platelet
aggregation, protecting endothelium from apoptosis, and improving
microcirculation (8,9). MDG-1, a water-soluble polysaccharide
extracted from O. japonicus, has been reported to protect
cardiomyocytes from hypoxia/reoxygenation-induced damage (10). In addition, MDG-1 presents
remarkable anti-ischemic activity and protects cardiomyocytes and
human microvascular endothelial cells (HMEC-1) from
ischemia-induced cell damage through the
sphingosine-1-phosphate/basic fibroblast growth
factor/Akt/extracellular signal-regulated kinase (ERK)/endothelial
nitric oxide synthase signaling pathway (11).
Human umbilical vein endothelial cells (HUVECs) are
the most widely used cell line to study the mechanisms of
cardiovascular diseases. Pyruvate protects HUVECs from
H2O2-induced dysfunction and improves
survival following oxidative stress via blocking the
mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB
pathways (12). Resveratrol
protects HUVECs from H2O2-induced oxidative
stress and senescence via sirtuin1 activation (13). However, although multiple
biological functions of MDG-1 have been identified to date, the
potential effect of MDG-1 to the H2O2-induced
endothelial injury has not been explored.
The specific aim of the present study was to
evaluate the potential protective effect of MDG-1 in HUVECs under
oxidative stress. H2O2 was used to induce
oxidative stress in HUVECs and to explore the effect of MDG-1 on
the endothelium following oxidative stress. The present findings
demonstrated that MDG-1 protected HUVECs against
H2O2-induced apoptosis and inflammation, by
inhibiting capspase-3 and BCL2 associated X (Bax)/BCL2 apoptosis
regulator (Bcl-2) ratio expression, as well as inhibiting the
secretion of inflammatory factors.
Materials and methods
Cultivation of HUVECs
HUVECs were purchased from the American Type Culture
Collection (Manassas, VA, USA). HUVECs were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin. Cultures were maintained in a 37°C incubator under a
humidified atmosphere of 5% CO2/95% air.
H2O2-induced
HUVECs injury
Cultured HUVECs were pre-incubated with MDG-1 (5, 10
or 50 mM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 12, 24
and 48 h prior to H2O2 treatment (100, 300 or
500 µM). Following the indicated time, the medium was removed, and
the cells were subjected to the subsequent experiments.
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
technologies, Inc., Rockville, MD, USA) was used to assess cell
viability in HUVECs. In brief, HUVECs in the logarithmic
growth-phase were collected, and 5×104 cells/well were
dispensed into 96-well culture plates with 100 µl culture medium.
After 24 h of culture, different concentrations of MDG-1 (5, 10 or
50 mM) were added to each well prior to H2O2
treatment (100, 300 or 500 µM). Each of the concentrations above
was regarded as one treatment group. Culture plates were then
incubated for 4, 6 and 12 h, and cell viability was evaluated by
CCK-8, following the manufacturer's instructions. Absorbance was
measured at 450 nm using a microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). The optical density of indicated groups
was used as a surrogate measurement for cell viability.
Apoptosis assay
HUVECs were treated with different concentrations of
MDG-1 (5, 10 or 50 mM) prior to H2O2
treatment (100, 300 or 500 µM) for 12 h. Cell apoptosis was then
analyzed using the Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis kit (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer's protocol.
In brief, HUVECs following treatment were washed three times with
PBS, trypsinized, centrifuged (400 × g at room temperature) for 10
min, and resuspended to a final concentration of 5×104
cells/ml in binding buffer containing Annexin V-FITC and PI.
Apoptotic cells were then analyzed using a BD Accuri C6 flow
cytometer equipped with BD Accuri C6 software (version 1.0.264)
(both from BD Biosciences).
Detection of ROS
HUVECs (3×103 cells/well) were cultured
on a slide in DMEM and the indicated treatments were performed.
Following treatment, the cells were washed three times with PBS,
trypsinized, centrifuged (400 × g at room temperature) for 10 min
and resuspended to a final concentration of 5×104
cells/ml. The dihydroethidium (DHE; 50 µM; Beyotime Institute of
Biotechnology) probe was then added to the cells at 37°C for 30
min, following which the cells were analyzed by flow cytometry (BD
Biosciences).
ELISA
HUVECs (5×104 cells/well) were seeded in
6-well culture plates and the indicated treatments were performed.
The concentration of each secreted inflammatory factor in the cell
supernatant was measured by ELISA, according to the manufacturer's
protocol. ELISA kits were purchased as following: Human tumor
necrosis factor (TNF-α; 070133h; Shanghai WuHao Trading Co., Ltd.,
Shanghai, China); human interleukin 1β (IL-1β; DL-IL1b-Hu; Wuxi
DonglinSci & Tech Development Co., Ltd., Wuxi, China); human
IL-6 (ab46042; Abcam, Cambridge, MA, USA); human cyclooxygenase-2
(Cox-2; ESK5229-48T; Sangon Biotech Co., Ltd., Shanghai, China).
ELISA kits were used according to the manufacturer's protocol.
Western blot analysis
HUVECs were harvested, washed twice with PBS, and
lyzed with radio immuno precipitation assay buffer (Beyotime
Institute of Biotechnology) with freshly added 0.01% protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA) on ice for 30 min.
