Introduction
Liver ischemia/reperfusion (I/R) injury is caused by
blood deprivation and subsequent reperfusion. It caused the release
of biological mediators contributing to liver dysfunction
eventually (1). Although Liver IR
injury is a main complication of hemorrhagic shock, resection and
transplantation, its mechanisms haven't been described adequately
(2). The pathophysiology of liver
I/R injury may include ATP depletion, caused by decrease in
oxidative phosphorylation, ROS (reactive oxygen species) creation,
cytokines and chemokines production by kupffer cells, neutrophil
accumulation, nitric oxide, apoptosis and necrosis (3). For example, liver I/R can induce
Kupffer cell activation releasing TNF α. The increasing serum TNF α
levels resulted in not only liver injury but also remote organ
insult (4). Effects on hepatic
secretory function and microsomal drug metabolizing systems varied
in duration of ischemia or reperfusion. These may be related to
lipid peroxidation rise (5). A lot
of research suggested that liver I/R injury was age-dependent,
which may be associated with neutrophil recruitment and function or
NF-kB activation (6,7). The age-related mechanism of NF-κB
activation in liver I/R injury could be related to recruitment of
phosphorylated and ubiquitinylated NF-κB-inhibitoryprotein, IκBα,
to the proteasome. This biological process can be stopped by
expression decline of proteasome subunit, non-ATPase 4 (PSMD4)
(8). Many methods and drugs had
been applied to ameliorate liver I/R injury (9–11).
Blood supply restoration was a primary step to treat ischemia
damage in clinical work. But reperfusion itself may exacerbate
organ injury induced by ischemia alone. Many therapeutic strategies
should be considered when applied to reduce tissue injury (12). Nowadays, pathways, pivotal genes or
cellular functions about liver ischemia and reperfusion, have not
been demonstrated clearly. In order to explore more theoretical
information about I/R injury precaution and treatment, we tried to
compare different molecular mechanisms between liver ischemia
followed by reperfusion and ischemia alone.
Materials and methods
Microarray data
Gene expression profile dataset GSE10657 was
obtained from the Gene Expression Omnibus database (GEO, https://www.ncbi.nlm.nih.gov/geo/), including 30
liver tissue samples (8). The
annotation platform was GPL1261 [Mouse430_2] Affymetrix Mouse
Genome 430 2.0 Array. A total of 30 liver tissue samples were
collected for analysis of whole mouse genome microarrays. We
selected the data of two groups (ischemia of 90 min and 90 min of
ischemia followed by 1 h of reperfusion) from 1-year-old mice. Each
group included 3 mice.
Data processing
The expression data were processed using the R
package limma in Bioconductor (http://www.bioconductor.org/), including background
