Introduction
Allergies are hypersensitivity reactions initiated
in response to various environmental allergens. During
sensitization to allergens through skin, and the respiratory and
digestive tracts, B cells differentiate into
immunoglobulin-producing cells and produce allergen-specific IgE,
which binds to high-affinity receptor (FcεRI) on surfaces of mast
cells in the connective or mucosal tissues (1). Subsequent exposure to the allergen
activates mast cells through the cross-linking of FcεRI-IgE
complexes, and activated mast cells then release amines with
inflammatory vascular effects (e.g. histamine), leukotrienes
derived from arachidonic acid, heparin, proteases, cytokines and
chemokines. These factors promote allergic immune response by
regulating the differentiations and activations of various immune
cells and induce lymphocyte infiltration to the inflamed site
(2). In addition,
allergen-specific CD4+ Th2 cells are known to play
important roles in the initiation and maintenance of allergic
response (3). Cytokines (e.g.
IL-4, IL-5, and IL-13) secreted by Th2 cells induce B cells to
produce IgE and activate immune cells, including mast cells,
basophils and eosinophils. On a macro scale, these responses cause
mucus hypersecretion, epithelium fibrosis and are even associated
with tissues damage (4).
Many medications used to treat allergic conditions,
including antihistamines and corticosteroids, can cause
side-effects. For example, long-term corticosteroid therapy may
cause Cushing's syndrome, osteoporosis and adrenal insufficiency
(5–7). Therefore, new therapeutic agents for
the allergic diseases are today's need. Numerous authors have
suggested that plant-derived drugs are intrinsically safer than
synthetic drugs (8,9).
Triticum aestivum sprouts germinate from seed
and contain large amounts of chlorophyll, minerals, enzymes, and
other functional entities (10).
Eosinophil accumulation and activation have been shown to play an
important role in the pathogenesis of allergic inflammation and
asthma (11,12). Studies have reported that in
thalassemic patients with eosinophilia, Triticum aestivum
sprouts reduced in the numbers of eosinophils in blood (13). We previously showed that a
dichloromethane fraction isolated from Triticum aestivum
sprouts (TDF) contains large amounts of sterols (e.g. β-sitosterol)
and polyunsaturated fatty acids (e.g. α-linolenic acid) and
glycolipids (14). Other studies
have reported that β-sitosterol has potential anti-allergic effects
(15,16), and increasing evidence indicates
polyunsaturated fatty acids alleviate allergic diseases (17,18).
However, the effects of TDF on allergic immune
response have not been elucidated. In the present study, we
examined the anti-allergic activity and mechanism of action of TDF
in an OVA-sensitized mouse model.
Materials and methods
Extraction and isolation of plant
material
Triticum aestivum were supplied by the Korean
National Institute of Crop Science and were germinated on sterile
organic feat moss (at 20±2°C). The dichloromethane fraction was
obtained from from Triticum aestivum sprouts (TDF) as
previously described (14).
Briefly, Triticum aestivum sprouts were cultivated for two
weeks after germination, harvested, lyophilized and crushed to
obtain a powder with a predetermined particle size. Frozen powder
(30 g) was then extracted with methanol. And the extract was
dissolved in 800 ml of water and partitioned sequentially with
hexane, dichloromethane (CH2Cl2), ethyl
acetate (EtOAc) and n-butanol. TDF was filtered through Whatman
filter paper (grade no. 1, diameter: 15 cm) and concentrated under
reduced pressure using a rotary evaporator (N-1000; EYELA, Tokyo,
Japan).
In vivo experiments
Female BALB/c mice (6 weeks old) were purchased from
Samtako (Osan, Korea), and acclimated in a pathogen-free facility
for 2 weeks prior to experiments. Animals were kept in an
air-conditioned room (22±2°C, 55±10% RH) and fed 5L79 rodent diet
(Orient Bio, Seongnam, Korea) throughout the experimental period.
The ovalbumin (OVA)-sensitized mouse model of this study was
previously described by Park et al (19). After acclimation to the facility's
environment, mice were divided into five groups (n=5 each): Group
1; 1% carboxymethyl cellulose (vehicle), group 2; OVA sensitization
+ vehicle, group 3; OVA sensitization + TDF 100 mg/kg, group 4; OVA
sensitization + TDF 200 mg/kg, group 5; OVA sensitization +
dexamethasone 0.5 mg/kg. On the first day, all mice were
intraperitoneally (i.p.) sensitized with 20 µg OVA (grade V;
Sigma-Aldrich, St. Louis, MO, USA) and 1 mg of aluminum hydroxide
(Imject® A lum; Thermo Scientific, Cramlington, UK)
dissolved in 100 µl of phosphate-buffered saline (PBS, pH 7.4);
except mice in the group 1. Two weeks later, mice were administered
a second i.p. injection of OVA and alum. To investigate in
vivo effect of TDF on allergic immune response, OVA-sensitized
mice were orally administered TDF or dexamethasone (p.o.) once
daily for 13 days following the second OVA injection. All animal
experiments were conducted after obtaining approval from the
Institutional Animal Care and Use Committee (IACUC) at Chonbuk
National University Laboratory Animal Center.
Ear swelling test
The ear swelling test was performed to analyze the
allergic response with reference to the previous studies (20,21).
Mice were injected subcutaneously with 20 µl of 0.1% (m/v) OVA
solution dissolved in PBS into ear skin after the 13 days treatment
period. Ear thicknesses of mice were measured using a thickness
gauge (Mitutoyo, Tokyo, Japan) at 6 and 24 h after OVA
injection.
