Introduction
Forkhead transcription factors of the O class
(FOXOs) are characterized by a conserved forkhead box DNA-binding
domain (1). The FOXO sub-family
contains four members (FOXO1, FOXO3, FOXO4 and FOXO6), which
activate or repress various genes, including B-cell lymphoma 2
(Bcl-2)-interacting mediator of cell death (Bim) and Fas ligand
(FasL), which are involved in apoptosis (2,3),
p27kip (4) and cyclin D
(5), which are involved in cell
cycle regulation, and growth arrest and DNA damage-inducible 45α
(GADD45a), which is involved in DNA damage repair (1–3,6). It
has been reported that FOXO factors regulate a variety of
physiological and pathological processes, and may be potential
targets in tumor therapy (7).
Previous studies have shown that the overexpression of FOXO3a may
inhibit tumor growth in vitro and tumor size in vivo
in breast cancer cells (8,9). Furthermore, the cytoplasmic location
of FOXO3a appears to correlate with poor survival rates in patients
with breast cancer (8). As the
first identified member of the FOXO subfamily, FOXO1 has been
demonstrated to modulate the expression of a number of genes,
including p27kip1 and p21cip1, which are
involved in cell cycle arrest, and Bim and FasL, involved in
apoptosis (10,11). Growth factor stimulates activation
of the phosphoinositide 3-kinase (PI3K)-AKT pathway, and induces
the phosphorylation of nuclear FOXO1 at specific sites (Thr24,
Ser256 and Ser319). Phosphorylated (p)-FOXO1 translocates into the
cytoplasm, where it is unable to affect the expression of its
target gene (12,13). The AKT-mediated phosphorylation of
FOXO1 on S256 facilitates interaction with the E3 ubiquitin ligase,
Skp2, resulting in its ubiquitination and proteasomal degradation
(14). It has been shown that the
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) signaling pathways can inhibit the
transcriptional activity of FOXO3 by phosphorylating FOXO3 at S294,
S344, S425. This mechanism is similar to that of the
phosphorylation of FOXO1 by PI3K/AKT (9). It has been demonstrated that the
PI3K/AKT and MAPK/ERK pathways are upregulated in non-small cell
lung cancer (NSCLC) cell lines (15).
Glucosamine, a naturally occurring monosaccharide,
acts as substrate for the biosynthesis of glycosaminoglycan and has
been used to treat osteoarthritis for >20 years (16). Glucosamine has also shown potential
anti-inflammatory effects by inhibiting neutrophil function,
including superoxide generation, phagocytosis, granule enzyme
release, chemotaxis and the expression of CD11b (17,18).
It has also been reported that glucosamine suppresses the
expression of intercellular adhesion molecule 1 (ICAM-1) and
monocyte chemoattractant protein-1 (MCP-1) in human umbilical vein
endothelial cells, showing anti-atherosclerotic activity (19,20).
Glucosamine has a neuroprotective effect through the suppressing
the production of inflammatory mediators, including interleukin-1β,
tumor necrosis factor-α, cyclooxygenase-2 and inducible nitric
oxide synthase, inhibiting the activation of nuclear factor (NF)-κB
in rat brain ischemia reperfusion injury, the glucosamine-mediated
induction of glucose-regulated protein 78, which protects renal
cells from hypoxia (21).
Glucosamine has shown antitumor activity in vivo and in
vitro (22,23). The possible mechanism of its
antitumor effect includes the translocation of cathepsin D and
downregulation of Bcl-extra large (24), inhibition of p70S6K (25), proteasome inhibition (26), and cell cycle arrest through
suppressing the expression of cyclin E (27). An increasing number of studies have
shown that glucosamine can also regulate the activities of multiple
proteins by suppressing specific-site amino acid phosphorylation,
including p53, cyclin E and AKT (27,28).
Lung cancer is the most common cause of
cancer-associated mortality worldwide and NSCLC accounts for 80% of
lung cancer cases (29). In the
present study, the normal human bronchial epithelial (HBE) cell
line and the A549 NSCLC cell line were used to examine the effect
of LY294002, a PI3K-specific inhibitor, UO126, an ERK-specific
inhibitor, and glucosamine on cell proliferation. The investigation
focused on the effect of glucosamine on the specific-site
phosphorylation of the FOXO1 protein, to attempt to elucidate the
mechanism underlying in its anti-lung cancer effect.
