Introduction
Atherosclerosis is the most common type of coronary
artery disease and the leading cause of morbidity and mortality
worldwide (1). It is characterized
by the accumulation of lipids in the arterial vessel wall (2). A number of studies have demonstrated
that the expression of cell adhesion molecules, such as
intercellular adhesion molecule-1 (ICAM-1) and vascular cell
adhesionmolecule-1 (VCAM-1), is increased in vascular smooth muscle
cells (VSMCs), leading to increased neointima or atherosclerotic
lesion formation (3–5). In addition, the expression of
adhesion molecules in vascular cells is affected by inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α) (6). Therefore, a promising therapeutic
approach for the treatment of pathological inflammation may be to
reduce the expression of adhesion molecules in VSMCs.
Myricitrin, a bioactive compound of Myrica
cerifera, has been demonstrated to exhibit a number of
pharmacological actions, including antinociceptive,
anti-inflammatory, anticancer and anti-oxidative activities
(7–9). Domitrović et al (10) reported that myricitrin
significantly reduced the carbon tetrachloride-induced increase in
cyclooxygenase-2 and TNF-α levels in the liver. In addition, a
previous study demonstrated that myricitrin exhibits
anti-atherogenic effects (11).
Administration of myricitrin in vivo decreased the vascular
wall thickness of the aortic arch in apolipoprotein E−/−
mice, and myricitrin treatment significantly attenuated oxidized
low-density lipoprotein-induced endothelial cell apoptosis
(11). However, the effect of
myricitrin on the expression of adhesion molecules in VSMCs remains
unknown. Therefore, the aim of the present study was to evaluate
the inhibitory effects of myricitrin on adhesion molecule
expression induced by TNF-α in VSMCs in vitro.
Materials and methods
Cell culture
The VSMC cell line MOVAS-1 was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin (Sigma; Merck KGaA, Darmstadt, Germany), 100 µg/ml
streptomycin (Sigma; Merck KGaA), and 200 mM L-glutamine (Sigma;
Merck KGaA) in a humidified 5% CO2 atmosphere at 37°C.
THP-1 cells (ATCC) were maintained in RPMI-1640 (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin in an incubator with a
humidified atmosphere of 5% CO2 at 37°C.
Cell adhesion assay
The cell adhesion assay was performed as previously
described (12). Briefly, MOVAS-1
cells were plated in 96-well plates at a density of
~1×104 cells/well, which were then pretreated with
varying concentrations (2.5, 5 and 10 µM) of myricitrin (Sigma;
Merck KGaA) for 2 h, followed by stimulation with or without TNF-α
(10 ng/ml; Sigma; Merck KGaA) for 8 h. The media was removed from
the wells and
2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein-labeled (5
µM; Sigma; Merck KGaA) THP-1 cells (1×105 cells/ml) in
0.2 ml medium were added to each well. Following incubation for 1 h
at 37°C in 5% CO2, the wells were washed three times
with 0.2 ml medium, and the number of adherent cells in 4 high
power fields of view were observed using a Nikon Eclipse E600
fluorescence microscope at ×100 magnification (Nikon Corporation,
Tokyo, Japan).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from MOVAS-1 cells
(1×104 cells/well) with TRIzol reagent (Abcam,
Cambridge, UK) according to the manufacturer's instructions. Total
RNA (5 µg) was reverse transcribed into cDNA using an oligo-(dT)
primer and M-MLV reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) for qPCR analysis. qPCR was performed in a final
volume of 10 µl, which consisted of 5 µl SsoFast™ EvaGreen Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), 1 µl cDNA (1:50
dilution) and 2 µl each of the forward and reverse primers (1 mM).