Histological examination
Animals were sacrificed by cervical dislocation
method under diethyl ether anesthesia. Ear tissues of animals were
harvested at 24 h after final OVA challenge for histological
examination. Tissues were fixed with 10% formalin and then embedded
in paraffin. Tissue sections (4 µm) were obtained using a microtome
for hematoxyin and eosin (H&E) staining, according to the
previous report (22), and stained
sections were examined under an optical microscope (CX21; Olympus,
Tokyo, Japan).
Serum analysis of immunoglobulins
After sacrifice, blood was collected from abdominal
inferior vena cava and centrifuged at 3,000 rpm for 10 min. Serum
levels of total immunoglobulin (Ig)E and IgG1 were evaluated using
commercial ELISA kits (BD Biosciences, San Jose, CA, USA), and the
serum levels of OVA-specific IgE and OVA-specific IgG1 were
measured using a detection kit obtained from Shibayagi (Gunma,
Japan).
Preparation of splenocytes
Spleens were removed from OVA-sensitized mice and
filtered using 100 µm cell strainers. Single-cell suspensions and
Histopaque® 1119 (Sigma-Aldrich) were mixed and
centrifuged at 1,600 rpm for 30 min to remove erythrocytes. The
cell pellets so obtained were washed twice with PBS, and cultured
in RPMI-1640 (HyClone Laboratories, Logan, UT, USA) supplemented
with heat-inactivated 10% fetal bovine serum (FBS), 100 U/ml of
penicillin and 100 µg/ml of streptomycin at 37°C in a humidified 5%
CO2 incubator. Splenocytes were used for experiment
after stabilization.
Reverse transcription polymerase chain
reaction (RT-PCR) and quantitative RT-PCR (qRT-PCR)
Splenocytes were treated with OVA and various
concentrations of TDF for 24 h, and total RNA was extracted from
splenocytes with TRIzol reagent (Invitrogen, Carlsbad, CA, USA).
cDNA synthesis were performed using the SuperScript™ III First
Synthesis system for RT-PCR kit (Invitrogen) and 1 µg of total RNA.
Template of cDNA (50 ng) was amplified using 1X reaction buffer, 10
mM dNTP mixture, 5 units of Taq polymerase (GeNet Bio, Seoul,
Korea) and RT-PCR primers. Amplified gene products were loaded into
1.5% agarose gel containing ethidium bromide, all gene expressions
were normalized vs. GAPDH of each sample. qRT-PCR method was
previously described (23). Primer
sequences are summarized in Table
I.
 | Table I.Primer sequences and product size
used for RT-PCR and qRT-PCR. |
Table I.
Primer sequences and product size
used for RT-PCR and qRT-PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Size (bp) |
|---|
| IFN-γ |
CTTCTTCAGCAACAGCAAGGCGAAAA |
CCCCCAGATACAACCCCGCAATCA | 456 |
| IL-12 |
AACCTCACCTGTGACACGCC |
CAAGTCCATGTTTCTTTGCACC | 309 |
| IL-4 |
GAGATCATCGGCATTTTGAAC |
CTTGGACTCATTCATGGTGCA | 267 |
| IL-5 |
GAAAGAGACCTTGACACAGCTG |
GAACTCTTGCAGGTAATCCAGG | 277 |
| IL-13 |
ATGAGTCTGCAGTATCCCG |
CCGTGGCAGACAGGAGTGTT | 194 |
| GAPDH (RT-PCR) |
GCCAAGGTCATCCATGACAAC |
GTCCACCACCCTGTTGCTGTA | 498 |
| T-bet |
ACTTTGAGTCCATGTACGCATCT |
AGGATACTGGTTGGATAGAAGAGGT | 113 |
| GATA-3 |
TACCACCTATCCGCCCTATGT |
ACACACTCCCTGCCTTCTGT | 138 |
| GAPDH
(qRT-PCR) |
CATGGCCTTCCGTGTTC |
CCTGGTCCTCAGTGTAGC | 152 |
Th1/Th2 cytokine assay
After preparation, splenocytes were incubated with
various concentration of TDF in presence or absence of OVA (100
µg/ml) for 48 h. Th1 (IFN-γ, IL-12) and Th2 (IL-4, IL-5, IL-13)
cytokines in cell culture supernatants were measured using the
following ELISA kits: mouse IFN-γ, IL-12 and IL-4 kits were
purchased from BioLegend (San Diego, CA, USA) and mouse IL-5 and
IL-13 kits from eBioscience (San Diego, CA, USA).
Statistical analysis
Results are presented as means ± SEMs. The Student's
t-test was carried out to verify the difference between the control
and experimental groups in GraphPad Prism software (version 5.0).
Statistical significance was accepted for p-values <0.05.
