Introduction
Cardiac extracellular matrix (ECM) remodeling is the
most severe clinical manifestations during the process of heart
failure (HF), which is characterized by myocytes hypertrophy,
excessive expansion and accumulation of ECM, less compliance, and
matrix metalloproteinases (MMPs) activation (1). Inflammation is evidently involved in
adverse cardiac remodeling and HF. Accumulating evidences have
shown that the elevated levels of pro-inflammatory cytokines
including interleukin-6 (IL-6) and IL-17, are significantly
correlated with the development of HF, especially with the ECM
deposit (2). Chronic IL-6
stimulation results in hypertrophy and dysfunction of left
ventricular (LV), accordingly, depletion of IL-6 could effectively
prevent LV hypertrophy induced by the infusion of angiotensin II
(AngII) (3). Besides the
production of cytokines, the imbalance of the CD4+
T-helper (Th) lymphocytes subtypes Th1/Th2, later the imbalance of
Th17/regulatory T (Treg) has been reported in the inflammatory
microenvironment. The ratios of T-bet/GATA-3 and IFN-γ/IL-4 were
markedly up-regulated in C57BL/6 mice treated with alcohol than
that of the control group, suggesting that activating a Th2-type
immune response is one of the underlying mechanisms of
alcohol-induced cardiac remodeling (4). Treatments that raise the expression
of IL-10, a Th2 cytokine, have been reported to be a promising
therapeutic strategy to prevent the progress of pressure
overload-induced adverse cardiovascular remodeling (5). Specifically, T lymphocytes were
accepted to play a critical role both in the noncardiac tissues
remodeling processes, and cardiac ECM remodeling and HF. Therefore,
medications effectively control inflammation and T cell balance in
the heart may provide therapeutic benefits (6).
Green tea was used to treat various diseases in the
traditional Chinese medicine. Later people realized that
Epigallocatechin gallate (EGCG) is the major catechin in
green tea and is regarded to have strong antioxidant activity,
which may prevent cell damage, exert numerous protective effects on
cancer growth, hyperlipidemia, diabetes, stroke, particularly on
the cardiovascular system (7–9).
Green tea consumers have lower death risk from cardiovascular
diseases. A recent meta-analysis of 18 published studies revealed
that the mortality of cardiovascular diseases decreased by 5% if
the patients' green tea consumption increased one cup per day
(10). Long-term consumption of
green tea may reduce the risk of attack and slightly drop the
systolic and diastolic blood pressures (11,12).
Consequently, EGCG has been accepted as a potential novel approach
for treating various cardiovascular diseases (13). We reported recently that EGCG
markedly inhibited the viability, proliferation and collagen
production of myocytes induced by AngII (14). EGCG may regulate cellular immunity
as reported that EGCG could target Th cells and natural killer cell
(15). Thus, we hypothesized that
EGCG inhibits the overload-induced ECM remodeling partially through
regulating T cell subtypes and corresponding pro-inflammatory
cytokines.
Materials and methods
Animals
Sprague-Dawley (SD) rat (male, 160±20 g) were
provided by the Experimental Animal Department of Anhui Medical
University, China (certificate no. SCXK [WAN] 2011-002). All rats
were fed in an SPF environment. All experiments were approved by
the Ethics Review Committee for Animal Experimentation of Anhui
Medical University and were conducted according to the Declaration
of Helsinki.
Materials and drugs
EGCG and AngII receptor inverse agonist valsartan
(Val) were both purchased from Sigma Chemical Co. (St. Louis, MO,
USA). Anti-rat CD4- FITC, CD3- PE, IL-17-PE, CD44-PE, CD62L-PE, PE
isotype, CD25-PE antibodies, anti-rat/mouse Foxp3-PECy5 antibody
and PECy5 isotype were the products of eBioscience, Inc. (San
Diego, CA, USA) or BD Biosciences. Phorbol myristate acetate (PMA)
and ionomycin were purchased from Sigma-Aldrich (St. Louis, MO,
USA). ELISA kits, anti-rat signal transducer and activator of
transcription 3 (STAT3) and anti-rat STAT5 were obtained from
R&D Systems, Inc., Minneapolis, MN, USA. MMPs activity assay
kit (Fluorometric-Red; Abcam, Hong Kong SAR, China).
Establishment of pressure
overload-induced cardiac remodeling model and treatment
SD rats were randomly subjected to either transverse
aortic constriction (TAC, to induce chronic pressure overload) or
sham operation following the protocol described previously
(16). Briefly, the rats were
anesthetized and the chest was opened, the intercostal muscles were
then blunt dissected and followed by the identification of thoracic
aorta, a 6.0 silk suture was placed around the transverse aorta,
and a 26-gauge blunt needle was put parallel to the transverse
aorta. To make a constriction, the needle was gently removed after
the suture was tied. Sham-operated rats (n=10) underwent a similar
surgical procedure without the ligation. TAC rats were randomly
divided into a model group which was treated with vehicle; three
EGCG-treatment groups (orally fed EGCG 25, 50 or 100 mg/kg B.W. day
for 6 weeks) and Val-treatment group (orally fed Val 30 mg/kg B.W.
day for 6 weeks) one week after the surgery.
Masson's Trichrome staining of heart
tissue
The hearts of rats were perfused with PBS and fixed
in formalin and then were embedded in paraffin and sectioned into
five-micron slices using Thermo HM355 Microtomes. The Masson's
Trichrome staining was performed according to the instruction. The
blue-stained areas in the sections were measured with Image J
software (NIH, Bethesda, MD, USA) to evaluate the collagen
deposition semi-quantitatively. The data from six regions of each
heart was analyzed.
Determination of cardiac ECM
The micrograms of collagen per milligram of the dry
heart were used to reflect the extent of cardiac ECM deposition.
Since collagen contains about 13.5% hydroxyproline (17), the level of total myocardial
hydroxyproline was determined with the standard
trans-hydroxyproline colorimetric curve (Sigma-Aldrich).
Analysis of
CD4+CD44+and CD4+CD62L+
T cells
Splenic lymphocytes were isolated by density
gradient centrifugation from splenic cells and then were stained
with CD4-FITC and CD44-PE, or CD4-FITC and CD62L-PE respectively.
The percentage of CD4+CD44+ and
CD4+CD62L+ T cells were analyzed by flow
cytometry on an FC500 flow cytometer (Beckman Coulter, Fullerton,
CA, USA). Data analysis was performed using Cell Quest™ analysis
software.