The specific primers (Invitrogen; Thermo Fisher Scientific, Inc.)
were as follows: VCAM-1 sense, 5′-CAAAGGTGGATCAGATTCAAG-3′ and
antisense, 5′-GGTGAGCATTATCACCCAGAA-3′; ICAM-1 sense,
5′-CAAAGGTGGATCAGATTCAAG-3′ and antisense,
5′-GGTGAGCATTATCACCCAGAA-3′; GAPDH sense,
5′-CAAAGGTGGATCAGATTCAAG-3′ and antisense,
5′-GGTGAGCATTATCACCCAGAA-3′. The thermal cycling procedure was as
follows: 95°C for 4 min, followed by 40 cycles of 95°C for 25 sec,
55°C for 30 sec and 72°C for 20 sec with 2 sec for plate reading,
and melting curve analysis from 65 to 95°C. GAPDH was used as the
control for normalizing gene expression. The relative
quantification of the gene of interest was determined using the
comparative ΔCq method (13).
Western blot analysis
MOVAS-1 cells (1×106 cells) were
harvested, washed twice with phosphate-buffered saline, and lysed
in radioimmunoprecipitation assay buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) containing 100 mM NaCl, 50 mM
Tris-HCl pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM
β-glycerophosphate, 2 mM sodium vanadate and protease inhibitors
(Cell Signaling Technology, Inc.) on ice for 10 min. Following 15
min, 0.5% Nonidet P (NP)-40 (Mairybio; Beijing Minhai Biotechnology
Co., Ltd., Beijing, China) was added to lyse the cells, which were
vortexed for 10 sec. Then, cytosolic cell extracts were obtained
following centrifugation at 1,500 × g for 10 min at 4°C. The
collected nuclei were re-suspended in 50 µl of Buffer C [20 mM
HEPES (pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA,
25% v/v glycerol, 0.5 mM PMSF and Protease Inhibitor Cocktail] and
were then incubated on ice for 20 min with intermittent agitation.
Nuclear cell extracts were obtained following centrifugation for 10
min at 13,000 × g and 4°C. Equal amounts (30 µg) of protein sample
were separated using a 10% SDS-PAGE gel and then electrotransferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). Following blocking in Tris-buffered saline buffer (50
mmol/l NaCl, 10 mmol/l Tris, pH 7.4) containing 5% non-fat milk for
1 h at room temperature, the membranes were then incubated with the
appropriate primary antibodies against VCAM-1 (1:3,000; cat. no.
sc-13160), ICAM-1 (1:3,000; cat. no. sc-8439), nuclear factor
(NF)-κB p65 (1:2,000; cat. no. sc-8008), nuclear factor of κ light
chain gene enhancer in B-cells inhibitor α (IκBα; 1:2,500; cat. no.
sc-1643) and GAPDH (1:3,000; cat. no. sc-47724) at 4°C overnight,
followed by incubation with a horseradish peroxidase-conjugated
anti-rabbit IgG secondary antibody (1:3,000; cat. no. sc-2005; all
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at
room temperature. The membranes were exposed and visualized using
enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). Densitometry was performed using Gel-Pro Analyzer software
version 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Measurement of intracellular reactive
oxygen species (ROS) accumulation
Intracellular ROS levels were measured using
dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich; Merck KGaA,)
staining. Briefly, MOVAS-1 cells (1×104 cells/well)
treated with myricitrin in the presence or absence of TNF-α (as
described in the Cell adhesion assay section) were incubated
with 5 µM DCFH-DA for 30 min at 37°C in the dark, and were then
washed with serum-free medium three times. The fluorescence
intensity was measured at an excitation and emission wavelength of
485 and 520 nm, respectively, using a fluorescence
spectrophotometer (Infinite M1000; Tecan Austria GmbH, Grödig,
Austria). The ROS level was expressed as units of fluorescence.