Results
Effect of TDF on allergic skin
response in OVA-sensitized mice
Allergic inflammation can be elicited on sensitized
subjects when exposure to a specific allergen (24). To investigate the inhibitory effect
of TDF on cutaneous allergic response, mice were sensitized with
OVA and administered TDF for 13 days, and then challenged with an
s.c. injection of OVA into ears. Swelling responses were quantified
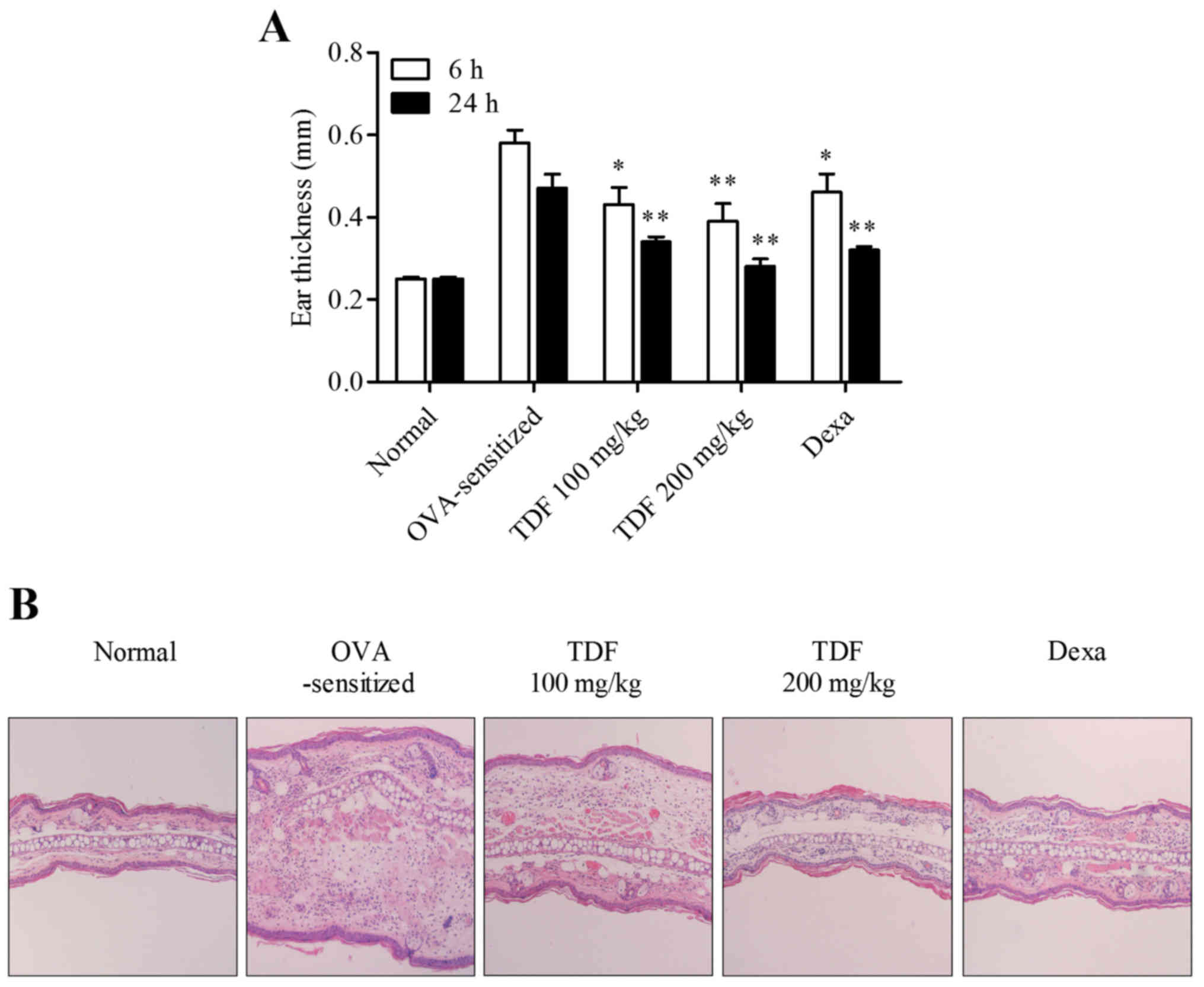
by measuring ear thicknesses. As shown in Fig. 1A, ear thickness increases were
significant compared to normal group at 6 and 24 h post-challenge.
And TDF reduced these increases.
Histological analyses of ear tissues revealed marked
increases in inflammatory cell infiltration into ear skin tissues
after challenge in OVA-sensitized group. But these inflammatory
cells infiltrations were inhibited dose-dependently by TDF
(Fig. 1B). These results
demonstrated that administration of TDF contributes to improving
the allergic inflammatory response.
Effect of TDF on levels of serum
immunoglobulins in OVA-sensitized mice
IgE production is considered the hallmark of
allergic diseases, and thus, we investigated whether TDF can
regulate the serum levels of immunoglobulins in OVA-sensitized
mice. It has been reported that IL-4 secreted by Th2 cells promotes
the productions of IgE and IgG4 in human and of IgE and IgG1 in
mice (25). We found significant
increases in the levels of serum total IgE and IgG1 in the
OVA-sensitized group (Table II).
Serum total IgE and IgG1 levels were about 6.6 and 2.8-fold
greater, respectively, in the OVA-sensitized group than in the
normal control group. Notably, serum levels of total IgE were
significantly lower in the TDF 100 and 200 mg/kg groups than in the
OVA-sensitized group, whereas total IgG1 levels were similar.
 | Table II.The level of total IgE and IgG1 in
serum of TDF treated OVA-sensitized mice. |
Table II.