Detection of Th17 cells
Splenic lyomphocytes were suspended in DMEM medium
at a concentration of 1×107 cells/ml and were stimulated
with PMA (final concentration is 50 ng/ml) and ionomycin (final
concentration is 1 µM) for 5 h in the incubator. Golgistop (final
concentration is 0.7 µl/ml) was added 1 h after culture. The
subpopulation of Th17 (CD4+ IL-17+) cells was
counted on an FC500 flow cytometer (Beckman Coulter) (18).
Determination of regulatory T cells
(Tregs)
Isolated splenic lymphocytes (100 µl)
(1×107 cells/ml) were stained with 0.125 µg anti-rat
CD4-FITC and 0.06 µg anti-rat CD25-PE antibodies in the dark at 4°C
for 30 min. The cells were then fixed and permeabilized, blocked
with 2% normal rat serum. 0.5 µg anti-mouse/rat Foxp3-PeCy5
antibody or rat IgG2a K isotype control PeCy5 was added to each
sample without washing after the blocking step and incubated at 4°C
for 30 min in the dark. 100,000 cells were counted on FC500 flow
cytometer (Beckman Coulter) (19).
Determination of MMP activity
The activity of MMP was evaluated with an MMP
activity assay kit. Briefly, 10 mg heart tissue was homogenized in
1 ml extraction buffer. Samples were spined and the supernatants
were then incubated with equal volume of 2 mM p-Aminophenylmercuric
acetate working (2x) solution for 15 min, then incubated with 50 µl
MMP red substrate solution for 0 min (for kinetic reading) or 1 h
(for end point reading), the fluorescence intensity was monitored
with absorbance reader (ELx808, BioTek, US) and the MMP activity
was analyzed as Ex/Em=540/590 nm.
The expression of TIMP-2, ANP, BNP,
T-bet, GATA-3, RORC and FoxP3 mRNA in heart tissue
The total RNA of splenic lymphocytes was extracted
using the traditional TRIzol method. The primers used for qPCR are
listed in Table I (20).
 | Table I.Primer sequences for qPCR. |
Table I.
Primer sequences for qPCR.
| Gene | Primer | Sequences |
|---|
| FoxP3 | F |
5′-TGAGCTGGCTGCAATTCTGG-3′ |
|
| R | 5′-A
TCTAGCTGCTCTGCATGAGGTGA-3′ |
| RORC | F | 5′-GGATGAGATTGCC
CTCTACAC-3′ |
|
| R |
5′-GGAGGCCTTGTCGATGAGTC-3′ |
| T-bet | F | 5′-AACCAGTATCCTG
TTCCCAGC-3′ |
|
| R |
5′-TGTCGCCACTGGAAGGATA G-3′ |
| GATA-3 | F |
5′-CTCTCCTTTGCTCACCTTTTC-3′ |
|
| R | 5′-AAGA
GATGCGGACTGGAGTG-3′ |
| TIMP-2 | F |
5′-GGATTCCGGGAATGACATCTAT-3′ |
|
| R |
5′-CGCCTTCCCTGCAATTAGATA-3′ |
| ANP | F |
5′-GAGGAGAAGATGCCGGTAG-3′ |
|
| R |
5′-CTAGAGAGGGAGCTAAGTG-3′ |
| BNP | F | 5′-
TGATTCTGCTCCTGCTTTTC-3′ |
|
| R |
5′-GTGGATTGTTCTGGAGACTG-3′ |
| GAPDH | F |
5′-TCAAGAAGGTGGTGAAGCAG-3′ |
|
| R |
5′-AGGTGGAAGAATGGGAGTTG-3′ |
Determination of serum IL-6, IL-7,
IL-15, IL-17, IFN-γ, and IL-10
Blood was centrifuged at 3000 × g for 30 min at 4°C,
and the serum was collected. IL-6, IL-7, IL-15, IL-17, IFN-γ and
IL-10 levels were measured using ELISA kits. Absorbance values were
read at 450 nm with an ELISA plate reader (ELx808; BioTek,
Winooski, VT, USA) (21).
Western blot analysis
Purified splenic lymphocytes were applied for
western blot assay to detect the expression level of STAT3 and
STAT5 following the conventional method. Briefly, the cells were
lysed with RIPA buffer and the proteins were separated in a running
gel. After the proteins had been transferred onto a PVDF membrane,
the membrane was blocked in 5% fat-free milk and stained with
anti-STAT3, anti-STAT5 or anti-β-actin primary antibody in 4°C
overnight, followed by the staining of secondary antibody. The
membranes were then scanned using an imaging densitometer (GS-700;
Bio-Rad, Berkeley, CA, USA). The images were quantified with ImageJ
software (NIH).
Statistical analysis
At least triplicate determinations were made for
each experiment. The representative results were shown in indicated
cases. All data are expressed as the mean and standard deviation
(SD). For multiple group comparisons, we used ANOVA and Tukey's
post test. P<0.05 was considered to indicate a statistically
significant difference.
Results
The effect of EGCG on cardiac
fibrosis
Masson's Trichrome staining was applied to
demonstrate the cardiac fibrosis and ECM deposition. As expected,
EGCG 100 mg/kg or Val 30 mg/kg treatment effectively ameliorated
the development of ventricular fibrosis (Fig. 1A). The data was semi-quantified in
Fig. 1B.