Materials and methods
Cell culture and cytotoxicity
assay
Human A549 lung adenocarcinoma cancer cells and HBE
cells (Jining Shiye, Shanghai, China) were cultured in high-glucose
DMEM with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), penicillin (100 U/ml) and streptomycin
(100 µg/ml). In separate experiments, LY294002 (PI3K inhibitor; 25
mM), UO126 (ERK inhibitor; 25 mM), and glucosamine sulfate (5 mM)
(all from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), were
added. All cells were incubated at 37°C in 5% CO2.
For the cell viability assay, the HBE cells and A549
cells were seeded in 96-well plates at 5×103 cells/well.
The cells were allowed to adhere for 24 h, and cell growth
inhibition was analyzed following incubation with LY294002 (25 mM),
UO126 (25 mM) or glucosamine sulfate (5 mM) for 24 h. The cells
were analyzed using a 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl
tetrazolium bromide (MTT) assay (Sigma-Aldrich; Merck KGaA).
Immunoblot and immunoprecipitation
analysis
The cell extracts were prepared using RIPA lysis
buffer with 0.5% protease inhibitor cocktail and 1% phosphatase
inhibitors. Protein samples were quantified using a Bradford
Protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China), and 40 µg/lane was separated on 10 or 12% SDS-PAGE gels and
transferred for 2.5 h onto PVDF membranes. The blotted PVDF
membranes were then probed with primary and secondary antibodies,
following which the bands were visualized using enhanced
chemiluminescence reagent (Sigma-Aldrich; Merck KGaA). The
membranes were blocked in 5% not-fat dry milk and washed in TTBS
(19,20). Anti-FOXO1 (1:2,000; cat no. 97635;
mouse), anti-p-FOXO1 (Ser 256; 1:2,000; cat no. 84192; rabbit),
anti-FOXO3 (1:2,000; cat no. 2497; rabbit), anti-p-FOXO3 (Ser 294;
1:2,000; cat no. 5538; rabbit) and horseradish peroxidase
(HRP)-conjugated horse anti-mouse IgG secondary antibody (1:1,500;
cat no. 7076) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-AKT (1:1,000; cat no. sc-5298; mouse),
anti-p-AKT (Ser 473; 1:1,000; cat no. sc-135651; rabbit), anti-ERK
(1:1,000; cat no. sc-135900; mouse), anti-p-ERK (1:1,000; cat no.
sc-81492; mouse), anti-GAPDH (1:500; cat no. sc-367714; rabbit) and
HRP-conjugated mouse anti-rabbit IgG secondary antibody (1:500; cat
no. sc-2357) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-β-O-linked N-acetylglucosamine (O-GlcNAc)
monoclonal antibody (1:2,000; cat no. MMS-248R; mouse) was
purchased from Covance, Inc. (Princeton, NJ, USA), and
HRP-conjugated goat anti-mouse IgG/IgM (1:2,000; cat no.
NBP1-75214) antibody was purchased from Novus Biologicals, LLC
(Littleton, CO, USA). The bands were detected and quantified using
the LAS-3000 image analyzer (Fujifilm Corporation, Tokyo, Japan).
The membranes were probed with the primary antibodies overnight at
4°C, and secondary antibodies were incubated with membranes for 1 h
at room temperature.
For immunoprecipitation analysis (27), the cell lysates were mixed with
Protein G Plus agarose for 30 min at 4°C. Primary antibody
(anti-O-GlcNAc monoclonal antibody; 1:50) and protein G agarose
beads (40 µl 50% bead slurry; Santa Cruz Biotechnology, Inc.) were
then added to 500 µl cell lysates and incubated for 2 h at 4°C.
Following five washes with cold lysis buffer, the
immunoprecipitated samples were analyzed using western blot
analysis on a 10% gel.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, and analyzed for significant differences using one-way
analysis of variance with a multiple comparison test or Student's
t-test (Prism 4; GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significance
difference.