The level of total IgE and IgG1 in
serum of TDF treated OVA-sensitized mice.
| Group | Total IgE
(µg/ml) | Total IgG1
(µg/ml) |
|---|
| Normal | 0.90±0.44 (15) |
847.18±181.42 (35) |
| OVA-sensitized |
6.02±1.03
(100) |
2389.45±206.82 (100) |
| TDF 100 mg/kg |
4.30±1.28
(71)a |
2374.91±60.66 (99) |
| TDF 200 mg/kg |
3.98±0.98
(66)a |
2316.73±156.32 (97) |
| Dexa |
4.12±1.28
(68)a |
1594.45±222.29 (67)b |
To add, we obtained similar result after examination
of OVA-specific IgE and IgG1 (Table
III). OVA-specific IgE and IgG1 levels were obvious in the
OVA-sensitized group but barely detectable in the normal control
group. TDF treatment concentration-dependently suppressed serum
OVA-specific IgE levels, but serum OVA-specific IgG1 levels
remained unchanged in the TDF treated group.
 | Table III.The level of OVA-specific IgE and
IgG1 in serum of TDF treated OVA-sensitized mice. |
Table III.
The level of OVA-specific IgE and
IgG1 in serum of TDF treated OVA-sensitized mice.
| Group | OVA-specific IgE
(U/ml) | OVA-specific
IgG1(U/ml) |
|---|
| Normal | ND | ND |
| OVA-sensitized |
105.74±14.68 (100) |
4741.24±1185.42 (100) |
| TDF 100 mg/kg |
71.58±10.43
(68)a |
4721.40±634.74 (100) |
| TDF 200 mg/kg |
57.84±5.35
(55)b |
4692.22±892.12 (99) |
| Dexa |
63.48±10.22
(60)a |
3432.45±793.27 (72)a |
Effect of TDF on Th2-related cytokine
level in splenocytes isolated from OVA-sensitized mice
By cellular interaction and cytokine secretion, Th
cells play a key role in the class switching of B cells. Of the
functional Th subsets, Th2 cells are important for enhancing
allergic immune response. To determine the effect of TDF on Th2
response, we examined the mRNA and protein levels of Th2-related
genes in splenocytes obtained from OVA-sensitized mice.
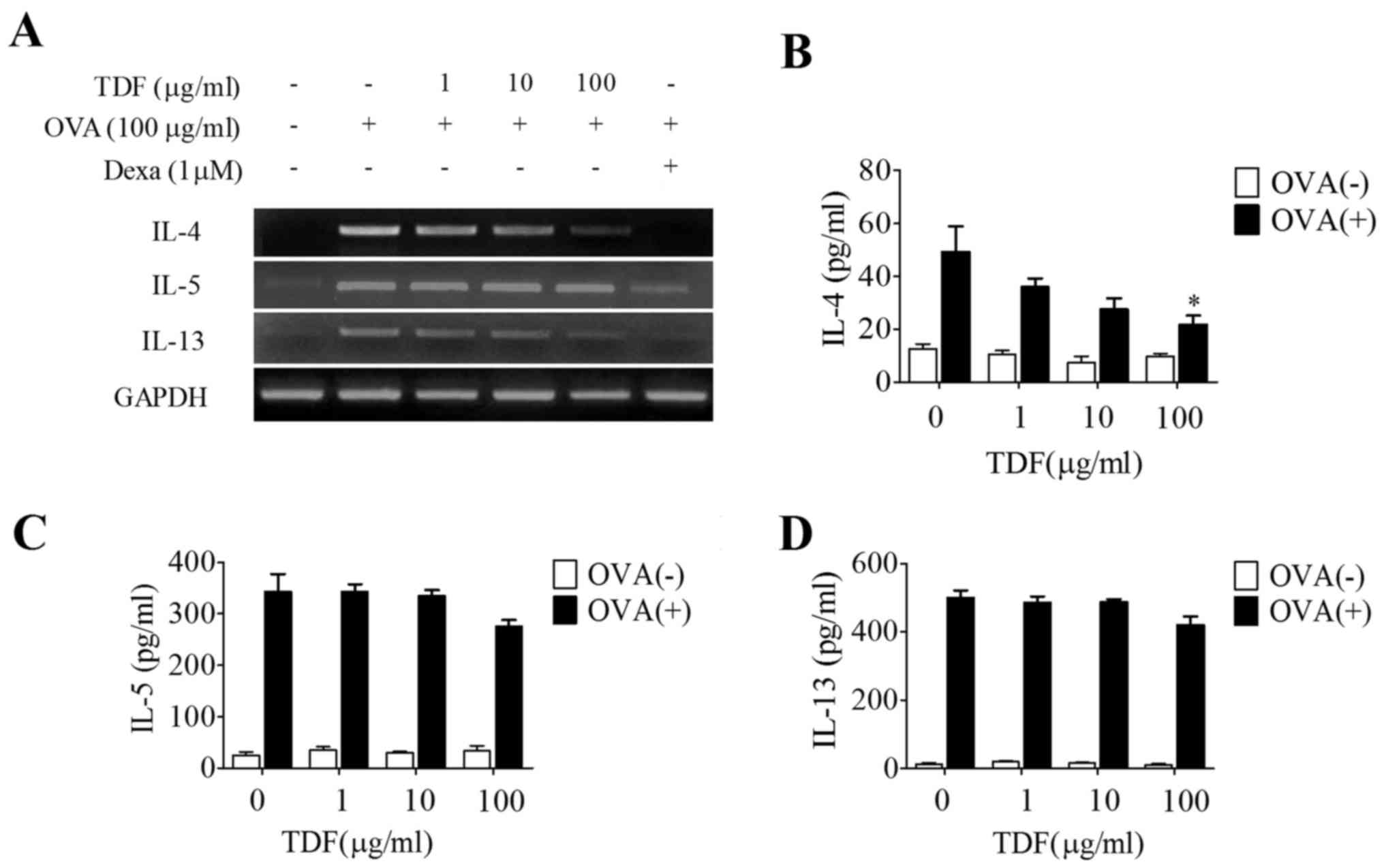
We observed increases of IL-4, IL-5 and IL-13 mRNA
and protein levels in OVA-treated cells. However, TDF treatment at
100 µg/ml attenuated IL-4 mRNA expression and decreased IL-4
secretion (Fig. 2A and B). IL-5
and IL-13 mRNA (Fig. 2A) and
protein levels tended (non-significantly) to be lower in TDF
treated cells than in OVA-treated cells (Fig. 2C and D).
