Introduction
Parkinson's disease (PD) is a common
neurodegenerative disorder, which is caused by degeneration of
dopaminergic neurons in the substantia nigra pars compacta (SNpc)
(1). Despite a number of studies,
the accurate pathogenesis of PD remains unclear and increasing
evidence has demonstrated that activation of the microglia, with
the attendant oxidative stress and neuroinflammation, may be
crucial events in the pathogenesis of PD (2,3).
Additionally, it has been demonstrated that the mitogen-activated
protein kinase (MAPK) pathway serves an important role in the
pathogenesis of PD (4). Modulating
the MAPK pathway represents a promising approach for the prevention
and treatment of PD.
In the past few years, several neurotoxic molecules,
including 6-hydroxydopamine (6-OHDA) and
methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), have been
utilized to establish PD models, by causing an inflammatory
response; however, it is difficult to confirm whether
neuroinflammation is the consequence or cause in injured
dopaminergic neurons (5). However,
a PD model induced by lipopolysaccharide (LPS), as an effective
glial cell activator, has demonstrated a gradual and selective
reduction in dopaminergic neurons in the SNpc (6). Several clinical and basic studies
have indicated that LPS-induced PD models demonstrate the basic
characteristics of PD pathology, including anti-inflammatory
actions (similar to nonsteroidal anti-inflammatory drugs,
cyclo-oxgenase-2 inhibitors, inducible nitric oxide synthase
inhibitors) and anti-oxidative properties, particularly NADPH
inhibitors (7–9).
In south China, DL-3n-butylphthalide (NBP) is
extracted from rapeseed and was approved for clinical use by the
China Food and Drug Administration in 2002 (Beijing, China). NBP
has been demonstrated to possess multiple neuroprotective effects
exerted by inhibiting the inflammatory process including reducing
oxidative stress, improving mitochondrial function and reducing
neuronal apoptosis (10,11). NBP also suppresses the generation
of peroxynitrite, superoxide and nitric oxide (12). Previously, NBP has been reported to
be able to prevent oxidative damage and reduce mitochondrial
dysfunction in a 1-methyl-4-phenylpyridinium+-induced
cellular model of PD (13).
Therefore, in the present study, the neuroprotective
effects of NBP on LPS-induced inflammatory nerve damage in a PD
mouse model were examined and the possible mechanisms were
investigated.
Materials and methods
Reagents
Anti-c-Jun N-terminal kinase (JNK), MAPK 14
(p38) and extracellular signal-regulated kinase (ERK),
phosphorylated (p)-p38, p-JNK, and p-ERK rabbit antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA;
cat. nos. 9252, 9212, 4695, 9211, 9251 and 9101, respectively).
Anti-β-actin rabbit polyclonal antibody was purchased from Beyotime
Institute of Biotechnology (Haimen, China; cat. no. AA128).
Protease inhibitor cocktail and extraction buffer
[radioimmunoprecipitation assay (RIPA) lysis buffer; cat. no.
P0013B] were purchased from the Beyotime Institute of
Biotechnology. Enhanced chemiluminescence (ECL) reagents were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
LPS was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) and NBP was obtained from CSPC Pharmaceutical Co., Ltd.
(Shijiazhuang, China).
Animals and treatment
Adult male C57BL/6 mice (n=30) weighing 18–20 g were
purchased from the Comparative Medicine Center at Yangzhou
University [Yangzhou, China; cat. no. scxk(Su) 2012-004] and housed
under pathogen-free conditions with a 12-h light/dark cycle, 60–70%
relative humidity and temperature of 20–22°C, and received food and
water ad libitum in their cages. All animals were ~10 weeks
of age at the start of administration. Prior to the experiment, the
animals were allowed 10 days to adapt to the new environment. All
mice were randomly separated into 3 groups (n=10/group) as follows:
i) The control group, which was treated with saline; ii) the
LPS-treatment group, which received a single intraperitoneal
injection of LPS at 5 mg/kg to generate the PD model; and iii) the
NBP-treatment group, which received gastric perfusions of NBP (120
mg/kg) dissolved in soybean oil once a day for 30 days, following
injection with LPS (similar to the LPS-treatment group). All
experiments commenced following 7 months of treatment. The present
study was approved by the ethics committee of Bengbu Medical
College (Bengbu, China).
Rotarod test
The rotarod test is widely used to assess motor and
coordination abilities, particularly bradykinesia in mice (14). In the present study, a rotarod
(3-cm diameter) was utilized at a fixed speed of 20 rpm. The
retention time for each mouse resting on the revolving rod was
recorded. The rotarod test was repeated 3 times for each mouse,
with 30-min rest periods in between the tests. The retention time
of each mouse was determined and used for comparison.