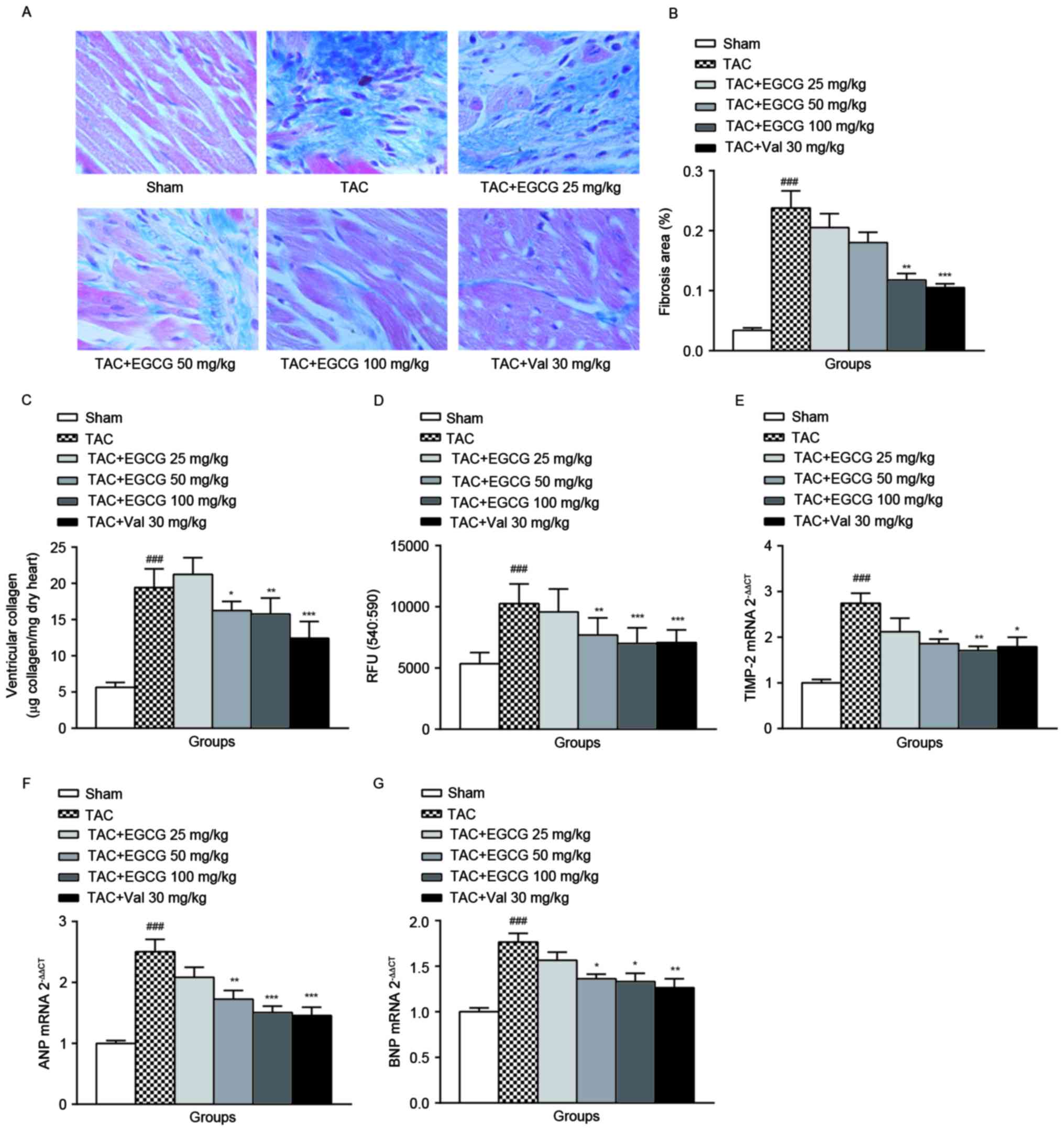 | Figure 1.The effect of EGCG on cardiac
fibrosis. (A) representative Masson's trichrome staining sections
for all experimental groups respectively, the regions stained blue
indicate collagen deposition (n=8). (B) Quantification of the
relative area of interstitial collagen by Image J program. (C)
Total cardiac fibrillar collagen was assayed by determination of
the hydroxyproline concentrations, assuming that collagen contains
an average of 13.5% hydroxyproline (n=8-10). (D) The fluorescence
signal of the activity of MMPs was monitored one hour after the
start of the reaction by using a microplate reader with a filter
set of Ex/Em=540/590 nm (n=8). The mRNA expression of cardiac
TIMP-2 (E), ANP (F), and BNP (G) was analyzed by qRT-PCR, and the
gene levels were analyzed by 2−ΔΔCq and normalized to
GAPDH. ###P<0.001 vs. sham-operated group;
*P<0.05, **P<0.01, ***P<0.001 vs. TAC group. EGCG,
Epigallocatechin gallate; MMP, matrix metalloproteinase;
TIMP, tissue inhibitor of metalloproteinase. |
Collagen is a major component of the cardiac ECM,
the amount of cardiac fiber detected by the determination of the
total amount of hydroxyproline represents the expression level of
collagen. As shown in Fig. 1C, the
collagen contents in heart tissues from TAC rats were increased
significantly compared with that of the sham-operated rats, the
administration of EGCG (100 mg/kg) or Val (30 mg/kg) decreased
total collagen content in overload-induced cardiac remodeling rats
with various degrees. This result confirmed that EGCG is an
effective preventer to against cardiac ECM accumulation. The
mechanism is not clearly understood.
As shown in Fig.
1D, the MMPs activity in the cardiac tissue of overload-induced
cardiac remodeling rats was evoked remarkably. The administration
of EGCG to rats with TAC dose-dependently blocked the activity of
MMPs. Val treatment slightly attenuated the increment of MMPs
activity (P=0.038). The tissue inhibitor of metalloproteinases
(TIMPs) specifically inhibit MMPs, we detected the mRNA level of
TIMP-2 in heart tissue by qRT-PCR. TIMP-2 mRNA expression increased
by 2.74 fold in TAC rats than that of normal rats. EGCG 50 and 100
mg/kg treatment, as well as Val administration significantly
reduced the expression of TIMP-2 (Fig.
1E).
Besides excessive expansion and accumulation of ECM,
MMPs activation, ECM remodeling is also characterized by myocytes
hypertrophy. ANP and BNP are common hypertrophic markers. As shown
in Fig. 1F and G, ANP
(2.503±0.456) and BNP (1.765±0.216) gene expression of the TAC rats
was significantly upregulated, EGCG 50 and 100 mg/kg treatment and
Val 30 mg/kg treatment markedly reduced both ANP and BNP
expression.