Cell lysates were centrifuged at 1,000 × g for 10 min at 4°C. The
supernatant (20–30 µg of protein) was separated on 10% SDS-PAGE and
transferred electrophoretically to a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The blots were
blocked with 5% skim milk overnight at 4°C, followed by incubation
with primary antibodies overnight at 4°C. Rabbit polyclonal
antibodies against Bcl-2 (cat. no. sc-492; 1:150) and Bax (cat. no.
sc-493; 1:100) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). A rabbit polyclonal antibody against caspase-3
(cat. no. ab2302; 1:500) was purchased from Abcam. A rabbit
monoclonal antibody against GAPDH (cat. no. 5174; 1:1,500) was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Blots were then incubated with goat anti-rabbit secondary antibody
(Beyotime Institute of Biotechnology) for 1 h at 37°C and
visualized using enhanced chemiluminescence reagents (EMD
Millipore). Quantity One 4.62 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to quantitatively analyze protein
expression levels.
Statistical analysis
Data were expressed as the mean ± standard deviation
of triplicate experiments. One-way analysis of variance, followed
by Tukey's post hoc test, was used to analyze the significance of
differences between groups with SPSS 19.0 (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
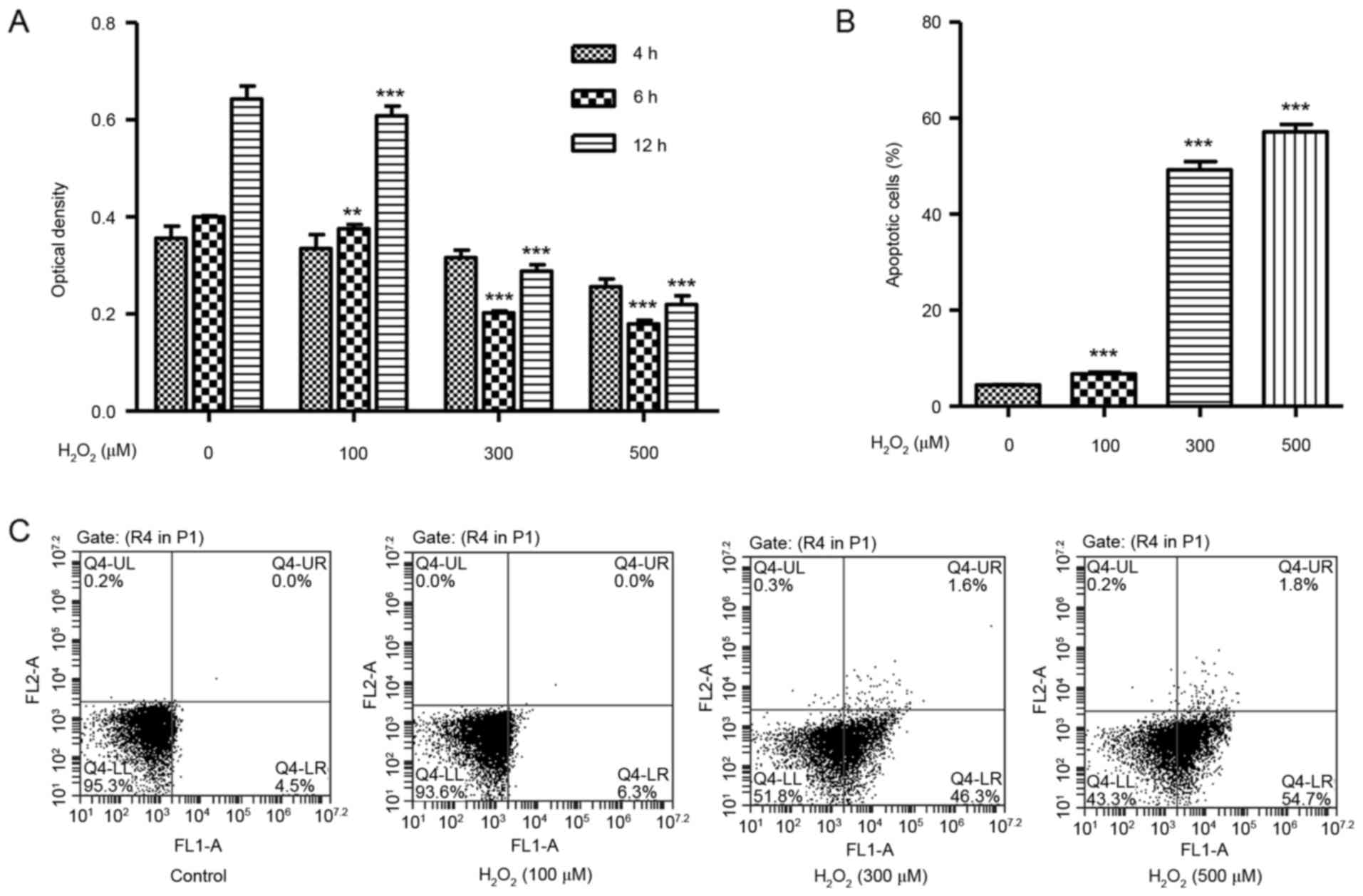
H2O2 induces
cell death and apoptosis in HUVECs
To identify whether H2O2 may
cause cytotoxicity in vitro, the effect of
H2O2 on HUVEC viability was determined using
the CCK-8 assay. HUVECs were treated with
H2O2 at concentrations of 100, 300 or 500 µM,
and exhibited significantly decreased cell viability in a
dose-dependent manner at 6 and 12 h (Fig. 1A). A flow cytometry assay was then
used in order to assess the effect of H2O2 on
HUVEC apoptosis. HUVECs were treated with increasing concentrations
of H2O2 for 12 h and exhibited a significant
dose-dependent increase in the % of apoptotic cells over total,
compared with untreated cells (Fig. 1B
and C).