correction, quantile normalization, log2 transformed and final
probe summarization (13,14). We compared the gene expression of
two groups of one-year old mice (ischemia of 90 min and 90 min of
ischemia followed by 1 h of reperfusion). The criterion for
differentially expressed genes (DEGs) are adjusted P-value <
0.05 and |log2fold-change (FC)|≥1.
Function annotation and KEGG pathway
analysis
To explore the biological function of DEGs, we
uploaded the target genes to the Database for Annotation,
Visualization and Integrated Discovery (DAVID) (https://david-d.ncifcrf.gov/). Gene Ontology (GO)
annotation (15) associated with
biological process (BP) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) (16) pathway enrichment
analysis were utilized to analyze the function and potential
pathways of these DEGs. The P-value <0.05 and gene counts >2
were criteria of the both.
PPI network construction
We aimed to identify the possible interaction
networks of DEGs by using STRING version 10.0, which covers over
2,000 organisms and provides direct (physical) and indirect
(functional) associations (17).
DEGs were put in STRING database to construct a PPI network. The
confidence score for selection was ≥0.4. Cytoscape (http://www.cytoscape.org/) software was used to
dispose the PPI network for visualization.
Results
Gene expression analysis
After comparing sample records from 1-year-old mice
subjected to different conditions (90 min of ischemia followed by 1
h of reperfusion or ischemia of 90 min) (n=3 each group), 114 DEGs
were selected to further analysis with the standard of|log2fold
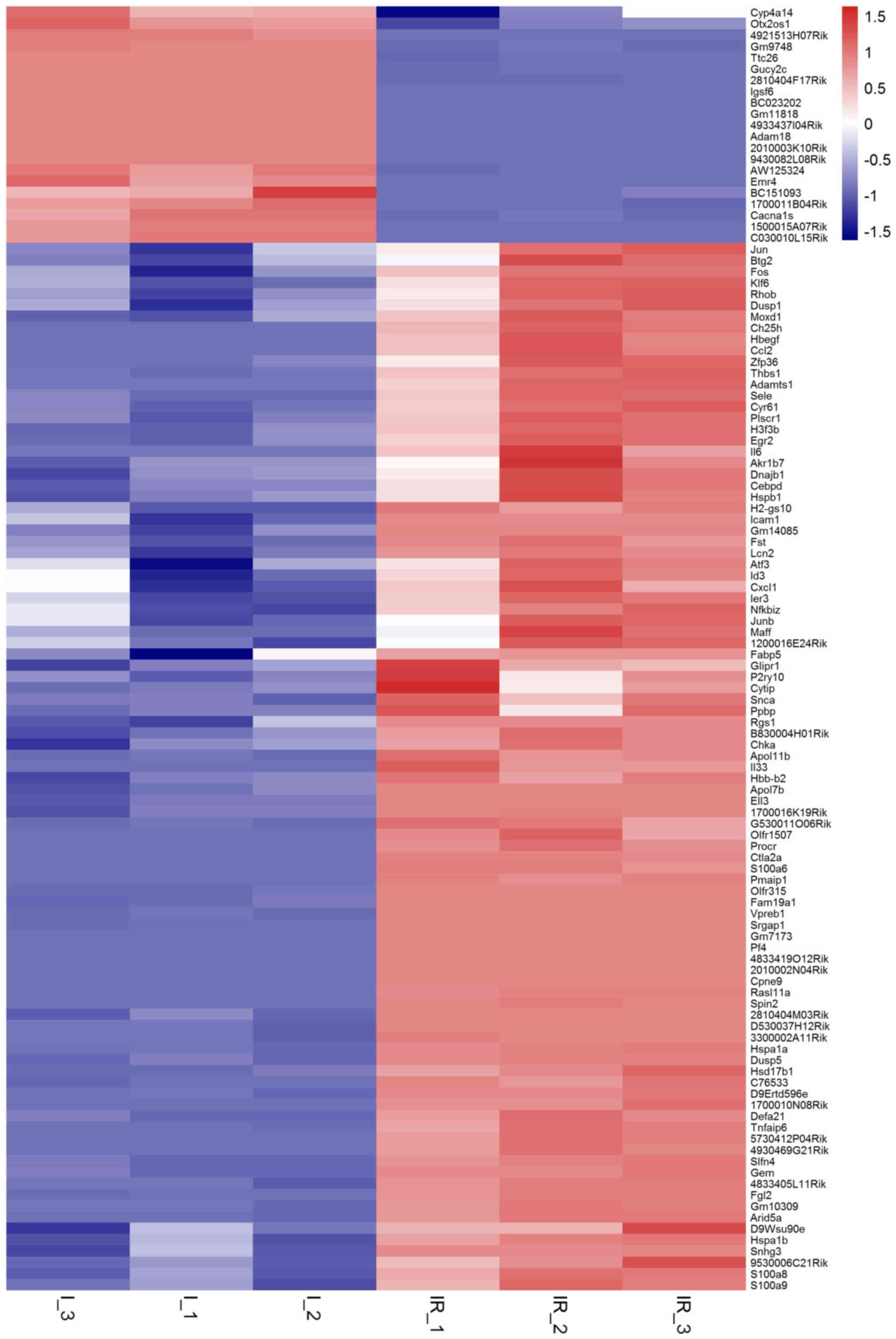
change (FC)|≥1 and adjusted P-values <0.05. (Table I and Fig. 1) Among the DEGs, 21 genes were
downregulated, while another 93 were upregulated. Cyp4a14, Igsf6
and Cacna1 s were most notably changed of the 21 downregulated
genes. Hspa1a, Il6, Hspa1b, Moxd1, Fos, S100a8, Atf3, S100a9, Thbs1
and Btg2 were the top ten increased of the 93 DEGs.
 | Table I.Differentially expressed genes. |
Table I.
Differentially expressed genes.
| Gene | logFC | P-value |
|---|
| Upregulated
genes |
|
|
|
Hspa1a | 4.189803495 | 4.40E-06 |
| Il6 | 3.414377201 | 0.007214798 |
|
Hspa1b | 2.835249411 | 0.002570444 |
|
Moxd1 | 2.792333639 | 0.005880914 |
| Fos | 2.635607507 | 0.008852822 |
|
S100a8 | 2.470587997 | 0.001612021 |
| Atf3 | 2.429293274 | 0.048994575 |
|
S100a9 | 2.382088918 | 0.00198724 |
|
Thbs1 | 2.366926809 | 0.002259069 |
| Btg2 | 2.024032519 | 0.036787526 |
|
Ctla2a | 1.987516668 | 6.94E-07 |
| Gem | 1.957385710 | 3.11E-05 |
|
Egr2 | 1.945932505 | 0.004711597 |
|
Ch25h | 1.945386244 | 0.00116547 |
|
Cyr61 | 1.884283907 | 0.00418327 |
|
Jun | 1.834974240 | 0.02738022 |
|
Dnajb1 | 1.834159651 | 0.017880087 |
|