Effect of TDF on Th1 cytokine levels
in splenocytes isolated from OVA-sensitized mice
Th1 and Th2 cells can inhibit each other by
secreting cytokines, and naïve T cells differentiate into Th1 cells
in the presence of IL-12, and Th1-derived cytokines (e.g. IFN-γ)
are able to suppress Th2 differentiation (26). To investigate the effect of TDF on
Th1 response, we examined Th1 response genes in splenocytes
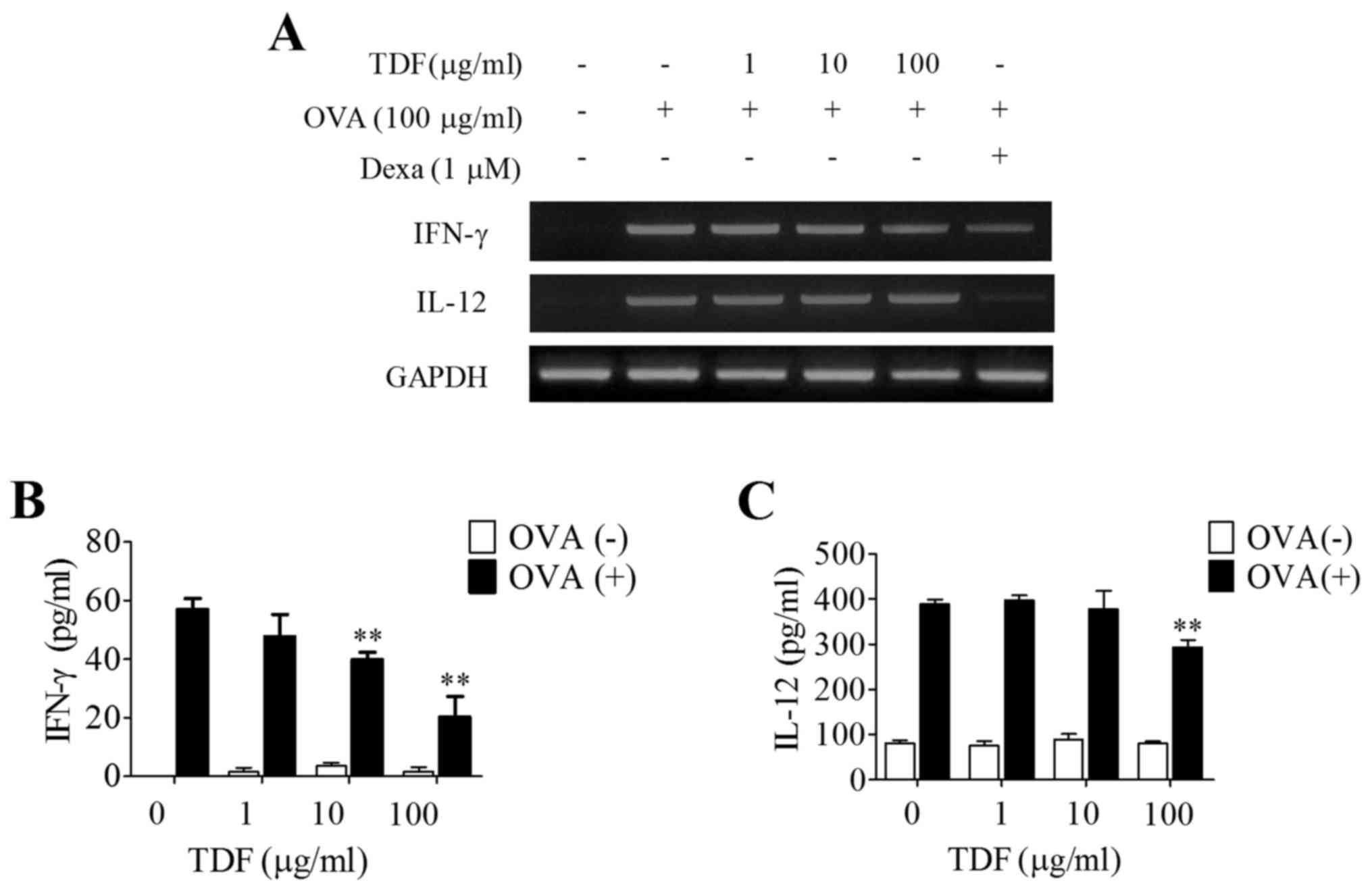
obtained from OVA-sensitized mice. Pre-treating splenocytes with
TDF had no effect on the mRNA expressions of IFN-γ and IL-12
(Fig. 3A). However, IFN-γ and
IL-12 secretions were significantly reduced by TDF, suggesting TDF
reduced the levels of proteins involved in Th1 response without
significantly changing mRNA levels (Fig. 3B and C).