Statistical analysis
The results were analyzed using SPSS software
version 13.0 (SPSS, Inc., Chicago, IL, USA) and data are expressed
as the mean ± standard deviation. Statistical significance was
assessed by one-way analysis of variance followed by a Tukey post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
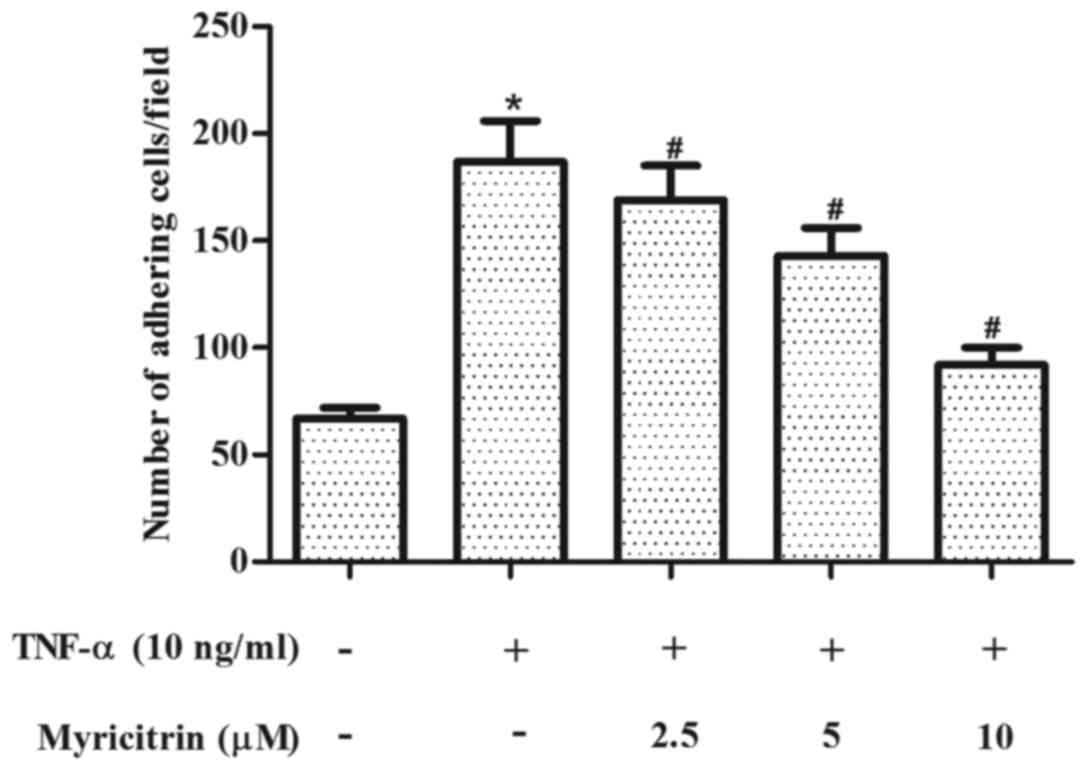
Myricitrin inhibited the adhesion of
THP-1 cells to TNF-α-stimulated MOVAS-1 cells
The effect of myricitrin on the adhesion of THP-1
cells to MOVAS-1 cells in response to TNF-α was first evaluated. As
shown in Fig. 1, treatment of
confluent MOVAS-1 cells with TNF-α for 8 h resulted in a 2.8-fold
increase in the adhesion of THP-1 monocytic cells when compared
with untreated MOVAS-1 cells (P<0.05). By contrast, pretreatment
with myricitrin significantly inhibited the adhesion of THP-1 cells
to TNF-α-stimulated MOVAS-1 cells in a dose-dependent manner (2.5
µM, P<0.05; 5 µM, P<0.05; 10 µM, P<0.05; Fig. 1).