Tnfaip6 | 1.783788316 | 0.000235748 |
|
Fgl2 | 1.713266954 | 1.40E-05 |
|
Rhob | 1.649879783 | 0.01784261 |
|
Junb | 1.559927741 | 0.048521262 |
|
Nfkbiz | 1.502336562 | 0.02315746 |
|
Apol11b | 1.465232692 | 3.76E-05 |
|
Pmaip1 | 1.457536521 | 1.95E-06 |
|
Snca | 1.446393501 | 0.002008527 |
|
G530011O06Rik | 1.443344075 | 0.000226511 |
|
Plscr1 | 1.441607653 | 0.003825377 |
|
Dusp1 | 1.421057162 | 0.018558258 |
|
Hspb1 | 1.415609621 | 0.012319322 |
|
Gm7173 | 1.414339093 | 1.21E-07 |
|
Cxcl1 | 1.410753622 | 0.044905834 |
|
Hbb-b2 | 1.351033985 | 0.000672398 |
|
Adamts1 | 1.329196641 | 0.003830208 |
|
Icam1 | 1.290995380 | 0.004126491 |
|
5730412P04Rik | 1.283988337 | 9.54E-05 |
|
Rasl11a | 1.282142362 | 6.19E-07 |
|
Maff | 1.272068458 | 0.037153789 |
|
2010002N04Rik | 1.271499304 | 1.21E-07 |
|
Rgs1 | 1.251181552 | 0.003312274 |
|
4833405L11Rik | 1.247575574 | 4.51E-05 |
|
Zfp36 | 1.234524892 | 0.011557402 |
|
Lcn2 | 1.231778515 | 0.001751743 |
|
Klf6 | 1.218441804 | 0.012152268 |
|
Chka | 1.213280213 | 0.002802906 |
|
Olfr1507 | 1.211292555 | 0.000350502 |
|
D530037H12Rik | 1.197701336 | 6.69E-06 |
|
H2-gs10 | 1.196542823 | 0.00144295 |
|
Fst | 1.193249187 | 0.000621087 |
|
Ell3 | 1.182028693 | 3.54E-05 |
|
P2ry10 | 1.171525584 | 0.017352901 |
|
2810404M03Rik | 1.167787654 | 5.67E-05 |
|
Ccl2 | 1.163350947 | 0.002313274 |
|
Hsd17b1 | 1.158145456 | 0.000301852 |
|
Il33 | 1.142985472 | 0.000393507 |
|
C76533 | 1.142247522 | 4.89E-05 |
|
Ppbp | 1.137612231 | 0.011227964 |
|
Id3 | 1.132539137 | 0.03950213 |
|
Ier3 | 1.130341285 | 0.014790191 |
|
1700016K19Rik | 1.128388132 | 0.000105373 |
|
D9Ertd596e | 1.117817849 | 1.34E-05 |
|
1200016E24Rik | 1.106239555 | 0.040908811 |
|
Sele | 1.106222576 | 0.002874116 |
|
Fam19a1 | 1.097266221 | 4.82E-06 |
|
Slfn4 | 1.091663043 | 2.98E-05 |
|
Snhg3 | 1.090971444 | 0.002765684 |
|
4833419O12Rik | 1.087463982 | 1.21E-07 |
|
Defa21 | 1.081747821 | 0.000252684 |
|
Gm10309 | 1.081623512 | 3.19E-05 |
|
Spin2 | 1.081365088 | 6.39E-07 |
|
3300002A11Rik | 1.078493847 | 8.17E-06 |
|
Pf4 | 1.078344289 | 1.21E-07 |
|
4930469G21Rik | 1.074420496 | 0.000104134 |
|
9530006C21Rik | 1.067140075 | 0.003784627 |
|
Procr | 1.060112014 | 3.41E-05 |
|
Cebpd | 1.056892489 | 0.009736321 |
|
Olfr315 | 1.054905005 | 1.28E-06 |
|
Vpreb1 | 1.049741265 | 6.09E-07 |
|
Fabp5 | 1.043946536 | 0.046394908 |
|
Hbegf | 1.041917009 | 0.002254356 |
|
Akr1b7 | 1.038117758 | 0.029888552 |
|
1700010N08Rik | 1.032603816 | 4.55E-05 |
|
D9Wsu90e | 1.031933317 | 0.013273235 |
|
S100a6 | 1.025678192 | 1.46E-05 |
|
Arid5a | 1.023214775 | 5.14E-05 |
|
Srgap1 | 1.020638687 | 1.71E-07 |
|
Dusp5 | 1.020438070 | 1.17E-05 |
|
Gm14085 | 1.018206352 | 0.000563399 |
|
H3f3b | 1.010059710 | 0.003098606 |
|
Cytip | 1.004873236 | 0.025437274 |
|
B830004H01Rik | 1.004683417 | 0.000757856 |