Open-field test
Following treatment, motor behavior was analyzed in
an open-field test. The apparatus consisted of a square (40×40 cm)
with a surrounding wall (height, 30 cm). The square floor was
divided into 16 (4×4) sub-squares using transverse and longitudinal
segments. Mice were placed in the center of the structure and then
the behavior of the mice was recorded for 5 min. When each mouse
repeated the test 3 times, their spontaneous activity was analyzed
for 30 min. The line crossings, grooming and rearing of each mouse
were recorded and analyzed.
Immunohistochemistry
Following treatment, 3 mice were randomly selected
from each group for the subsequent study. Mice were deeply
anesthetized with 10% chloral hydrate (3 ml/kg; Sigma-Aldrich) at
room temperature and perfused through the ventriculus sinister with
100 ml saline, followed by 4% paraformaldehyde for 20 min. The
midbrains were collected and fixed with 4% paraformaldehyde at 4°C
for 24 h. Subsequently, the midbrains were embedded in paraffin and
cut (35-µm-thick) and processed for immunohistochemistry. The
sections were blocked with 5% bovine serum albumin (cat. no. P0007;
Beyotime Institute of Biotechnology) dissolved in PBS at room
temperature for 1 h.
The sections were incubated with primary monoclonal
antibodies at 37°C overnight [rabbit anti-tyrosine hydroxylase
(TH); 1:1,000; cat. no. 2791; Cell Signaling Technology, Inc.].
Then, the sections were rinsed with PBS, incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies at room
temperature for 50 min (goat anti-rabbit Immunoglobulin G, 1:800
(cat. no. GB23303; Goodbio Technology Co., Ltd., Wuhan, China).
Sections were subsequently incubated for 30 min in peroxidase
substrate mixing liquid and then washed and detected with a DAB
staining kit (cat. no. K5007; Dako; Agilent Technologies, Santa
Clara, CA, USA) at room temperature for 3–10 min, terminating the
coloration when brown granules (positive cells) were observed.
Finally, the sections were counterstained with
hematoxylin at room temperature for 3 min, dehydrated with
deionized water, dried and sealed. The immunopositive cells in the
stained sections were analyzed under an optical microscope (Nikon
Corporation, Tokyo, Japan). The number of TH-positive cells
(magnification, ×40) in every sixth section was counted by two
researchers blinded to the treatment.
Western blot analysis
RIPA lysis buffer was used to extract proteins from
the ventral mesencephalon. Tissues were lyzed in RIPA buffer
supplemented with protease inhibitor cocktail. Then, the lysates
were centrifuged (8,000 × g for 10 min at 4°C) and the protein
concentration in the extracts was determined using the Bradford
assay.
Equal amounts (30 µg) of protein samples were
electrophoresed on 12% denaturing polyacrylamide gels and then
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MD, USA) using a semi-dry blotting apparatus (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 2 h at 4°C. The membrane
was then blocked with 5% bovine serum albumin (cat. no. P0007;
Beyotime Institute of Biotechnology) dissolved in TBS containing
Tween [TBST; 10 mM Tris-HCl (pH 7.5), 150 mM NaCl and 0.1%
Tween-20] at room temperature for 1 h. Following washing with TBST
buffer 3 times, membranes were incubated with primary antibodies
against JNK, ERK, p38, p-JNK, p-ERK and p-p38 (all 1:1,000) at 4°C
overnight. Then, the membranes were washed 3 times with TBST for 10
min and incubated with the HRP-conjugated secondary antibodies
(cat. no. GB23303; Goodbio Technology Co., Ltd.) at room
temperature for 1 h (1:5,000). An anti-β-actin antibody (1:1,000)
was used as an internal control.
Band intensities were detected with ECL reagents
(cat. no. KGP1123; Nanjing KeyGen Biotech Co., Ltd.) and measured
with Quantity one Software version 25.0.0 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All values were expressed as the mean ± standard
deviation. Results were analyzed using one-way analysis of variance
followed by Tukey post-hoc tests using SPSS software version 18.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
NBP attenuates behavioral impairment
in intraperitoneal LPS-induced PD mice
To investigate whether NBP protects mice from
LPS-induced neurotoxicity, the behavioral tests were conducted,
including the rotarod and the open-field test.
