Introduction
Cardiac fibrosis is central to the development of
cardiac dysfunction in a variety of cardiovascular diseases,
including myocardial infarction, cardiac hypertrophy and heart
failure (1). It is characterized
by the proliferation of cardiac fibroblasts (CFs) and abundant
accumulation of extracellular matrix (ECM) proteins in the
myocardium (2). However, the
molecular mechanisms underlying cardiac fibrosis remain to be
elucidated.
CFs are the primary cardiac cells present in the
myocardium. Accumulating evidence has suggested that the
transdifferentiation of CFs into myofibroblasts is important in ECM
deposition and cardiac fibrosis (3–5).
High glucose or hyperglycemia is a factor, which promotes collagen
deposition by inducing CF proliferation and activation in
vitro, and can lead to cardiac fibrosis (6,7).
Thus, intervention of high glucose-induced myofibroblast
differentiation may be an effective method to improve and assist in
curing cardiac fibrosis.
Betulinic acid (BA) is an active compound isolated
from the bark of the birch tree Betula spp. (Betulaceae).
Various biological and pharmacological effects of BA have been
demonstrated, including anti-inflammatory, anti-viral, anti-oxidant
and anti-tumor activities (8–10).
For example, Xia et al (11) reported that BA prevents
cardiomyocyte apoptosis and eventually improves cardiac function.
In addition, BA attenuates hepatic fibrosis via suppressing
thioacetamide-mediated increases in liver tissue hydroxyproline and
α-smooth muscle actin (α-SMA) (12). However, the effect of BA on the
high glucose-induced fibrotic response in CFs remains to be
elucidated. Therefore, the present study investigated the effect of
BA on high glucose-induced CFs and examined the possible mechanism
underlying the effect of BA on CF transdifferentiation.
Materials and methods
Culture of cardiac fibroblasts
Neonatal Sprague-Dawley rats (age, 1–3 days; weight,
180–200 g) were obtained from the Animal Breeding Center of Henan
Provincial People's Hospital (Zhengzhou, China) and were maintained
at a constant temperature (21±2°C) and 60% humidity in a holding
facility under a 12-h light/dark cycle, with free access to food
and water. The CFs were isolated from neonatal rats as described
previously (13). The cells were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Bio-Rad Laboratories, Inc., Hercules, CA, USA), 100
IU/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). The CFs were maintained in growth
media and incubated in humidified atmosphere of 5% CO2
at 37°C. All experimental protocols were approved by the Ethical
Committee of Henan Provincial People's Hospital for Animal Care and
Use (Henan, China).
Cell proliferation assay
Cell proliferation was measured using an MTT assay.
Briefly, the CFs were seeded in 96-well culture plates at a density
of 1×104 cells per well. Following starvation in
serum-free medium for 24 h, the CFs were pre-treated with various
concentrations of BA (1, 5 and 10 µM) for 24 h at room temperature,
and exposed to high glucose (25 mM). After 24 h, 20 µl of MTT
solution (5 mg/ml; Sigma-Aldrich; Merck Millipore) was added to
each well and incubated at 37°C for 4 h, following which the
culture medium was removed and 150 µl of DMSO (Sigma-Aldrich; Merck
Millipore) was added. The absorbance was measured at 490 nm using a
microplate spectrophotometer (Bio-Rad Laboratories, Inc.).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the CFs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (~5
µg) was then subjected to TaqMan one-step reverse transcription
(Applied Biosystems; Thermo Fisher Scientific, Inc.), followed by
qPCR using an ABI PRISM 7700 sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RT-qPCR was performed in a final volume of
10 µl, which consisted of 5 µl SsoFast™ EvaGreen Supermix (Bio-Rad
Laboratories, Inc.), 1 µl cDNA (1:50 dilution) and 2 µl each of the
forward and reverse primers (1 mM). The primers used were as
follows: α-smooth muscle actin (SMA) forward,
5′-GCTATTCAGGCTGTGCTGTC-3′ and reverse, 5′-GGTAGTCGGTGAGATCTCGG-3′;
transforming growth factor (TGF)-β1 forward,
5′-CCAACTATTGCTTCAGCTCCA-3′ and reverse, 5′-GTGTCCAGGCTCCAAATGT-3′;
and GAPDH forward, 5′-ACTCCCATTCTTCCACCTTTG-3′ and reverse,
5′-CCCTGTTGCTGTAGCCATATT-3′. Thermocycling conditions were as
follows: 94°C for 2 min for initial denaturation; 94°C for 20 sec,
59°C for 15 sec, and 72°C for 20 sec; 2 sec for plate reading (35
cycles); with a melt curve between 65 and 95°C. GAPDH was used as
an internal control. The relative expression levels of genes were
calculated using control GAPDH mRNA and the 2−ΔΔCq
method (14).