|
Glipr1 | 1.003361817 | 0.010390201 |
|
Apol7b | 1.002402297 | 9.71E-05 |
|
Cpne9 | 1.000076745 | 1.21E-07 |
| Downregulated
genes |
|
|
|
Cyp4a14 | −1.491686323 | 0.046208079 |
|
Igsf6 | −1.357451711 | 1.21E-07 |
|
Cacna1s | −1.339250877 | 0.000204248 |
|
Gucy2c | −1.310550662 | 1.88E-07 |
|
Emr4 | −1.269668108 | 0.000244481 |
|
C030010L15Rik | −1.235720965 | 3.00E-05 |
|
1500015A07Rik | −1.137485728 | 2.58E-05 |
|
AW125324 | −1.097415017 | 7.03E-05 |
|
BC023202 | −1.096162784 | 1.21E-07 |
|
Gm11818 | −1.083128913 | 1.21E-07 |
|
2810404F17Rik | −1.081564746 | 1.21E-07 |
|
BC151093 | −1.076581311 | 0.005019689 |
|
1700011B04Rik | −1.044376574 | 0.000123774 |
|
Otx2os1 | −1.042838602 | 0.001185282 |
|
Ttc26 | −1.039636118 | 8.52E-07 |
|
4933437I04Rik | −1.036693263 | 1.21E-07 |
|
4921513H07Rik | −1.032871132 | 3.95E-06 |
|
2010003K10Rik | −1.031683970 | 1.21E-07 |
|
Gm9748 | −1.026995861 | 1.92E-06 |
|
Adam18 | −1.024258697 | 1.21E-07 |
|
9430082L08Rik | −1.006312751 | 1.21E-07 |
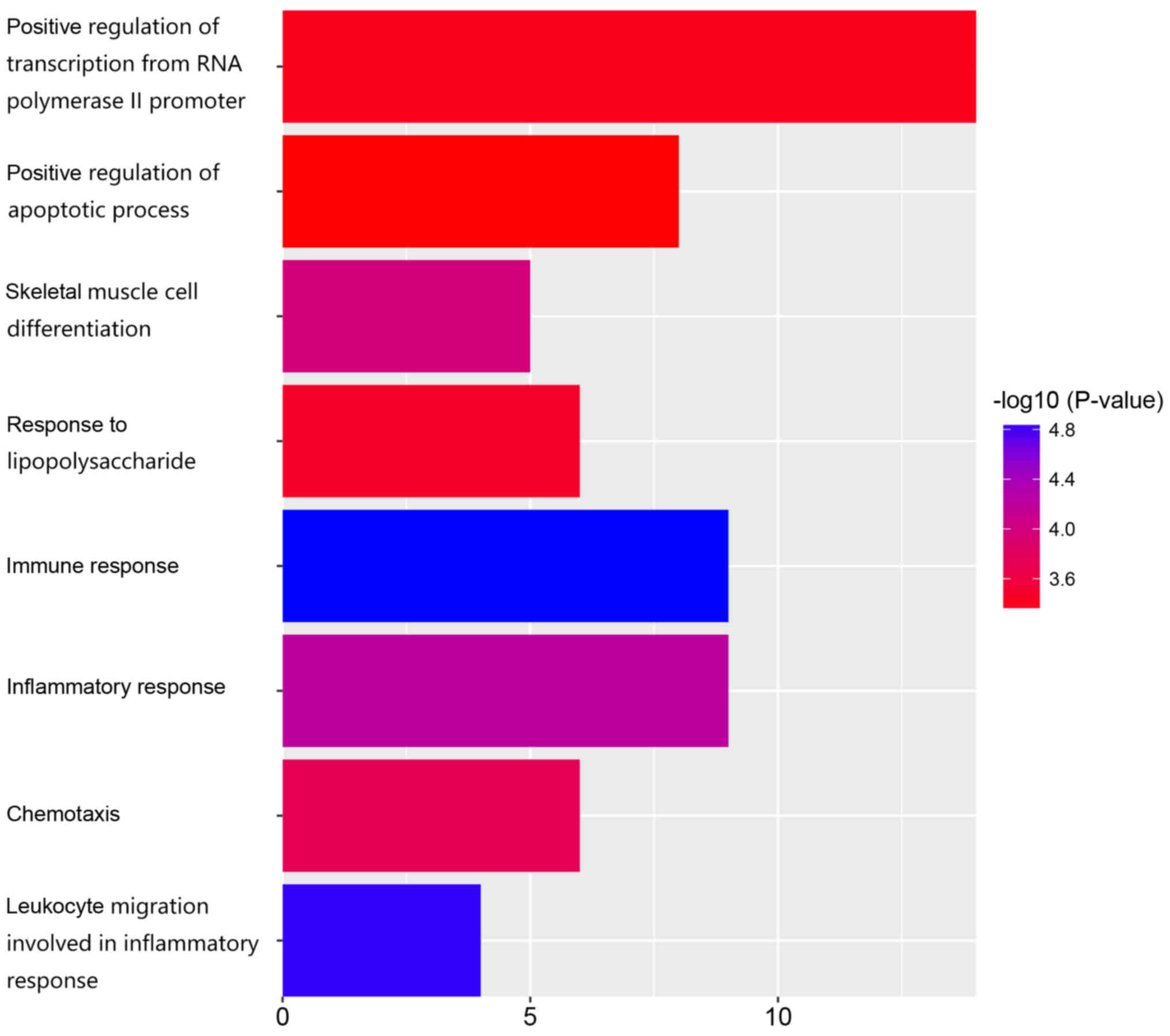
GO analysis and KEGG pathway
According to function annotation, the most
significant biological processes included immune response
(GO:0006955, P=1.37E-05), leukocyte migration involved in
inflammatory response (GO:0002523, P=1.48E-05), inflammatory
response (GO:0006954, P=5.96E-05), skeletal muscle cell
differentiation (GO:0035914, P=1.08E-04), chemotaxis (GO:0006935,
P=1.82E-04), response to lipopolysaccharide (GO:0032496,
P=3.58E-04), positive regulation of transcription from RNA
polymerase II promoter (GO:0045944, P=4.11E-04), and positive
regulation of apoptotic process (GO:0043065, P=4.96E-04) (Table II and Fig. 2).
 | Table II.GO biological process for DEGs (top
10). |
Table II.
GO biological process for DEGs (top
10).
| GO ID | GO Term | Count | P-value |
|---|
| GO:0006955 | Immune
response | 9 | 1.37E-05 |
| GO:0002523 | Leukocyte migration
involved in inflammatory response | 4 | 1.48E-05 |
| GO:0006954 | Inflammatory
response | 9 | 5.96E-05 |
| GO:0035914 | Skeletal muscle
celldifferentiation | 5 | 1.08E-04 |
| GO:0006935 | Chemotaxis | 6 | 1.82E-04 |
| GO:0032496 | Response to
lipopolysaccharide | 6 | 3.58E-04 |
| GO:0045944 | Positive regulation
oftranscription from RNApolymerase II promoter | 14 | 4.11E-04 |
| GO:0043065 | Positive regulation
of apoptoticprocess | 8 | 4.96E-04 |
| GO:0006366 | Transcription from
RNApolymerase II promoter | 7 | 8.04E-04 |
| GO:0070098 |
Chemokine-mediatedsignaling pathway | 4 | 0.001213338 |
As for highly enriched pathways, TNF signaling
pathway (P=1.57E-06), Malaria (P=5.41E-06), Influenza A
(P=3.28E-05), and MAPK signaling pathway (P=3.72E-04) were detected
(Table III).
 | Table III.KEGG pathway analysis for DEGs (top
10). |
Table III.
KEGG pathway analysis for DEGs (top
10).