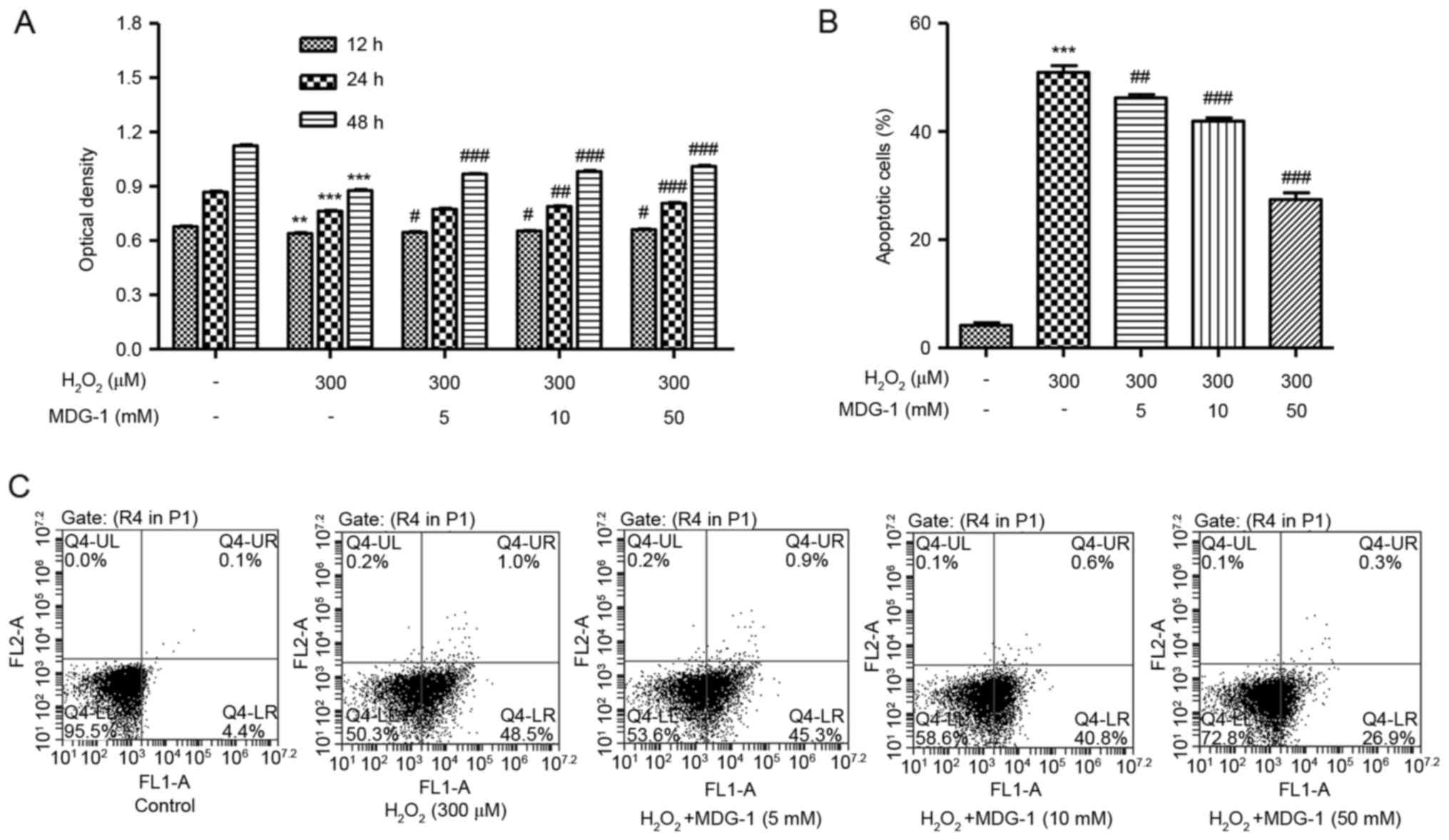
MDG-1 protects from
H2O2-induced cell death and apoptosis in
HUVECs
To investigate the effect of MDG-1 on
H2O2-induced cytotoxicity, HUVECs were
pretreated with MDG-1 at different concentrations prior to exposure
to 300 µM H2O2 for 12 h. As presented in
Fig. 2A, pretreatment of HUVECs
with 5, 10 or 50 µM MDG-1 for 12, 24 or 48 h significantly
increased cell viability in a dose-dependent manner, compared with
cells treated with H2O2 alone. In addition,
pretreatment of HUVECs with MDG-1 at concentrations of 5, 10 or 50
mM for 24 h significantly decreased cell apoptosis in a
dose-dependent manner, compared with cells treated with
H2O2 alone (Fig.
2B and C). These results indicate that MDG-1 pretreatment
protected HUVECs against H2O2-induced
toxicity.