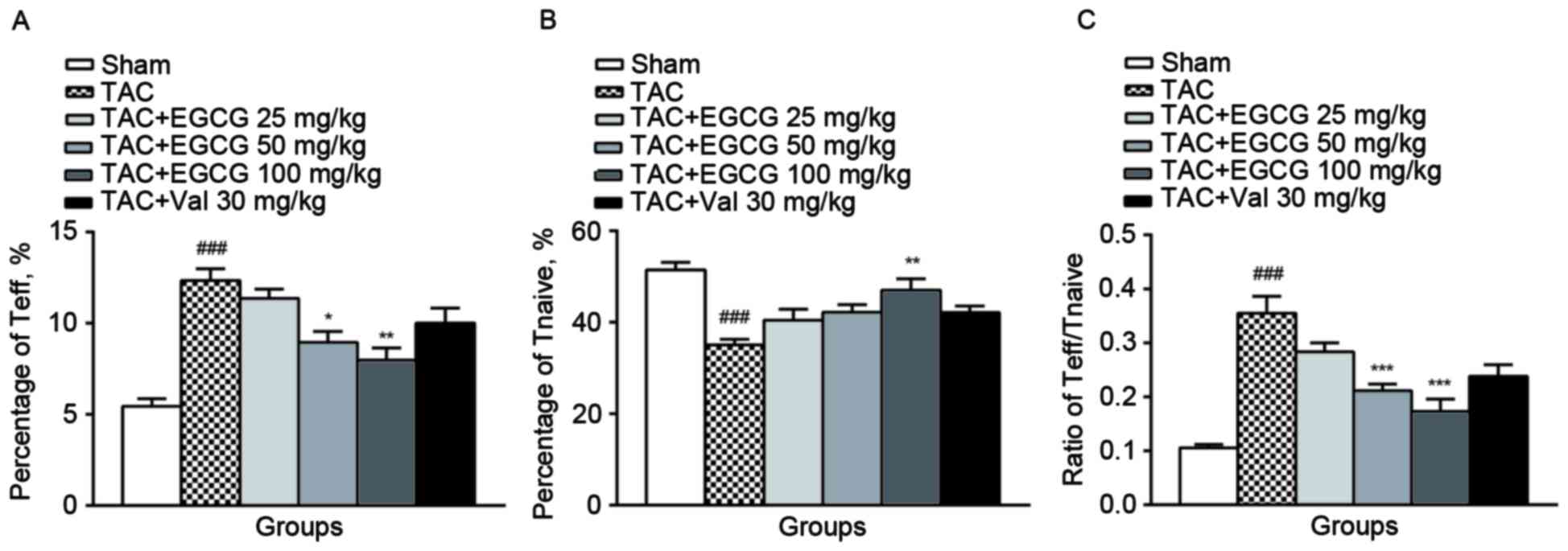
The effect of EGCG on T cell
activation
We found that T cells were activated and
differentiated into CD4+CD44+ effector T
cells (Teff) in splenic lymphocytes from TAC rats, meanwhile, the
population of CD4+CD62L+ naïve T cells
(Tnaive) was diminished. EGCG (50, 100 mg/kg) treatment decreased
the percentage of Teff subset and EGCG (100 mg/kg) rescued the
subpopulation of Tnaive (Fig. 2A and
B). As a result, EGCG restored the balance of Teff/Tnaive
(Fig. 2C), suggesting that it
effectively inhibited T cells activation.
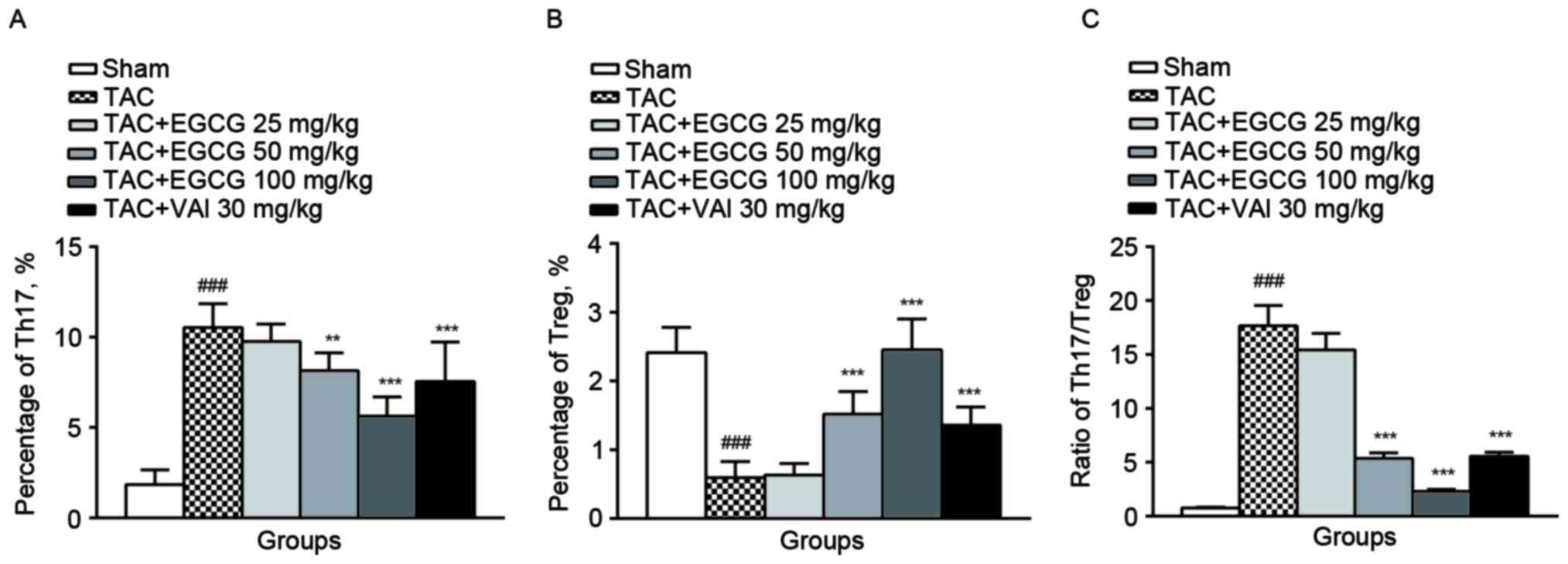
The effect of EGCG on the percentage
of Th17 and Treg cells
Th17 cells were identified by expression of
intracellular IL-17 and surface marker CD4 from rat spleens by flow
cytometry (22). It turned out
that the percentages of Th17 cells represented a much higher value
in overload-induced cardiac remodeling rats (13.4%) compared with
the sham-operated rats (0.98%). As expected, Th17 subpopulation was
substantially reduced in EGCG (50 mg/kg) (7.77%), EGCG (100 mg/kg)
(3.32%) or Val (30 mg/kg) (5.63%) treatment groups (Fig. 3A).
We next counted the subpopulation of Treg subsets by
identifying the expression of nucleic transcriptional factor Foxp3
in CD4+ CD25+ rat splenic lymphocytes. Unlike
the changes of Th17, the percentage of Treg cells was decreased
obviously in rats with overload-induced cardiac remodeling
(0.59±0.15%) compared with control rats (2.15±0.39%). The
administration of EGCG (50 mg/kg) restored the content of Treg to
1.22±0.36%. In particular, the administration of EGCG (100 mg/kg)
significantly increased the percentage of Treg cells (2.35±0.48%).
Val exerted a similar effect as EGCG (1.29±0.28%, P=0.021)
(Fig. 3B). Comparingwith the sham
rats, TAC group exhibited an increase in the ratio of Th17/Treg.
EGCG (50, 100 mg/kg) and Val (30 mg/kg) restored the imbalance of
Th17/Treg ratio significantly (Fig.
3C).