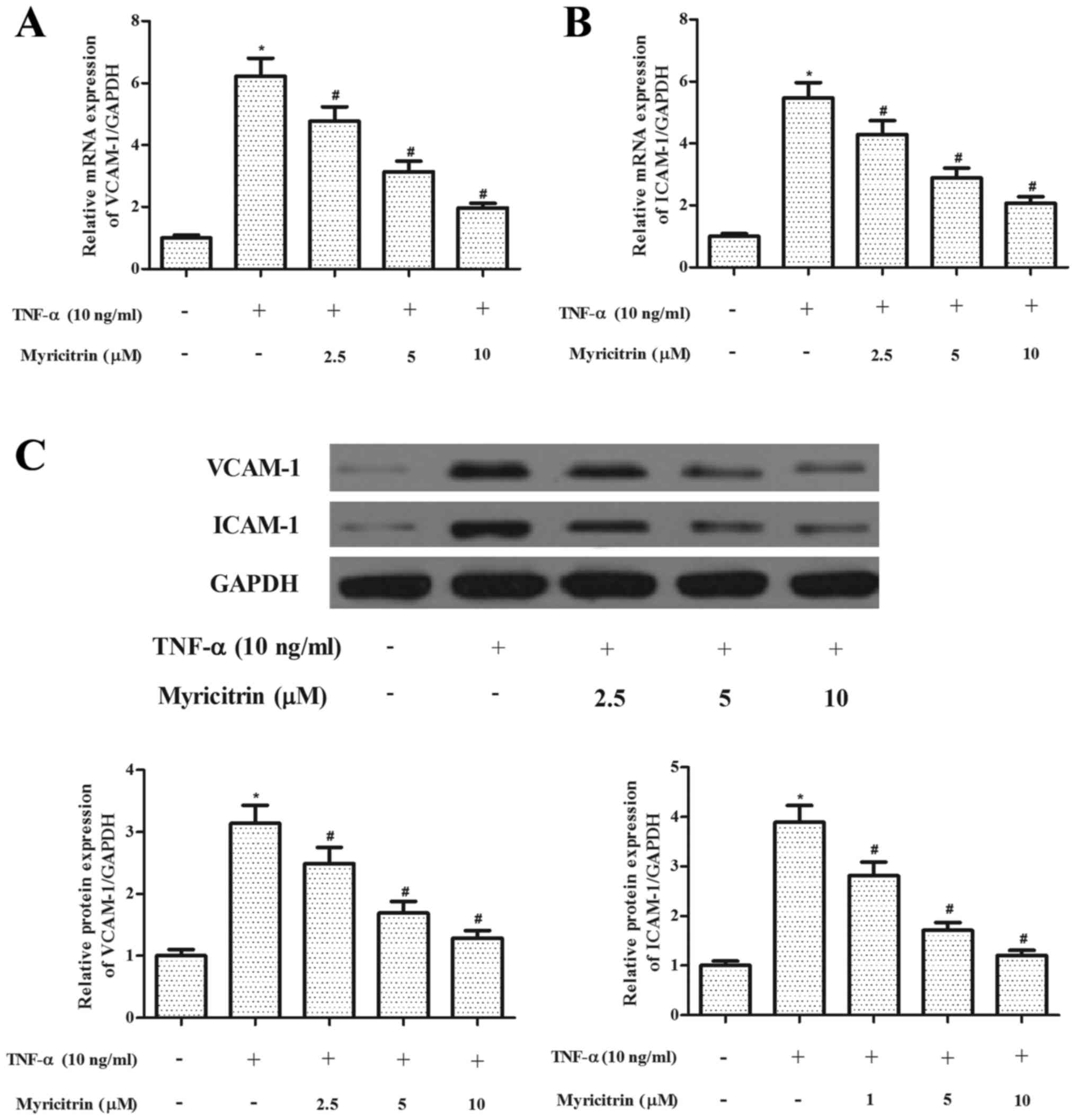
Myricitrin inhibited the expression of
adhesion molecules in TNF-α-stimulated MOVAS-1 cells
The increased expression of adhesion molecules is
thought to serve an important role in the pathogenesis of
atherosclerosis (4). Therefore,
the present study investigated the effect of myricitrin on the
expression of adhesion molecules in MOVAS-1 cells in response to
TNF-α treatment. The RT-qPCR analysis results revealed that TNF-α
significantly increased the mRNA expression levels of VCAM-1 and
ICAM-1 in MOVAS-1 cells when compared with untreated controls.