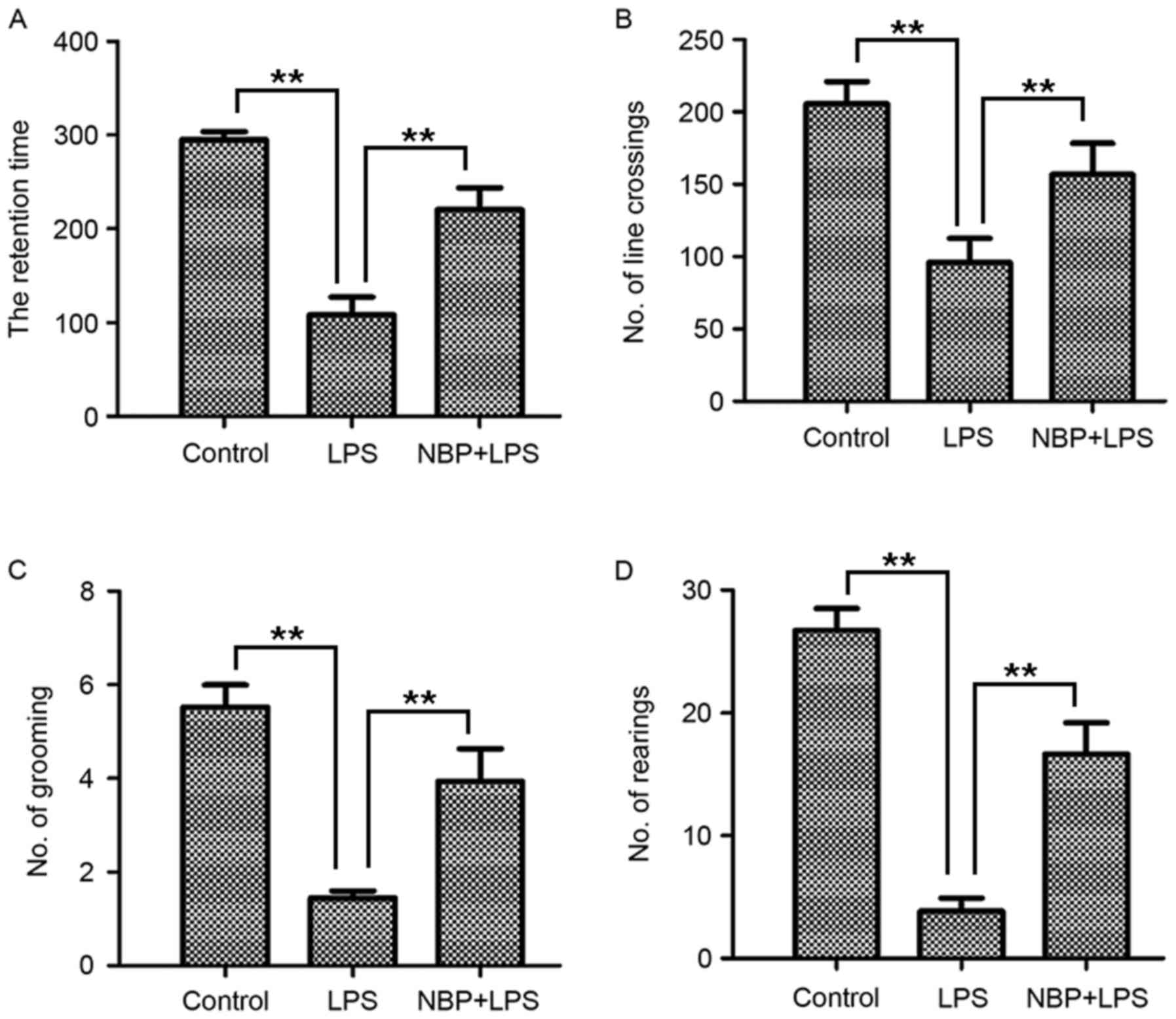
Rotarod test results demonstrated that the retention
time of the control group was 298.61±2.94 sec (Fig. 1A). Compared with the control group,
the LPS-treatment group (103.78±46.01 sec) had a significantly
reduced retention time (P<0.01). However, the NBP-treatment
group, who demonstrated marked improvement in retention time
(Fig. 1A), had significantly
enhanced retention times (221.83±53.88 sec) compared with mice in
the group treated with LPS (P<0.01).
As presented in Fig.
1B-D, the open field test, which assessed the degree of
locomotory capacity demonstrated that the number of line crossing,
rearing and grooming actions in the control group was 205.56±26.82,
27.06±3.59 and 5.50±0.84, respectively. In the LPS-treatment group,
the line crossing (96.00±28.65), rearing (3.83±1.87) and grooming
(1.44±0.27) activities were decreased significantly (all P<0.01;
Fig. 1B-D). In the NBP treatment
group, the line crossing (156.67±37.23), rearing (16.78±4.64) and
grooming (3.94±1.22) behaviors were significantly increased
compared with the LPS-treatment group (all P<0.01; Fig. 1B-D). Therefore, the mice treated
with NBP demonstrated a significant improvement in behavioral
performance in the open field test compared with the LPS-treatment
group.