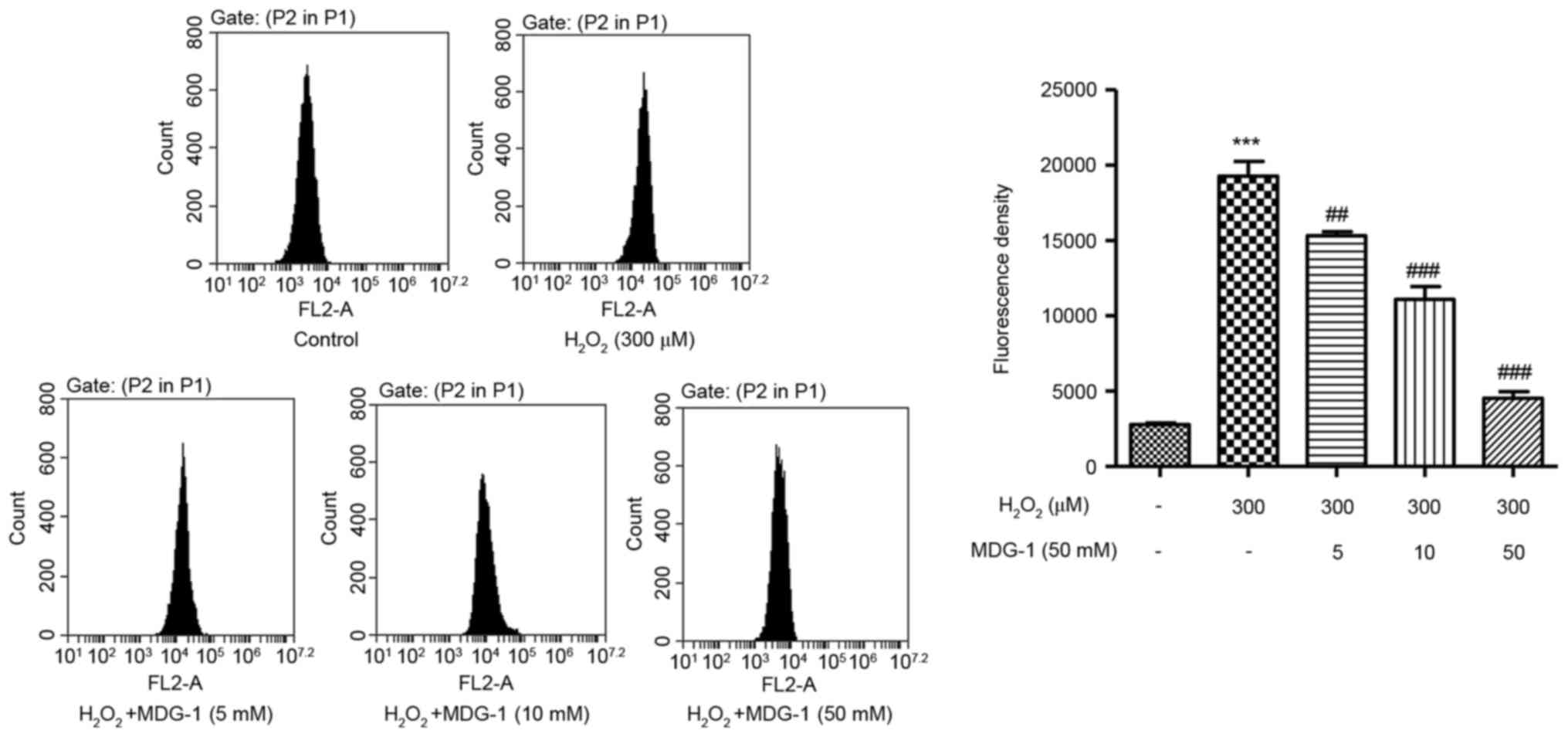
MDG-1 inhibits
H2O2-induced ROS generation in HUVECs
To elucidate the possible mechanisms by which MDG-1
prevented H2O2-induced HUVEC apoptosis, ROS
generation was measured in HUVECs that were treated with
H2O2 and/or MDG-1. Exposure of HUVECs to 300
µM H2O2 for 12 h significantly enhanced ROS
generation compared with untreated cells (Fig. 3). However, pretreatment with MDG-1
for 24 h (at concentrations of 5, 10 or 50 mM) significantly
attenuated the H2O2-induced increase in ROS
generation in a dose-dependent manner (Fig. 3). These findings demonstrated that
the protective function of MDG-1 on HUVEC
H2O2-induced toxicity may be associated
through its antioxidant effect.