Results
Glucosamine inhibits lung cancer cell
proliferation
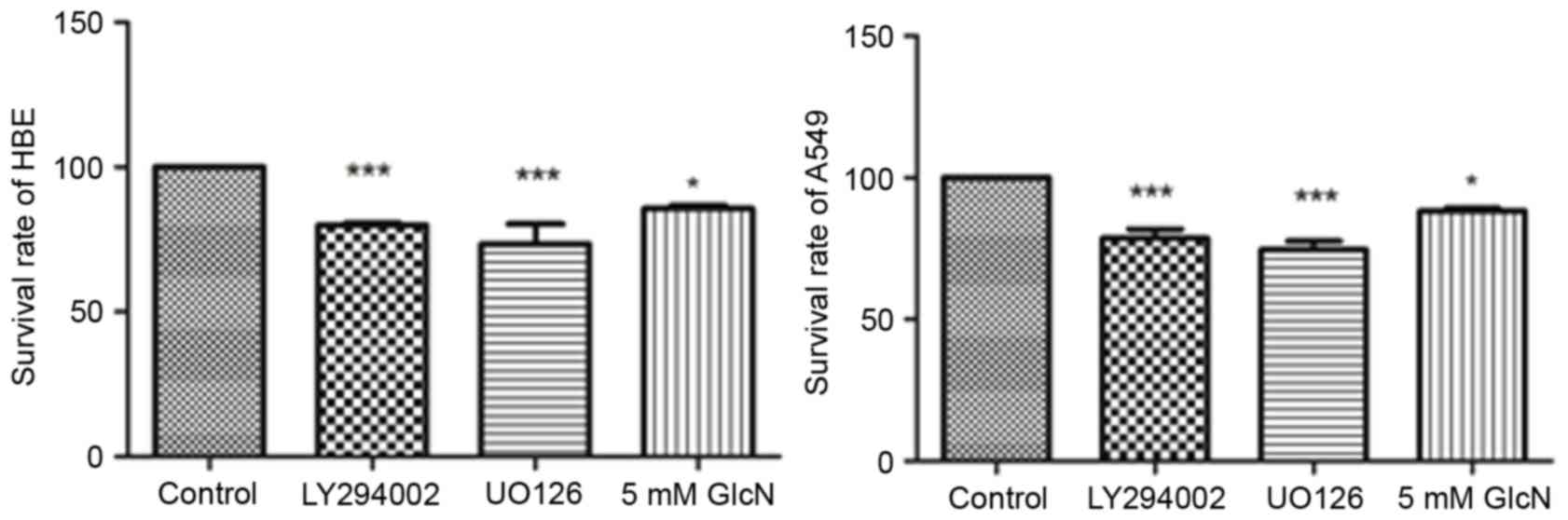
To evaluate the effect of glucosamine on cell line
proliferation, HBE cells and A549 cells were incubated with
LY294002 (25 mM), UO126 (25 mM) or 5 mM glucosamine for 24 h. As
shown in Fig. 1, LY294002, UO126
and glucosamine inhibited the rates of cell proliferation by
~15–30%, showing significant inhibition and indicating the
antitumor effect of glucosamine on A549 cells.
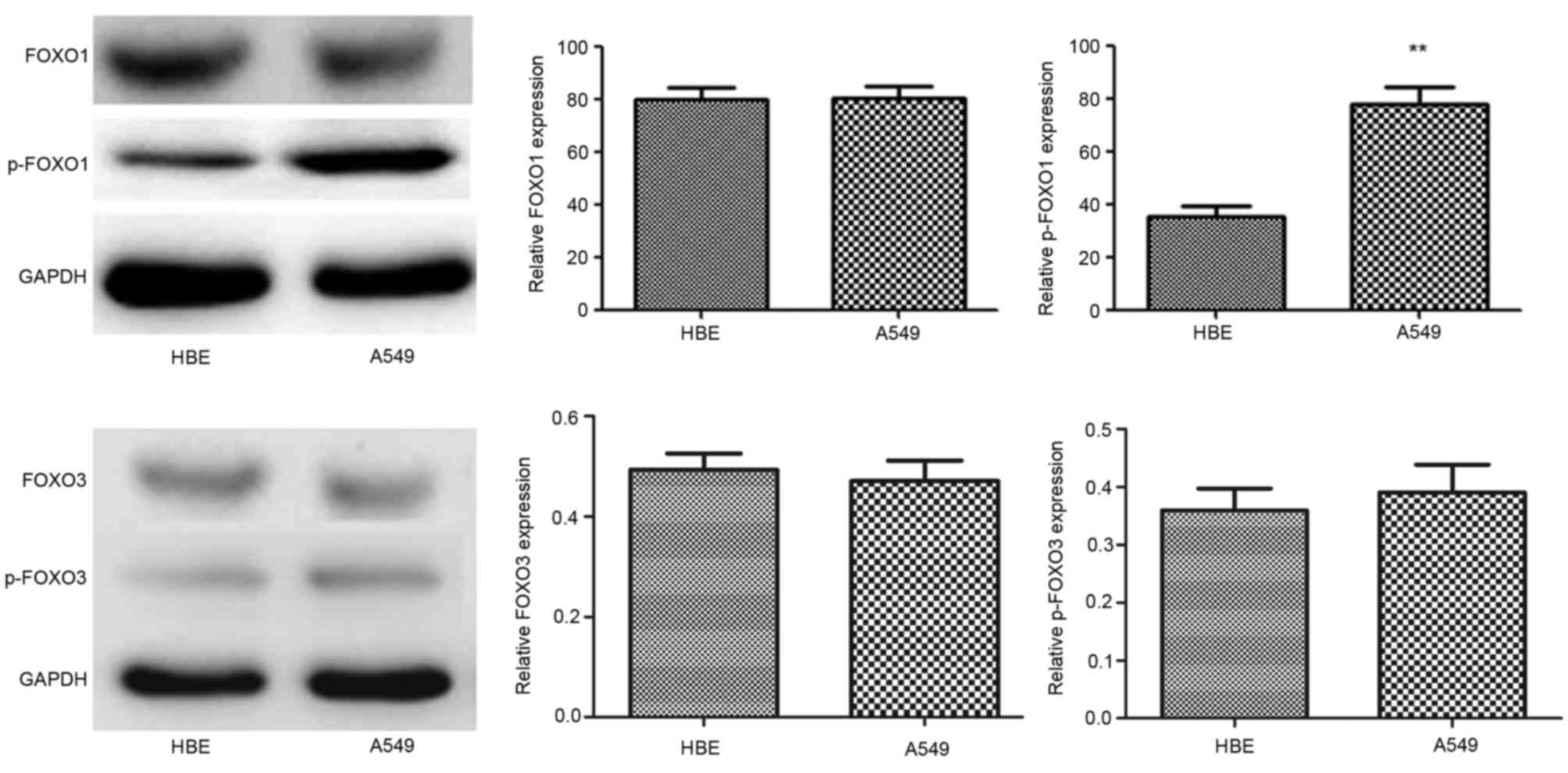
Expression of FOXO1, p-FOXO1, FOXO3
and p-FOXO3 in HBE and A549 cells
The expression levels of FOXO1 and FOXO3 were almost
identical in the two cell lines, however, p-FOXO1 was significantly