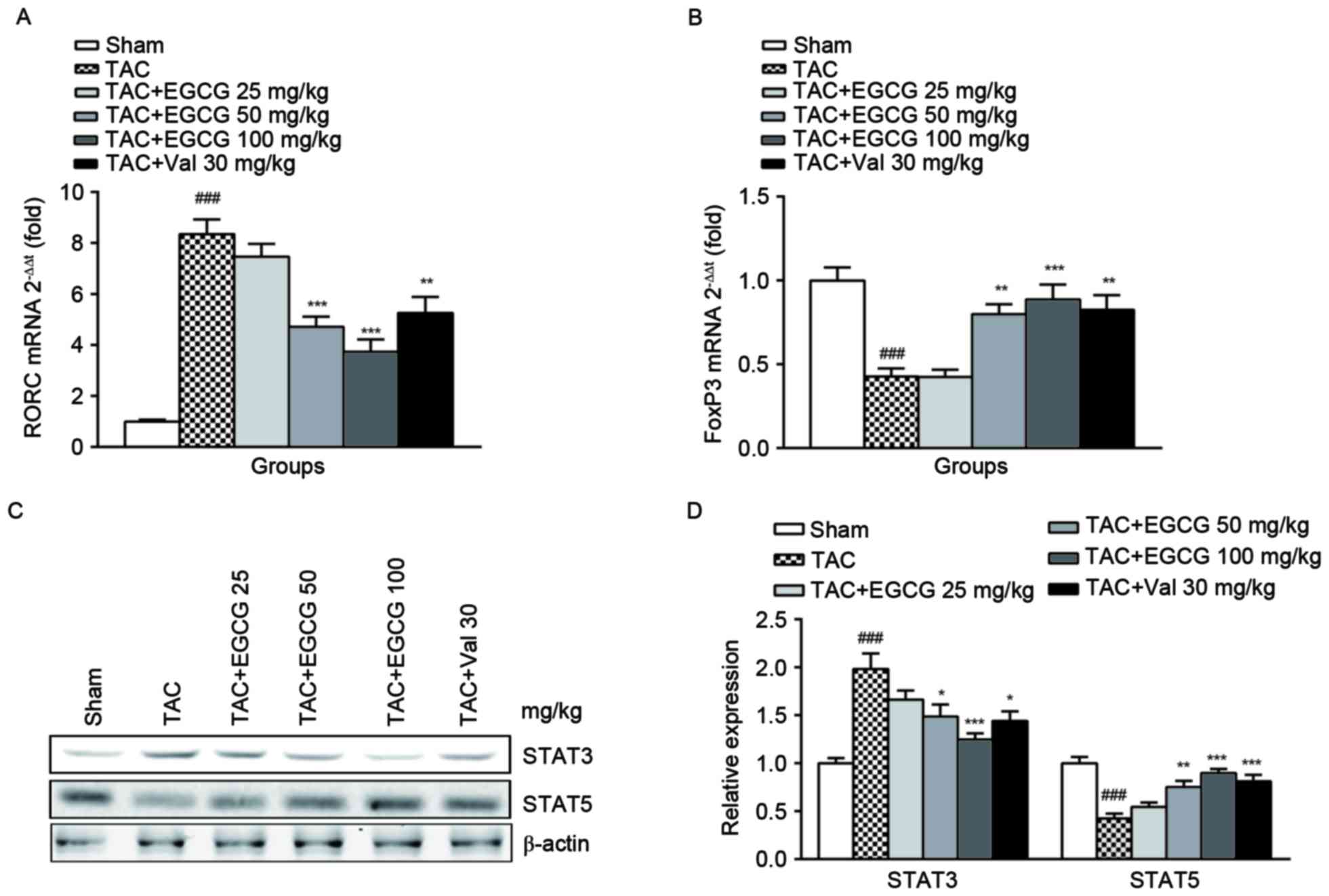
The effect of EGCG on the expressions
of regulators for Th17 and Treg cells differentiation
Besides the percentage determination, we further
analyzed the expressions of transcription factor RORC for Th17 and
FoxP3 for Treg cells, respectively. RORC encoding retinoid-related
orphan receptor γt (RORγt) which is identified as the master
transcription factor designating Th17 cell subpopulation. In
accordance with the results of flow cytometry, we found RORC was
upregulated in splenic lymphocytes from TAC rats, EGCG treatment
restored its expression in a dose-dependent manner (Fig. 4A). The polarization and maturation
of Treg are tightly controlled by FoxP3, which expression was shown
to be inhibited in the TAC group, EGCG and Val significantly
rescued FoxP3 expression (Fig.
4B). The differentiation of Th17 and Treg cells is mediated by
downstream regulator STAT3 and STAT5 respectively (23). As expected, the expression of STAT3
was substantially increased and STAT5 was obviously decreased in
splenic lymphocytes of TAC mice. The administration of EGCG and Val
was found to have distinct restorative effects on the expression of
both regulators (Fig. 4C and
D).