However, myricitrin significantly suppressed the mRNA expression
levels of VCAM-1 and ICAM-1 in TNF-α-stimulated MOVAS-1 cells
(VCAM-1, P<0.05; ICAM-1, P<0.05; Fig. 2A and B). In addition, myricitrin
significantly suppressed the protein expression levels of VCAM-1
and ICAM-1 in TNF-α-stimulated MOVAS-1 cells (P<0.05 and
P<0.05, respectively; Fig.
2C).
Myricitrin suppressed TNF-α-induced
NF-κB activation in MOVAS-1 cells
NF-κB has been reported to serve a critical role in
the regulation of adhesion molecule expression (14). Therefore, the present study
investigated the effects of myricitrin on NF-κB activation in
MOVAS-1 cells in response to TNF-α treatment. As indicated in
Fig. 3, TNF-α significantly
increased NF-κB p65 protein expression and IκBα degradation (NF-κB,
P<0.05; IκBα, P<0.05). However, myricitrin pretreatment
significantly prevented the TNF-α-induced increase in NF-κB p65 and
the TNF-α-induced degradation of IκBα in MOVAS-1 cells in a
dose-dependent manner (NF-κB, P<0.05 at 2.5, 5 and 10 µM
myricitrin; IκBα, P<0.05 at 2.5, 5 and 10 µM myricitrin;
Fig. 3).
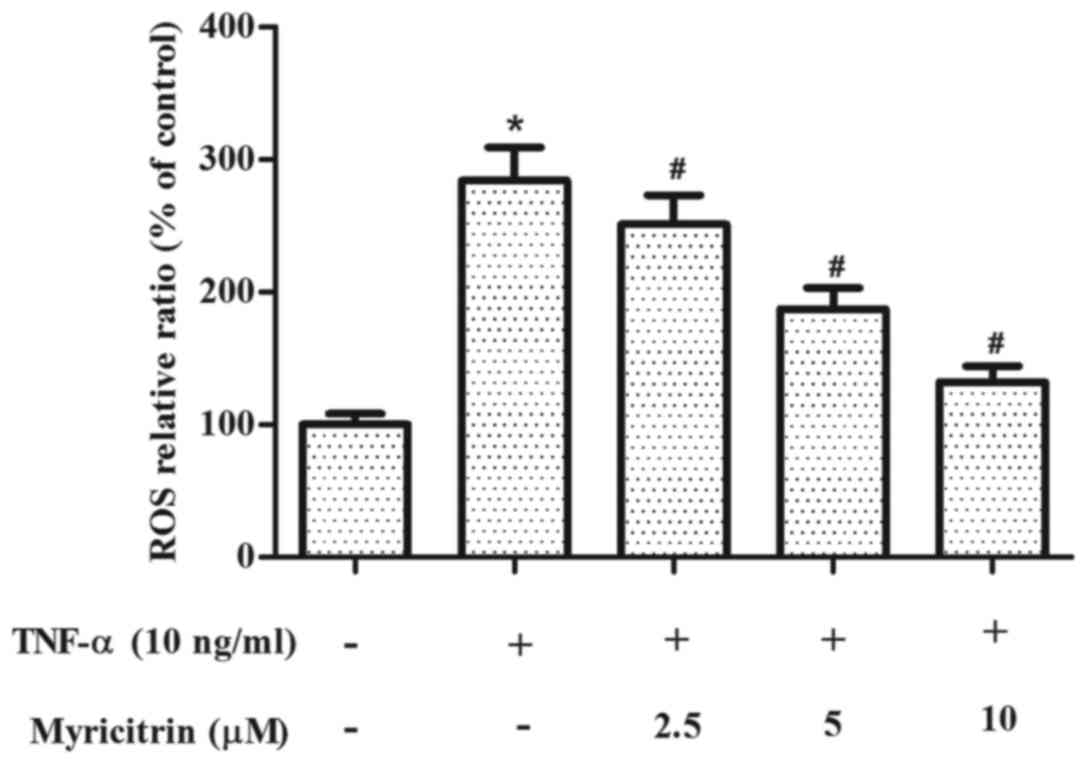
Myricitrin inhibited ROS production in
TNF-α-stimulated MOVAS-1 cells
ROS is involved in the development of
atherosclerosis (15). Therefore,
the present study examined the effect of myricitrin on ROS
production in MOVAS-1 cells in response to TNF-α. As shown in
Fig. 4, treatment with TNF-α
significantly increased the production of ROS when compared to that
of the untreated control cells (P<0.05). By contrast,
pretreatment with myricitrin significantly inhibited TNF-α-induced
ROS production in MOVAS-1 cells in a dose-dependent manner (2.5 µM,
P<0.05; 5 µM, P<0.05; 10 µM, P<0.05; Fig. 4).