These results demonstrated that NBP could protect
mice from LPS-induced behavioral impairment.
NBP inhibits LPS-induced dopaminergic
neuronal loss
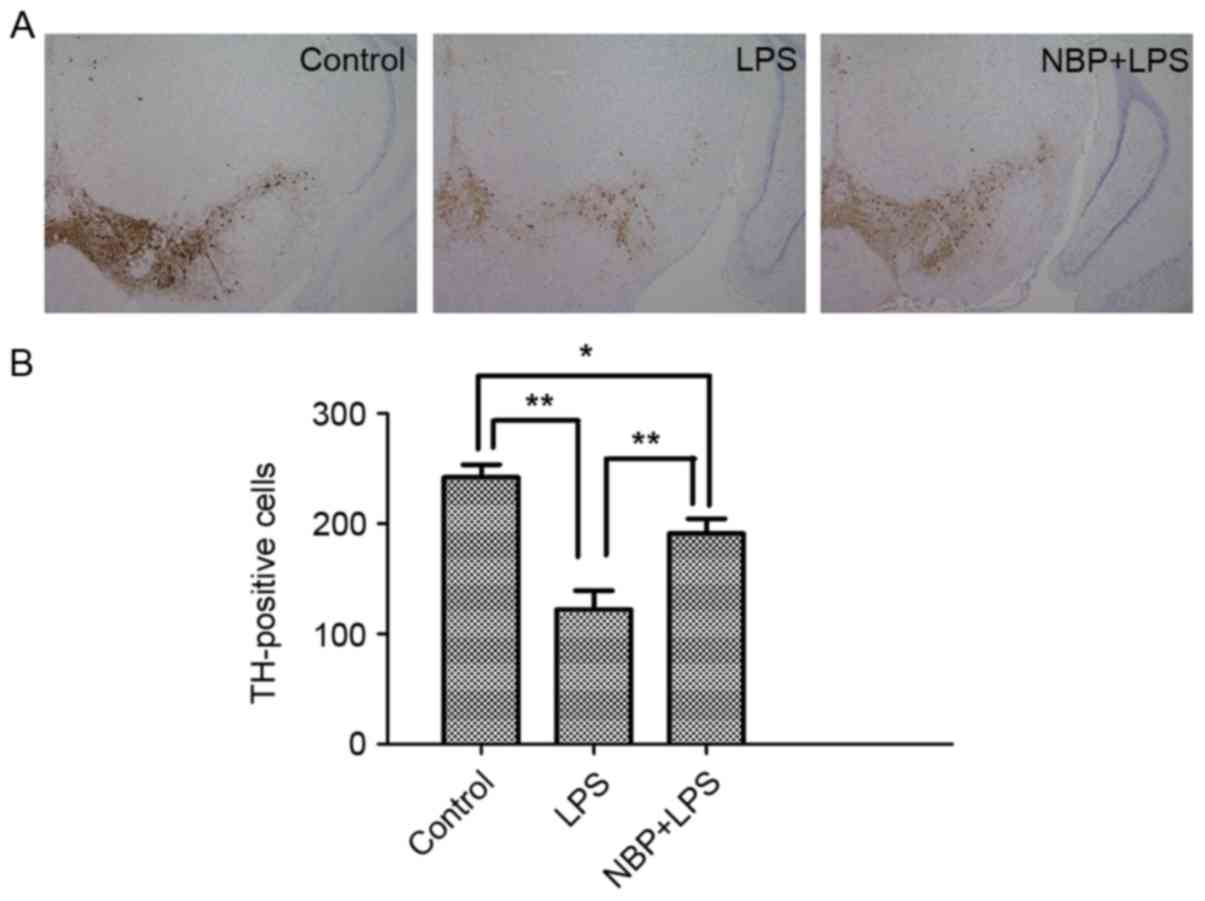
TH-positive cells are an important pathological
indicator for assessing whether a drug exhibits protective effects
on dopaminergic neurons (15).
Immunoblotting was also used to analyze the number of TH-positive
cells in the SNpc (Fig. 2). In the
LPS-treatment group, the number of TH-positive cells in the SNpc
(122.33±30.29) was significantly reduced compared with the control
group (242.33±19.14; P<0.01). Compared with the LPS-treatment
group, the number of TH-positive cells in the NBP treatment group
(191.67±23.12) were significantly increased (P<0.01), indicating
that NBP could inhibit the loss of TH.