Effect of TDF on the expressions of Th
specific transcription factors in splenocytes isolated from
OVA-sensitized mice
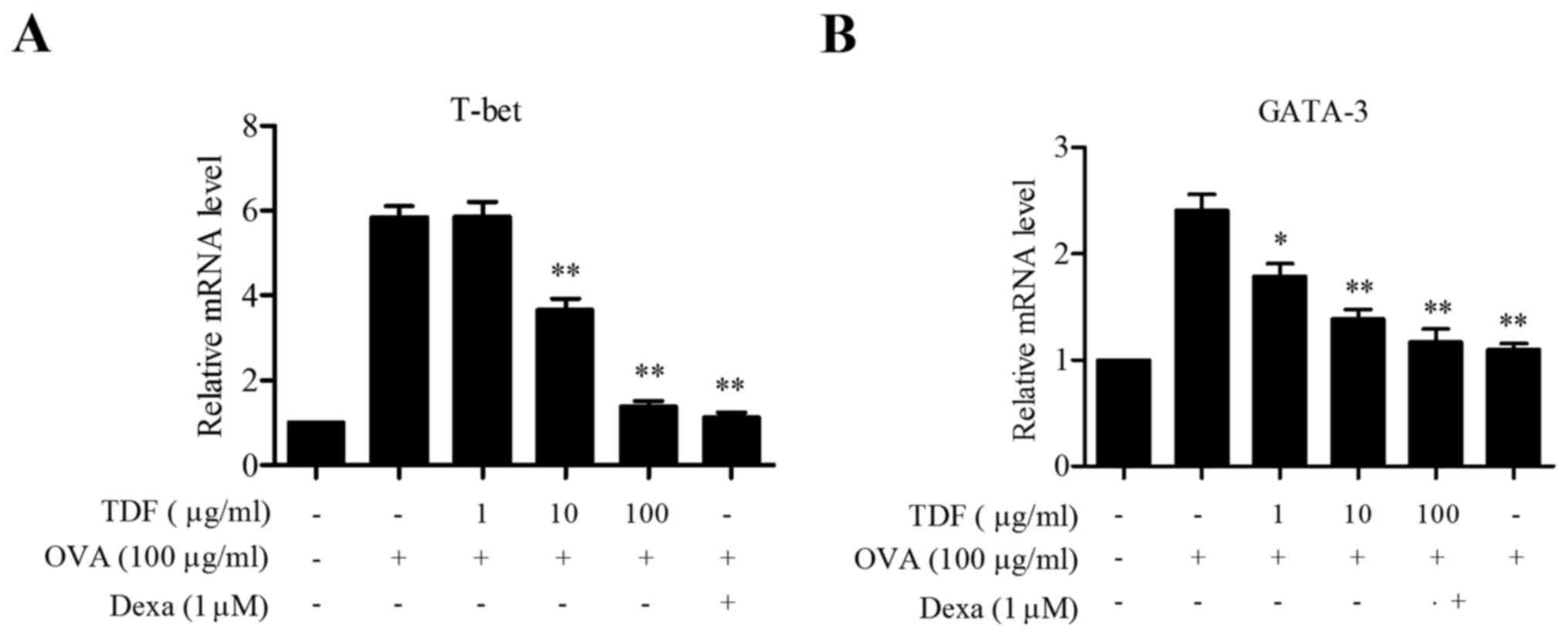
Due to the observed inhibition of Th1/Th2 related
cytokine production by TDF in splenocyte culture, we further
examined the expressions of transcription factors involved in
skewing T cell polarization toward Th1 and Th2 cells (Fig. 4). As shown in Fig. 4A, TDF markedly inhibited the mRNA
expression of T-bet, which plays a key role in Th1 differentiation.
Likewise, the mRNA expression of the Th2 specific transcription
factor GATA-3 was also inhibited dose-dependently by TDF (Fig. 4B).
Discussion
The prevalence of allergic diseases has increased in
association with the western lifestyle in recent decades.
Furthermore, it has been reported more than 25% of the populations
of industrialized countries are under the influence of allergic
diseases. The clinical manifestations of allergic diseases such as
allergic rhinitis, asthma, atopic dermatitis, food allergy,
allergic conjunctivitis and anaphylaxis can occur alone or in
combination (27–29). OVA-sensitized animal models are
commonly used to mimic chronic allergic diseases such as asthma and
atopic dermatitis in human (30,31).
In the present study, we investigated the potential ameliorative
role of TDF in allergic immune response using OVA-sensitized mouse
model.
IgE mediated-mast cell degranulation induces an
immediate hypersensitivity reaction to causes allergic
inflammation. For example, edema is caused by the movement of
immune cells on inflamed site, the expansion of blood vessels, and
increased vascular permeability (32). In the present study, allergic
cutaneous reaction was suppressed in TDF treated groups as compared
with to the OVA-sensitized group. And this effect was comparable to
that induced by dexamethasone (the positive control).
Allergic reactions are initiated by enhanced IgE
production induced by activation of the Th2 pathway. Especially,
antigen-specific IgE have a key role on mast cells. Mast cells
which have FcεRI-bound IgE produce a diverse array of biologically
active mediators when re-exposed to the antigens (33). We observed that TDF treatment
significantly inhibited the total and OVA-specific IgE, when
compared to the OVA-sensitized group. Our in vivo results
suggest the observed suppression of allergic inflammation by TDF
was due to IgE down-regulation.
We also investigated T cell regulation by TDF to
explore further the natures of these IgE reductions. After an
antigen encountered and bound by antigen presenting cells (APCs) in
the body presents, the antigen to naive CD4+ T cells,
which then differentiate into functionally distinct subsets as
determined by the micro-environmental background.