upregulated in the A549 cells. Additionally, p-FOXO3 protein was
also upregulated in A549 cells, however, this was not significant
(Fig. 2). p-FOXO1 and p-FOXO3 are
located in the cytoplasm, and their transcriptional activities on
p27kip1 and p21cip1, which are involved in cell cycle arrest, and
Bim and FasL, which are involved in apoptosis, are suppressed.
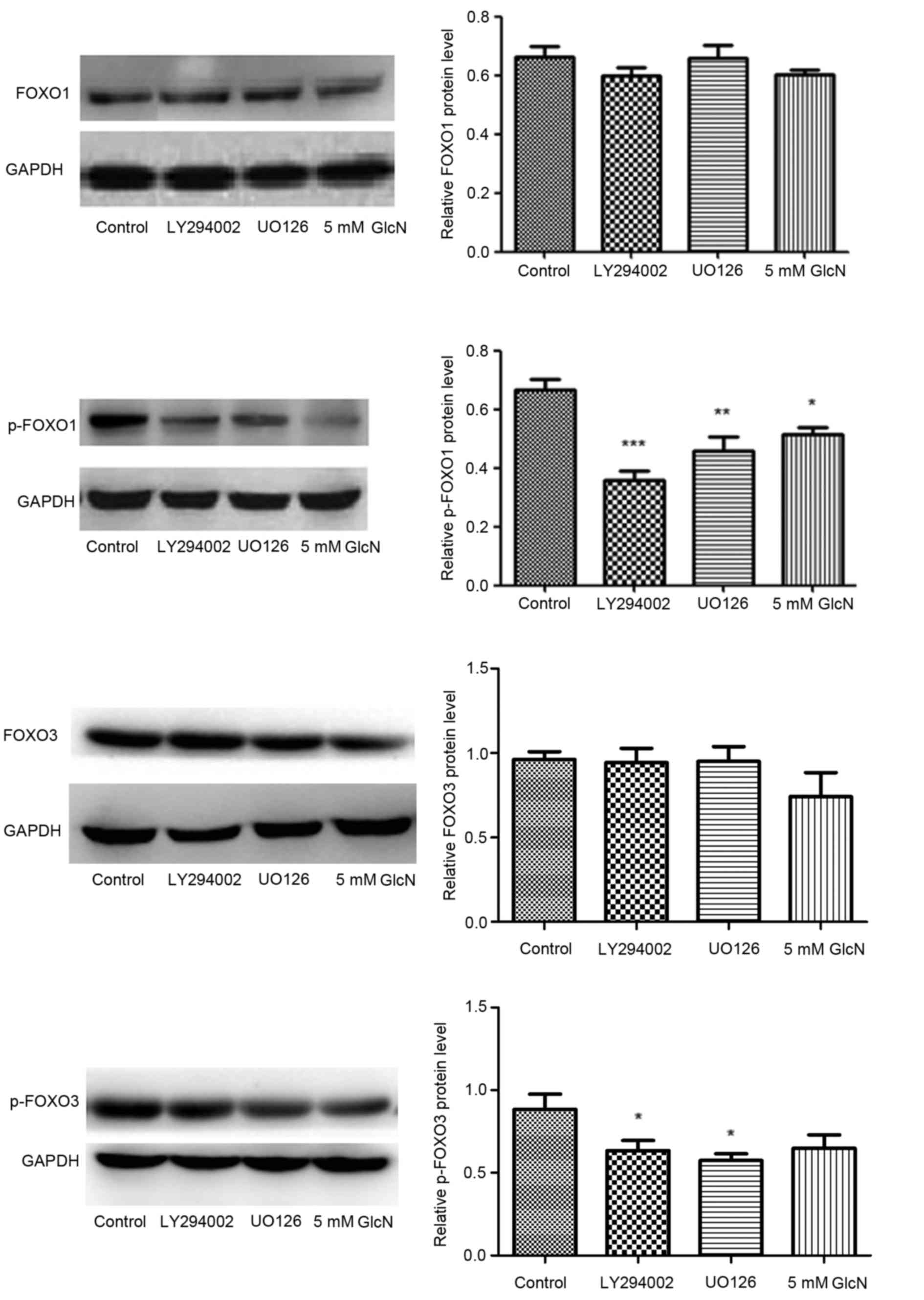
The protein expression levels of FOXO1, p-FOXO1,
FOXO3 and p-FOXO3 were evaluated using western blot analysis in the
presence of the PI3K and ERK inhibitors, LY294002 and UO126. FOXO1
and FOXO3 have previously been demonstrated to regulate the
expression of a number of genes. The transcriptional activity may