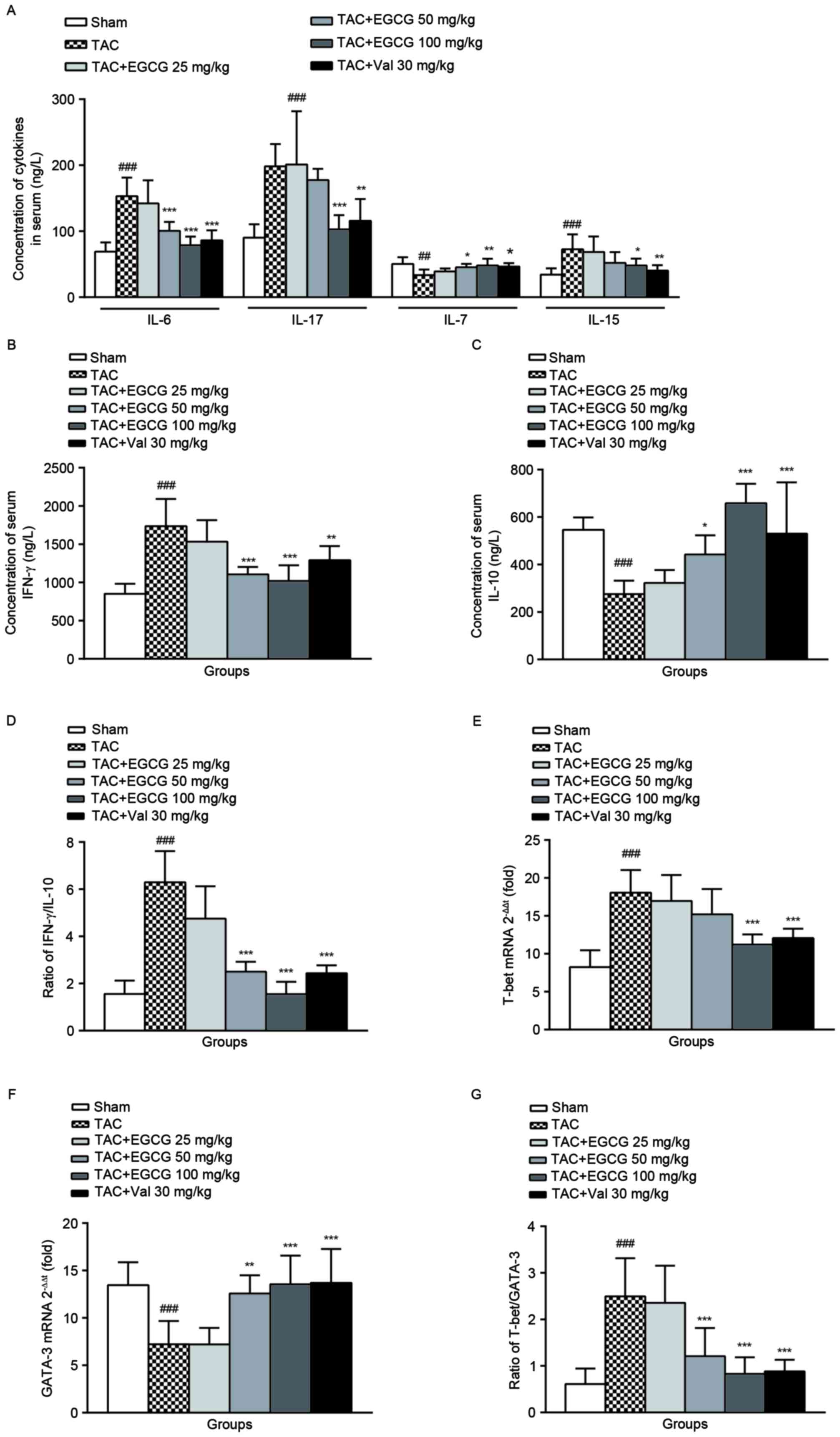
The effect of EGCG on the levels of
serum Th1/Th2 cytokines
As shown in Fig.
5A, IL-7 expression was obviously reduced, while IL-15 level
was increased in TAC rats comparing to that of normal rats. EGCG
and Val administration restored IL-7 production, and decreased the
level of IL-15 in TAC rats at different degrees. The concentrations
of serum IL-6, IL-17, and IFN-γ were significantly elevated
(Fig. 5A and B), while serum IL-10
was decreased in TAC rats (Fig.
5C). EGCG (50 mg/kg) inhibited the upregulation of IL-6 and
IFN-γ, EGCG (100 mg/kg) reduced the production of IL-6, IL-17 and
IFN-γ, and the IL-10 synthesis was restored to almost the normal
level. Similarly, Val treatment modulated the levels of serum IL-6,
IL-17, IFN-γ and IL-10 with varying degrees. These data indicated
that rats with overload-induced cardiac remodeling have more Th1
cytokine IFN-γ and less Th2 cytokine IL-10. Administration of EGCG
or Val ameliorated the disequilibrium of IFN-γ/IL-10 ratio
(Fig. 5D), which suggests an
imbalanced ratio of Th1/Th2 subsets, consequently improved disease
symptoms.
Both Th1 and Th2 populations could be differentiated
from naive Th cells, each of them has distinct functions and
cytokine profiles (24). Comparing
with sham-operated control, the mRNA levels of T-bet, a T-box
transcription factor required for Th1 cell development, in splenic
lymphocytes from TAC rats was significantly increased
(2−ΔΔCq=18.34). The treatment of EGCG (100 mg/kg) or Val
(30 mg/kg) mildly decreased T-bet gene expressions (Fig. 5E). However, the level of GATA-3
mRNA, a critical regulator of Th2 polarization, was decreased in
model rats. Oral administered EGCG (50, 100 mg/kg) or Val to TAC
rats positively changed the GATA-3 mRNA expression (Fig. 5F). In TAC model rats, the ratio of
T-bet/GATA-3 was found to be elevated, which could be balanced by
EGCG treatment (Fig. 5G).
Discussion
In accordance with published articles, we observed
that the collagen was accumulated in the heart of TAC rats, the
MMPs were also found to be activated significantly, MMPs inhibitor
TIMP-2 was induced simultaneously. The ECM may expand under
microenvironmental stimuli including inflammatory cytokines
(25). The increased cardiac
collagen expression paralleled to the ventricular stiffness was
described by Badenhorst et al (26). As expected, heart failure
parameters ANP and BNP were elevated in TAC rats. In addition, Yu
et al (27) reported that
the ventricular stiffness is associated with T lymphocyte response
in 2006, indicating that cellular immunity may contribute to ECM
production. Consistently, we found T cells were activated, and the
ratio of Teff/Tnaive was up-regulated in TAC rats.
The Th1/Th2 populations which are characterized
based on the unique patterns of surface markers, cytokine profiles,
and immune functions are induced by specific ligands. In chronic
alcohol-induced cardiac fibrosis, alcohol consumption contributes
to the imbalance of Th1/Th2 subsets with an increased Th2 activity
(4). Th1 and Th17 cytokine
productions were increased, and Th2 cytokine was reduced in Chagas
disease cardiomyopathy (28).
Hypertensive rats induced by Ang II infusion were observed to have
upregulation of IFN-γ (Th1 cytokine) and downregulation of IL-4
(Th2 cytokine) (29). It was also
reported that selectively induce Th1 leads to cardiac collagen
deposit and collagen cross-linking, however, selectively induce Th2
subtype has the opposite effect in mice. As a result, the different
Th cell phenotype distinctly influences the expression of
pro-collagen and pro-MMP genes in cardiac fibroblasts (CFs),
modulates the MMPs activity and collagen accumulation, contributes
to the alteration of ECM composition, therefore, affects the
diastolic function of the heart. Accordingly, regulation of the
profile and function of Th lymphocyte could promote the adaptive
ventricular remodeling in postmyocardial infarction and HF
(6). In the present study, we
found the mRNA level of T-bet in splenic lymphocytes of TAC rats
was significantly increased, however, the level of GATA-3 mRNA was
decreased, and the ratio of T-bet/GATA-3 was elevated. In parallel
with the expression of the transcription factor, IFN-γ (a Th1
cytokine) was elevated, while IL-10 (a Th2 cytokine) was diminished
in the model rats. We illustrated that the imbalance of Th1/Th2
function exists in the cardiac remodeling process.