Th2-biased immune responses commonly results in
atopy, and is observed in blood and lung in the presence of
asthmatic conditions, and thus, medications targeting Th cells are
viewed as promising therapeutic strategies for the treatment of
allergic diseases (34).
Differentiation of naïve Th precursor cells into Th2 cells is
characterized by the productions of Th2 cytokines (e.g. IL-4, IL-5
and IL-13). During Th2-development, Th2 signaling initiates
activation of signal transducer and activator of transcription
(STAT)-6 in the presence of IL-4, which is followed by the
expressional up-regulation of GATA-3 (35). We observed TDF decreased the
production of IL-4 and the mRNA expression of transcription factor
GATA-3 induced by OVA stimulation in splenocyte, which result shows
that TDF suppressed Th2 differentiation by inhibiting IL-4
production.
On the other hand, during the development of Th1
lineage cells, antigen stimulates APCs to secret Th1-promoting
cytokines such as IL-12 and IFN-γ. In naïve Th precursor cells
during T cell antigen receptor (TCR) engagement, IFN-γ activates
STAT-1 and then downstream T-bet expressed in T cells. Furthermore,
T-bet is known as a specific regulator for Th1 differentiation and
to be related to IFN-γ production (36). Our results show that TDF inhibited
the productions of the Th1 cytokines IFN-γ and IL-12 and decreased
T-bet expression. Although Th1 and Th2 responses can block each
other, this result confirmed that TDF does not participate in the
induction of Th1 response and suggested that there are other
mechanisms for inhibition of Th2 response. In addition, we examined
the effect of TDF on other cell subsets such as regulatory T cells
which have protective roles in the presence of excessive immune
response. However, TDF was not found to have a significant effect
on this subset (data not shown).
Triticum aestivum sprouts are known as
contain flavonoids such as isoorientin, isoscoparin and luteolin as
well as sterols and polyunsaturated fatty acids (37,38).
These constituents have been reported to have the potential to
ameliorate allergic diseases. For example, isoorientin was reported
to suppress the release of histamine and leukotrienes in guinea pig
lung mast cells activated by OVA (39), and luteolin was found to inhibit
mast cell-mediated allergic inflammation and to attenuate immediate
and late-phase asthmatic responses (40,41).
Consistent to these studies, these factors could be evidences for
beneficial effect of Triticum aestivum sprouts on allergic
diseases.
In conclusion, our data reveal that TDF reduces
OVA-induced allergic immune response by inhibiting Th2
differentiation mediated by the activation of GATA-3 and IL-4, and
suggest that TDF could be useful for the treatment of allergic
diseases. Further studies are required to elucidate the molecular
targets associated with the TDF mediated inhibition of allergic
immune response.
Acknowledgements
The present study was financially supported by
grants from Wonkwang University (2017).
References
|
1
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Larché M, Akdis CA and Valenta R:
Immunological mechanisms of allergen-specific immunotherapy. Nat
Rev immunol. 6:761–771. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosnjak B, Stelzmueller B, Erb KJ and
Epstein MM: Treatment of allergic asthma: Modulation of Th2 cells
and their responses. Respir Res. 12:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romagnani S: The role of lymphocytes in
allergic disease. J Allergy Clin Immunol. 105:399–408. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simons FE: Advances in H1-antihistamines.
N Engl J Med. 351:2203–2217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buchman AL: Side effects of corticosteroid
therapy. J Clin Gastroenterol. 33:289–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warrington TP and Bostwick JM: Psychiatric
adverse effects of corticosteroids. Mayo Clin Proc. 81:1361–1367.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petry JJ and Hadley SK: Medicinal herbs:
Answers and advice, part 1. Hosp Pract (1995). 36:57–60. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balandrin MF, Klocke JA, Wurtele ES and
Bollinger WH: Natural plant chemicals: Sources of industrial and
medicinal materials. Science. 228:1154–1160. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mujoriya R and Bodla RB: A study on wheat
grass and its Nutritional value. Food Sci Qual Manag. 2:2224–6088.
2011.
|
|
11
|
Wardlaw AJ, Brightling C, Greesn R,
Woltmann G and Pavord I: Eosinophils in asthma and other allergic
diseases. Br Med Bull. 56:985–1003. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Till S, Dickason R, Huston D, Humbert M,
Robinson D, Larché M, Durham S, Kay AB and Corrigan C: IL-5
secretion by allergen-stimulated CD4+ T cells in primary
culture: Relationship to expression of allergic disease. J Allergy
Clin Immunol. 99:563–569. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Desai TR, Solanki JK, Buch Pankaj and
Goyal RK: Triticum aestivum (Wheatgrass): An alternate
treatment for the patients with Thalassemia. Orient Pharm Exp Med.
7:466–476. 2008. View Article : Google Scholar
|
|
14
|
Luyen BT, Thao NP, Tai BH, Lim JY, Ki HH,
Kim DK, Lee YM and Kim YH: Chemical constituents of Triticum
aestivum and their effects on adipogenic differentiation of
3T3-L1 preadipocytes. Arch Pharm Res. 38:1011–1018. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han NR, Kim HM and Jeong HJ: The potential
anti-proliferative effect of β-sitosterol on human mast cell line-1
cells. Can J Physiol Pharmacol. 93:979–983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahajan SG and Mehta AA: Suppression of
ovalbumin-induced Th2-driven airway inflammation by β-sitosterol in
a guinea pig model of asthma. Eur J Pharmacol. 650:458–464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arm JP, Boyce JA, Wang L, Chhay H, Zahid
M, Patil V, Govindarajulu U, Ivester P, Weaver KL, Sergeant S, et
al: Impact of botanical oils on polyunsaturated fatty acid
metabolism and leukotriene generation in mild asthmatics. Lipids
Health Dis. 12:1412013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barros R, Moreira A, Fonseca J, Delgado L,
Castel-Branco MG, Haahtela T, Lopes C and Moreira P: Dietary intake
of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated
with decreased exhaled NO and improved asthma control. Br J Nutr.