Discussion
To the best of the authors' knowledge, the present
study is the first to delineate the effects of myricitrin on the
expression of adhesion molecules in TNF-α-stimulated MOVAS-1 cells.
The results indicated that myricitrin inhibits the adhesion of
THP-1 cells to TNF-α-stimulated MOVAS-1 cells, as well as the
expression of adhesion molecules. The underlying mechanism may
involve the NF-κB signaling pathway.
Leukocyte adhesion to VSMCs during atherosclerotic
progression is primarily mediated by cell adhesion molecules, such
as VCAM-1 and ICAM-1 (16). It has
been demonstrated that VCAM-1 and ICAM-1 are expressed by VSMCs and
are prominent in the fibrous caps of advanced atherosclerotic
plaques (17). In addition, a
number of studies have reported increased expression of VCAM-1 and
ICAM-1 in coronary atherosclerotic tissues (18,19).
Furthermore, inflammatory cytokines, such as interleukin-1β and
TNF-α, may induce the expression of VCAM-1 and ICAM-1 in VSMCs
(20–22). In accordance with previous reports,
the present study demonstrated that TNF-α significantly increased
the adhesion of THP-1 cells to MOVAS-1 cells and the expression of
VCAM-1 and ICAM-1 in MOVAS-1 cells. By contrast, myricitrin
inhibited the adhesion of THP-1 cells to TNF-α-stimulated MOVAS-1
cells, as well as the expression of VCAM-1 and ICAM-1. These
results indicate that myricitrin may exhibit an inhibitory effect
on the expression of adhesion molecules in TNF-α-stimulated MOVAS-1
cells.
The NF-κB signaling pathway serves a critical role
in the regulation of adhesion molecule expression (23,24).
Translocation of NF-κB to the nucleus is preceded by the
phosphorylation, ubiquitination and proteolytic degradation of IκBα
(25). It has been reported that
activation of the NF-κB transcription factor by TNF-α is required
for the transcriptional activation of muscle cell adhesion
molecules (26,27). Furthermore, a previous study
reported that oral treatment with myricitrin induced
anti-inflammatory effects in dextran sulfate sodium-induced acute
colitis in mice by inhibiting Akt/phosphatidylinositol-3
kinase-dependent phosphorylation and the NF-κB signaling pathway
(28). In addition, myricitrin was
able to prevent the formation of advanced glycation end products by
suppressing NF-κB activation and translocation triggered by
methylglyoxal in SH-SY5Y cells (29). Similarly, myricitrin pretreatment
prevented NF-κB p65 activation and IκBα degradation in
TNF-α-stimulated MOVAS-1 cells in the present study. Therefore,
these data indicate that the inhibitory effect of myricitrin on the
expression of adhesion molecules may be mediated, in part, through
suppression of the NF-κB signaling pathway.
ROS, which are synthesized by nicotinamide adenine
dinucleotide phosphate oxidase, serve as secondary messengers that
activate a number of signaling pathways, including the NF-κB
pathway (30). Activation of these
signaling cascades leads to the induction of various genes that
serve critical roles in the development of atherosclerosis
(31). In addition, it has been
reported that TNF-α induces ROS production in VSMCs (20,32,33).
The present study demonstrated that TNF-α significantly increased
the production of ROS in MOVAS-1 cells. By contrast, pretreatment
with myricitrin significantly inhibited ROS production in a
concentration-dependent manner. These results indicate that
myricitrin may inhibit the activation of NF-κB via suppression of
ROS production in TNF-α-stimulated MOVAS-1 cells.