Western blot analysis
The whole-cell proteins were collected in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) with protease inhibitor PMSF, and
the protein concentration was quantified using a Bradford assay.
Equal quantities of protein sample (20 µg) were separated via 12%
SDS-PAGE and transferred onto nitrocellulose membranes (Amersham;
GE Healthcare Life Sciences, Little Chalfont, UK). Following
blocking with 5% non-fat dry milk at room temperature for 1 h, the
membranes were incubated with primary antibodies against α-SMA
(1:3,000; cat. no. PA5-19465; Invitrogen; Thermo Fisher Scientific,
Inc.), TGF-β1 (1:2,000; cat. no. sc-146; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), Smad3 (1:2,500; cat. no. PA5-34774;
Invitrogen; Thermo Fisher Scientific, Inc.), phosphorylated
(p-)Smad3 (1:3,000; cat. no. 44-246G; Invitrogen; Thermo Fisher
Scientific, Inc.) and GAPDH (1:3,000; cat. no. sc-25778; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membranes were washed
(3×5 min) in TBS containing 0.1% Tween-20 and incubated for 1 h at
room temperature in the presence of horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
sc-2005; Santa Cruz Biotechnology, Inc.) diluted in blocking
solution. Finally, the membranes were washed again with TBST, and
the blots were examined using an enhanced chemiluminescence
detection system (GE Healthcare Life Sciences, Uppsala, Sweden).
The optical densities of the bands were quantified using Gel-Pro
Analyzer version 4.0 (Media Cybernetics, Inc. Rockville, MD,
USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation of 3 independent experiments. Statistical analysis was
performed using one-way analysis of variance followed by Tukey's
multiple comparisons test using GraphPad Prism software version
5.01 (GraphPad software, Inc., San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
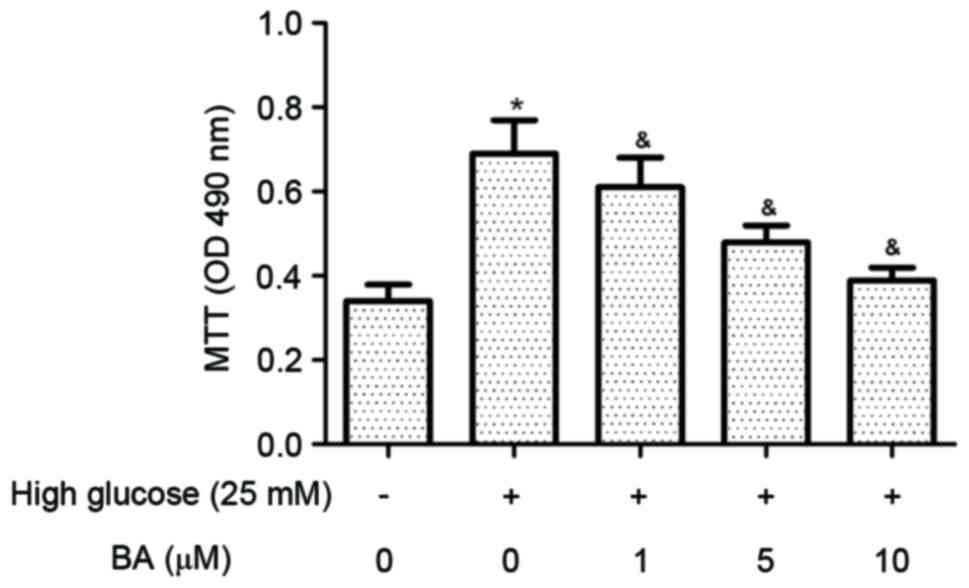
BA attenuates high glucose-induced CF
proliferation
High glucose has been shown to promote the
proliferation of CFs. The present study examined the effect of BA
on CF proliferation induced by high glucose using an MTT assay. The
results indicated that glucose at a high concentration
significantly increased the proliferation of CFs, compared with
glucose at a normal concentration. However, the high glucose
induced-CF proliferation was significantly inhibited by BA
treatment (Fig. 1).