be regulated by multiple posttranslational modifications, for
example phosphorylation, acetylation and ubiquitination (7). LY294002 and UO126 significantly
inhibited the phosphorylation of FOXO1 and FOXO3, but did not
affect their expression. Glucosamine significantly suppressed
phosphorylation of FOXO1, and reduced the expression of FOXO3 and
p-FOXO3, although this was not statistically significant for FOXO3
and p-FOXO3, indicating its multiple functions on translation and
signal transduction (Fig. 3).
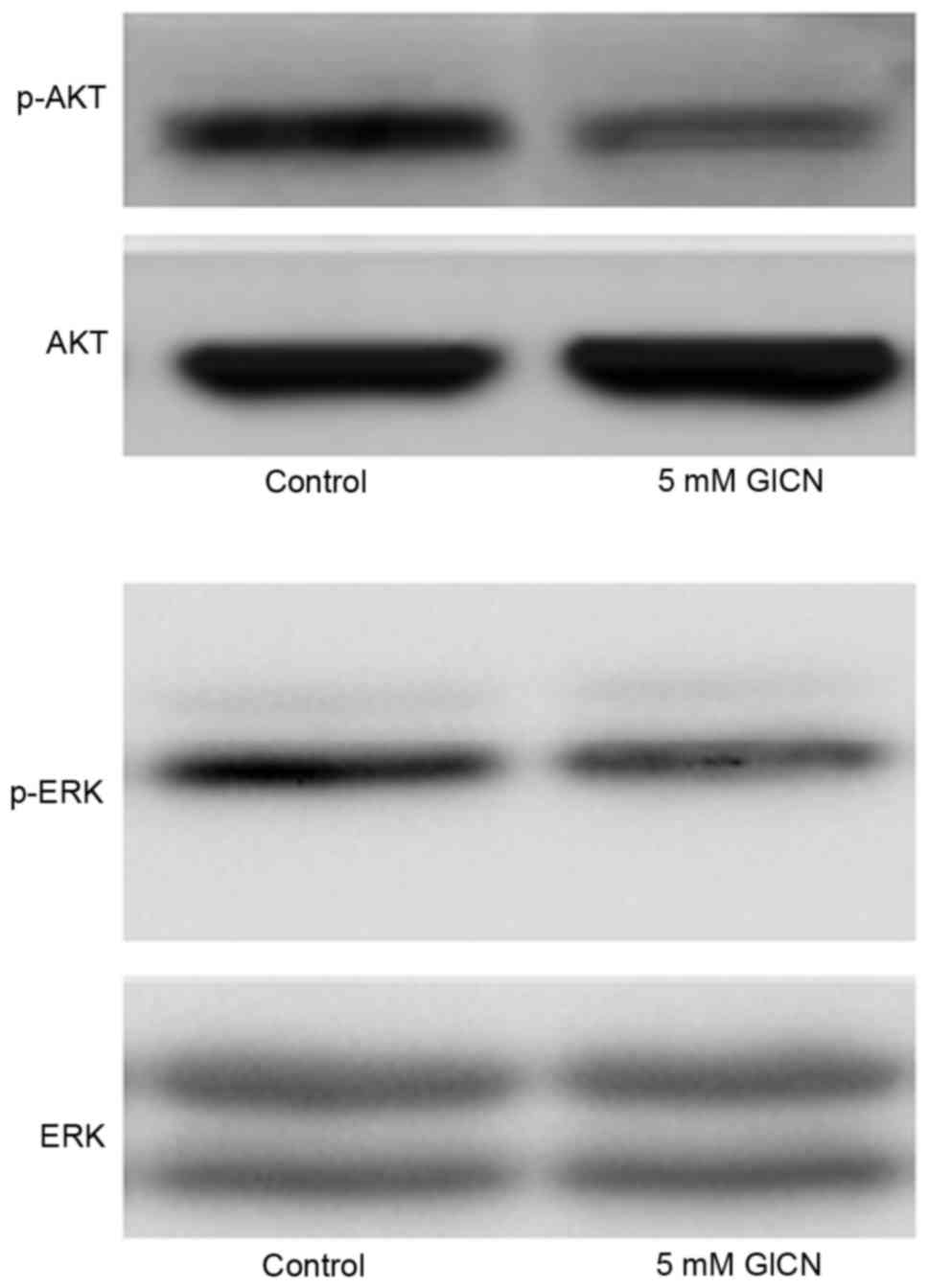
Glucosamine suppresses the
phosphorylation of ERK and AKT
The present study evaluated the effect of
glucosamine on the phosphorylation of ERK and AKT, which are two