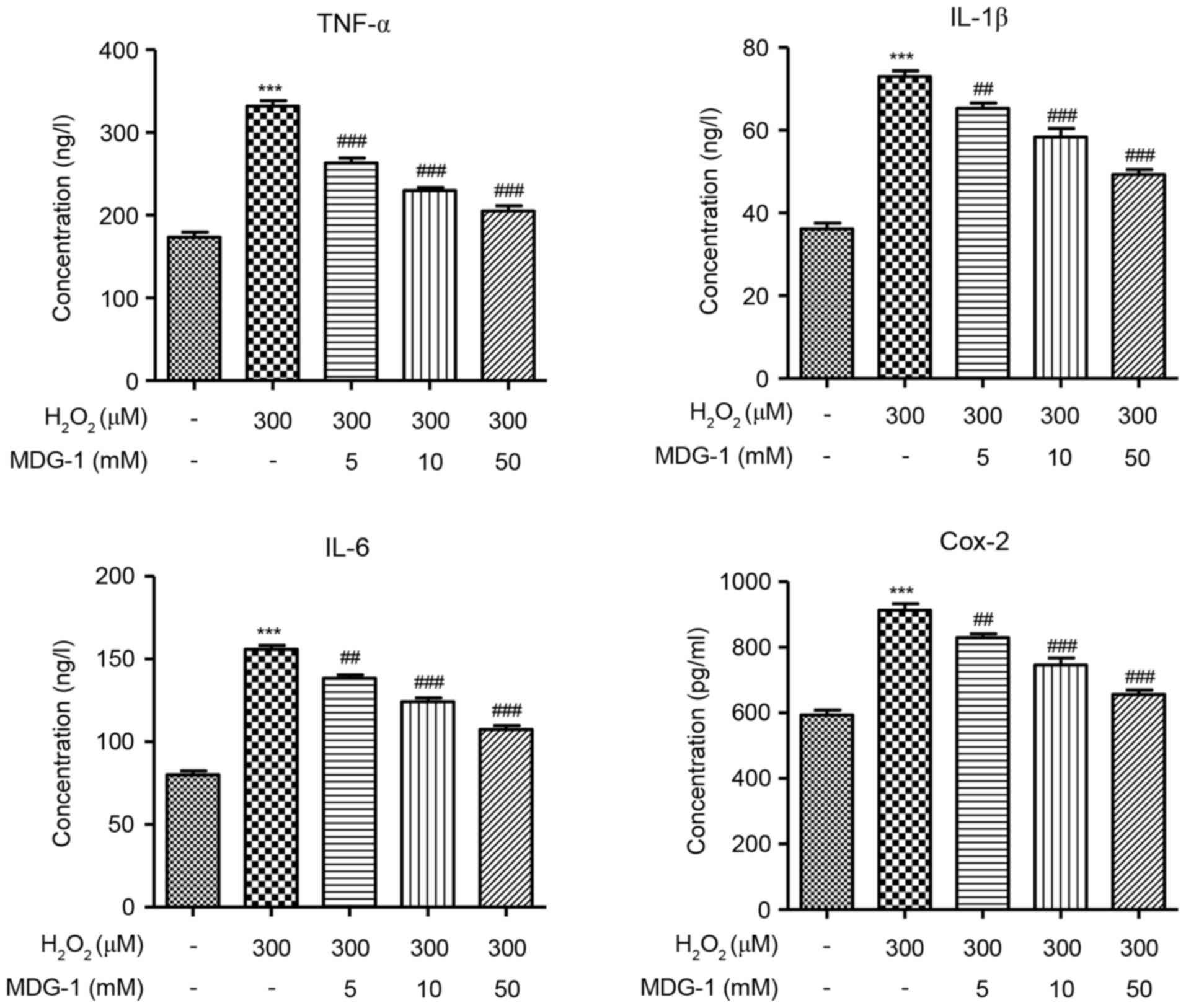
MDG-1 inhibits the
H2O2-induced secretion of inflammatory
factors in HUVECs
To determine the effect of MDG-1 in the inflammatory
response of endothelial cells, secretion of the inflammatory
factors TNF-α, IL-1β, IL-6 and Cox-2 was measured by ELISA in
HUVECs that were treated with H2O2 and/or
MDG-1. The concentration of TNF-α, IL-1β, IL-6 and Cox-2 was
significantly increased in H2O2-induced
HUVECs, compared with untreated cells (Fig. 4). However, pretreatment with MDG-1
for 24 h resulted in a significant and dose-dependent decrease in
TNF-α, IL-1β, IL-6 and Cox-2 secretion, compared with cells induced
with H2O2 alone (Fig. 4). These results indicated that
MDG-1 pretreatment protected against
H2O2-induced inflammatory responses in
HUVECs.
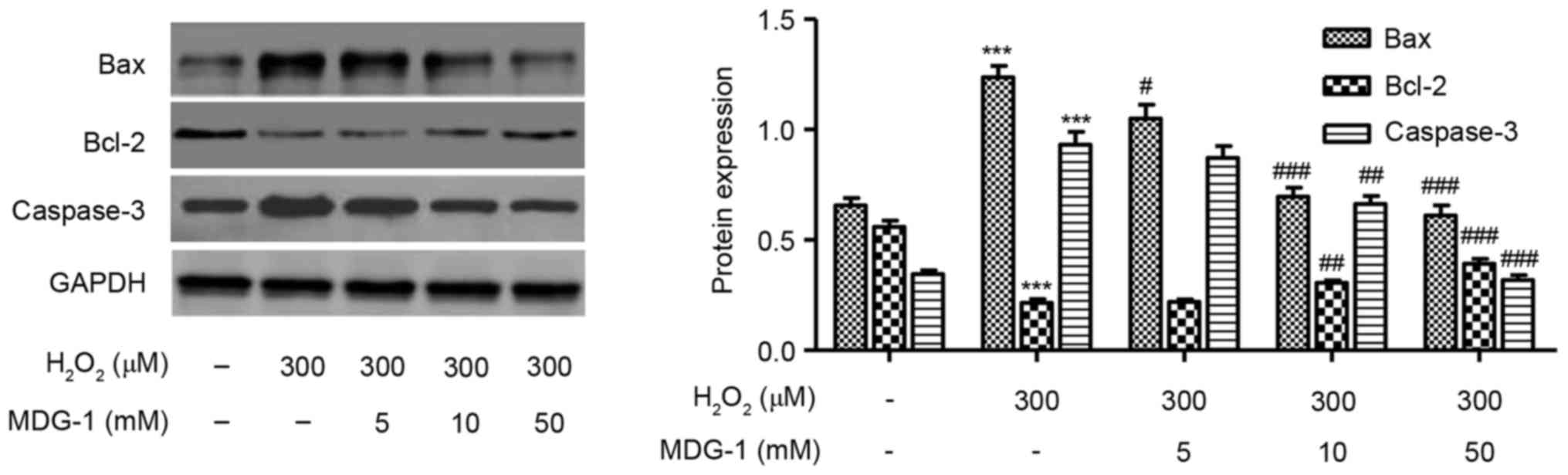
Effect of MDG-1 on expression of
apoptosis-related proteins
Western blot analysis was performed to detect the
protein expression levels of Bax, Bcl-2 and caspase-3 in HUVECs
treated with 300 µM H2O2 for 12 h.