NBP inhibits LPS-induced inflammatory
signaling pathways involving JNK and p38
A previous study demonstrated that the MAPK
signaling pathway serves an important role in the pathogenesis of
PD (4). To evaluate whether the
MAPK pathway is involved in the protective effect exerted by NBP,
the alterations in JNK, p38 and ERK in mice treated with LPS and
NBP were investigated.
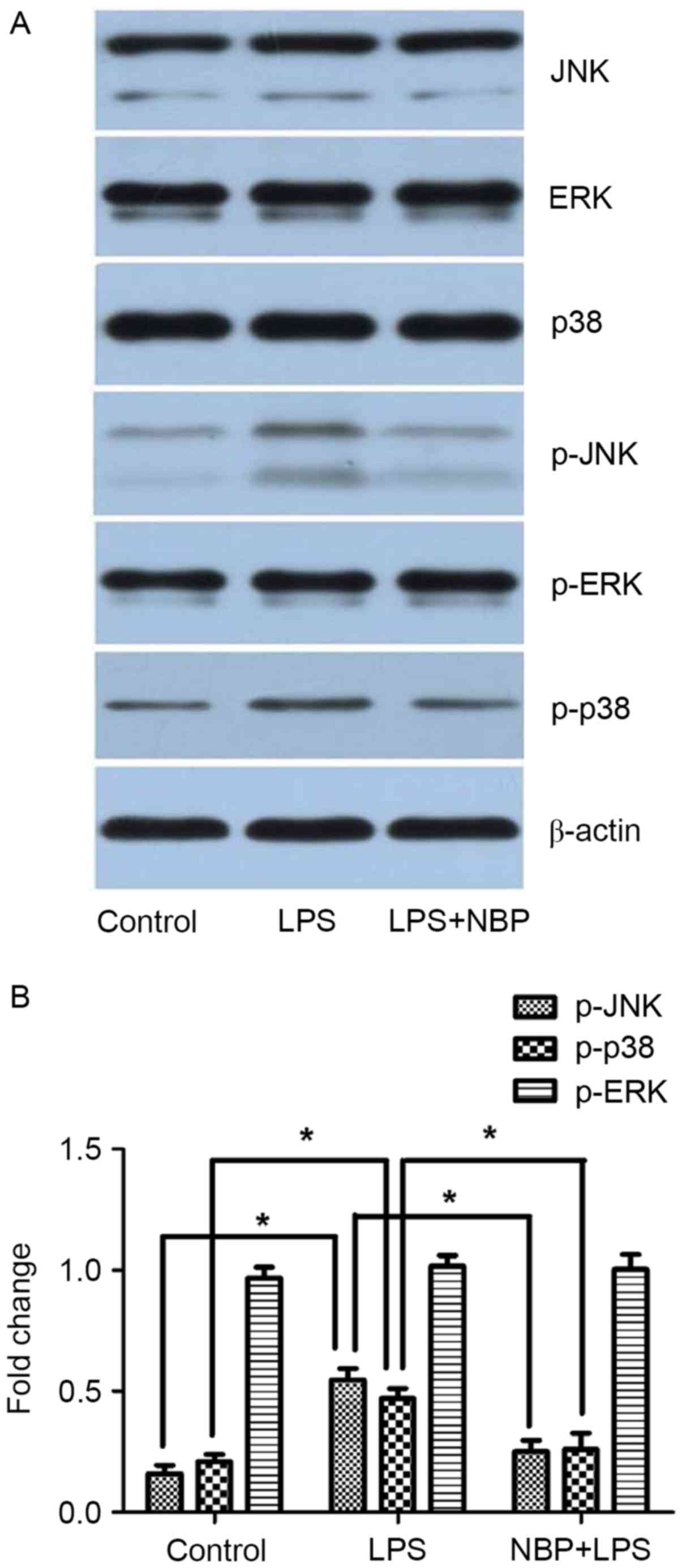
As demonstrated in Fig.
3, the levels of p-JNK, p-p38 and p-ERK in the control group
were 0.23±0.08, 0.28±0.08 and 0.99±0.24, respectively. The levels
of p-JNK, p-p38 and p-ERK in the LPS-treatment group were
0.56±0.13, 0.52±0.17, and 1.0±0.27, respectively, which indicated
phosphorylation of JNK, p38, and ERK, although no significant
difference was observed with ERK. The levels of p-JNK, p-p38 and
p-ERK in the NBP-treatment group were 0.31±0.09, 0.34±0.11, and
0.85±0.21, respectively. Compared with the LPS-treatment group,
treatment with NBP significantly inhibited phosphorylation of JNK
and p38 (P<0.05), but had little effect on ERK. Compared with
the control group, treatment with NBP had no significant effect on
the phosphorylation of JNK and p38 (P>0.05).