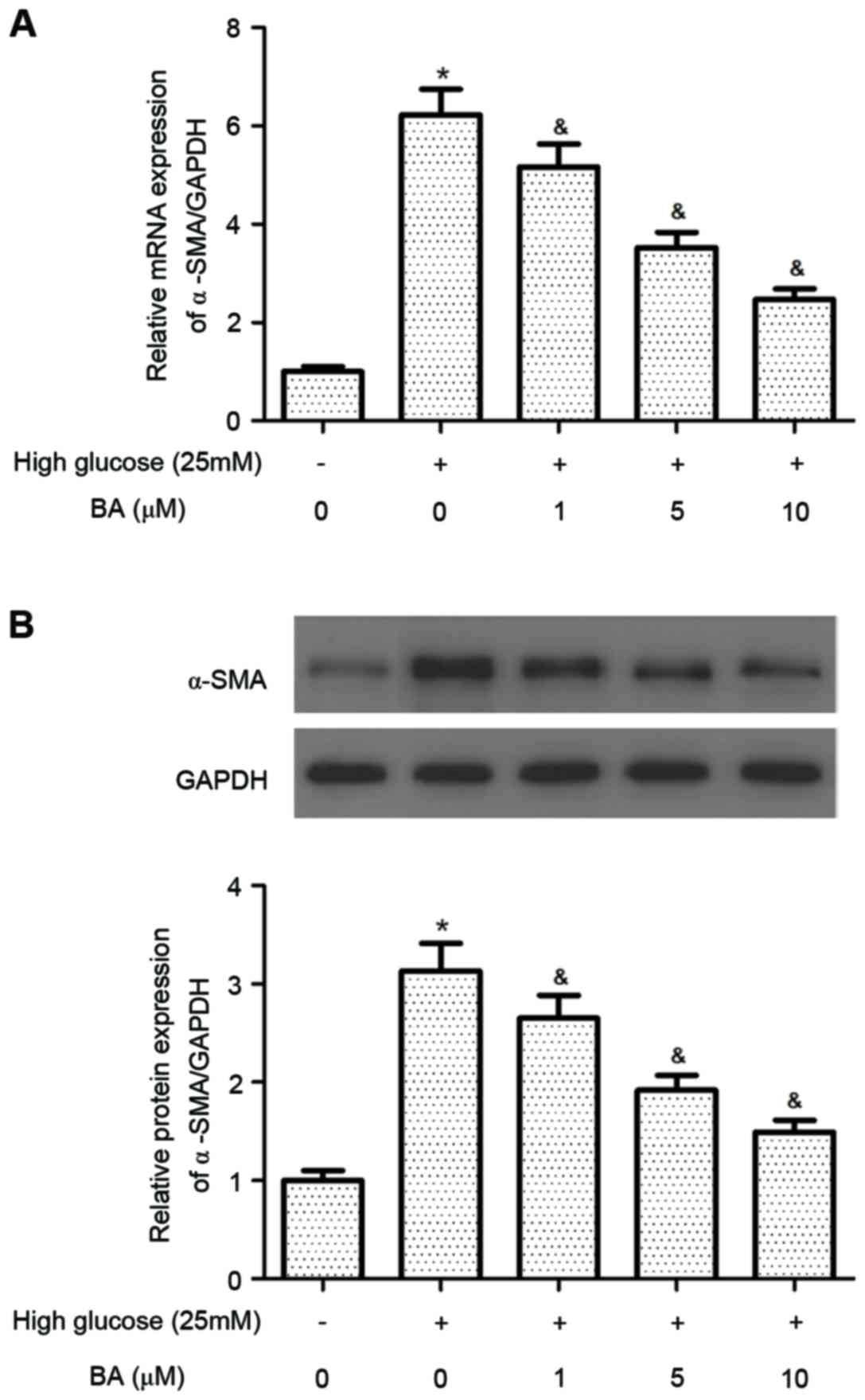
BA reduces the differentiation of CFs
into myofibroblasts
The expression of α-SMA is a major morphological
marker for myofibroblasts. Therefore, the present study evaluated
the effect of BA on the expression of α-SMA in CFs stimulated by
high glucose. The results of the RT-qPCR analysis demonstrated that
the mRNA expression of α-SMA was significantly increased in the
high glucose-treated CFs, compared with the control CFs. However,
pre-treatment of the CFs with BA inhibited the high glucose-induced
mRNA expression of α-SMA in the CFs (Fig. 2A). Consistent with the results of
the RT-qPCR analysis, the results of the western blot analysis also
indicated that BA inhibited the high glucose-induced increase of
α-SMA in the CFs (Fig. 2B).