H2O2 treatment decreased the expression of
the antiapoptotic protein Bcl-2, while it increased the expression
of the proapoptotic proteins caspase-3 and Bax, compared with
untreated cells (Fig. 5). Notably,
MDG-1 pretreatment at concentrations of 5, 10 and 50 mM markedly
reversed the effects induced by H2O2
treatment on apoptosis-related protein expression, compared with
cells treated with H2O2 alone (Fig. 5).
Discussion
Hypoxia-induced injury occurs in many diseases,
including sickle cell disease (14), cardiovascular disease (15) and cerebrovascular disease (16). Oxidative stress and increased
inflammatory response are two key risk factors for these diseases.
MDG-1, a drug extracted from O. japonicus, exerts various
effects in vivo, including anti-ischemic properties
(17), cytoprotective and
proangiogenic effects (11).
Therefore, the present study tested the hypothesis that MDG-1 may
confer protective effects against
H2O2-induced vascular injuries.
To investigate whether MDG-1 mayprotect HUVECs
against H2O2-induced cytotoxicity, HUVECs
were pretreated with MDG-1 at concentrations 5–50 mM for 24 h prior
to exposure to 300 µM H2O2. Notably, the
results demonstrated that pretreatment with MDG-1 significantly
enhanced HUVEC viability and attenuated HUVEC apoptosis induced by
H2O2. The present results also indicated that
the protective effects of MDG-1 were time and dose-dependent. The
anticytotoxic and antiapoptotic effect of MDG-1 has also been
previously reported in high-fat diet-induced obese C57BL/6 mice
(18) and ischemia-induced HMEC-1
cells (11).
Another important finding of the present study was
that MDG-1 inhibited oxidative stress induced by
H2O2 in HUVECs. Treatment with
H2O2 elicited a marked increase in ROS
generation in HUVECs and this increased ROS production was
significantly abrogated by pretreatment with MDG-1. The oxidative
stress response may be a potential mechanism by which MDG-1 affects
the viability of HUVECs, as it has been recently recognized as a
mediator of cell apoptosis. Scavenging of intracellular ROS, such
as the hydroxyl radical, or increasing the intracellular levels of
reduced glutathione with membrane-permeable antioxidants,
significantly blocks apoptosis in endothelial cells (19). Intracellular ROS has been
demonstrated to function as a second messenger activating a set of
MAPK family members, including ERK1/2 (20), c-Jun N-terminal kinase (21) and p38 MAPK (22). Further studies will be required to
elucidate other pathways mediating the inhibition of ROS generation
by MDG-1, such as the phosphoinositide 3-kinase/Akt pathway
(23).
The inflammatory response is an important
contributing factor in H2O2-induced injury.
Several inflammatory cytokines are induced by
H2O2 in endothelial cells (24). Ophiopogonin D inhibits
H2O2-induced secretion of inflammatory
factors, such as TNF-α and IL-6, in HUVECs (25). Therefore,
H2O2 is a powerful proinflammatory mediator
in endothelial cells. In the present study, besides cytotoxicity
and oxidative stress, H2O2 treatment also
affected the inflammatory response in HUVECs, as evidenced by an
increase in the secretion of TNF-α, IL-1β, IL-6 and Cox-2. Of note,
MDG-1 pretreatment significantly attenuated this
H2O2-stimulated increase in TNF-α, IL-1β,
IL-6 and Cox-2 secretion in HUVECs, suggesting that MDG-1 protected
HUVECs against H2O2-induced inflammatory
response. Cox-2 is a potent proinflammatory mediator that promotes
the production of multiple inflammatory factors (26). The Huang Qi herb, which is
frequently used in traditional Chinese medicine, has been reported
to reduce ischemia/reperfusion-induced injury partly by inhibition
of Cox-2 (27). In addition, Cox-2
inhibition by NS-398 can confer anti-inflammatory effects,
decreasing IL-6 secretion and increasing IL-10 secretion in a liver
damage rat model (28). These data
suggest that IL-6 and Cox-2 are proinflammatory factors. However,
IL-6 confers cytoprotective effects by diminishing oxidant-mediated
endothelial cell injury, mediated partly by a signal transducer and
activator of transcription 3 and MAPK kinase 1 signaling (29). These results imply that different
cell type, disease model and experimental conditions may affect the
precise function of IL-6. Thus, the biological effects of IL-6 on
H2O2-induced inflammation require further
research in vitro and in vivo.