| KEGG ID | KEGG Term | Count | P-value |
|---|
| mmu04668 | TNF signaling
pathway | 8 | 1.57E-06 |
| mmu05144 | Malaria | 6 | 5.41E-06 |
| mmu05164 | Influenza A | 8 | 3.28E-05 |
| mmu04010 | MAPK signaling
pathway | 8 | 3.72E-04 |
| mmu05166 | HTLV-I
infection | 8 | 6.71E-04 |
| mmu05143 | African
trypanosomiasis | 4 | 8.72E-04 |
| mmu05323 | Rheumatoid
arthritis | 5 | 8.93E-04 |
| mmu04915 | Estrogen signaling
pathway | 5 | 0.001899238 |
| mmu05134 | Legionellosis | 4 | 0.003588248 |
| mmu05169 | Epstein-Barr virus
infection | 6 | 0.005803772 |
Interaction network construction
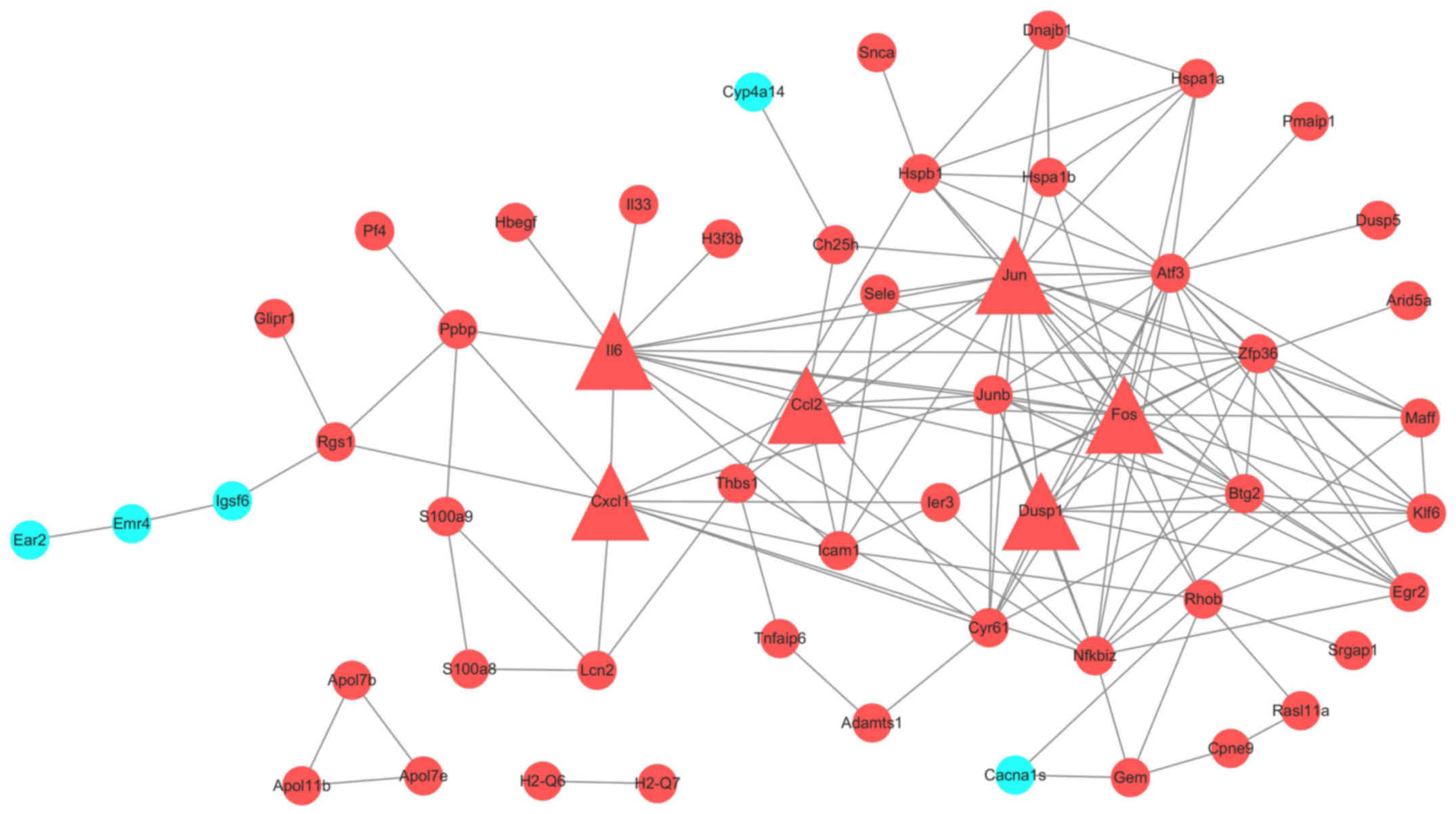