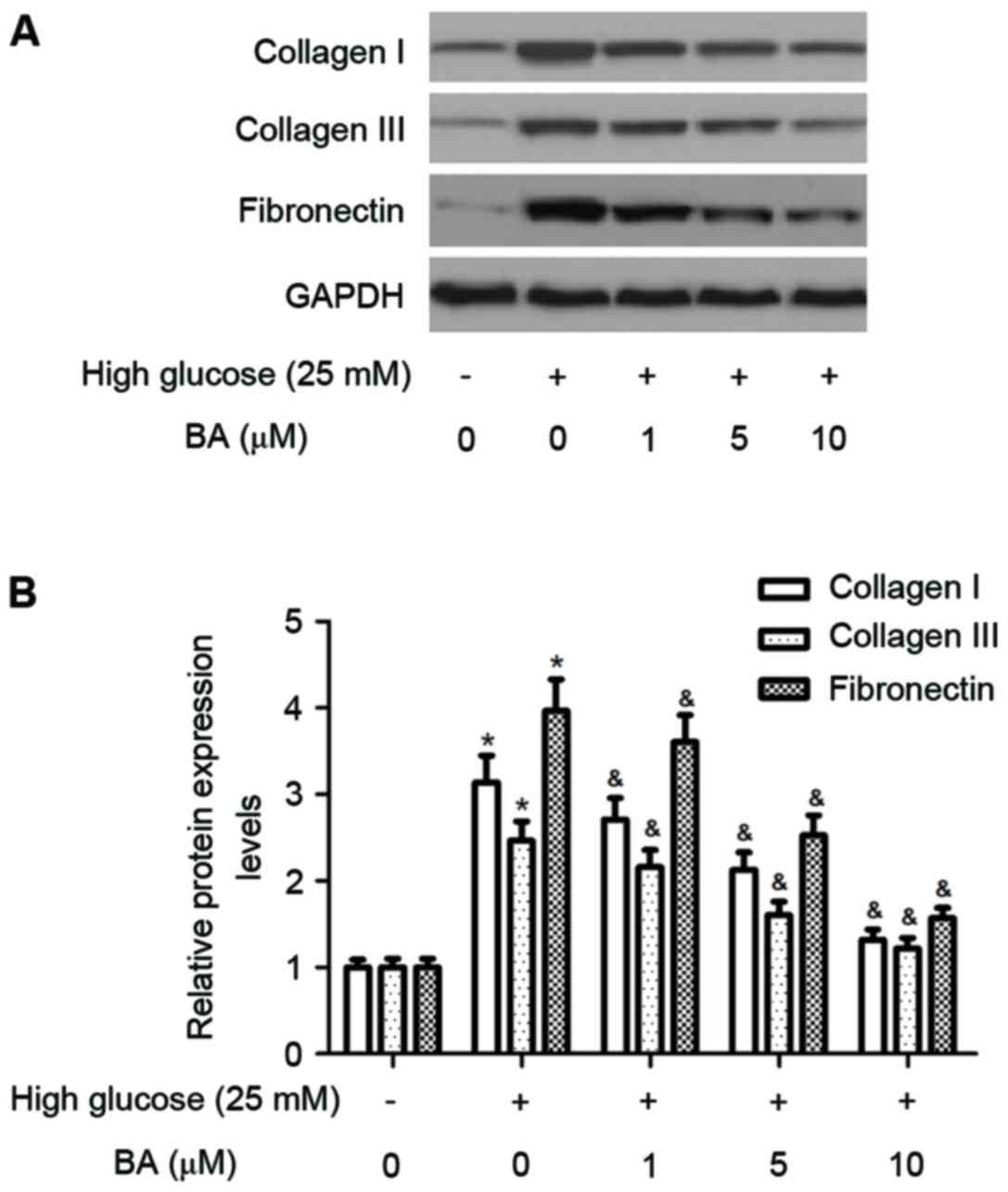
BA inhibits high glucose-induced
expression of ECM in CFs
It has been reported that high glucose induces
cardiac fibrosis by increasing the expression of ECM proteins,
including collagen I, collagen III and fibronectin, therefore, the
present study investigated the effect of BA on the expression of
ECM proteins in CFs induced by high glucose. As shown in Fig. 3A and B, treatment of the cultured
CFs with high glucose for 24 h caused significant increases in the
protein levels of collagen I, collagen III and fibronectin.
However, pre-treatment of the CFs with BA reduced the stimulatory
effects of high glucose.