Pretreatment of HUVECs with catalpol increases
expression of Bcl-2, decreases expression of Bax, induces Akt
activation and BCL2 associated agonist of cell death (Bad)
phosphorylation, and ultimately results in reduced
H2O2-induced apoptosis (30). Among the Bcl-2 family, several
members, such as Bcl-2 and Bcl-extra large induce cell survival,
while other members, such as Bad and Bax, promote cell death
(31). Furthermore, it has been
demonstrated that members of the Bcl-2 family, which are located on
the mitochondrial membrane, can alter mitochondrial membrane
permeability and trigger apoptosis (32). H2O2 has been
reported to induce cell death in U937 myeloid cells by decreasing
the Bcl-2/Bax ratio (33).
H2O2 has also been reported to induce
apoptosis in PC12 rat adrenal pheochromocytoma cells via activation
of caspase-3 (34). In the present
study, H2O2 treatment decreased the
expression of Bcl-2, while it increased the expression of the
proapoptotic proteins caspase-3 and Bax in HUVECs, compared with
untreated cells. Notably, MDG-1 pretreatment markedly reversed
these H2O2-induced effects.
In conclusion, the present results indicated that
MDG-1 may be a potential candidate for preventing oxidative
stress-induced damage to endothelial cells. Further studies are
required to fully elucidate the potential utility of MDG-1 in
protection against cardiovascular dysfunction.
Acknowledgements
The present study was supported by the National
Natural Foundation of China (grant no. 81501376) and the
Fundamental Research Funds for the Central Universities (grant no.
2042017kf0111).
References
|
1
|
Heitzer T, Schlinzig T, Krohn K, Meinertz
T and Münzel T: Endothelial dysfunction, oxidative stress, and risk
of cardiovascular events in patients with coronary artery disease.
Circulation. 104:2673–2678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong F, Zhang X, Wold LE, Ren Q, Zhang Z
and Ren J: Endothelin-1 enhances oxidative stress, cell
proliferation and reduces apoptosis in human umbilical vein
endothelial cells: Role of ETB receptor, NADPH oxidase and
caveolin-1. Brit J Pharmacol. 145:323–333. 2005. View Article : Google Scholar
|
|
3
|
Assmus B, Urbich C, Aicher A, Hofmann WK,
Haendeler J, Rössig L, Spyridopoulos I, Zeiher AM and Dimmeler S:
HMG-CoA reductase inhibitors reduce senescence and increase
proliferation of endothelial progenitor cells via regulation of
cell cycle regulatory genes. Circ Res. 92:1049–1055. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clapp BR, Hingorani AD, Kharbanda RK,
Mohamed-Ali V, Stephens JW, Vallance P and MacAllister RJ:
Inflammation-induced endothelial dysfunction involves reduced
nitric oxide bioavailability and increased oxidant stress.
Cardiovasc Res. 64:172–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown DI and Griendling KK: Regulation of
signal transduction by reactive oxygen species in the
cardiovascular system. Circ Res. 116:531–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ceriello A and Motz E: Is oxidative stress
the pathogenic mechanism underlying insulin resistance, diabetes,
and cardiovascular disease? The common soil hypothesis revisited.
Arterioscl Throm Vas Biol. 24:816–823. 2004. View Article : Google Scholar
|
|
7
|
Duan CL, Kang ZY, Lin CR, Jiang Y, Liu JX
and Tu PF: Two new homoisoflavonoids from the fibrous roots of
Ophiopogon japonicus (Thunb.) Ker-Gawl. J Asian Nat Prod
Res. 11:876–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu
B and Xu Q: Anti-inflammatory activities of aqueous extract from
Radix Ophiopogon japonicus and its two constituents. Biol
Pharma Bull. 28:1234–1238. 2005. View Article : Google Scholar
|
|
9
|
Kou J, Tian Y, Tang Y, Yan J and Yu B:
Antithrombotic activities of aqueous extract from Radix
Ophiopogon japonicus and its two constituents. Biol Pharm
Bull. 29:1267–1270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Q, Feng Y, Xu DS, Lin X and Chen YZ:
Influence of sulfation on anti-myocardial ischemic activity of
Ophiopogon japonicus polysaccharide. J Asian Nat Prod Res.
11:306–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Zhang Z, Lin X, Xu DS, Feng Y and
Ding K: A polysaccharide, MDG-1, induces S1P1 and bFGF expression
and augments survival and angiogenesis in the ischemic heart.
Glycobiology. 20:473–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee YJ, Kang IJ, Bünger R and Kang YH:
Enhanced survival effect of pyruvate correlates MAPK and NF-kappaB
activation in hydrogen peroxide-treated human endothelial cells. J
Appl Physiol (1985). 96:792–801. 2004. View Article : Google Scholar
|
|
13
|
Kao CL, Chen LK, Chang YL, Yung MC, Hsu
CC, Chen YC, Lo WL, Chen SJ, Ku HH and Hwang SJ: Resveratrol
protects human endothelium from H(2)O(2)-induced oxidative stress
and senescence via SirT1 activation. J Atheroscl Throm. 17:970–979.
2010. View
Article : Google Scholar
|
|
14
|
Pritchard KA, Ou J, Ou Z, Shi Y, Franciosi
JP, Signorino P, Kaul S, Ackland-Berglund C, Witte K, Holzhauer S,
et al: Hypoxia-induced acute lung injury in murine models of sickle
cell disease. Am J Physiol Lung Cell Mol Physiol. 286:L705–L714.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsushita H, Morishita R, Nata T, Aoki M,
Nakagami H, Taniyama Y, Yamamoto K, Higaki J, Yasufumi K and
Ogihara T: Hypoxia-induced endothelial apoptosis through nuclear
factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: In vivo
evidence of the importance of NF-kappaB in endothelial cell
regulation. Circ Res. 86:974–981. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chrissobolis S and Faraci FM: The role of
oxidative stress and NADPH oxidase in cerebrovascular disease.