106:441–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park EJ, Kim B, Eo H, Park K, Kim Y, Lee
HJ, Son M, Chang YS, Cho SH, Kim S and Jin M: Control of IgE and
selective TH1 and TH2 cytokines by PG102 isolated from Actinidia
arguta. J Clin Immunol. 116:1151–1157. 2005. View Article : Google Scholar
|
|
20
|
Schouten B, van Esch BC, Hofman GA, van
den Elsen LW, Willemsen LE and Garssen J: Acute allergic skin
reactions and intestinal contractility changes in mice orally
sensitized against casein or whey. Int Arch Allergy Immunol.
147:125–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hogenkamp A, Thijssen S, van Vlies N and
Garssen J: Supplementing pregnant mice with a specific mixture of
nondigestible oligosaccharides reduces symptoms of allergic asthma
in male offspring. J Nutr. 145:640–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poudel B, Yoon DS, Lee JH, Lee YM and Kim
DK: Collagen I enhances functional activities of human
monocyte-derived dendritic cells via discoidin domain receptor 2.
Cell Immunol. 278:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poudel B, Nepali S, Xin M, Ki HH, Kim YH,
Kim DK and Lee YM: Flavonoids from Triticum aestivum inhibit
adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol
Med Rep. 12:3139–3145. 2015.PubMed/NCBI
|
|
24
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aversa G, Punnonen J, Cocks BG, de Waal
Malefyt R, Vega F Jr, Zurawski SM, Zurawski G and de Vries JE: An
interleukin 4 (IL-4) mutant protein inhibit both IL-4 or
IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and
B cell proliferation: Support for a common component shared by IL-4
and IL-13 receptors. J Exp Med. 178:2213–2218. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations and implications for health and disease. Altern Med
Rev. 8:223–246. 2003.PubMed/NCBI
|
|
27
|
Akdis CA: Allergy and hypersensitivity
Mechanisms of allergic disease. Curr Opin Immunol. 18:718–726.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yazdanbakhsh M, Kremsner PG and van Ree R:
Allergy, parasites, and the hygiene hypothesis. Science.
296:490–494. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holgate ST and Polosa R: Treatment
strategies for allergy and asthma. Nat Rev Immunol. 8:218–230.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nials AT and Uddin S: Mouse models of
allergic asthma: Acute and chronic allergen challenge. Dis Models
Mech. 1:213–220. 2008. View Article : Google Scholar
|
|
31
|
Jin H, He R, Oyoshi M and Geha RS: Animal
models of atopic dermatitis. J Invest Dermatol. 129:31–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zgraggen S, Ochsenbein AM and Detmar M: An
important role of blood and lymphatic vessels in inflammation and
allergy. J Allergy (Cairo). 2013:6723812013.PubMed/NCBI
|
|
33
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heijink IH and Van Oosterhout AJ:
Strategies for targeting T-cells in allergic diseases and asthma.
Pharmacol Ther. 112:489–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chatila TA, Li N, Garcia-Lloret M, Kim HJ
and Nel AE: T-cell effector pathways in allergic diseases:
Aanscriptional mechanisms and therapeutic targets. J Allergy Clin
Immunol. 121:812–823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szabo SJ, Kim ST, Costa GL, Zhang X,
Fathman CG and Glimcher LH: A novel transcription factor, T-bet,
directs Th1 lineage commitment. Cell. 100:655–669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SH, Xin M, Luyen BT, Cha JY, Im JY,
Kwon SU, Lim SW, Suh JW, Kim YH, Kim DK and Lee YM: Inhibitory
effect of Triticum aestivum ethanol extract on lipid
accumulation in 3T3-L1 preadipocytes. Yakhak Hoeji. 55:478–484.
2011.
|
|
38
|
Luyen BT, Tai BH, Thao NP, Cha JY, Lee YM
and Kim YH: A new phenolic component from Triticum aestivum
sprouts and its effects on LPS-stimulated production of nitric
oxide and TNF-α in RAW 264.7 cells. Phytother Res. 28:1064–1070.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim DS, Son EJ, Kim M, Heo YM, Nam JB, Ro
JY and Woo SS: Antiallergic herbal composition from Scutellaria
baicalensis and Phyllostachys edulis. Planta Med.
76:678–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kritas SK, Saggini A, Varvara G, Murmura
G, Caraffa A, Antinolfi P, Toniato E, Pantalone A, Neri G, Frydas
S, et al: Luteolin inhibits mast cell-mediated allergic
inflammation. J Biol Regul Homeost Agents. 27:955–959.
2013.PubMed/NCBI
|
|
41
|
Lee JY, Kim JM and Kim CJ: Flavones
derived from nature attenuate the immediate and late-phase
asthmatic responses to aerosolized-ovalbumin exposure in conscious
guinea pigs. Inflamm Res. 63:53–60. 2014. View Article : Google Scholar : PubMed/NCBI
|