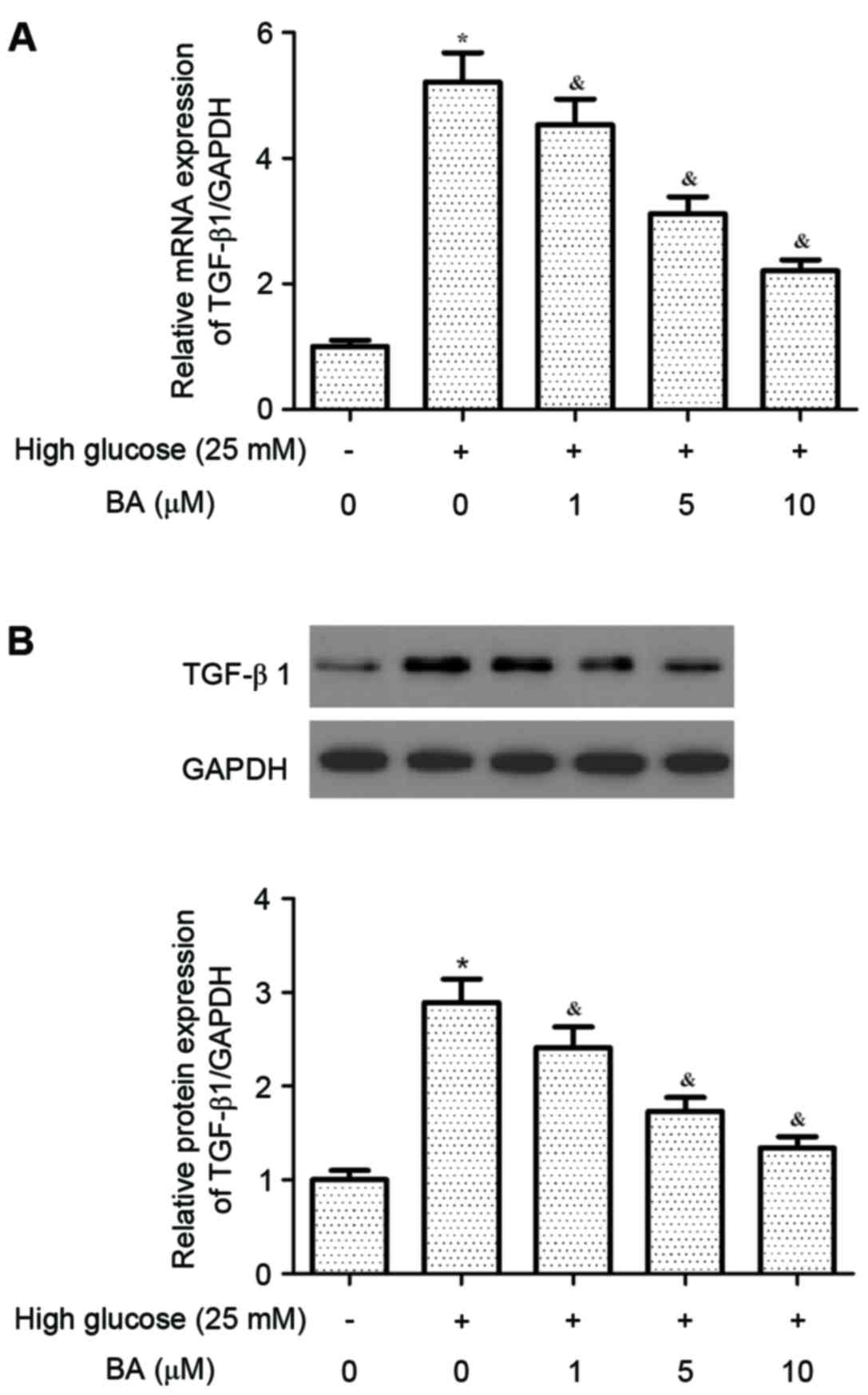
BA inhibits high glucose-induced
expression of TGF-β1 in CFs
TGF-β1 can induce cardiac fibrosis by activating
CFs. Thus, the present study investigated the effect of BA on the
expression of TGF-β1 in CFs induced by high glucose. As shown in
Fig. 4A and B, high glucose
treatment significantly increased the expression of TGF-β1 at the
mRNA and protein levels in the CFs, compared with the normal group.
However, BA inhibited the high glucose-induced expression of TGF-β1
in the CFs in a dose-dependent manner.