signal transduction pathways associated with cell proliferation and
usually upregulated in cancer cells. As shown in Fig. 4, glucosamine inhibited the
phosphorylation of ERK and AKT, indicating the potential inhibitory
activity of glucosamine on these two signal transduction
pathways.
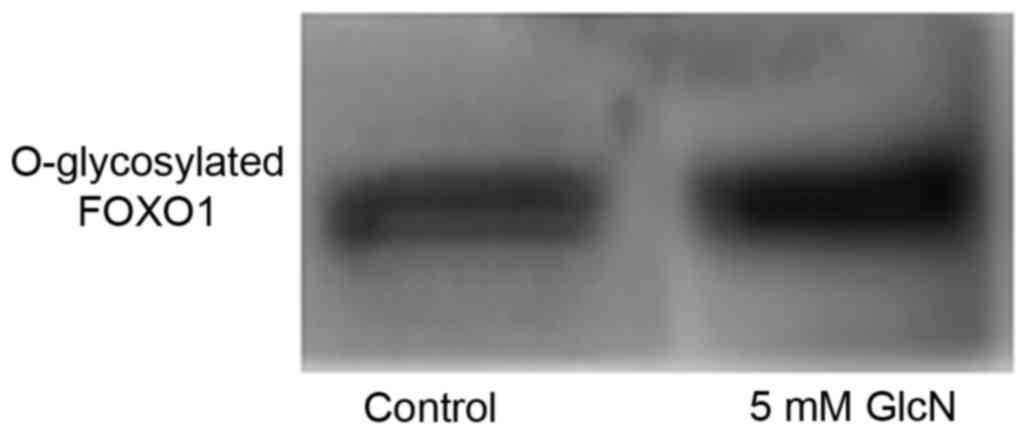
Glucosamine induces O-GlcNAc
modification of FOXO1
Glucosamine induced O-GlcNAc modification in the
A549 cells; O-GlcNAc modification may have affected the protein
phosphorylation of FOXO to alter its biological functions. The
immunoprecipitation of FOXO1 in the present study confirmed that
FOXO1 O-GlcNAc was modified by glucosamine (Fig. 5).