In conclusion, myricitrin inhibited the expression
of VCAM-1 and ICAM-1 in TNF-α-stimulated MOVAS-1 cells potentially
via the NF-κB signaling pathway. Therefore, myricitrin may be an
effective pharmacological agent for the prevention or treatment of
atherosclerosis.
References
|
1
|
Zernecke A, Shagdarsuren E and Weber C:
Chemokines in atherosclerosis: An update. Arterioscler Thromb Vasc
Biol. 28:1897–1908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munro J and Cotran R: The pathogenesis of
atherosclerosis: Atherogenesis and inflammation. Lab Invest.
58:249–261. 1988.PubMed/NCBI
|
|
3
|
Tschoepe D: Adhesion molecules influencing
atherosclerosis. Diabetes Res Clin Pract. 30 Suppl:S19–S24. 1996.
View Article : Google Scholar
|
|
4
|
Galkina E and Ley K: Vascular adhesion
molecules in atherosclerosis. Arterioscler Thromb Vasc Biol.
27:2292–2301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien KD, McDonald TO, Chait A, Allen MD
and Alpers CE: Neovascular expression of E-selectin, intercellular
adhesion molecule-1 and vascular cell adhesion molecule-1 in human
atherosclerosis and their relation to intimal leukocyte content.
Circulation. 93:672–682. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huo Y and Ley K: Adhesion molecules and
atherogenesis. Acta Physiol Scand. 173:35–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Chen P, Tang C, Wang Y, Li Y and
Zhang H: Antinociceptive and anti-inflammatory activities of
extract and two isolated flavonoids of Carthamus tinctorius L. J
Ethnopharmacol. 151:944–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu R, Zhang Y, Ye X, Xue S, Shi J, Pan J
and Chen Q: Inhibition effects and induction of apoptosis of
flavonoids on the prostate cancer cell line PC-3 in vitro. Food
Chem. 138:48–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Q, Gao B, Wang L, Hu YQ, Lu WG, Yang
L, Luo Z-J and Liu J: Protective effects of myricitrin against
osteoporosis via reducing reactive oxygen species and
bone-resorbing cytokines. Toxicol Appl Pharmacol. 280:550–560.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Domitrović R, Rashed K, Cvijanović O,
Vladimir-Knežević S, Škoda M and Višnić A: Myricitrin exhibits
antioxidant, anti-inflammatory and antifibrotic activity in carbon
tetrachloride-intoxicated mice. Chem Biol Interact. 230:21–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin M, Luo Y, Meng XB, Wang M, Wang HW,
Song SY, Ye JX, Pan RL, Yao F, Wu P, et al: Myricitrin attenuates
endothelial cell apoptosis to prevent atherosclerosis: An insight
into PI3K/Akt activation and STAT3 signaling pathways. Vascul
Pharmacol. 70:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen C, Chou C, Sun Y and Huang W: Tumor
necrosis factor alpha-induced activation of downstream NF-kappaB
site of the promoter mediates epithelial ICAM-1 expression and
monocyte adhesion. Involvement of PKCalpha, tyrosine kinase, and
IKK2, but not MAPKs, pathway. Cell Signal. 13:543–553. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng JC, Cheng HP, Tsai IC and Jiang MJ:
ROS-mediated downregulation of MYPT1 in smooth muscle cells: A
potential mechanism for the aberrant contractility in
atherosclerosis. Lab Invest. 93:422–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rains JL and Jain SK: Hyperketonemia
increases monocyte adhesion to endothelial cells and is mediated by
LFA-1 expression in monocytes and ICAM-1 expression in endothelial
cells. Am J Physiol Endocrinol Metab. 301:E298–E306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Libby P and Li H: Vascular cell adhesion
molecule-1 and smooth muscle cell activation during atherogenesis.