BA inhibits high glucose-induced
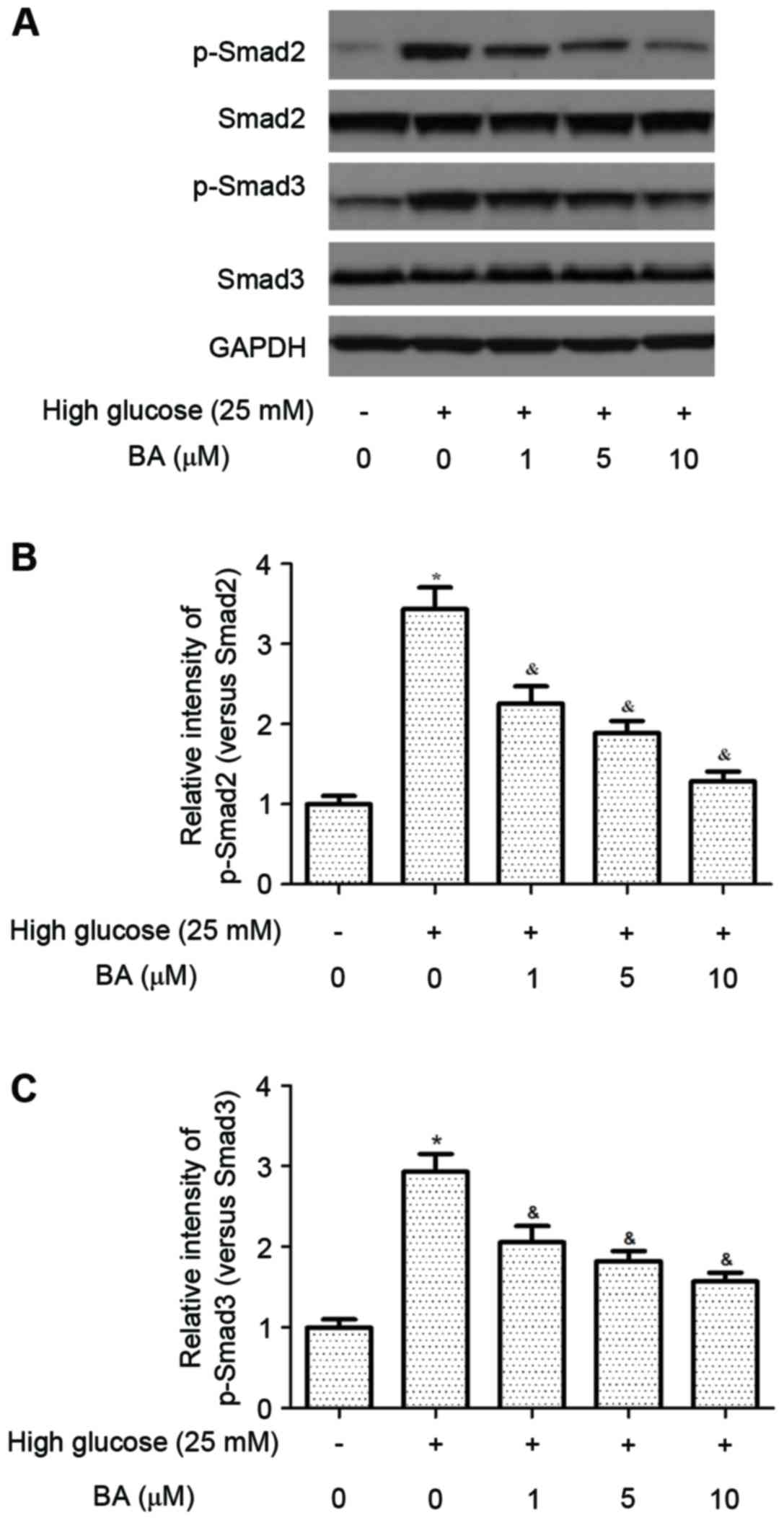
activation of the TGF-β1/Smad pathway in CFs
The TGF-β1/Smad signaling pathway is critical for
the development of cardiac fibrosis. Therefore, the present study
examined the effects of BA on the levels of p-Smad2/3 in CFs. As
shown in Fig. 5, the protein
levels of Smad 2/3 were increased in CFs cultured in high glucose,
which may facilitate the development of a pro-fibrotic phenotype.
By contrast, BA inhibited the high glucose-induced phosphorylation
of Smad2/3 in the CFs.
Discussion
It is well-known that hyperglycemia is an inducer of
cardiac fibrosis, therefore, the present study cultured neonatal
CFs with high glucose and observed the effect of BA on the high
glucose-induced CFs, to elucidate the possible mechanism for BA on
CF transdifferentiation. The results of the present study revealed
that BA attenuated high glucose-induced CF proliferation and
myofibroblast differentiation. In addition, BA inhibited the high
glucose-induced expression of ECM proteins in the CFs. It was also
found that the effect of BA on myofibroblast differentiation and
the excessive expression of ECM involved the TGF-β1/Smad signaling
pathway.