Discussion
Lung cancer is the leading cause of
cancer-associated mortality worldwide. Although early stage disease
can be cured with surgery, rates of recurrence remain high. The
PI3K/AKT and MAPK/ERK signaling pathways are upregulated in lung
cancer, including tissues and cell lines. The transcriptional
activity of FOXO1 and FOXO3 can be regulated by multiple
posttranslational modifications, including phosphorylation,
acetylation and ubiquitination (12). Phosphorylated FOXO translocates
into the cytoplasm, where it is unable to affect the expression of
its target gene (13). Regulatory
genes include p27kip1 and p21cip1, which are
involved in cell cycle arrest, GADD45, which is involved in DNA
damage repair, and Bim and FasL, which are involved in apoptosis
(11). The results of the present
study demonstrated that the levels of p-FOXO1 and p-FOXO3 were
upregulated in A549 cells, and that PI3K, an MAPK inhibitor,
suppressed their expression. This indicated that the two signaling
pathways were associated with tumorigenesis via FOXO1 and
FOXO3.
Glucosamine is a naturally occurring monosaccharide,
which exerts biological effects, including anti-inflammatory and
antitumor effects (2,3). In the present study, an MTT assay
indicated the anti-lung cancer effect of glucosamine on A549 cells.
The levels of protein O-GlcNAc modification of lung cancer cells
induced by glucosamine were also examined, and the resulting data
indicated that FOXO1 and FOXO3 O-GlcNAc modification was induced by
glucosamine incubation. The glucosamine-induced protein O-GlcNAc
modification sites are the same or close to sites of
phosphorylation, therefore, certain proteins can be modulated by
these two types of modification. In this context, it has been found
that glucosamine inhibits the expression of ICAM-1 and MCP-1 by
interfering with p38-MAPK- and NF-κB-specific site phosphorylation
(19,20). The translocation of FOXO is
controlled by the phosphorylation of specific amino acids. The
present study investigated the suppressive effect of glucosamine on
the expression of FOXO and p-FOXO, and the inhibition of
phosphorylation appeared to be one of the mechanisms underlying the
anti-lung cancer effect of glucosamine. The decreased protein level
of FOXO3 induced by glucosamine, although not statistically
significant, appeared to conflict with the anti-lung cancer
effects, therefore, nucleoprotein, functioning as a transcription
factor, was extracted and the protein level of FOXO was examined.
The data indicated that there was no reduction in the number of
nuclei, despite suppression of the protein (data not shown). The
data also showed that glucosamine inhibited the PI3K/AKT and
MAPK/ERK signaling pathways, which were activated in A549 cells.
Our previous study indicated that the interference of the G1/S
checkpoint by cyclin E was a possible mechanism underlying the
anti-lung cancer effects (27).
The present study showed that glucosamine inhibited lung cancer
cell proliferation via multiple signal transduction pathways and by
affecting the expression of various genes, including transcription,
translation and post-translational degradation. In conclusion,
glucosamine was found to suppress lung cancer cell proliferation,
possibly by affecting the transcriptional activity of FOXO1 and
p-FOXO3. The results of the present study supplement current
knowledge of the mechanism underlying the anti-lung cancer effect
of glucosamine and provide theoretical evidence for the clinical
application of glucosamine.
Acknowledgements
This study was supported by a grant from the
Department of Science and Technology of Liaoning Province (grant
no. 2015020258).
References
|
1
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finnberg N and El-Deiry WS: Activating
FOXO3a, NF-kappaB and p53 by targeting IKKs: An effective
multi-faceted targeting of the tumor-cell phenotype? Cancer Biol
Ther. 3:614–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tran H, Brunet A, Griffith EC and
Greenberg ME: The many forks in FOXO's road. Sci STKE.
2003:RE52003.PubMed/NCBI
|
|
4
|
Dijkers PF, Medema RH, Pals C, Banerji L,
Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW,
Koenderman L and Coffer PJ: Forkhead transcription factor FKHR-L1
modulates cytokine-dependent transcriptional regulation of
p27(KIP1). Mol Cell Biol. 20:9138–9148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt M, de Fernandez Mattos S, van der
Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM and Medema RH:
Cell cycle inhibition by FoxO forkhead transcription factors
involves downregulation of cyclin D. Mol Cell Biol. 22:7842–7852.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang JY, Xia W and Hu MC: Ionizing
radiation activates expression of FOXO3a, Fas ligand, and Bim, and
induces cell apoptosis. Int J Oncol. 29:643–648. 2006.PubMed/NCBI
|
|
7
|
Maiese K, Chong ZZ, Shang YC and Hou J:
Clever cancer strategies with FoxO transcription factors. Cell
Cycle. 7:3829–3839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang
F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al: IkappaB kinase
promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell.
117:225–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding
Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, et al: ERK promotes
tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation.