Therefore, these results indicated that inhibition
of the JNK and p38 pathway is involved in the protective effect of
NBP against LPS-induced neurotoxicity.
Discussion
PD is a common age-associated neurodegenerative
disorder, characterized by dopaminergic neuron loss in the SNpc. In
the later stages of human PD, there are clear signs of microglial
activation and inflammation that may contribute to the progression
of the disease (16,17).
The LPS model has been widely used in studies of the
biochemical, neurochemical and inflammatory markers, and functional
behaviors (18). It has previously
been reported that administration of LPS induced symptoms of PD,
dopaminergic neuron death and microglial activation (19,20).
NBP has been reported to possess a range of
pharmacological effects. It has been demonstrated that NBP protects
the brain from ischemia, inhibits platelet aggregation, reduces the
area of cerebral infarcts and decreases neuronal apoptosis and
oxidative brain damage (10, 21-26). Additionally, NBP has also been
demonstrated to serve a potential therapeutic role in Alzheimer's
and can decrease the cytotoxicity of MPTP in a cellular model of PD
(27).
Therefore, in the present study, a purely
inflammation-driven (LPS-induced) mouse model of PD was employed.
It was also examined whether NBP can suppress inflammatory nerve
damage induced by LPS in a mouse model and further investigated the
possible mechanisms.
Behavioral tests, including the open-field test and
the rotarod test, can be used to observe movement disorders in the
mouse. TH is the restricting enzyme in dopamine synthesis;
immunohistochemical analysis of TH allows assessment of the number
and function of dopaminergic neurons in the SN (28). Behavioral tests are crucial to
evaluate whether a drug exhibits protective effects on dopaminergic
neurons, while the number of TH-positive cells is an important
pathological indicator. In the present study, the mice treated with
LPS exhibited the basic characteristics of PD. The mice exhibited
movement disorder. The retention times of mice treated with LPS was
markedly reduced and the numbers of line crossing, rearing, and
grooming actions of mice treated with LPS were decreased. These
mice demonstrated a decrease in TH-positive cells. However, the
group that received NBP treatment demonstrated an improvement in
behavior and increase in TH-positive cells. These results suggested
that NBP markedly improved behavior performance and pathological
indicator test results in the LPS-induced mouse model of PD, but
the exact underlying mechanism remains unclear.
The MAPK superfamily comprises 3 major branches,
including JNK, p38 and ERK (29).
These subfamilies serve key roles in a variety of cellular
responses, including immunity, apoptosis, autophagy, cell survival,
cell growth and cell division (29, 30–32).
JNK is markedly activated in models of PD induced by
LPS, 6-OHDA and MPTP (33,34). A previous study demonstrated that
activation of the JNK pathway is associated with neural cell
apoptosis in models of PD (34).
The inhibitor of JNK, SR-3306, can reduce the loss of neurons in a
rat model of PD, suggesting that JNK inhibitors possess a
protective effect on neurons in PD (35). p38 is also activated in neurotoxic
PD models (36). Furthermore,
inhibitors of p38 are effective in the treatment of PD (4). ERK is involved in regulating cell
proliferation, development and apoptosis. In a cell model of PD,
chronic treatment with MPTP can decrease mitochondrial oxidative
phosphorylation in dopaminergic neural cells, and inhibiting ERK
activation by adding the inhibitor U0126 reversed the mitochondrial
morphological alterations and the loss of mitochondrial proteins
observed in cells chronically stressed with MPTP (37). A study has also revealed that the
ERK signaling pathway may be an important target for the treatment
of PD (38).
In the present study, the expression of p-JNK, p-p38
and p-ERK in mice treated with LPS and NBP was observed. The
results of the present study revealed that mice treated with NBP
demonstrated inhibited LPS-induced phosphorylation of JNK and p38,
but had no effect on ERK. These results indicated that
neuroprotection mediated by NBP involves the JNK and p38
pathways.
In conclusion, the present study demonstrated that
NBP may serve a valuable neuroprotective role, due to its ability
to regulate MAPK pathways in models of PD. These results on the
effect of NBP may lay the basis for the development of novel
clinical drugs for treating PD.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Anhui Province in 2014 (grant no.