CF proliferation is the basic function in the
response to pro-fibrotic stimuli, which can lead to excessive ECM
production and subsequent cardiac fibrosis (15,16).
Hyperglycemia was shown to promote the proliferation of CFs and
these results are consistent with previous studies showing that CF
proliferation was significantly increased by high glucose (17–19).
In addition, the present study showed that BA significantly
inhibited high glucose-induced fibroblast proliferation. These data
suggested that BA has a critical role in CF proliferation.
The differentiation of CFs into myofibroblasts and
the exclusive deposition of ECM components, including α-SMA,
collagen and fibronectin, are essential for the development of
cardiac fibrosis (20). Previous
evidence has suggested that the differentiation of CFs occurs in
response to high glucose, TGF-β1 and angiotensin-II, which are
important in this process (21,22).
In the present study, it was found that the expression of α-SMA was
significantly increased by high glucose. In addition, the results
of the present study showed that BA prevented the high
glucose-induced expression of α-SMA in the CFs. It was also found
that BA inhibited the high glucose-induced levels of collagen I,
collagen III and fibronectin in the CFs. These data suggested that
BA is important in the phenotypic transformation of CFs into
myofibroblasts.
TGF-β1 is known as a major stimulator of fibrous
tissue deposition in the heart, and can induce cardiac fibrosis by
activating fibroblasts and producing collagen. Inhibiting TGF-β1
effectively reverses CF transdifferentiation and reduces ECM
deposition, and it may be a potential therapeutic target for
cardiac fibrosis (23–25). In the present study, it was found
that high glucose increased the protein levels of TGF-β1 in CFs.
These observations are in agreement with those reported by Singh
et al (26), in which
neonatal rat CFs cultured in high glucose showed increased protein
expression levels of TGF-β1. In addition, BA partially suppressed
the high glucose-induced increases in the expression of ECM in the
CFs. The Smad signaling pathway acts as a downstream mediator of
TGF-β1. Smad2/3 translocate into the nucleus accompanied by Smad4.
In the nucleus, this protein complex acts in conjunction with Sp1
and enhances the transcription of several genes, including collagen
I, collagen III and fibronectin. In addition, it has been shown
that high glucose increases the expression of TGF-β1 and induces
activation of the TGF-β1/Smad signaling pathway, leading to
upregulated expression of ECM in CFs (27). In accordance with these results,
the results of the present study showed that the protein levels of
Smad2/3 increased, whereas those of Smad7 were suppressed in CFs
cultured in high glucose, which may facilitate the development of a
pro-fibrotic phenotype. BA inhibited the high glucose-induced
phosphorylation of Smad2/3 in the CFs. Thus, the findings of the
present study suggested that BA suppressed the high glucose-induced
increases in the proliferation of CFs and collagen synthesis, which
may be associated with inhibition of the TGF-β/Smad signaling
pathway.
In conclusion, the present study demonstrated that
BA suppressed high glucose-induced increases in the proliferation
of CFs and the expression of ECM via inhibition of the TGF-β/Smad
signaling pathway. Thus, BA may offer therapeutic potential towards
the treatment of cardiac fibrosis.
References
|
1
|
Kong P, Christia P and Frangogiannis NG:
The pathogenesis of cardiac fibrosis. Cell Mol Life Sci.
71:549–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teunissen BE, Smeets PJ, Willemsen PH, De
Windt LJ, Van der Vusse GJ and Van Bilsen M: Activation of
PPARdelta inhibits cardiac fibroblast proliferation and the
transdifferentiation into myofibroblasts. Cardiovasc Res.
75:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan D, Takawale A, Lee J and Kassiri Z:
Cardiac fibroblasts, fibrosis and extracellular matrix remodeling
in heart disease. Fibrogenesis Tissue Repair. 5:152012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian K, Liu Z, Wang J, Xu S, You T and Liu
P: Sirtuin-6 inhibits cardiac fibroblasts differentiation into
myofibroblasts via inactivation of nuclear factor κB signaling.
Transl Res. 165:374–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bugyei-Twum A, Advani A, Advani SL, Zhang
Y, Thai K, Kelly DJ and Connelly KA: High glucose induces Smad
activation via the transcriptional coregulator p300 and contributes
to cardiac fibrosis and hypertrophy. Cardiovasc Diabetol.
13:892014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang M, Zhang W, Lin H, Jiang H, Dai H and
Zhang Y: High glucose promotes the production of collagen types I
and III by cardiac fibroblasts through a pathway dependent on
extracellular-signal-regulated kinase 1/2. Mol Cell Biochem.