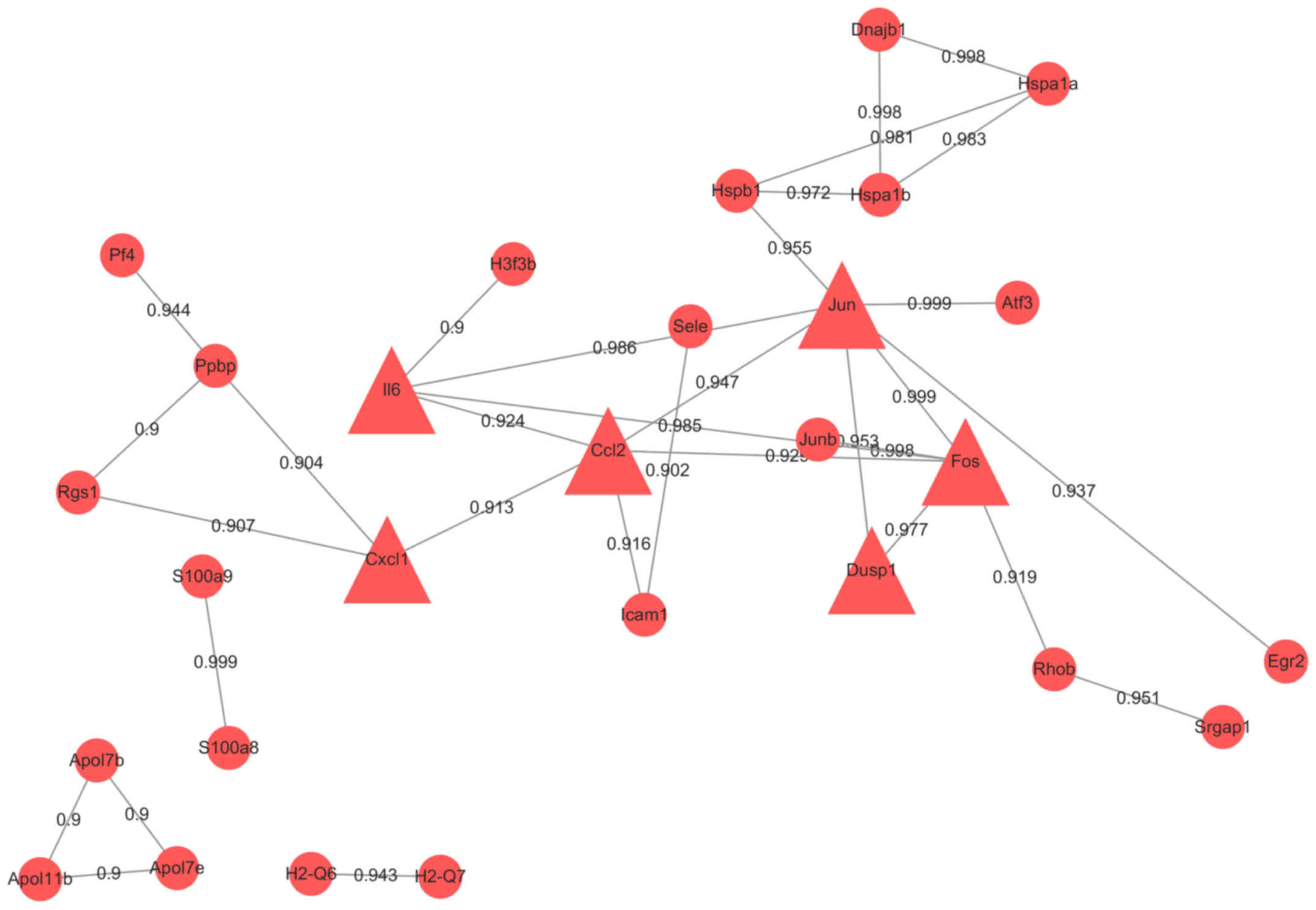
All 114 DEGs were put in the String database. A PPI
network included 94 nodes and 145 edges was constructed. We
analyzed the network by Cytoscape. (Fig. 3) To get more useful information,
PPI sub-networks were generated. Nodes with edges more than 6 were
CCL2, JUN, CYR61, DUSP1, KLF6, BTG2, ZFP36, IL6, CXCL1, JUNB,
NFKBIZ, MAFF, FOS, EGR2 and ATF3 (Fig.
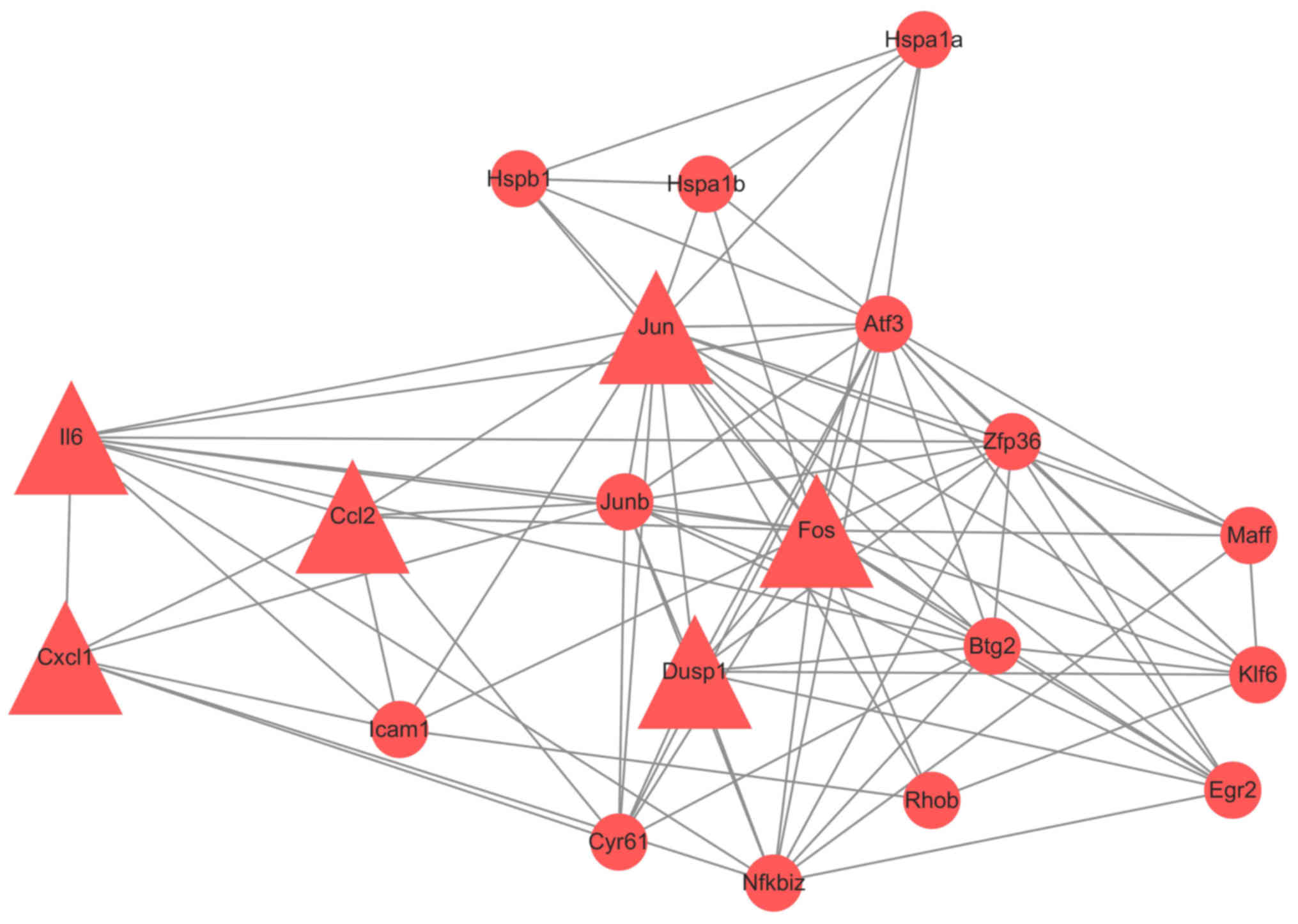
4). Genes with interaction combined-score ≥0.9 were selected to
form a PPI sub-network (Fig. 5).
Hub proteins were FOS, CCL2, CXCL1, JUN, IL6 and DUSP1, all of
which were upregulated.