KJ2014A163), the National Natural Science Fund of China in 2017
(grant no. 81641050), and the Postgraduate Research and Innovation
Project of Bengbu Medical College in 2015 (grant no. Byycxz1506).
The authors would like to thank Editage (Cactus Communications,
Mumbai, India) for English language editing.
Glossary
Abbreviations
Abbreviations:
|
NBP
|
DL-3-n-butylphthalide
|
|
PD
|
Parkinson's disease
|
|
TH
|
tyrosine hydroxylase
|
|
SNpc
|
substantia nigra pars compacta
|
|
MAPK
|
mitogen-activated protein kinase
|
|
LPS
|
lipopolysaccharide
|
|
MPTP
|
methyl-4-phenyl-1,2,3,6-tetrahydropyridine
|
|
6-OHDA
|
6-hy-droxydopamine
|
References
|
1
|
Pezzoli G and Zini M: Levodopa in
Parkinson's disease: From the past to the future. Expert Opin
Pharmacother. 11:627–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson JR and Hossain MM: Microglial
ion channels as potential targets for neuroprotection in
Parkinson's disease. Neural Plast. 2013:5874182013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
AlDakheel A, Kalia LV and Lang AE:
Pathogenesis-targeted, disease-modifying therapies in Parkinson
disease. Neurotherapeutics. 11:6–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang G, Pan J and Chen SD: Kinases and
kinase signaling pathways: Potential therapeutic targets in
Parkinson's disease. ProgNeurobiol. 98:207–221. 2012.
|
|
5
|
Whitton PS: Inflammation as a causative
factor in the aetiology of Parkinson's disease. Br J Pharmaco.
150:963–976. 2007. View Article : Google Scholar
|
|
6
|
Dutta G, Zhang P and Liu B: The
lipopolysaccharide Parkinson's disease animal model: Mechanistic
studies and drug discovery. Fundam Clin Pharmacol. 22:453–464.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hines DJ, Choi HB, Hines RM, Phillips AG
and MacVicar BA: Prevention of LPS-induced microglia activation,
cytokine production and sickness behavior with TLR4 receptor
interfering peptides. PLoS One. 8:e603882013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pereira MD, Ksiazek K and Menezes R:
Oxidative stress in neurodegenerative diseases and ageing. Oxid Med
Cell Longev. 2012:7963602012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imai Y, Venderova K, Park DS, Cai H and
Schmidt E: Animal models of Parkinson's disease. Parkinsons Dis.
2011:3643282011.PubMed/NCBI
|
|
10
|
Chang Q and Wang XL: Effects of chiral
3-n-butylphthalide on apoptosis induced by transient focal cerebral
ischemia in rats. Acta Pharmacol Sin. 24:796–804. 2003.PubMed/NCBI
|
|
11
|
Dong GX and Feng YP: Effects of NBP on
ATPase and anti-oxidant enzymes activities and lipid peroxidation
in transient focal cerebral ischemic rats. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 24:93–97. 2002.PubMed/NCBI
|
|
12
|
Li L, Zhang B, Tao Y, Wang Y, Wei H, Zhao
J, Huang R and Pei Z: DL-3-n-butylphthalide protects endothelial
cells against oxidative/nitrosative stress, mitochondrial damage
and subsequent cell death after oxygen glucose deprivation in
vitro. Brain Res. 1290:91–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang JZ, Chen YZ, Su M, Zheng HF, Yang
YP, Chen J and Liu CF: DL-3-n-butylphthalide prevents oxidative
damage and reduces mitochondrial dysfunction in an MPP(+)-induced
cellular model of Parkinson's disease. Neurosci Lett. 475:89–94.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian Y, Zhang Y, Zhang R, Qiao S and Fan
J: Resolvin D2 recovers neural injury by suppressing inflammatory
mediators expression in lipopolysaccharide-induced Parkinson's
disease rat model. Biochem Biophys Res Commun. 460:799–805. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W, Chen YH, Liu H and Qu HD:
Neuroprotective effects of piperine on the
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's
disease mouse model. Int J Mol Med. 36:1369–1376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cebrián C, Loike JD and Sulzer D:
Neuroinflammation in Parkinson's disease animal models: A cell
stress response or a step in neurodegeneration? Curr Top Behav
Neurosci. 22:237–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kannarkat GT, Boss JM and Tansey MG: The
role of innate and adaptive immunity in Parkinson's disease. J
Parkinsons Dis. 3:493–514. 2013.PubMed/NCBI
|
|
18
|
Tai W, Ye X, Bao X, Zhao B, Wang X and
Zhang D: Inhibition of Src tyrosine kinase activity by squamosamide
derivative FLZ attenuates neuroinflammation in both in vivo and in
vitro Parkinson's disease models. Neuropharmacology. 75:201–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou HF, Liu XY, Niu DB, Li FQ, He QH and
Wang XM: Triptolide protects dopaminergic neurons from
inflammation-mediated damage induced by lipopolysaccharide
intranigral injection. Neurobiol Dis. 18:441–449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu B and Hong JS: Role of microglia in
inflammation-mediated neurodegenerative diseases: Mechanisms and
strategies for therapeutic intervention. J Pharmacol Exp Ther.
304:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Y, Zeng X, Feng Y and Wang X:
Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in
rats. J Cardiovasc Pharmacol. 43:876–881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan CH, Feng YP and Zhang JT: Effects of
dl-3-n-butylphthalide on regional cerebral blood flow in right
middle cerebral artery occlusion rats. Zhongguo Yao Li Xue Bao.
19:117–120. 1998.PubMed/NCBI
|
|
23
|
Liu CL, Liao SJ, Zeng JS, Lin JW, Li CX,
Xie LC, Shi XG and Huang RX: DL-3n-butylphthalide prevents stroke
via improvement of cerebral microvessels in RHRSP. J Neurol Sci.
260:106–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Yu WH, Wang YX, Wang C, Zhao F,
Qi W, Chan WM, Huang Y, Wai MS, Dong J and Yew DT:
DL-3-n-butylphthalide, an anti-oxidant agent, prevents neurological
deficits and cerebral injury following stroke per functional
analysis, magnetic resonance imaging and histological assessment.
Curr Neurovasc Res. 9:167–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Li Y, Ogle M, Zhou X, Song M, Yu SP
and Wei L: DL-3-n-butylphthalide prevents neuronal cell death after
focal cerebral ischemia in mice via the JNK pathway. Brain Res.
1359:216–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan CH, Feng YP and Zhang JT: Effects of
dl-3-n-butylphthalide on regional cerebral blood flow in right
middle cerebral artery occlusion rats. Zhongguo Yao Li XueBao.
19:117–120. 1998.
|
|
27
|
Peng Y, Xing C, Lemere CA, Chen G, Wang L,
Feng Y and Wang X: L-3-n-butylphthalide ameliorates
beta-amyloid-induced neuronal toxicity in cultured neuronal cells.
Neurosci Lett. 434:224–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang XH, Lu G, Hu X, Tsang KS, Kwong WH,
Wu FX, Meng HW, Jiang S, Liu SW, Ng HK and Poon WS: Quantitative
assessment of gait and neurochemical correlation in a classical
murine model of Parkinson's disease. BMC Neurosci. 13:1422012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corrêa SA and Eales KL: The role of p38
MAPK and its substrates in neuronal plasticity and
neurodegenerative disease. J Signal Transduct. 2012:6490792012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaul T, Credle J, Haggerty T, Oaks AW,
Masliah E and Sidhu A: Region-specific tauopathy and
synucleinopathy in brain of the alpha-synuclein overexpressing
mouse model of Parkinson's disease. BMC Neurosci. 12:792011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Q, Du X, He X, Yu Q, Hu K,
Breitwieser W, Shen Q, Ma S and Li M: JNK-mediated activation of
ATF2 contributes to dopaminergic neurodegeneration in the MPTP
mouse model of Parkinson's disease. Exp Neurol. 277:296–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crocker CE, Khan S, Cameron MD, Robertson
HA, Robertson GS and Lograsso P: JNK inhibition protects dopamine
neurons and provides behavioral improvement in a rat
6-hydroxydopamine model of Parkinson's disease. ACS Chem Neurosci.
2:207–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang CJ, Lee HP, Choi DY, Jeong HS, Kim
TH, Lee TH, Kim YM, Moon DB, Park SS, Kim SY, et al: Inhibitory
effect of thiacremonone on MPTP-induced dopaminergic
neurodegeneration through inhibition of p38 activation. Oncotarget.
7:46943–46958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu JH, Gusdon AM, Cimen H, Van Houten B,
Koc E and Chu CT: Impaired mitochondrial biogenesis contributes to
depletion of functional mitochondria in chronic MPP+
toxicity: Dual roles for ERK1/2. Cell Death Dis. 3:e3122012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amiri E, Ghasemi R and Moosavi M: Agmatine
protects against 6-OHDA-induced apoptosis, and ERK and Akt/GSK
disruption in SH-SY5Y cells. Cell Mol Neurobiol. 36:829–838. 2016.
View Article : Google Scholar : PubMed/NCBI
|