301:109–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kasperczyk H, La Ferla-Brühl K, Westhoff
MA, Behrend L, Zwacka RM, Debatin KM and Fulda S: Betulinic acid as
new activator of NF-κB: Molecular mechanisms and implications for
cancer therapy. Oncogene. 24:6945–6956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yogeeswari P and Sriram D: Betulinic acid
and its derivatives: A review on their biological properties. Curr
Med Chem. 12:657–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pavlova N, Savinova O, Nikolaeva S, Boreko
E and Flekhter O: Antiviral activity of betulin, betulinic and
betulonic acids against some enveloped and non-enveloped viruses.
Fitoterapia. 74:489–492. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia A, Xue Z, Li Y, Wang W, Xia J, Wei T,
Cao J and Zhou W: Cardioprotective effect of betulinic acid on
myocardial ischemia reperfusion injury in rats. Evid Based
Complement Alternat Med. 2014:5737452014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan Y, Wu YL, Lian LH, Xie WX, Li X,
Ouyang BQ, Bai T, Li Q, Yang N and Nan JX: The anti-fibrotic effect
of betulinic acid is mediated through the inhibition of NF-κB
nuclear protein translocation. Chem-Biol Interact. 195:215–223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner NA, Das A, Warburton P, O'Regan DJ,
Ball SG and Porter KE: Interleukin-1alpha stimulates
proinflammatory cytokine expression in human cardiac
myofibroblasts. Am J Physiol Heart Circ Physiol. 297:H1117–H1127.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen HN, Wang DJ, Ren MY, Wang QL and Sui
SJ: TWEAK/Fn14 promotes the proliferation and collagen synthesis of
rat cardiac fibroblasts via the NF-кB pathway. Mol Biol Rep.
39:8231–8241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Driesen RB, Nagaraju CK, Abi-Char J,
Coenen T, Lijnen PJ, Fagard RH, Sipido KR and Petrov VV: Reversible
and irreversible differentiation of cardiac fibroblasts. Cardiovasc
Res. 101:411–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang P, Li H, Wang Y, Chen H and Zhang P:
Effects of recombinant human relaxin upon proliferation of cardiac
fibroblast and synthesis of collagen under high glucose condition.
J Endocrinol Invest. 32:242–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neumann S, Huse K, Semrau R, Diegeler A,
Gebhardt R, Buniatian GH and Scholz GH: Aldosterone and D-glucose
stimulate the proliferation of human cardiac myofibroblasts in
vitro. Hypertension. 39:756–760. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou H, Zhang KX, Li YJ, Guo BY and Wang M
and Wang M: Fasudil hydrochloride hydrate, a Rho-kinase inhibitor,
suppresses high glucose-induced proliferation and collagen
synthesis in rat cardiac fibroblasts. Clin Exp Pharmacol Physiol.
38:387–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fedak PW, Bai L, Turnbull J, Ngu J, Narine
K and Duff HJ: Cell therapy limits myofibroblast differentiation
and structural cardiac remodeling basic fibroblast growth
factor-mediated paracrine mechanism. Circ Heart Fail. 5:349–356.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Gao G, Liu J, Guo R, Lin Y, Chu Y,
Han F, Zhang W and Bai Y: Identification of differentially
expressed genes induced by angiotensin II in rat cardiac
fibroblasts. Clin Exp Pharmacol Physiol. 33:41–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim H and Zhu Y: Role of transforming
growth factor-beta in the progression of heart failure. Cell Mol
Life Sci. 63:2584–2596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bujak M and Frangogiannis NG: The role of
TGF-beta signaling in myocardial infarction and cardiac remodeling.
Cardiovasc Res. 74:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuwahara F, Kai H, Tokuda K, Kai M,
Takeshita A, Egashira K and Imaizumi T: Transforming growth
factor-beta function blocking prevents myocardial fibrosis and
diastolic dysfunction in pressure-overloaded rats. Circulation.
106:130–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh VP, Baker KM and Kumar R: Activation
of the intracellular renin-angiotensin system in cardiac
fibroblasts by high glucose: Role in extracellular matrix
production. Am J Physiol Heart Circ Physiol. 294:H1675–H1684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Zhuo X, Liu W, Wan Z, Liang X, Gao
S, Yuan Z and Wu Y: Resveratrol inhibits high glucose induced
collagen upregulation in cardiac fibroblasts through regulating
TGF-β1-Smad3 signaling pathway. Chem Biol Interact. 227:45–52.
2015. View Article : Google Scholar : PubMed/NCBI
|