Discussion
In the current study, 114 DEGs were recognized in
the liver tissue from two groups of 1-year-old mice. The expression
was significantly different between 90 min of ischemia and 90 min
of ischemia followed by 1 h of reperfusion. Based on the pathway
enrichment analysis, most DEGs enriched in immune response,
leukocyte migration involved in inflammatory response, and
inflammatory response, including genes like CXCL1, PLSCR1, IL6,
CCL2, PROCR, PPBP, VPREB2, VPREB1, PF4, S100A8, S100A9, NFKBIZ,
THBS1, and SELE. TNF signaling pathway and MAPK signaling pathway
were recognized with highest count and low P-value. In PPI network,
CXCL1, CCL2, IL6, JUN, FOS and DUSP1 were hub proteins.
In our results, the expression of CXCL1 and IL6
increased rapidly in 90 min of ischemia followed by 1 h of
reperfusion, suggesting that reperfusion could induce severer
damage or more organs dysfunction. CXCL1, also known as GRO-α,
could be a therapeutic target with further research. For instance,
depletion of CXCL1 can lessen angiogenesis activity and reduce
tumor growth. AS a member of the CXC chemokine family, it involved
in recruitment of leukocytes and their migration, and many other
inflammatory conditions (18).
Gomez-Rodriguez et al (19)
discovered that the expression of CXCL1 can be regulated by MMP-10.
The latter was necessary for tissue repair by inhibiting CXCL1.
In vivo, pre-emptive hypoxia-regulated Haem oxygenase-1
(pHRE-HO-1) could reduce the level of IL6 and CXCL. It was helpful
for tissue regeneration and thus alleviating critical limb ischemia
injury (20). Ahuja et al
(21) first proved that serum IL6
had an essential role in AKI-mediated lung neutrophil accumulation
and lung injury by stimulating CXCL1 production in lung, which
indicated that inhibition of CXCL1 may be a possible therapy of
lung injury after AKI. Hepatic stellate cells (HSCs) had a
significant effect on I/R- and endotoxin-induced acute hepatocyte
injury. When suppressing the function of HSCs, the expression of
TNF α, neutrophil chemoattractant CXCL1 and endothelin-A receptor
were all decreased (22).
Our study also identified that CCL2 was upregulated
in I/R group. It might indicate that reperfusion could aggravate
inflammation reaction. Much research had tried to confirm the
relationship between CCL2 and inflammation. For example, CCL2-CCR2
signaling could accelerate liver I/R injury, for the reason that
CCL2 attracted inflammatory monocytes and CCR2-expressing
neutrophil to move into liver from bone marrow (23). Heil et al (24) stated that CCL2, was related to the
accumulation of macrophages in growing collateral vessels. In mouse
femoral artery excision model, CCL2 and CCR2, played an important
role in post-ischemic regenerative processes of skeletal muscle
(25). CCL2/CCR2 dominated
post-ischemic vessel growth (26).
Zhang et al (27) found
that in retinal vascular inflammation, the production of CCL2
required NAD (P) H oxidase activity.
The other three key genes in this study are JUN, FOS
and DUSP1. Expression of FOS and DUSP1 were substantially elevated
in stroke patients (28).
We analyzed the gene microarray data from a new
point, the damage of reperfusion per se, while Huber et al
(8) studied liver I/R injury
emphasizing on the impact of age. There were some limitations of
our study. Firstly, for the lack of preconditioning data, we can't
continue to mine biological function under the circumstance of
precondition or other more relations. Kapoor et al (29) proposed that liver ischemic
preconditioning activated MAPK signaling pathway, permitting
hepatocytes to sustain secondary damage. Oyaizu et al
(30) suggested that in rat
pulmonary ischemia-reperfusion models, Src PTK activation was the
major reason for reperfusion-induced lung injury but not gene
expression alteration. Secondly, GSE10657 only consisted of
reperfusion data of one time point. We couldn't compare gene
expression changes between different time points of
reperfusion.
In conclusion, our study provides supplementary
evidence for the hypothesis that Reperfusion itself creates injury
during liver I/R. We identified 114 DEGs between Reperfusion
following Ischemia and Ischemia alone. CXCL1, CCL2, IL6, JUN, FOS
and DUSP1 were key genes in I/R injury. These genes may be the
potential therapeutic target. However, more experimental researches
are needed to verify.
Acknowledgements
This study was supported by The Natural Scientific
Foundation of Guangdong Province (2016A030313255) and The
Foundation of Sun Yat-sen University for Young Teachers
(16ykpy36).
Glossary
Abbreviations
Abbreviations:
|
I/R
|
ischemia and reperfusion
|
|
DEGs
|
differentially expressed genes
|
|
DAVID
|
Database for Annotation Visualization
and Integrated Discovery
|
|
PPI
|
Protein-protein interaction
network
|
|
GEO
|
network Gene Expression Omnibus
database
|
|
GO
|
Gene Ontology
|
|
BP
|
biological process
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Li Y, Yang Y, Feng Y, Yan J, Fan C, Jiang
S and Qu Y: A review of melatonin in hepatic ischemia/reperfusion
injury and clinical liver disease. Ann Med. 46:503–511. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhai Y, Petrowsky H, Hong JC, Busuttil RW
and Kupiec-Weglinski JW: Ischaemia-reperfusion injury in liver
transplantation-from bench to bedside. Nat Rev Gastroenterol
Hepatol. 10:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papadopoulos D, Siempis T, Theodorakou E
and Tsoulfas G: Hepatic ischemia and reperfusion injury and trauma:
Current concepts. Arch Trauma Res. 2:63–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka Y, Maher JM, Chen C and Klaassen
CD: Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via
NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 71:817–825.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SM, Park MJ, Cho TS and Clemens MG:
Hepatic injury and lipid peroxidation during ischemia and
reperfusion. Shock. 13:279–284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calkins CM, Bensard DD, Moore EE, McIntyre
RC, Silliman CC, Biffl W, Harken AH, Partrick DA and Offner PJ: The
injured child is resistant to multiple organ failure: A different
inflammatory response? J Trauma. 53:1058–1063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okaya T, Blanchard J, Schuster R, Kuboki
S, Husted T, Caldwell CC, Zingarelli B, Wong H, Solomkin JS and
Lentsch AB: Age-dependent responses to hepatic ischemia/reperfusion
injury. Shock. 24:421–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huber N, Sakai N, Eismann T, Shin T,
Kuboki S, Blanchard J, Schuster R, Edwards MJ, Wong HR and Lentsch
AB: Age-related decrease in proteasome expression contributes to
defective nuclear factor-kappaB activation during hepatic
ischemia/reperfusion. Hepatology. 49:1718–1728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaeschke H and Woolbright BL: Current
strategies to minimize hepatic ischemia-reperfusion injury by
targeting reactive oxygen species. Transplant Rev (Orlando).