The homeostasis of T cells is regulated by two
members of the common gamma chain family of cytokines, IL-7 and
IL-15 (30). Interestingly, in the
present study, we observed an upregulation of serum IL-15 and a
downregulation of serum IL-7 in TAC rats. Literature also reported
that plasma IL-7 level dropped in chronic heart failure patients
(31). EGCG restored the
homeostasis of T cells by rebalancing the expression of IL-7 and
IL-15. IL-6 is considered an important cytokine in promoting the
differentiation of Th17 cells from precursor Th cells. We revealed
that in overload-induced cardiac remodeling rats, serum IL-6
production was increased significantly, resulting in the
upregulation of both the percentage of Th17 and the level of IL-17.
Previous studies have been conducted to exmine the role of IL-17 in
inflammation-induced cardiac remodeling. It was reported that in
IL-17-deficient mice, the interstitial myocardial fibrosis was
attenuated, the expressions of MMP-2 and MMP-9 was reduced and the
activity of gelatinase was inhibited. Anti-IL-17A monoclonal
antibody could ameliorate both cardiac fibrosis and heart function
when administered after the onset of myocarditis in BALB/c mice
(32). Th17 cells and Treg cells
develop reciprocally from naive Th cells, and exert opposite
functions. The differentiation of Th17 depends on the activation of
STAT3, while the development of Treg is mediated by the activation
of STAT5 (23). The imbalance of
Th17/Treg is an important feature of inflammation and autoimmune
diseases (33). As expected, in
TAC rats, we found a disequilibrated Th17/Treg balance with the
expanded Th17 population and the shrinking Treg population compared
with the sham-operated controls, indicating that the imbalance of
Th17/Treg ratio potentially involved in the pathogenesis of cardiac
ECM remodeling, and restoring this balance may be a promising
therapeutic approach to attenuate cardiac remodeling.
Evidence shows that EGCG has multiple protective
effects against many kinds of cardiovascular diseases, including
cardiac fibrosis. However, how does ECCG prevent collagen deposit
in the heart is still unknown. Cai and colleagues recently reported
that reducing collagen production, fibronectin (FN) expression, and
CFs proliferation by inhibiting NF-κB activation in rats challenged
with AngII is the underlying molecular mechanism of EGCG (34). Besides cardiac fibrosis, EGCG is
also a promising therapeutic drug for systemic sclerosis (SSc)
induced oxidant stress and fibrosis (35).
In the present series of studies, we investigated
whether the inhibitory effects of EGCG on collagen accumulation and
heart remodeling were associated with the modulation of T
lymphocytes subsets. As shown in the results, EGCG reduced total
collagen content and MMPs activity in remodeled cardiac tissue
indicating EGCG is a potential treatment for preventing cardiac
remodeling in rats with overload induced cardiac ECM remodeling. We
further showed that EGCG augmented Th2 cytokine production and
diminished Th1 and Th17 cytokine production, that is in accordance
with the modulation of the expression of T-bet and GATA-3, which
are the master regulators for Th1 and Th2 differentiation
respectively. It also decreased Th17 and increased Treg populations
in the spleen through restoring the expression of Th17 and Treg
downstream regulators STAT3 and STAT5. Moreover, EGCG inhibited T
cell activation by rebalanced the population of Teff and
Tnaive.
In conclusion, based on the current studies, EGCG is
proved to be an effective therapeutic natural medicine in treating
cardiac ECM remodeling and improving heart function, and restoring
the balances of Th1/Th2, Th17/Treg, Teff/Tnaive and regaining the
immune homeostasis are the presumed mechanisms of its action.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (81202541), the Anhui Provincial
Natural Science Foundation (1208085QH146 and 1408085MH173), the
Grants for Scientific Research of BSKY (XJ201213), University
Science Research Project of Anhui Province (KJ2017A176), Training
Programme Foundation for the Young Talents of Higher Education
(gxyqZD2017025), the Foundation for Young Academic Backbone of
Anhui Medical University, the Grants for Young Talents of Anhui
Medical University (2013), Young Outstanding Doctor Research
Program, Anhui Provincial Hospital (2015).
References
|
1
|
Morita H and Komuro I: Periostin isoforms
and cardiac remodeling after myocardial infarction: Is the dispute
settled? Hypertension. 67:504–505. 2016.PubMed/NCBI
|
|
2
|
Xu X, Pang J, Chen Y, Bucala R, Zhang Y
and Ren J: Macrophage migration inhibitory factor (MIF) deficiency
exacerbates aging-induced cardiac remodeling and dysfunction
despite improved inflammation: Role of autophagy regulation. Sci
Rep. 6:224882016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melendez GC, McLarty JL, Levick SP, Du Y,
Janicki JS and Brower GL: Interleukin 6 mediates myocardial
fibrosis, concentric hypertrophy, and diastolic dysfunction in
rats. Hypertension. 56:225–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu W, Li J, Tian W, Xu T and Zhang Z:
Chronic alcohol consumption induces cardiac remodeling in mice from
Th1 or Th2 background. Exp Mol Pathol. 91:761–767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwon WY, Cha HN, Heo JY, Choi JH, Jang BI,
Lee IK and Park SY: Interleukin-10 deficiency aggravates
angiotensin II-induced cardiac remodeling in mice. Life Sci.
146:214–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu Q, Vazquez R, Zabadi S, Watson RR and
Larson DF: T-lymphocytes mediate left ventricular fibrillar
collagen cross-linking and diastolic dysfunction in mice. Matrix
Biol. 29:511–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang CS, Chen G and Wu Q: Recent
scientific studies of a traditional chinese medicine, tea, on
prevention of chronic diseases. J Tradit Complement Med. 4:17–23.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Xu ZL, Sun L, Liu Y, Li CC, Li HM,
Zhang W, Li CJ and Qin W: (−)-Epigallocatechin-3-gallate induces
apoptosis in human pancreatic cancer cells via PTEN. Mol Med Rep.
14:599–605. 2016.PubMed/NCBI
|
|
9
|
Zeng X and Tan X:
Epigallocatechin-3-gallate and zinc provide anti-apoptotic
protection against hypoxia/reoxygenation injury in H9c2 rat cardiac
myoblast cells. Mol Med Rep. 12:1850–1856. 2015.PubMed/NCBI
|
|
10
|
Tang J, Zheng JS, Fang L, Jin Y, Cai W and
Li D: Tea consumption and mortality of all cancers, CVD and all
causes: A meta-analysis of eighteen prospective cohort studies. Br
J Nutr. 114:673–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xuan F and Jian J: Epigallocatechin
gallate exerts protective effects against myocardial
ischemia/reperfusion injury through the PI3K/Akt pathway-mediated
inhibition of apoptosis and the restoration of the autophagic flux.