Trends Mol Med. 14:495–502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Lin X, Wang LY, Ruan KF, Feng Y
and Li XY: A polysaccharides MDG-1 augments survival in the
ischemic heart by inducing S1P release and S1P1 expression. Int J
Biol Macromol. 50:734–740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi LL, Li Y, Wang Y and Feng Y: MDG-1, an
Ophiopogon polysaccharide, regulate gut microbiota in
high-fat diet-induced obese C57BL/6 mice. Int J Biol Macromol.
81:576–583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abello PA, Fidler SA, Bulkley GB and
Buchman TG: Antioxidants modulate induction of programmed
endothelial cell death (apoptosis) by endotoxin. Arch Surg.
129:134–141. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe N, Zmijewski JW, Takabe W,
Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J,
Arai H, et al: Activation of mitogen-activated protein kinases by
lysophosphatidylcholine-induced mitochondrial reactive oxygen
species generation in endothelial cells. Am J Pathol.
168:1737–1748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin SJ, Shyue SK, Liu PL, Chen YH, Ku HH,
Chen JW, Tam κB and Chen YL: Adenovirus-mediated overexpression of
catalase attenuates oxLDL-induced apoptosis in human aortic
endothelial cells via AP-1 and C-Jun N-terminal
kinase/extracellular signal-regulated kinase mitogen-activated
protein kinase pathways. J Mol Cell Cardiol. 36:129–139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Usatyuk PV, Vepa S, Watkins T, He D,
Parinandi NL and Natarajan V: Redox regulation of reactive oxygen
species-induced p38 MAP kinase activation and barrier dysfunction
in lung microvascular endothelial cells. Antioxid Redox Sign.
5:723–730. 2003. View Article : Google Scholar
|
|
23
|
Wang LY, Wang Y, Xu DS, Ruan KF, Feng Y
and Wang S: MDG-1, a polysaccharide from Ophiopogon
japonicus exerts hypoglycemic effects through the PI3K/Akt
pathway in a diabetic KKAy mouse model. J Ethnopharmacol.
143:347–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai H: Hydrogen peroxide regulation of
endothelial function: Origins, mechanisms, and consequences.
Cardiovasc Res. 68:26–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian J, Jiang F, Wang B, Yu Y, Zhang X,
Yin Z and Liu C: Ophiopogonin D prevents
H2O2-induced injury in primary human
umbilical vein endothelial cells. J Ethnopharmacol. 128:438–445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang C, Yang Z, Zhang M, Dong Q, Wang X,
Lan A, Zeng F, Chen P, Wang C and Feng J: Hydrogen sulfide protects
against chemical hypoxia-induced cytotoxicity and inflammation in
HaCaT cells through inhibition of ROS/NF-κB/COX-2 pathway. PLoS
One. 6:e219712011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chi Y and Kim H: Suppression of
cyclooxygenase-2 expression of skin fibroblasts by wogonin, a plant
flavone from Scutellaria radix. Prostaglandins Leukot Essent
Fatty Acids. 72:59–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Li YM, Li X, Shi B, He MY, Zhu XL,
Zhou WC, Wachtel MS and Frezza E: COX-2 inhibition improves immune
system homeostasis and decreases liver damage in septic rats. J
Surg Res. 157:43–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Waxman AB, Mahboubi K, Knickelbein RG,
Mantell LL, Manzo N, Pober JS and Elias JA: Interleukin-11 and
interleukin-6 protect cultured human endothelial cells from
H2O2-induced cell death. Am J Respir Cell Mol
Biol. 29:513–522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu L, Sun Y and Hu J: Catalpol inhibits
apoptosis in hydrogen peroxide-induced endothelium by activating
the PI3K/Akt signaling pathway and modulating expression of Bcl-2
and Bax. Eur J Pharmacol. 628:155–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olivetti G, Abbi R, Quaini F, Kajstura J,
Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski
S, et al: Apoptosis in the failing human heart. New Engl J Med.
336:1131–1141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun XM, Bratton SB, Butterworth M,
MacFarlane M and Cohen GM: Bcl-2 and Bcl-xL inhibit CD95-mediated
apoptosis by preventing mitochondrial release of Smac/DIABLO and
subsequent inactivation of X-linked inhibitor-of-apoptosis protein.
J Biol Chem. 277:11345–11351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fukamachi Y, Karasaki Y, Sugiura T, Itoh
H, Abe T, Yamamura K and Higashi K: Zinc suppresses apoptosis of
U937 cells induced by hydrogen peroxide through an increase of the
Bcl-2/Bax ratio. Biochem Biophys Res Commun. 246:364–369. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamakawa H, Ito Y, Naganawa T, Banno Y,
Nakashima S, Yoshimura S, Sawada M, Nishimura Y, Nozawa Y and Sakai
N: Activation of caspase-9 and-3 during
H2O2-induced apoptosis of PC12 cells
independent of ceramide formation. Neurol Res. 22:556–564. 2000.
View Article : Google Scholar : PubMed/NCBI
|