Nat Cell Biol. 10:138–148. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Keizer PL, Packer LM, Szypowska AA,
Riedl-Polderman PE, van den Broek NJ, de Bruin A, Dansen TB, Marais
R, Brenkman AB and Burgering BM: Activation of forkhead box O
transcription factors by oncogenic BRAF promotes
p21cip1-dependent senescence. Cancer Res. 70:8526–8536.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JY and Hung MC: A new fork for
clinical application: Targeting forkhead transcription factors in
cancer. Clin Cancer Res. 15:752–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vogt PK, Jiang H and Aoki M: Triple layer
control: Phosphorylation, acetylation and ubiquitination of FOXO
proteins. Cell Cycle. 4:908–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rena G, Prescott AR, Guo S, Cohen P and
Unterman TG: Rols of the forkhead in rhabdomyosarcoma (FKHR)
phosphorylation sites in regulating 14–3-3 binding, transactivation
and nuclear targeting. Biochem J. 354:605–612. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riquelme E, Behrens C, Lin HY, Simon G,
Papadimitrakopoulou V, Izzo J, Moran C, Kalhor N, Lee JJ, Minna JD
and Wistuba II: Modulation of EZH2 expression by MEK-ERK or
PI3K-AKT signaling in lung cancer is dictated by different KRAS
oncogene mutations. Cancer Res. 76:675–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crolle G and D'Este E: Glucosamine
sulphate for the management of arthrosis: A controlled clinical
investigation. Curr Med Res Opin. 7:104–109. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meininger CJ, Kelly KA, Li H, Haynes TE
and Wu G: Glucosamine inhibits inducible nitric oxide synthesis.
Biochem Biophys Res Commun. 279:234–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hua J, Sakamoto K and Nagaoka I:
Inhibitory actions of glucosamine, a therapeutic agent for
osteoarthritis, on the functions of neutrophils. J Leukoc Biol.
71:632–640. 2002.PubMed/NCBI
|
|
19
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Glucosamine, a naturally occurring amino monosaccharide
modulates LL-37-induced endothelial cell activation. Int J Mol Med.
22:657–662. 2008.PubMed/NCBI
|
|
20
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Modulation of TNF-α-induced endothelial cell activation
by glucosamine, a naturally occurring amino monosaccharide. Int J
Mol Med. 22:809–815. 2008.PubMed/NCBI
|
|
21
|
Dalirfardouei R, Karimi G and Jamialahmadi
K: Molecular mechanisms and biomedical applications of glucosamine
as a potential multifunctional therapeutic agent. Life Sci.
152:21–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brasky TM, Lampe JW, Slatore CG and White
E: Use of glucosamine and chondroitin and lung cancer risk in the
VITamins And Lifestyle (VITAL) cohort. Cancer Causes Control.
22:1333–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang MS and Baek WK: Glucosamine induces
autophagic cell death through the stimulation of ER stress in human
glioma cancer cells. Biochem Biophys Res Commun. 399:111–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Liang R, Huang GS, Piao Y, Zhang
YQ, Wang AQ, Dong BX, Feng JL, Yang GR and Guo Y: Glucosamine
sulfate-induced apoptosis in chronic myelogenous leukemia K562
cells is associated with translocation of cathepsin D and
downregulation of Bcl-xL. Apoptosis. 11:1851–1860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh HJ, Lee JS, Song DK, Shin DH, Jang BC,
Suh SI, Park JW, Suh MH and Baek WK: D-glucosamine inhibits
proliferation of human cancer cells through inhibition of p70S6K.
Biochem Biophis Res Commun. 360:840–845. 2007. View Article : Google Scholar
|
|
26
|
Liu BQ, Meng X, Li C, Gao YY, Li N, Niu
XF, Guan Y and Wang HQ: Glucosamine induces cell death via
proteasome inhibition in human ALVA41 prostate cancer cell. Exp Mol
Med. 43:487–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ju Y, Yu A, Sun X, Wu D and Zhang H:
Glucosamine, a naturally occurring amino monosaccharide, inhibits
A549 and H446 cell proliferation by blocking G1/S transition. Mol
Med Rep. 8:794–798. 2013.PubMed/NCBI
|
|
28
|
Ozcan S, Andrali SS and Cantrell JE:
Modulation of transcription factor function by O-GlcNAc
modification. Biochim Biophys Acta. 1799:353–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Y, Jaklitsch MT and Bueno R:
Neoadjuvant therapy in non-small cell lung cancer. Surg Oncol Clin
N Am. 25:567–584. 2016. View Article : Google Scholar : PubMed/NCBI
|