J Clin Invest. 92:538–539. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang PY, Rui YC, Lu L, Li TJ, Liu SQ, Yan
HX and Wang HY: Time courses of vascular endothelial growth factor
and intercellular adhesion molecule-1 expressions in aortas of
atherosclerotic rats. Life Sci. 77:2529–2539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davies MJ, Gordon JL, Gearing AJ, Pigott
R, Woolf N, Katz D and Kyriakopoulos A: The expression of the
adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human
atherosclerosis. J Pathol. 171:223–229. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Park HJ, Um SH, Sohn EH, Kim BO,
Moon EY, Rhee DK and Pyo S: Sulforaphane suppresses vascular
adhesion molecule-1 expression in TNF-α-stimulated mouse vascular
smooth muscle cells: Involvement of the MAPK, NF-κB and AP-1
signaling pathways. Vascul Pharmacol. 56:131–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi KW, Park HJ, Jung DH, Kim TW, Park
YM, Kim BO, Sohn EH, Moon EY, Um SH and Rhee DK: Inhibition of
TNF-α-induced adhesion molecule expression by diosgenin in mouse
vascular smooth muscle cells via downregulation of the MAPK, Akt
and NF-κB signaling pathways. Vascul Pharmacol. 53:273–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hakonarson H, Halapi E, Whelan R, Gulcher
J, Stefansson K and Grunstein MM: Association between
IL-1beta/TNF-alpha-induced glucocorticoid-sensitive changes in
multiple gene expression and altered responsiveness in airway
smooth muscle. Am J Respir Cell Mol Biol. 25:761–771. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia P, Gamble JR, Rye KA, Wang L, Hii CS,
Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ and Vadas MA: Tumor
necrosis factor-alpha induces adhesion molecule expression through
the sphingosine kinase pathway. Proc Natl Acad Sci USA.
95:14196–14201. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang WJ and Frei B: Alpha-lipoic acid
inhibits TNF-alpha-induced NF-kappaB activation and adhesion
molecule expression in human aortic endothelial cells. FASEB J.
15:2423–2432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collins T, Read MA, Neish AS, Whitley MZ,
Thanos D and Maniatis T: Transcriptional regulation of endothelial
cell adhesion molecules: NF-kappa B and cytokine-inducible
enhancers. FASEB J. 9:899–909. 1995.PubMed/NCBI
|
|
27
|
Beg AA, Finco T, Nantermet PV and Baldwin
AS Jr: Tumor necrosis factor and interleukin-1 lead to
phosphorylation and loss of I kappa B alpha: A mechanism for
NF-kappa B activation. Mol Cell Biol. 13:3301–3310. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwanke RC, Marcon R, Meotti FC, Bento
AF, Dutra RC, Pizzollatti MG and Calixto JB: Oral administration of
the flavonoid myricitrin prevents dextran sulfate sodium-induced
experimental colitis in mice through modulation of PI3K/Akt
signaling pathway. Mol Nutr Food Res. 57:1938–1949. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang YH, Yu HT, Pu XP and Du GH:
Myricitrin alleviates methylglyoxal-induced mitochondrial
dysfunction and AGEs/RAGE/NF-κB pathway activation in SH-SY5Y
cells. J Mol Neurosci. 53:562–570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang GG, Bai YP, Chen MF, Shi RZ, Jiang
DJ, Fu QM, Tan GS and Li YJ: Asymmetric dimethylarginine induces
TNF-alpha production via ROS/NF-κappaB dependent pathway in human
monocytic cells and the inhibitory effect of reinioside C. Vascul
Pharmacol. 48:115–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Griendling KK, Sorescu D, Lassègue B and
Ushio-Fukai M: Modulation of protein kinase activity and gene
expression by reactive oxygen species and their role in vascular
physiology and pathophysiology. Arterioscler Thromb Vasc Biol.
20:2175–2183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang HS and Wang SQ: Salvianolic acid B
from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha
(TNF-alpha)-induced MMP-2 upregulation in human aortic smooth
muscle cells via suppression of NAD(P)H oxidase-derived reactive
oxygen species. J Mol Cell Cardiol. 41:138–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|