Int J Mol Med. 38:328–336. 2016.PubMed/NCBI
|
|
12
|
Tian C, Huang Q, Yang L, Légaré S,
Angileri F, Yang H, Li X, Min X, Zhang C, Xu C, et al: Green tea
consumption is associated with reduced incident CHD and improved
CHD-related biomarkers in the Dongfeng-Tongji cohort. Sci Rep.
6:243532016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai Y, He SQ, Hong HQ, Cai YP, Zhao L and
Zhang M: High doses of (−)-epigallocatechin-3-gallate from green
tea induces cardiac fibrosis in mice. Biotechnol Lett.
37:2371–2377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han YS, Lan L, Chu J, Kang WQ and Ge ZM:
Epigallocatechin gallate attenuated the activation of rat cardiac
fibroblasts induced by angiotensin II via regulating β-arrestin1.
Cell Physiol Biochem. 31:338–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YH, Won YS, Yang X, Kumazoe M,
Yamashita S, Hara A, Takagaki A, Goto K, Nanjo F and Tachibana H:
Green tea catechin metabolites exert immunoregulatory effects on
CD4 (+) T cell and natural killer cell activities. J Agric Food
Chem. 64:3591–3597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Zhang L and Liang J: Unraveling the
expression profiles of long noncoding RNAs in rat cardiac
hypertrophy and functions of lncRNA BC088254 in cardiac hypertrophy
induced by transverse aortic constriction. Cardiology. 134:84–98.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo K, Lan CZ, Yu TT, Huang LL, Wang XH,
Pan C and Gao S: Effects of Xin-Ji-Er-Kang formula on 2K1C-induced
hypertension and cardiovascular remodeling in rats. J
Ethnopharmacol. 155:1227–1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen Y, Zeng Z, Gui C, Li L and Li W:
Changes in the expression of Th17 cell-associated cytokines in the
development of rheumatic heart disease. Cardiovasc Pathol.
24:382–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang B, Wang QT, Song SS, Wu YJ, Ma YK,
Zhang LL, Chen JY, Wu HX, Jiang L and Wei W: Combined use of
etanercept and MTX restores CD4*/CD8* ratio and Tregs in spleen and
thymus in collagen-induced arthritis. Inflamm Res. 61:1229–1239.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Feng X and Huang Q: Modulation of
T-Bet and GATA-3 expression in experimental autoimmune thyroiditis
rats through ginsenoside treatment. Endocr Res. 41:28–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang QT, Zhang LL, Wu HX and Wei W: The
expression change of β-arrestins in fibroblast-like synoviocytes
from rats with collagen-induced arthritis and the effect of total
glucosides of paeony. J Ethnopharmacol. 133:511–516. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terrazas C, Varikuti S, Kimble J, Moretti
E, Boyaka PN and Satoskar AR: IL-17A promotes susceptibility during
experimental visceral leishmaniasis caused by Leishmania donovani.
FASEB J. 30:1135–1143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YJ, Hyung KE, Yoo JS, Jang YW, Kim SJ,
Lee DI, Lee SJ, Park SY, Jeong JH and Hwang KW: Effects of exposure
to extremely low-frequency electromagnetic fields on the
differentiation of Th17 T cells and regulatory T cells. Gen Physiol
Biophys. 35:487–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stellato C, Gubin MM, Magee JD, Fang X,
Fan J, Tartar DM, Chen J, Dahm GM, Calaluce R, Mori F, et al:
Coordinate regulation of GATA-3 and Th2 cytokine gene expression by
the RNA-binding protein HuR. J Immunol. 187:441–449. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moore-Morris T, Guimarães-Camboa N, Yutzey
KE, Pucéat M and Evans SM: Cardiac fibroblasts: From development to
heart failure. J Mol Med (Berl). 93:823–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Badenhorst D, Maseko M, Tsotetsi OJ,
Naidoo A, Brooksbank R, Norton GR and Woodiwiss AJ: Cross-linking
influences the impact of quantitative changes in myocardial
collagen on cardiac stiffness and remodelling in hypertension in
rats. Cardiovasc Res. 57:632–641. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Q, Horak K and Larson DF: Role of T
lymphocytes in hypertension-induced cardiac extracellular matrix
remodeling. Hypertension. 48:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nogueira LG, Santos RH, Fiorelli AI,
Mairena EC, Benvenuti LA, Bocchi EA, Stolf NA, Kalil J and
Cunha-Neto E: Myocardial gene expression of T-bet, GATA-3, Ror-γt,
FoxP3, and hallmark cytokines in chronic Chagas disease
cardiomyopathy: An essentially unopposed TH1-type response.
Mediators Inflamm. 2014:9143262014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao J, Nangaku M, Miyata T, Inagi R,
Yamada K, Kurokawa K and Fujita T: Imbalance of T-cell subsets in
angiotensin II-infused hypertensive rats with kidney injury.
Hypertension. 42:31–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung YW, Kim HG, Perry CJ and Kaech SM:
CCR7 expression alters memory CD8 T-cell homeostasis by regulating
occupancy in IL-7- and IL-15-dependent niches. Proc Natl Acad Sci
USA. 113:8278–8283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cappuzzello C, Di Vito L, Melchionna R,
Melillo G, Silvestri L, Cesareo E, Crea F, Liuzzo G, Facchiano A,
Capogrossi MC and Napolitano M: Increase of plasma IL-9 and
decrease of plasma IL-5, IL-7 and IFN-γ in patients with chronic
heart failure. J Transl Med. 9:282011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Machino-Ohtsuka T, Tajiri K, Kimura T,
Sakai S, Sato A, Yoshida T, Hiroe M, Yasutomi Y, Aonuma K and
Imanaka-Yoshida K: Tenascin-C aggravates autoimmune myocarditis via
dendritic cell activation and Th17 cell differentiation. J Am Heart
Assoc. 3:e0010522014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim SM, Kang GD, Jeong JJ, Choi HS and Kim
DH: Neomangiferin modulates the Th17/Treg balance and ameliorates
colitis in mice. Phytomedicine. 23:131–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai Y, Yu SS, Chen TT, Gao S, Geng B, Yu
Y, Ye JT and Liu PQ: EGCG inhibits CTGF expression via blocking
NF-κB activation in cardiac fibroblast. Phytomedicine. 20:106–113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dooley A, Bruckdorfer KR and Abraham DJ:
Modulation of fibrosis in systemic sclerosis by nitric oxide and
antioxidants. Cardiol Res Pract. 2012:5219582012. View Article : Google Scholar : PubMed/NCBI
|