26:103–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abu-Amara M, Yang SY, Seifalian A,
Davidson B and Fuller B: The nitric oxide pathway-evidence and
mechanisms for protection against liver ischaemia reperfusion
injury. Liver Int. 32:531–543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaouali MA, Boncompagni E, Reiter RJ,
Bejaoui M, Freitas I, Pantazi E, Folch-Puy E, Abdennebi HB,
Garcia-Gil FA and Roselló-Catafau J: AMPK involvement in
endoplasmic reticulum stress and autophagy modulation after fatty
liver graft preservation: A role for melatonin and trimetazidine
cocktail. J Pineal Res. 55:65–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eltzschig HK and Collard CD: Vascular
ischaemia and reperfusion injury. Br Med Bull. 70:71–86. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai J, Perkumas KM, Qin X, Hauser MA,
Stamer WD and Liu Y: Expression profiling of human schlemm's canal
endothelial cells from eyes with and without glaucoma. Invest
Ophthalmol Vis Sci. 56:6747–6753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40:(Database
issue). D109–D114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:(Database issue). D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bechara C, Chai H, Lin PH, Yao Q and Chen
C: Growth related oncogene-alpha (GRO-alpha): Roles in
atherosclerosis, angiogenesis and other inflammatory conditions.
Med Sci Monit. 13:RA87–RA90. 2007.PubMed/NCBI
|
|
19
|
Gomez-Rodriguez V, Orbe J,
Martinez-Aguilar E, Rodriguez JA, Fernandez-Alonso L, Serneels J,
Bobadilla M, Perez-Ruiz A, Collantes M, Mazzone M, et al:
Functional MMP-10 is required for efficient tissue repair after
experimental hind limb ischemia. FASEB J. 29:960–972. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jazwa A, Stepniewski J, Zamykal M,
Jagodzinska J, Meloni M, Emanueli C, Jozkowicz A and Dulak J:
Pre-emptive hypoxia-regulated HO-1 gene therapy improves
post-ischaemic limb perfusion and tissue regeneration in mice.
Cardiovasc Res. 97:115–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahuja N, Andres-Hernando A, Altmann C,
Bhargava R, Bacalja J, Webb RG, He Z, Edelstein CL and Faubel S:
Circulating IL-6 mediates lung injury via CXCL1 production after
acute kidney injury in mice. Am J Physiol Renal Physiol.
303:F864–F872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart RK, Dangi A, Huang C, Murase N,
Kimura S, Stolz DB, Wilson GC, Lentsch AB and Gandhi CR: A novel
mouse model of depletion of stellate cells clarifies their role in
ischemia/reperfusion- and endotoxin-induced acute liver injury. J
Hepatol. 60:298–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Xu P, Song P, Wang H, Zhang Y, Hu
Q, Wang G, Zhang S, Yu Q, Billiar TR, et al: CCL2-CCR2 signaling
promotes hepatic ischemia/reperfusion injury. J Surg Res.
202:352–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heil M, Ziegelhoeffer T, Wagner S,
Fernández B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann
G and Schaper W: Collateral artery growth (arteriogenesis) after
experimental arterial occlusion is impaired in mice lacking
CC-chemokine receptor-2. Circ Res. 94:671–677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Contreras-Shannon V, Ochoa O, Reyes-Reyna
SM, Sun D, Michalek JE, Kuziel WA, McManus LM and Shireman PK: Fat
accumulation with altered inflammation and regeneration in skeletal
muscle of CCR2-/−mice following ischemic injury. Am J Physiol Cell
Physiol. 292:C953–C967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cochain C, Rodero MP, Vilar J, Récalde A,
Richart AL, Loinard C, Zouggari Y, Guérin C, Duriez M, Combadière
B, et al: Regulation of monocyte subset systemic levels by distinct
chemokine receptors controls post-ischaemic neovascularization.
Cardiovasc Res. 88:186–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Rojas M, Lilly B, Tsai NT,
Lemtalsi T, Liou GI, Caldwell RW and Caldwell RB: NAD(P)H
oxidase-dependent regulation of CCL2 production during retinal
inflammation. Invest Ophthalmol Vis Sci. 50:3033–3040. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adamski MG, Li Y, Wagner E, Yu H,
Seales-Bailey C, Soper SA, Murphy M and Baird AE: Expression
profile based gene clusters for ischemic stroke detection.
Genomics. 104:163–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kapoor S, Berishvili E, Bandi S and Gupta
S: Ischemic preconditioning affects long-term cell fate through DNA
damage-related molecular signaling and altered proliferation. Am J
Pathol. 184:2779–2790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oyaizu T, Fung SY, Shiozaki A, Guan Z,
Zhang Q, dos Santos CC, Han B, Mura M, Keshavjee S and Liu M: Src
tyrosine kinase inhibition prevents pulmonary
ischemia-reperfusion-induced acute lung injury. Intensive Care Med.
38:894–905. 2012. View Article : Google Scholar : PubMed/NCBI
|